Abstract
Poor ovarian response (POR) is a challenge in reproductive medicine, often leading to suboptimal outcomes in IVF/ICSI cycles. Autologous platelet-rich plasma (PRP) injections have emerged as a potential therapy to enhance ovarian function. This study aims to compare the efficacy of single versus double PRP ovarian injections in improving ovarian reserve, response to stimulation, and IVF/ICSI outcomes in women with POR. This before-and-after study was conducted at the Reproductive Center of the Sixth Affiliated Hospital of Sun Yat-sen University. Seventy-one women diagnosed with POR (POSEIDON group 3 or 4), characterized by anti-Müllerian hormone (AMH) levels below 1.2 ng/mL and fewer than five antral follicles, who had completed at least one IVF/ICSI cycle before and after PRP treatment were included. Participants received intraovarian injections of autologous PRP (2–2.5 mL per ovary) via transvaginal ultrasound guidance, either once or twice as determined clinically. We evaluated changes in ovarian reserve markers (AMH and antral follicle count [AFC]) and IVF/ICSI outcomes, such as the number of retrieved oocytes and quality embryos. Comparative analysis between single and double injections utilized the difference (Δ) between post- and pre-treatment values. PRP treatment resulted in significant improvements in AMH levels (from 0.33 ± 0.24 ng/mL to 0.43 ± 0.29 ng/mL, p = 0.005) and AFC (from 2.62 ± 1.09 to 3.80 ± 1.95, p < 0.001). Both single and double PRP injections significantly increased the number of retrieved oocytes (2.32 ± 1.80 vs. 3.59 ± 2.00, p < 0.001) and high-quality embryos (0.73 ± 1.08 vs. 1.28 ± 1.21, p = 0.002). Subgroup analysis indicated no significant differences in ΔAMH, ΔAFC, or IVF/ICSI outcomes between single and double treatments. However, the increase in AMH levels reached statistical significance only after double PRP injection, not after single injection. Autologous PRP ovarian injections significantly improve ovarian reserve parameters, the number of oocytes retrieved and high-quality embryos in women with POR. A single PRP injection is as effective as double injections, suggesting a more cost-effective and simpler protocol for clinical application.
Similar content being viewed by others
Background
Poor ovarian response (POR) presents a significant challenge in assisted reproductive technology (ART), often leading to suboptimal outcomes in ovarian stimulation, reduced oocyte yield, and compromised embryo quality1,2. Currently, there is no universally accepted treatment for POR3, and novel strategies to improve clinical outcomes for these patients are urgently needed.
Autologous platelet-rich plasma (PRP), enriched with growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF), has shown promise in regenerative medicine4. In reproductive medicine, PRP intraovarian injections have been reported to enhance ovarian reserve, improve ovarian response, and increase the number of retrieved oocytes and high-quality embryos in POR patients5,6,7.
However, Previous studies primarily focused on the effects of PRP injections, with limited data on whether repeated injections provide additional benefits. A Prospective controlled, non-randomized comparative study suggests that triple autologous PRP ovarian injections are effective and safe to improve markers of low ovarian reserve prior to ART, although further evidence is required to evaluate the impact of PRP on pregnancy outcomes8. Additionally, some researchers have questioned the necessity of repeated PRP treatments and their study suggested that a single autologous PRP ovarian injection can significantly increased AFC but did not demonstrate improvement in embryo production9,10.
Thus, this study aimed to evaluate the effects of one-time versus two-time PRP injections on ovarian reserve and in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) outcomes in POR patients, providing novel insights into optimizing PRP protocols.
Methods
Study population and design
This before-and-after study was conducted at the Reproductive Center of the Sixth Affiliated Hospital of Sun Yat-sen University from June 2022 to July 2023. The present study obtained approvals from the Ethics Committees at the Sixth Affiliated Hospital of Sun Yat-sen University (E2022233). The study was conducted in accordance with the Declaration of Helsinki. The written informed consents were waived by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University because it is a retrospective study.
A total of 71 women diagnosed with POR (POSEIDON group 3 or 4) were included. Inclusion criteria required patients to have: AMH levels < 1.2 ng/mL, fewer than five antral follicles, and Completed at least one IVF/ICSI cycle before and after PRP treatment. Exclusion criteria included chromosomal abnormalities, preimplantation genetic testing requirements, and single ovary cases. For patients with multiple pre-PRP IVF cycles, the most recent cycle was compared to the first cycle after PRP treatment.
We compared ovarian reserve, ovarian response, and embryo outcomes of IVF/ICSI cycles before and after autologous PRP ovarian injection to assess the efficacy in improving poor ovarian response. In addition, subgroup analyses were performed based on the number of PRP injections (single versus double), aiming to evaluate whether additional injections provided further benefit.
Autologous platelet-rich plasma ovarian injection
Autologous PRP was freshly prepared on the day of injection using the method detailed in previous studies11. Approximately 30 mL of peripheral blood was collected from each patient, followed by initial platelet counting. The sample underwent two sequential centrifugations to separate red blood cells and concentrate platelets, producing approximately 4–5 mL PRP solution with a mean platelet concentration of approximately (9.62 ± 1.73)×10¹¹ platelets/L.
PRP activation was achieved via mechanical oscillation by placing the PRP solution in a vortex mixing apparatus at 2800 RPM per minute for 5 min. Once activated, 2–2.5 mL PRP per ovary was injected into the ovarian stroma under transvaginal ultrasound (TVS) guidance using an 18-gauge needle. The procedure was conducted by an infertility specialist under intravenous anesthesia. Injection timing was either on the day of oocyte retrieval or between days 3 and 7 post-menstruation, based on patient preference and clinical considerations. If the patient opted for PRP treatment during the controlled ovarian stimulation process, the injection was administered at the time of oocyte retrieval. If the decision was made after oocyte retrieval, the injection was scheduled 3–7 days following menstruation.And only one autologous PRP ovarian injection was performed per menstrual cycle.
Patient assessment of ovarian reserve
Baseline antral follicle count (AFC) and follicle-stimulating hormone (FSH) levels were determined on days 2–3 of the menstrual cycle during IVF/ICSI treatment. Anti-Müllerian hormone (AMH) levels were assessed within three months prior to autologous PRP ovarian injection and again on days 2–3 of menstruation during IVF/ICSI cycles following the PRP treatment.
AMH, FSH, luteinizing hormone (LH), and estradiol (E2) levels were measured using chemiluminescent immunoassays (Cobas, Roche, Switzerland). Serum was isolated by centrifugation, and hormone assays were performed in duplicate to ensure accuracy. Inter- and intra-assay coefficients of variation were maintained within acceptable limits, with quality control samples included in each run.
Controlled ovarian stimulation protocol and embryo evaluation
A controlled ovarian stimulation protocol was tailored based on individual characteristics, such as AMH, baseline FSH, LH, E2, and AFC on days 2–3 of the menstrual cycle. Protocols included progestin-primed ovarian stimulation (PPOS), antagonist protocol, and microstimulation, with gonadotropin doses ranging from 150 to 300 IU/day. Antagonists or progesterone were administered to prevent premature LH surges. Human chorionic gonadotropin (hCG) was administered when ≥ 3 follicles reached ≥ 17 mm in diameter or ≥ 2 follicles reached ≥ 18 mm in diameter. Oocyte retrieval occurred 36–38 h post-hCG administration.
Fresh embryos were transferred primarily on day 3; however, embryos were cryopreserved under specific conditions, such as PPOS protocol, severe adenomyosis, ovarian endometriomas, advanced maternal age with only one embryo, thin endometrial lining, or elevated progesterone levels. Cleavage-stage embryos were evaluated according to Scott’s criteria12: embryos classified as grades I–II with ≥ 4 cells were deemed usable, and those with ≥ 6 cells were defined as high quality.
Outcome measures
The primary outcome was the number of retrieved oocytes. Secondary outcomes included baseline AFC, FSH, AMH levels, peak estradiol levels, number of follicles ≥ 14 mm on trigger day, number of two-pronuclear (2PN) embryos, usable cleavage embryos, and good-quality embryos. Subgroup analyses based on single or double PRP injections were performed To further investigate the impact of different treatment numbers on efficacy, we defined “Δ” as the post-treatment result minus the pre-treatment result, enabling a comparison between the effectiveness of single versus double treatments.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics (version 26.0; IBM, USA). Continuous variables were expressed as means ± standard deviations, while categorical variables were presented as counts and percentages. Paired t-tests or Wilcoxon signed-rank tests were used for pre- and post-treatment comparisons within groups. Between-group comparisons (single versus double injections) utilized independent t-tests or Mann-Whitney U tests. Statistical significance was set at a P-value < 0.05.
Result
Patient characteristics
The average age of the 71 infertile women undergoing autologous PRPvintraovarian injections was 37.94 ± 5.45 years. The mean body mass index (BMI) was 22.12 ± 2.99 kg/m², and the average duration of infertility was 4.31 ± 3.30 years, with 49.30% of cases classified as primary infertility. The infertility factors identified included isolated POR (26.76%), POR combined with tubal factors (19.72%), endometriosis (15.49%), male factor infertility (16.90%), and multiple complex factors (21.13%) (Table 1).
Evaluation of the intraovarian PRP infusion outcome
We assessed changes in ovarian reserve in POR women before and after PRP injection. Post-PRP treatment, significant increases were observed in AMH levels (0.33 ± 0.24 vs. 0.43 ± 0.29, p = 0.005) and AFC (2.62 ± 1.09 vs.3.80 ± 1.95, p < 0.001).However, baseline hormone levels, including FSH, LH, and E2, did not differ significantly (Table 2).
In terms of IVF/ICSI outcomes before and after PRP injection were also shown in Table 2. There were no significant differences in the duration of controlled ovarian stimulation or the total drug dosage. However, on the trigger day, both the peak estradiol levels (712.40 ± 496.10 vs. 1004.12 ± 697.87, p < 0.001) and the number of follicles ≥ 14 mm in diameter (2.52 ± 1.48 vs. 3.51 ± 1.78, p < 0.001) significantly increased after autologous PRP ovarian injection. Additionally, the number of oocytes retrieved (2.32 ± 1.80 vs. 3.59 ± 2.00, p < 0.001), normal fertilized zygotes (1.37 ± 1.45 vs. 2.03 ± 1.59, p = 0.007), usable cleavage embryos (1.03 ± 1.21 vs. 1.54 ± 1.23, p = 0.008), and high-quality cleavage embryos (0.73 ± 1.08 vs. 1.28 ± 1.21, p = 0.002) all showed significant increases.
Comparison of one vs. two treatments of autologous PRP ovarian injection
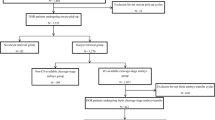
To further investigate the impact of the frequence of autologous PRP ovarian injections on outcomes for POR patients, we conducted a subgroup analysis, dividing patients into one-treatment group and two-treatment group. And the mean interval was 27.5 ± 9.5 days between the first and second PRP treatments for the 30 women in the double PRP treatment group. Figure 1 illustrates the process of autologous PRP ovarian injection treatment for different subgroups.
Our results demonstrated significant improvements in ovarian reserve for POR patients who received either one or two autologous PRP ovarian injections (Table 3). After a single PRP injection, while the increase in serum AMH levels (0.37 ± 0.28 vs. 0.42 ± 0.25, p = 0.215) did not achieve statistical significance, the number of antral follicles (2.66 ± 1.06 vs. 3.83 ± 1.96, p = 0.002) increased significantly, and basal FSH levels (12.43 ± 9.46 vs. 10.23 ± 6.14, p = 0.029) decreased significantly. In patients receiving two injections, both serum AMH levels (0.27 ± 0.18 vs. 0.44 ± 0.34, p = 0.006) and the number of antral follicles (2.57 ± 1.14 vs. 3.77 ± 1.96, p = 0.005) were significantly increased, while basal FSH levels remained unchanged (11.02 ± 5.46 vs. 10.80 ± 5.21, p = 0.862).
Regarding IVF/ICSI treatment outcomes, the peak estradiol levels (752.46 ± 571.63 vs. 926.70 ± 688.83, p = 0.046; 657.64 ± 371.47 vs. 1109.93 ± 707.85, p = 0.002), number of follicles ≥ 14 mm on the trigger day (2.59 ± 1.47 vs. 3.56 ± 2.05, p = 0.014; 2.43 ± 1.52 vs. 3.43 ± 1.36, p = 0.009), number of oocytes retrieved (2.41 ± 1.95 vs. 3.68 ± 2.18, p = 0.006; 2.20 ± 1.61 vs. 3.47 ± 1.76, p = 0.001), and number of high-quality cleavage embryos (0.78 ± 1.19 vs. 1.32 ± 1.31, p = 0.043; 0.67 ± 0.92 vs. 1.23 ± 1.07, p = 0.009) all showed significant improvement following one or two autologous PRP injections. Furthermore, there was a notable increase in the number of normal fertilized zygotes (1.27 ± 1.20 vs. 2.20 ± 1.67, p = 0.008) and usable cleavage embryos (1.03 ± 1.07 vs. 1.60 ± 1.10, p = 0.011) in patients receiving two ovarian injections of PRP (Table 3).
We further compared whether there were any differences in efficacy between single and double treatments. The changes in parameters following one treatment were compared with those following two treatments, including ΔAMH, Δantral follicle count, Δbasal FSH, Δpeak estradiol, Δnumber of follicles ≥ 14 mm, Δnumber of oocytes retrieved, Δnumber of normal fertilized zygotes, Δnumber of usable cleavage embryos, and Δnumber of high-quality cleavage embryos. No statistically significant differences were found.(Table 4).
Discussion
Summary of key findings
In this study, we evaluated the effects of autologous PRP ovarian injections on women with POR, particularly investigating whether the frequency of PRP injections influences ovarian reserve and IVF/ICSI outcomes. Our findings demonstrated significant improvements in AMH levels and AFC, accompanied by better ovarian responsiveness and enhanced IVF/ICSI outcomes, including the number of retrieved oocytes and high-quality embryos. These results highlight PRP as a promising therapeutic intervention for women with diminished ovarian reserve or POR.
Nevertheless, we were unable to reliably evaluate key pregnancy outcomes. Given the specific nature of our study population (POR patients), the number of embryos formed per retrieval cycle was relatively low and not all participants had available pregnancy data during the analysis, complicating outcome assessment. Furthermore, confounding factors arose due to simultaneous embryo transfers from different cycles—a challenge inherent to the before-and-after study design. After excluding confounders, the already limited sample size (n = 71) became smaller, further restricting the robustness of our conclusions regarding pregnancy outcomes. Future studies will specifically address the effects of autologous PRP ovarian injections on pregnancy outcomes using more robust study designs.
Mechanistic insights into PRP action
Although the precise mechanisms by which PRP enhances ovarian function remain unclear, its key components are well-documented. PRP contains an array of growth factors, including PDGF, TGF-β, and VEGF, which collectively promote tissue regeneration, angiogenesis, and cellular proliferation4. Additionally, PRP exhibits anti-inflammatory properties that create a favorable ovarian microenvironment, potentially enhancing follicular development and improving oocyte quality13,14,15,16. These mechanisms likely underlie the observed improvements in ovarian reserve and IVF/ICSI outcomes. Furthermore, PRP has been shown to activate dormant primordial follicles, expand the pool of ovulatory follicles, and improve ovarian cell proliferation, all of which are critical for optimal ovarian function17. This aligns with prior studies suggesting that PRP may rejuvenate ovarian tissue and enhance its regenerative capacity5,6,7.
Comparison with previous studies
Our findings are consistent with previous studies reporting the efficacy of PRP in improving ovarian reserve parameters and the number of oocytes retrieved and high-quality embryos in women with POR. For instance, prior research has demonstrated significant increases in AMH levels, AFC, and clinical pregnancy rates following PRP treatment in poor responders8,18. Reports of successful live births further underscore the potential of PRP as an effective tool for managing infertility in women with POR7. A systematic review and meta-analysis have also highlighted improvements in ovarian response metrics and pregnancy outcomes, reinforcing the growing interest in PRP as an innovative therapeutic approach in reproductive medicine19.
Innovation: impact of treatment frequency
A key innovation of this study lies in its comparative analysis of single versus double PRP injections. While previous studies have explored the effects of PRP on ovarian reserve and IVF outcomes, limited data exist on whether repeated treatments provide additional benefits20. Our results demonstrated that both single and double PRP injections significantly improved ovarian reserve and embryo quality, with no statistically significant differences in treatment efficacy between the two groups. This finding is particularly noteworthy, as it suggests that a single PRP injection may be sufficient to achieve meaningful therapeutic benefits. This has important clinical implications, potentially simplifying treatment protocols, reducing patient burden, and lowering costs, thereby enhancing the practicality and accessibility of PRP interventions.
However, a notable nuance emerged: while double PRP injections led to statistically significant increases in AMH levels, the increases observed after a single injection were not statistically significant. Furthermore, among patients receiving two PRP injections, there was a significant increase in the number of normally fertilized eggs and available cleavage-stage embryos. Patients who received a single injection, in contrast, exhibited only a non-significant trend toward improvement. Given the relatively small sample size, these findings indicate the necessity of further research to definitively determine the potential incremental benefits of multiple injections.
Our observations parallel findings from other areas of regenerative medicine, where increasing treatment frequency does not necessarily translate into better clinical outcomes. For instance, in studies of joint disorders and soft tissue repair, fewer PRP treatments have proven equally effective21,22,23. These parallels further support the notion that less intensive PRP regimens may be viable without compromising efficacy.
Influence of platelet concentration on PRP efficacy in ovarian response
Another critical variable explored in this study was platelet concentration, standardized at (9.62 ± 1.73)×10¹¹ platelets/L. The consistent efficacy observed in both single and double injection groups suggests a potential saturation effect, wherein a single injection delivered sufficient bioactive molecules to stimulate follicular recruitment and angiogenesis. While theoretically higher platelet concentrations could amplify growth factor release, further studies are required to explore varying platelet concentrations, refine PRP protocols, and tailor interventions to individual patients, thereby potentially improving treatment success rates in POR patients.
Limitations and future directions
Despite these promising results, several limitations warrant consideration. First, the relatively small sample size may limit the generalizability of our findings, and a larger cohort is needed to validate these results. Second, the retrospective nature of the study and the lack of a randomized controlled design introduce potential biases, including selection bias, which could influence the observed outcomes. Third, timing of PRP injections varied among patients—some received injections simultaneously with oocyte retrieval, others at 3–7 days post-menstruation—which could have influenced outcomes. Fourth, There may be selection bias in determining the number of treatments. Physicians decided on one or two autologous PRP ovarian injections based on clinical presentation, introducing potential selection bias. A second treatment may be chosen due to physiological ovarian cysts or scheduling difficulties for multiple follow-up visits, resulting in a one-month delay before IVF. Fifth, Our study focused more on comparing the efficacy of single versus double PRP injections, so a blank control group was not included. However, the lack of a blank control group prevented us from fully attributing observed improvements solely to PRP, as mechanical ovarian stimulation during injection could confound results. Last, our study did not assess long-term outcomes, such as live birth rates, which are critical for evaluating the ultimate success of PRP treatment.
Future studies should address these limitations by employing larger, well-designed prospective trials with randomized controlled designs. Investigating the long-term effects of PRP on live birth rates and exploring the molecular mechanisms underlying its action will provide valuable insights. Additionally, further research is needed to refine treatment protocols, including the optimal frequency, timing, and dosage of PRP injections, to maximize therapeutic benefits.
Conclusion
In conclusion, this study demonstrates that autologous PRP ovarian injections significantly enhance ovarian reserve, the number of oocytes retrieved and high-quality embryos in women with POR. Importantly, the efficacy of a single PRP injection is comparable to that of two injections, suggesting that a one-time treatment may be sufficient to achieve desired results. This finding not only simplifies treatment protocols but also reduces costs and patient burden, offering a practical and effective approach for managing infertility in poor responders. However, our findings also suggest potential incremental benefits of repeated injections on specific outcomes, such as fertilization rates and embryo availability. Given our limited sample size, further research with larger, robustly-designed trials is necessary to confirm these observations and determine the optimal PRP treatment strategy for women with diminished ovarian reserve.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- POR:
-
Poor ovarian response
- PRP:
-
Platelet-rich plasma
- AMH:
-
Anti-Müllerian hormone
- AFC:
-
Antral follicle count
- ART:
-
Assisted reproductive technology
- PDGF:
-
Platelet-derived growth factor
- TGF-β:
-
Transforming growth factor-beta
- VEGF:
-
Vascular endothelial growth factor
- TVS:
-
Transvaginal ultrasound
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- E2:
-
Estradiol
- HCG:
-
Human chorionic gonadotropin
- PPOS:
-
Progestin-primed ovarian stimulation
- 2PN:
-
Two-pronuclear
- BMI:
-
Body mass index
References
Ferraretti, A. P. et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 26 (7), 1616–1624. https://doi.org/10.1093/humrep/der092 (2011).
Busnelli, A. et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum. Reprod. 30 (2), 315–322. https://doi.org/10.1093/humrep/deu319 (2015).
Jeve, Y. B. & Bhandari, H. M. Effective treatment protocol for poor ovarian response: A systematic review and meta-analysis. J. Hum. Reprod. Sci. 9(2):70–81. https://doi.org/10.4103/0974-1208.183515 (2016).
Amable, P. R. et al. Platelet-rich plasma Preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell. Res. Ther. 4 (3), 67. https://doi.org/10.1186/scrt218 (2013).
Sills, E. S., Rickers, N. S., Li, X. & Palermo, G. D. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol. Endocrinol. 34 (9), 756–760 (2018).
Sfakianoudis, K. et al. A case series on Platelet-Rich plasma revolutionary management of poor responder patients. Gynecol. Obstet. Invest. 84 (1), 99–106 (2019).
Farimani, M., Heshmati, S., Poorolajal, J. & Bahmanzadeh, M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol. Biol. Rep. 46(2), 1611–1616. https://doi.org/10.1007/s11033-019-04609-w (2019).
Melo, P., Navarro, C., Jones, C., Coward, K. & Coleman, L. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J. Assist. Reprod. Genet. 37 (4), 855–863. https://doi.org/10.1007/s10815-020-01710-z. (2020).
Cakiroglu, Y. et al. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY). 12 (11), 10211–10222. https://doi.org/10.18632/aging.103403 (2020).
Barrenetxea, G. et al. Intraovarian platelet-rich plasma injection and IVF outcomes in patients with poor ovarian response: a double-blind randomized controlled trial. Hum. Reprod. 39(4), 760–769. (2024). https://doi.org/10.1093/humrep/deae038.
Chang, Y. et al. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltim). 98 (3), e14062. https://doi.org/10.1097/MD.0000000000014062 (2019).
Scott, L. A. & Smith, S. The successful use of pronuclear embryo transfers the day following oocyte retrieval. Hum. Reprod. 13 (4), 1003–1013 (1998).
Tuknayat, A., Thami, G. P., Bhalla, M. & Sandhu, J. K. Autologous intralesional platelet rich plasma improves melasma. Dermatol. Ther. 34 (2), e14881. https://doi.org/10.1111/dth.14881 (2021).
Tian, J. et al. Correlation of bioactive components of platelet rich plasma derived from human female adult peripheral blood and umbilical cord blood with age. Sci. Rep. 13 (1), 18428. https://doi.org/10.1038/s41598-023-45747-3 (2023).
Ozcan, P. et al. The protective effect of platelet-rich plasma administrated on ovarian function in female rats with Cy-induced ovarian damage. J. Assist. Reprod. Genet. 37 (4), 865–873. https://doi.org/10.1007/s10815-020-01689-7 (2020).
Wang, X. et al. Therapeutic roles of platelet-rich plasma to restore female reproductive and endocrine dysfunction. Front. Endocrinol. (Lausanne). 15, 1374382. https://doi.org/10.3389/fendo.2024.1374382 (2024).
Seckin, S., Ramadan, H., Mouanness, M., Kohansieh, M. & Merhi, Z. Ovarian response to intraovarian platelet-rich plasma (PRP) administration: hypotheses and potential mechanisms of action. J. Assist. Reprod. Genet. 39 (1), 37–61. https://doi.org/10.1007/s10815-021-02385-w (2022).
Hosseinisadat, R., Farsi Nejad, A. & Mohammadi, F. Intra-ovarian infusion of autologous platelet-rich plasma in women with poor ovarian reserve: A before and after study. Eur J Obstet Gynecol Reprod Biol. 280, 60–63. https://doi.org/10.1016/j.ejogrb.2022.11.001 (2023).
Vahabi Dastjerdi, M. et al. Efficacy of intra-ovarian injection of autologous platelet-rich plasma in women with poor responders: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 309 (6), 2323–2338. https://doi.org/10.1007/s00404-024-07442-0 (2024).
Li, X., Liu, H., Lin, G. & Xu, L. The effect of ovarian injection of autologous platelet rich plasma in patients with poor ovarian responder: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne). 14, 1292168. https://doi.org/10.3389/fendo.2023.1292168 (2023).
Rodriguez, J. A. Corticosteroid versus platelet-rich plasma injection in epicondylitis. Orthop. Nurs. 33(5), 257–65; quiz 266–7. https://doi.org/10.1097/NOR.0000000000000081. (2014).
Zhuang, W. et al. The varying clinical effectiveness of single, three and five intraarticular injections of platelet-rich plasma in knee osteoarthritis. J. Orthop. Surg. Res. 19 (1), 284. https://doi.org/10.1186/s13018-024-04736-6 (2024).
Moraes, V. Y., Lenza, M., Tamaoki, M. J., Faloppa, F. & Belloti, J. C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst. Rev. 2014 (4), CD010071. https://doi.org/10.1002/14651858.CD010071.pub3 (2014).
Acknowledgements
Not applicable.
Funding
This study was initiated by the researchers without financial support.
Author information
Authors and Affiliations
Contributions
QL analyzed and interpreted the patient data, and was a major contributor to the manuscript. JYG, JWL, JL, LNZ and CF collected the patients’ clinical data and contributed to the essay writing. XYL designed the study and took part in the result interpretation. All authors read and approved the final manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study obtained approvals from the Ethics Committees at the Sixth Affliated Hospital of Sun Yat-sen University (E2022233) in accordance with the Declaration of Helsinki. The written informed consents were waived by the ethics committees because it is a retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Q., Guo, Jy., Liu, Jw. et al. Comparative efficacy of single vs. double autologous platelet-rich plasma ovarian injections for improving ovarian response in poor ovarian responders. Sci Rep 15, 18292 (2025). https://doi.org/10.1038/s41598-025-02689-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02689-2