Abstract
Marine plastic pollution is increasing in the world’s ocean, with the Indian Ocean understudied compared to the Pacific and Atlantic Oceans. This study investigates plastic pollution in the Southwest Indian Ocean, focusing on a size range from large debris to microplastics (> 500 μm). Using visual surveys and manta trawling, we assessed plastic concentrations, compositions, and polymer types across 19 oceanographic campaigns. A total of 11,438 litter items were identified, with over 70% consisting of plastics. Larger plastic debris was predominantly observed near Glorieuses Islands during visual surveys, while microplastics were more prevalent offshore, collected through manta trawling. We observed a gradient of increasing plastic concentrations along the 30°/33°S latitudes, from 40°E (macroplastics: 10 items/km²; microplastics: 103 items/km²) to 65°E (macroplastics: 102 items/km²; microplastics: 105 items/km²). The majority of plastic debris consisted of hard fragments, primarily polyethylene (45.7%) and polypropylene (26.7%). Our findings provide new insights into microplastic concentrations in offshore regions, highlight the significant degradation of plastic debris, and emphasize the need for further research to identify and map the Indian Ocean’s garbage patch along these latitudes. Keys words: Indian Ocean, Marine litter, Visual survey, Manta trawling, Microplastics.
Similar content being viewed by others
Introduction
Plastic pollution is accumulating in marine and coastal ecosystems1,2,3,4,5, causing significant socio-economic impacts and threatening wildlife worldwide6,7,8. Despite ongoing negotiations for a global plastics treaty aimed at ending plastic pollution, the continued mass production of plastics and inadequate waste management systems contribute to substantial emissions into the marine environment9. Marine litter enters ecosystems through various pathways, including maritime10, aerial, and terrestrial sources1,2,11, and can travel large distances via ocean currents, meanwhile breaking down into smaller fragments. There is an urgent need to identify zones of plastic accumulation and understand their long-term fate9,10,11,12.
Floating plastics, carried by wind and ocean currents, accumulate in certain areas, forming so-called “garbage patches.” While five such patches have been identified globally: two in the Pacific (North14,15,16; South16, two in the Atlantic (North17,18; South19,20,21, and one in the Southern Indian Ocean22,23,24,25, the exact location of the latter is still debated. Some studies suggest it lies in the southwest26,27,28, while others place it in the southeast4,23,29,30 or central parts of the basin31. Numerical models predict the Indian Ocean garbage patch to be the second largest accumulation of floating plastics in the world22, as a result of countries bordering this ocean being major emitters of pollution (e.g. Southeast Asia)10,32. For example, the top 10 rivers contributing to ocean pollution are located in this ocean basin2. In these countries, waste management infrastructure is lacking, and the importation of consumable products and waste from other countries worsens the situation33. Despite this, direct observational data from this region remain limited22,23,34,35,36. Most research to date has focused on beach plastic debris, with evidence pointing to Southeast Asia as a primary source5,10,37,38,39,40,41,42,43. In contrast, offshore studies are scarce, and little is known about the concentration, distribution, composition, and polymer types of floating plastic pollution in the Southwest Indian Ocean. Increasing in situ surface ocean data, including both the concentration and composition of plastic debris, is essential for developing and improving plastic debris dispersion models in the Indian Ocean, as field observations provide the foundational input needed for accurate modeling.
This study aims to fill this knowledge gap by conducting extensive in situ sampling across the Southwest Indian Ocean. Our objectives are to: (i) estimate the concentration of plastic debris across the region using visual surveys and manta trawling, (ii) characterize the composition of plastic debris in terms of shape, size class, and (iii) determine the polymer types. By understanding where plastic is most likely to accumulate in this region, targeted cleanup efforts, like large-scale removal operations or technologies designed to collect plastic from the ocean, can be more efficiently deployed. Additionally, evidence of plastic accumulation hotspots is valuable for identifying the sources of pollution and informing upstream reduction strategies.
Methods
Study site
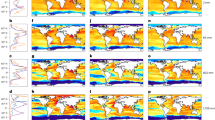
This research utilizes data collected during 19 oceanographic campaigns that have been conducted in the Southwest Indian Ocean (SWIO, 4°/40°S − 38°/82°E, Fig. 1) between 2019 and 2023. During these opportunistic campaigns, data on the concentration of plastic debris at the ocean surface was collected using visual sea surface surveys and manta trawls in offshore sites (> 20 km from the coast) and near to remote islands (< 20 km). Campaign details, such as date, location, type of vessel, are listed in Supplementary Table S1 and Figures S1, S2.
Map of the Southwest Indian Ocean showing the different field trips for plastic collection at sea and on beaches. Circles correspond to manta trawl samples (N = 204). The blue line corresponds to visual surveys (N = 1,884 h effort). All information about oceanographic campaigns and programs are included in the Supplementary information Tables S1 and Figure S1.
Visual sea surface surveys
Before each opportunistic offshore campaign, we conducted training sessions for each observer on board the vessel, focusing on the identification of floating plastic debris at the sea surface. Observations were made during daylight hours while the vessel was moving at constant speed34,44,45,46,47. At least two observers were located on the bridge to allow a visual survey area of 180 degrees. Observers used both naked eye and binoculars to make observations. There was no standard survey duration or distance, but observers recorded start/end GPS coordinates for the starting and ending points of the survey, duration of the survey, vessel speed, platform height, number of observers, and environmental parameters (sea state and cloud coverage). During these surveys, observers counted plastic debris larger than 2.5 cm up to 1 m (referred to as macroplastics48 and those exceeding 1 m (referred to as megaplastics48 along a 10 m wide transect. For each observation, observers noted : number of items, general type of items according to protocols48,49 (e.g., clothing, rubber, ceramic, fishing gear, health materials, metal, paper, glass, wood, hard plastic, soft plastic, various and other) and sub category for each type (i.e. : soft plastic : polystyrene, more details on the Supplementary Table S2).
The plastic debris concentration was calculated with:
Where Ci is the concentration plastic debris observed (item.km−2), Cs corresponds to the raw concentration of plastic debris, t is the sampling duration (h), v is the vessel speed (km.h−1) and 0.01 is the individual transect width in km14.
Manta trawl sampling
On board, the manta net was deployed at > 30 m behind the vessel to avoid the vessel’s wake to collected plastic debris at the sea surface15 (all information about dimensions of nets and flowmeters are given in Supplementary Table S1). At each site, three consecutive 30-minute transects were conducted at a speed of 2 knots using an individual single-use cod-end for each transect15,50. Between transects, the net was rinsed with seawater on the outside of the net to move any missed plastic debris towards the cod-end. The cod-end was then removed, placed into an annotated Ziplock bag (date, campaign, cod-end identification number), and stored in a freezer until transferred for analysis in the laboratory ashore. For the next deployment, a new code-end was placed. For each manta trawling, the following environmental parameters were recorded: wave height (m), wind speed (m.s−1), atmospheric pressure (hPa), year, month, season (Dec-Feb: Wet season; Mar-May: Interseason 1; Jun-Aug: Dry season; Sept-Nov: Interseason 2), surface area of sampling (km−2, flowmeters, gps coordinates) and vessels characteristics. The manta net was not deployed if wave height exceeded 2 m. Once at the laboratory, each manta trawl cod-end was externally rinsed to deposit all the content on a sieve with 500 μm. Debris smaller than 500 μm was not included in the study to remove any potential contamination (e.g. microfibers).
Under light and a magnifying glass, all plastic debris were collected with ultra-fine tweezers (300 μm point diameter) and placed in a Petri dish until analysis and characterization. When all plastic debris were placed on the Petri dish, an image was taken with a camera and used to count the number of items (Nikon D7500 - lens: AF-S MICRO NIKKOR 105 mm). Then we determined for each item: (i) the shape (hard plastic: fragment, film; foam; pellet; line; non-synthetic); (ii) the dry weight (10−5 g precision balance); (iii) the size class (SC1: 0.05–0.15 cm, SC2: 0.15–0.5 cm, SC3: 0.5–1.5 cm, SC4: 1.5–5 cm, ImageJ software 1.5 K51. The concentration of plastic debris was calculated by incorporating the effect of wind mixing into the calculation of the concentration of plastic debris at the sea surface52:
Where Ci is the depth-integrated concentration for the upper 5 m of the water column (item/km−2), Cs corresponds to the raw concentration of plastic debris type and size class as measured in the laboratory linked with the sampling surface area (km−2), d is the depth of the manta net, Wb is the rising velocity by plastic type and size (m.s−1 determined by Lebreton et al.15, ρa is the air density (kg.m−3), ρw is the seawater density (kg.m−3), Cd is the drag coefficient (0.0012), U is the sea surface wind speed during sampling (m.s−1), k is the Karman constant (0.4), g is the gravitational constant (9.81 m.s−2) and σ is the wave age equal to the constant 35.
Infrared spectroscopy ATR-FTIR
Among all items collected by manta sampling, we determined the polymer type for 1,176 items using Fourier Transform InfraRed (FTIR) spectroscopy at the UMR Softmat laboratory, in University Paul Sabatier II, France. This was done with a Thermo Nicolet Nexus 6700 instrument equipped with a diamond crystal Attenuated Total Reflection (ATR) mode and a deuterated triglycine sulphate detector. During the analysis, white background and sample spectra were obtained using 16 scans covering the wavelength range of 400 cm⁻¹ to 4,000 cm⁻¹ with a resolution of 4 cm⁻¹. A white background spectrum was taken every 2 h to ensure accuracy. Each piece of plastic debris was pressed between the diamond crystal and the base. The diamond crystal was cleaned between each measurement to avoid any bias between spectra. The obtained spectra were corrected using the ATR thermo-correction method to obtain transmission-like spectra53. The final infrared spectra were observed using the Omnic software (version 9.9.0.473). Only spectra with more than 80% similarity to one of the spectra in the spectra database54 created by the laboratory SOFTMAT at the University of Toulouse Paul Sabatier, were validated. In the cases where the similarity was less than 80%, the assignment to a specific polymer type was not made to avoid identification errors.
Index oxidative carbonyl
To provide additional information on the level of degradation of plastic debris, a carbonyl index was determined for polyethylene (PE) and polypropylene (PP) polymer particles. These particles were matched with a similarity of over 80%. For the PE carbonyl index, the ratio between the integrated absorbance of the carbonyl peak in the range of 1,850 cm−1 to 1,650 cm−1 and the methylene scissoring peak in the range of 1,500 cm−1 to 1,420 cm−1 was calculated. The Specified Area Under these two bands (SAUB) provided by Almond et al.55 was calculated using Omnic software with the options band analysis tool. For the PP carbonyl index, the ratio between the peak height at 1,715 cm−1 and the area under the band in the range of 1,500 cm−1 to 1,450 cm−1 was also calculated. The area under the band and peak height were calculated by the same software using the options peak analysis tool. For each measurement, a flat baseline was applied using the data between 4,000 cm−1 and 2,000 cm−1.
Statistical analysis
Before each test, normality and homoscedasticity were assessed using the Shapiro and Levene tests, respectively, for each quantitative variable of item concentration. None of our data follow a normal distribution. For each method (visual sea surface surveys and manta net), the quantity of items, expressed as an integer percentage (%, Yn), was modeled based on explanatory variables. For visual observation, only the category was considered, while for the Manta net, the variables category, polymer, and size were used. These models were fitted using a generalized linear model (GLM) with a Poisson distribution.
Regarding total concentration, after visualizing our data using Q-Q plots and the Shapiro-Wilk test, we found that our data was not normally distributed (Script on the supplementary data Figure S3). Therefore, we decided to examine the influence of dependent qualitative explanatory variables (Xn) including continuous (latitude, longitude) and categorical (season, platform height [m: 0–3, 3–6, 6–9, 9–12], cloud coverage [%: 0, 25, 50, 75, 100], and sea state), on the quantitative variable ‘abundance of plastic debris’ observed from visual survey effort using a generalized additive model (GAM) with a Poisson family. A GAM with a Poisson family was also used to explain the concentration of plastic debris collected from manta trawl sampling based on the following explanatory variables: latitude, longitude seasons (Dec-Feb: Wet; Mar-May: Interseason1; Jun-Aug: Dry; Sep-Nov: Interseason2) and distance (offshore, onshore).
For each GAM, we selected models with Akaïke’s Information Criteria adjusted for small sample sizes (AICc). All statistical tests were conducted in the R computing environment (R Core Team, 2023).
Results
Overall
A total of 11,438 marine debris items were recorded, from sea surface survey (75.7%) and manta trawling (24.3%).
Visual survey composition
During the 1,884 h of sea surface survey, 8,655 pieces of marine litter were observed, of which 97% were plastic debris. Hard plastic was the most frequently recorded marine debris category in all surveys with averages of 70% (Fig. 2.A), followed by soft plastic (film, sheet), fishing gear (line, rope, bucket, drum) and foam, while glass, ceramic, and healthcare-related items were generally less frequently observed (GLM, Table S3.1). The category, “Hard Plastics” was predominantly composed of “fragment” with 64% for floating plastic debris (Fig. 2.B), followed by “PET bottles” and containers with 15% and 6% of observations respectively (GLM, Table S3.2).
Manta sampling composition
A total of 2,745 items were collected by manta trawl, among them 43% (N = 1,176) where analyzed using ATR-FTIR. A large proportion of this items, 84% (N = 985) were successfully matched to the database with a score > 80%, while 16% (N = 191) remained unidentified. Items that were successfully identified were composed of hard plastic (81.2 ± 1.51%), non-synthetic items (25.9 ± 5.99%), line (23.8 ± 1.57%), foam (18.6 ± %) and pellet (19.6 ± 1.51%, Fig. 3.A, GLM, Table S3.3). These microplastics were predominantly composed of PE (45.7%) followed by PP (26.7%) of various shapes and size classes (Fig. 3.B, Table 1, GLM, Table S3.4). The size classes SC2 (0.15–0.5 cm) has been mainly collected (Fig. 3.C, Table 1; GLM, Table S3.5). Foam-shaped plastics exhibited a diverse range of polymers. Plastic debris found floating inshore (< 12 miles, N = 220) consisted of 40% PE, 34% PP, and 1.8% (PVA, PS, PDMS), whereas offshore (> 12 miles, N = 956) debris was composed of 62% PE, and 19% PP and 1.5% (PVA, PES, PS, PA, PET). There was no significant difference in polymer composition with distance from the coastline. PE and PP carbonyl index showed no significant difference between onshore and offshore samples. Nevertheless, there was a noticeable trend indicating increased degradation of PP with distance from the coast towards offshore (Supplementary Figure S4, Table S4).
Concentration of plastic debris from macro to micro
We observed a mean (± sd) macroplastics concentration of 90.8 (± 411) items.km−2 (Fig. 4.A). The maximum concentration, reaching 4,585 items.km−2, was observed around Glorieuses Islands in the north of the Mozambique Channel. A gradient of macroplastics was also observed from the west (100 items.km−2) to the east (102 items.km−2) on the latitude 30/33°S. The average abundance at 32°S latitudes was 40 (± 869) items.km−2 (max: 237 items.km−2, 32°S and 60°E). The GAM confirmed the influence of the wet season and the interseasonal period in October, which had an influence on the increase in observed macroplastic concentration, as well as good weather conditions characterized by little or no cloud coverage. However, the higher the vessel platform height, the lower the concentration of smaller sizes of plastic debris was observed. The longitude and latitude variables also show significant relationships, with significant smooth terms (p < 2e-16, n = 178). The model explains 65.9% of the deviance, with an adjusted R² of 0.72, indicating a good fit of the model to the data. (Figure S5.1)
The concentration of plastic debris by abundance recorded from (A) manta trawl sampling (size class: 500 μm − 5 mm) and (B) visual surveys (size class: 2.5–100 cm). *The South Indian Subtropical Gyre boundary is adapted from Lebreton2.
Regarding microplastics, the highest concentration was collected on the northwest of Reunion Island from the IOTA campaigns with 107 items.km−2. The lowest was recorded around Aldabra with 100 items.km−2 (Fig. 4.B). Overall, the microplastic concentration exhibits a noticeable increasing longitudinal gradient along latitude 30°/33°S, ranging from 103 items.km−2 at 40°E to 105 items.km−2 at 65°E. The GAM revealed significant effects of several factors on the variable microplastics concentration. The seasonal effects indicate that both the interseason 1 and interseason 2 periods, as well as the wet season, were associated with a significant decrease in microplastics concentration, as well as the distance from the shore (p < 2e-16, n = 206, ScriptR results from GAM test, Supplementary Figure S5.2). The smooth terms for longitude and latitude were highly significant, indicating that both geographical coordinates are important predictors of macroplastic concentration (p < 2e-16, n = 206 for ScriptR results from GAM test, Supplementary Figure S5.2). The model explained 46.3% of the deviance, with an adjusted R² of 0.416, suggesting a reasonable fit to the data.
Discussion
The offshore concentration gradient
Our study confirms the presence of sea surface plastic pollution across the Southwest Indian Ocean. We observed a gradient of increasing plastic concentrations along the 30°/33°S latitudes, from 40°E (macroplastics: 10 items.km−²; microplastics: 103 items.km−2) to 65°E (macroplastics: 102 items.km−²; microplastics: 105 items.km−²). In this region, the macroplastic concentration observed at the 65°E is in the same order of magnitude as previous research34. In the North Pacific15, macroplastics (> 5 cm) concentrations range from 10 to 103 items.km−2. In other oceans, data is reported for macroplastic concentrations22,28,56, but the classification of size categories is inconsistent or not standardized. Most authors adopt a threshold of 0.5 cm, considering anything larger than microplastics as macroplastics, while others use > 2.5 cm or > 5 cm. Although macroplastics are a major source of secondary microplastics by degradation, their monitoring in the ocean remains limited to mostly visual observations that may underestimate concentrations57,while larger net device may be more appropriate to study their distribution yet at this stage only data from the North Pacific was reported using such methods25.
Microplastics surface concentrations observed in the Indian Ocean are higher than those observed in the South Atlantic (103 items.km−2)21 or in the South Pacific (104 items.km−2)16, in similar orders of magnitude of maximum concentrations reported in the North Atlantic (105 items.km−2)58 but lower than those in the North Pacific (> 106 items.km−2)58. Regional studies in the Southern Indian Ocean22,23,34 and Bay of Bengal59 have reported concentrations ranging from 102 to 106 items.km−², however, differences in sampling methods and mesh sizes (ranging from 0.02 to 0.09 cm) make comparisons challenging, highlighting the value of regional scientific expeditions for gaining robust information on this issue. As we identified a concentration gradient between 30° and 33°S, further field work is needed to continue sampling along this gradient further east and accurately delineate the accumulation area.
Inshore concentrations
High concentrations of macroplastics (103 items.km−2), primarily composed of fragmented hard plastics, followed by caps, water bottles, and flip-flops, were observed in the northern Mozambique Channel, particularly around the Glorieuses Islands, while microplastics were more prevalent farther offshore. Studies identified water bottles as the predominant sources of pollution close to urbanized areas (e.g., Mayotte60, Seychelles10,37, Madagascar43, Reunion Island42, while flip-flop and fishing-related debris were more prevalent in more remote locations (e.g., Saint Brandon38, Tromelin, Juan de Nova61, Glorieuses, Europa, Sainte-Marie43. However, by modeling sea currents and identifying sources of plastic debris beached on remote islands by the Brand Audit method in the region62, studies have identified Southeast Asia as a major origin of plastic bottles32,37,43,63,64. These macroplastics are likely driven across the Indian Ocean by the South Equatorial Current65, and can degrade into microplastics through exposure to sunlight and wind. They may then accumulate in mesogyre, such as those in the Mozambique Channel, or be trapped by mesoscale anticyclonic eddies generated by island mass effects66,67, like the one northwest of Reunion Island, potentially explaining the extremely high microplastics concentrations we observed 107 items.km−². The threat of plastic pollution is not limited to the ocean surface, it can also travel long distances and wash up on remote coastal regions with unique ecosystems that are considered biodiversity hotspots61,68.
Consequences of this plastic pollution in the region
Depending on the size of plastic debris (from macro to microplastics) and their location (inshore or offshore), they can impact biodiversity in various ways. In our study region, the Chagos Archipelago69, Reunion Island70, Mayotte70, or the Eparses Islands71 are all defined as hotspots of biodiversity, with the hosting of marine megafauna like sea turtles72, seabirds73, and cetaceans74,75. Previous studies in the region have reported entanglement of post hatchling sea turtles on nesting beaches during emergence76,77,69, as well as ingestion by sea turtles43,78 and seabirds79 in pelagic environment. A study on Loggerhead sea turtle by-catch in the south west Indian ocean, indicated that ingested plastic debris was primarily composed of plastic bottle caps43 that were likely originating from Southeast Asia. This finding agrees with visual surveys of floating macroplastics reported here. Two other studies have reported high ingestion risk for seabirds, specifically petrels, around latitudes 30/33° S in the Indian Ocean80,81. Adding to risks of ingestion, plastics can adsorb toxic substances82, as well as pathogenic bacteria42, which can cause diseases in tropical ecosystems83,84 such as those found in the Southwest Indian Ocean.
Limitation and perspective
From 198886 to 2024, studies in the Indian Ocean have investigated floating plastic debris, using either visual surveys of macroplastics or in situ sampling of microplastics, with various collection protocols and size classification methods64. In our study, we collaborated with research groups from multiple countries or regions (Madagascar, Seychelles, Reunion Island, Eparses Islands) and organizations, aiming to harmonize protocols and size classes as much as possible. Naturally, some differences remained, such as the number of observers on board, the size of the nets used, and the sampling periods (e.g., during or outside the rainy season). All relevant details are provided in the supplementary data. For future research, it would be essential to establish a standardized nomenclature and clearly define size classes, and make raw data files openly available48,62. Concerning the concentrations observed around remote islands (e.g., the Éparses Islands), it would be valuable to conduct seasonal monitoring of beached marine litter and determine its origin. Similarly, at latitudes 30°−33° South, further research is needed to define the extent of the Indian Ocean plastic patch and to precisely locate it, particularly to assess whether higher concentrations may be found further East. A large quantity of water bottle caps was identified during our surveys, it is difficult to know whether these come from bottles that were originally discarded on land or at sea, from shipping or fishing vessels. As it could well be a result of both, it is therefore recommended to improve access to potable water in countries neighboring the Indian Ocean to reduce the consumption and discard of bottled water, as well as enforcing stricter controls of waste management aboard vessels crossing the region. These actions could be integrated into the United Nations international treaty on plastic pollution to end plastic pollution, including in the marine environment.
Conclusion
Our sampling represents the largest geographical survey and robust sampling of floating macro and microplastics in the Southwest Indian Ocean region to date. This provides valuable in situ data for improving the understanding of plastic particle distribution and the location of the floating plastic accumulation in the region. As we identified a concentration gradient at latitude 30/33°S, we suggest conducting further research toward the central and eastern parts of the Indian Ocean basin to investigate and potentially delineate the presence of plastic patch. These results offer a first step in understanding plastic dispersion in the region, helping to guide future research, cleanup efforts, and strategy development to reduce pollution before it reaches the ocean and eventually degrades into smaller particles.
Data availability
The datasets generated during the sea surface survey and manta trawling are available at Figshare in the [Raw data of plastic debris collected in the Southwest Indian Ocean by manta trawling] repository by DOI: 10.6084/m9.figshare.26828164 and [Raw data of plastic debris collected in the Southwest Indian Ocean by visual survey respectively] by DOI: 10.6084/m9.figshare.28451912.
References
Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. Science 347 (2015).
Meijer, L. J. J., van Emmerik, T., van der Ent, R., Schmidt, C. & Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 7, 1–14 (2021).
Zhang, Y. et al. Atmospheric microplastics: A review on current status and perspectives. Earth Sci. Rev. 203, 103118 (2020).
Lebreton, L. C. M., Greer, S. D. & Borrero, J. C. Numerical modelling of floating debris in the world’s oceans. Mar. Pollut Bull. 64, 653–661 (2012).
Ryan, P. G., Dilley, B. J., Ronconi, R. A. & Connan, M. Rapid increase in Asian bottles in the South Atlantic Ocean indicates major debris inputs from ships. Proc. Natl. Acad. Sci. U. S. A. 116, 20892–20897 (2019).
Beaumont, N. J. et al. Global ecological, social and economic impacts of marine plastic. Mar. Pollut Bull. 142, 189–195 (2019).
Lincoln, S. et al. Interaction of climate change and marine pollution in Southern India: implications for coastal zone management practices and policies. Sci Total Environ 902, (2023).
Egbeocha, C. O., Malek, S., Emenike, C. U. & Milow, P. Feasting on microplastics: ingestion by and effects on marine organisms. Aquat. Biol. 27, 93–106 (2018).
Syberg, K. et al. Informing the plastic treaty negotiations on science - experiences from the scientists’ coalition for an effective plastic treaty. Microplastics Nanoplastics 4, (2024).
Vogt-Vincent, N. S. et al. Sources of marine debris for Seychelles and other remote Islands in the Western Indian ocean. Mar Pollut Bull 187, (2023).
Weiss, L. Evaluation Des Apports Fluviaux De Microplastiques Et Modélisation De Leur Dispersion En Mer Méditerranée (Université de Perpignan, 2021).
Lebreton, L. et al. Seven years into the North Pacific garbage patch: legacy plastic fragments rising disproportionally faster than larger floating objects. Under Rev (2024).
Ryan, P. G., Moore, C. J., Van Franeker, J. A. & Moloney, C. L. Monitoring the abundance of plastic debris in the marine environment. 1999–2012 (2012). https://doi.org/10.1098/rstb.2008.0207
Egger, M., Sulu-Gambari, F. & Lebreton, L. First evidence of plastic fallout from the North Pacific garbage patch. Sci. Rep. 10, 7495 (2020).
Lebreton, L. et al. Evidence that the great Pacific garbage patch is rapidly accumulating plastic. Sci. Rep. 8, 1–15 (2018).
Eriksen, M. et al. Plastic pollution in the South Pacific subtropical Gyre. Mar. Pollut Bull. 68, 71–76 (2013).
Debroas, D., Mone, A. & Ter Halle, A. Plastics in the North Atlantic garbage patch: A boat-microbe for hitchhikers and plastic degraders. Sci. Total Environ. 599–600, 1222–1232 (2017).
Pham, C. K. et al. Plastic ingestion in oceanic-stage loggerhead sea turtles (Caretta caretta) off the North Atlantic subtropical Gyre. Mar. Pollut Bull. https://doi.org/10.1016/j.marpolbul.2017.06.008 (2017).
Ryan, P. G. Litter survey detects the South Atlantic ‘garbage patch’. Mar. Pollut Bull. 79, 220–224 (2014).
Kanhai, L. D. K., Officer, R., Lyashevska, O., Thompson, R. C. & O’Connor, I. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic ocean. Mar. Pollut Bull. 115, 307–314 (2017).
Suaria, G., Cappa, P., Perold, V., Aliani, S. & Ryan, P. G. Abundance and composition of small floating plastics in the Eastern and Southern sectors of the Atlantic ocean. Mar. Pollut Bull. 193, 115109 (2023).
Cozar, A. et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. 111, 10239–10244 (2014).
Eriksen, M. et al. Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One 9, (2014).
Pearce, A., Jackson, G. & Cresswell, G. R. Marine debris pathways across the Southern Indian ocean. Deep Res. Part. II Top. Stud. Oceanogr. 166, 34–42 (2019).
Lebreton, L. The status and fate of oceanic garbage patches. Nat. Rev. Earth Environ. 3, 730–732 (2022).
Maximenko, N., Hafner, J. & Niiler, P. Pathways of marine debris derived from trajectories of lagrangian drifters. Mar. Pollut Bull. 65, 51–62 (2012).
Van Sebille, E. et al. A global inventory of small floating plastic debris. Environ Res. Lett 10, (2015).
van der Mheen, M., Pattiaratchi, C. & van Sebille, E. Role of Indian ocean dynamics on accumulation of buoyant debris. J. Geophys. Res. Ocean. 124, 2571–2590 (2019).
Maes, C. et al. A surface superconvergence pathway connecting the South Indian ocean to the subtropical South Pacific Gyre. Geophys. Res. Lett. 1915–1922. https://doi.org/10.1002/2017GL076366 (2018).
Mountford, A. Eulerian modeling of the Three - Dimensional distribution of seven popular microplastic types in the global ocean. J. Geophys. Res. Ocean. 8558–8573. https://doi.org/10.1029/2019JC015050 (2019).
Chenillat, F., Huck, T., Maes, C., Grima, N. & Blanke, B. Fate of floating plastic debris released along the Coasts in a global ocean model. Mar. Pollut Bull. 165, 112116 (2021).
van der Mheen, M. & Pattiaratchi, C. Plastic debris beaching on two remote Indian ocean Islands originates from handful of Indonesian rivers. Environ Res. Lett 19, (2024).
Borrelle, S. B. et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Sci. (80-). 1518, 1515–1518 (2020).
Connan, M. et al. The Indian ocean ‘garbage patch’: empirical evidence from floating macro-litter. Mar. Pollut Bull. 169, 112559 (2021).
Li, J., Gao, F., Zhang, D., Cao, W. & Zhao, C. Zonal distribution characteristics of microplastics in the Southern Indian ocean and the influence of ocean current. J Mar. Sci. Eng 10, (2022).
Eriksen, M. et al. A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans—Urgent solutions required. PLoS ONE vol. 18 at (2023). https://doi.org/10.1371/journal.pone.0281596
Duhec, A. V., Jeanne, R. F., Maximenko, N. & Hafner, J. Composition and potential origin of marine debris stranded in the Western Indian ocean on remote alphonse Island, Seychelles. Mar. Pollut Bull. 96, 76–86 (2015).
Bouwman, H., Evans, S. W., Cole, N., Kwet Yive, C., Kylin, H. & N. S. & The flip-or-flop boutique: marine debris on the Shores of St Brandon’s rock, an isolated tropical Atoll in the Indian ocean. Mar. Environ. Res. 114, 58–64 (2016).
Dunlop, S. W., Dunlop, B. J. & Brown, M. Plastic pollution in Paradise: daily accumulation rates of marine litter on Cousine Island, Seychelles. Mar. Pollut Bull. 151, 110803 (2020).
Mattan-Moorgawa, S., Chockalingum, J. & Appadoo, C. A first assessment of marine meso-litter and microplastics on beaches: where does Mauritius stand? Mar. Pollut Bull. 173, 112941 (2021).
Okuku, E. O. et al. Marine macro-litter composition and distribution along the Kenyan Coast: the first-ever documented study. Mar. Pollut Bull. 159, 111497 (2020).
Sabadadichetty, L. et al. Microplastics in the insular marine environment of the Southwest Indian ocean carry a Microbiome including antimicrobial resistant (AMR) bacteria: a case study from reunion Island. Preprint 198, 1–43 (2024).
Thibault, M. et al. Do loggerhead sea turtle (Caretta caretta) gut contents reflect the types, colors and sources of plastic pollution in the Southwest Indian ocean?? Mar. Pollut Bull. 194, 115343 (2023).
Matsumura, S. & Nasu, K. Distribution of floating debris in the north pacific ocean: sightinh surveyz 1986–1991. Marine Debris (1997).
Thiel, M., Hinojosa, I. & Macaya, E. Floating debris of coastal waters of SE Pacific Chile. Mar. Pollut Bull. 46, 224–231 (2003).
Pichel, W. G. et al. Marine debris collects within the North Pacific subtropical convergence zone. Mar. Pollut Bull. 54, 1207–1211 (2007).
Ryan, P. G. A simple technique for counting marine debris at sea reveals steep litter gradients between the Straits of Malacca and the Bay of Bengal. Mar. Pollut Bull. 69, 128–136 (2013).
IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA. Guidelines for the monitoring and assessment of plastic litter in the ocean. Rep. Stud. GESAMP. no 99, 138 (2019).
Barnardo, T., Marine, A., Network, W. & Litter, M. WIOMSA MARINE LITTER MONITORING PROGRAMME REPORT. (2019).
Egger, M. et al. A spatially variable scarcity of floating microplastics in the Eastern North Pacific ocean. Environ Res. Lett 15, (2020).
Schneider, F., Parsons, S., Clift, S., Stolte, A. & McManus, M. C. Collected marine litter — A growing waste challenge. Mar. Pollut Bull. 128, 162–174 (2018).
Kukulka, T., Proskurowski, G., Morét-Ferguson, S., Meyer, D. W. & Law, K. L. The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 39, 1–6 (2012).
ter Halle, A. et al. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 227, 167–174 (2017).
Nono Almeida, F. et al. Among-colony variation in plastic ingestion by Yellow-legged gulls (Larus michahellis) across the Western mediterranean basin. Mar Pollut Bull 204, (2024).
Almond, J., Sugumaar, P., Wenzel, M. N., Hill, G. & Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. E-Polymers 20, 369–381 (2020).
Gallitelli, L. & Scalici, M. Riverine macroplastic gradient along watercourses: A global overview. Front. Environ. Sci. 10, 1–13 (2022).
Jang, Y. L. et al. Ship-based visual observation underestimates plastic debris in marine surface water. Mar. Pollut Bull. 209, 117245 (2024).
Law, K. L., Morét-Ferguson, S. & Maximenko, N. A. Plastic accumulation in the North Atlantic subtropical Gyre. Sci. (80-). 329, 1185–1188 (2010).
Eriksen, M. et al. Microplastic sampling with the AVANI trawl compared to two Neuston trawls in the Bay of Bengal and South Pacific. Environ. Pollut. 232, 430–439 (2018).
Mulochau, T., Sere, M. & Lelabousse, C. Estimations des densités en macro-déchets sur les platiers et récifs frangeants de Mayotte - Impacts sur les communautés coralliennes. (2019).
Jaquemet, S. A first assessment of marine litter on a beach of an uninhabited Island in the Mozambique channel. West. Indian Ocean. J. Mar. Sci. 23, 1–7 (2024).
Barnardo, T. & Ribbink, A. Afr. Mar. Litter Monit. Man. 152 (2020).
Ryan, P. G., Weideman, E. A., Perold, V., Hofmeyr, G. & Connan, M. Message in a bottle: assessing the sources and origins of beach litter to tackle marine pollution. Environ Pollut 288, (2021).
Thibault, M. Composition, Abundance, Origin and Distribution of Plastic Pollution Accumulated in the Southern Indian Ocean Gyre (University of Reunion Island, 2024). https://theses.fr/2024LARE0001
Van der Mheen, M. Transport and accumulation of buoyant marine plastic debris in the Indian Ocean. (2020).
Chandelier, G. Etude de L ’ influence des Îles Océaniques Sur les écosystèmes hauturiers: contribution à La production Océanique et Anthropisation Du Milieu Marin Guillaume Chandelier To Cite this Version : HAL Id : tel-04925946 Étude De L ’ Influence D ’ Une Ile Océan. (University of Reunion Island, (2025).
Chandelier, G. et al. Isotopic niche partitioning of co-occurring large marine vertebrates around an Indian ocean tropical oceanic Island. Mar Environ. Res 183, (2023).
Burt, A. J. et al. The costs of removing the unsanctioned import of marine plastic litter to small Island States. Sci. Rep. 10, 1–10 (2020).
Hoare, V. et al. Spatial variation of plastic debris on important turtle nesting beaches of the remote Chagos Archipelago, Indian ocean. Mar Pollut Bull 181, (2022).
Dalleau, M. et al. Modeling the emergence of migratory corridors and foraging hot spots of the green sea turtle. Ecology and Evolution vol. 9 10317–10342 at (2019). https://doi.org/10.1002/ece3.5552
Lauret-Stepler, M. et al. Reproductive seasonality and trend of Chelonia mydas in the SW Indian Ocean: a 20 year study based on track counts. Endanger. Species Res. 3, 217–227 (2007).
Dalleau, M. Ecologie Spatiale Des Tortues Marines Dans Le Sud-Ouest De l’Océan Indien. Apport De La Géomatique Et De La Modélisation Pour La Conservation (Université de La Réunion, 2013).
Le Corre, M. et al. Tracking seabirds to identify potential marine protected areas in the tropical Western Indian ocean. Biol. Conserv. 156, 83–93 (2012).
Webber, T. et al. Cetaceans of the Saya de Malha bank region, Indian Ocean: A candidate important marine mammal area. Reg. Stud. Mar. Sci. 66, 103164 (2023).
Dulau-Drouot, V., Boucaud, V. & Rota, B. Cetacean diversity off La réunion Island (France). J. Mar. Biol. Assoc. United Kingd. 88, 1263–1272 (2008).
Botterell, Z. L. R. et al. A global assessment of microplastic abundance and characteristics on marine turtle nesting beaches Eyup Bas ay Daniela Rojas-Ca N y ˜ or Casta N i. Mar Pollut Bull 215, (2025).
Ryan, P. G. et al. Impacts of plastic ingestion on post-hatchling loggerhead turtles off South Africa. Mar. Pollut Bull. 107, 155–160 (2016).
Hoarau, L., Ainley, L., Jean, C. & Ciccione, S. Ingestion and defecation of marine debris by loggerhead sea turtles, Caretta Caretta, from by-catches in the South-West Indian ocean. Mar. Pollut Bull. 84, 90–96 (2014).
Cartraud, A. E., Le Corre, M., Turquet, J. & Tourmetz, J. Plastic ingestion in seabirds of the Western Indian ocean. Mar. Pollut Bull. https://doi.org/10.1016/j.marpolbul.2019.01.065 (2019).
Clark, B. L. et al. Global assessment of marine plastic exposure risk for oceanic birds. Nat. Commun. 14, 3665 (2023).
Thibault, M. et al. Barau’s petrel, Pterodroma Baraui, as a bioindicator of plastic pollution in the South-West Indian Ocean: A multifaceted approach. Mar Environ. Res 202, (2024).
Teuten, E. L. et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R Soc. B Biol. Sci. 364, 2027–2045 (2009).
Lamb, J. B. et al. Plastic waste associated with disease on coral reefs. Sci. (80-). 359, 460–462 (2018).
Koh, J. et al. Sediment-driven plastisphere community assembly on plastic debris in tropical coastal and marine environments. Environ. Int. 179, 108153 (2023).
Ryan, P. G. The characteristics and distribution of plastic particles at the Sea-surface off the Southwestern cape Province, South Africa. Mar. Environ. Res. 25, 249–273 (1988).
Gove, J. M. et al. Prey-size plastics are invading larval fish nurseries. Proc. Natl. Acad. Sci. U S A. 116, 24143–24149 (2019).
Acknowledgements
We would like to express our gratitude to the donors of The Ocean Cleanup for funding the doctoral project and the Western Indian Ocean Marine Science Association for the Marine Litter Monitoring Project in Madagascar (MALIMO). We also thank the French government (DEAL) for the DEMARRE project, for their support under the 2019-2022 Convergence, Transformation Contract (CCT) and by Région Réunion. We thank the European Regional Development Fund and Region Reunion for OSIRIS campaign precisely for COMBAVA and PRIMO projects. We extend our thanks to all the volunteers and organizations who have contributed to the expeditions cruises: Madagascar, MALIMO project (Cetamada association, Alida Tihelle, Givio Mareva, Jonesse Lydivano, Antonio Fotaka, Angelico Monrose, Florian Hoarau); Reunion Island, IOTA2 supported by the Flotte Oceanographique Française, PLAST, DEMARRE projects (BESTRUN association, Christopher Graziano, Valentin Lauféron, Daniel Rasbash, Loïc Sabadadichetty, Captain Michel Guillemard of the Lys vessels from “Travaux sous Marins de l’Océan Indien”, Master students from BESTALI 2022-2023 at the University of Reunion Island, Lisa Rolland and Amanda Lejeune, Ifremer); offshore expeditions: MADCAPS project (S.A Aghullas II vessel, Exploration de Monaco, Chloé Thibault artist onboard), SIOM1 (Antsiva shooner, Captain Nicolas Tisné), Ecole Bleue Outre Mer 2022 (Marion Dufresne II, Ifremer); We are also grateful to GLOBICE association and Directive Marine South Indian Ocean (DMSOI) for their commitment to monitoring plastic observation from OSIRIS II vessel patrols and volunteers (Paul Lallement, Alain Dubois (MMCO), Emmanuelle Leroy, Michael Mwang’ombe (Watamu Marine Association), Tristan Simille (Goodbye Plastic), Ina Madler, Vincent Quinquempois, Rémi Trimouille, Lana Barteneva (MMCO), Bernard Rota, Erwan Bailby (Cetamada), and Marine Malen). Thank you very much to Lisa Weiss for her review in improving the paper.
Funding
Part of this study is funded by the donors of The Ocean Cleanup. The DEMARRE project is also supported by the French government (DEAL) under the 2019–2022 Convergence and Transformation Contract (CCT) and by Region Reunion. OSIRIS campaigns from 1 to 5 was funded by European Regional Development Fund and Region Reunion for COMBAVA project. OSIRIS campaigns from 6 to 9 was funded by European Regional Development Fund and Region Reunion for PRIMO project.
Author information
Authors and Affiliations
Contributions
M.T. and L.L. designed the study; M.T., A.F., A.R., A.S., G.F., M.C., P.M., M.A., S.J., P.J., A.B., S.J.R., M.E., A.T.H., M.L.C., L.L managed expeditions cruises or beach monitoring or directly facilitated the expeditions; M.T., A.F., A.R., G.F., V.M., M.C., T.M., S.J collected the samples; M.T., A.R., M.C., V.M., J.G., analyzed the samples at laboratory; M.T. conducted the data analyses and the calculations, and prepared figures and tables; M.T. wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.T., L.L., M.E., S.J.R. are employed by The Ocean Cleanup, a non-for-profit developing and scaling technologies to rid the oceans of plastics, headquartered in the Netherlands. A.F., A.R., A.S., G.F., M.C., P.M.,A.B., M.A., S.J., T.M., P.J., A.T.H., M.L.C declare no competing interests. All the remaining authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Thibault, M., Fajeau, A., Ramanampananjy, A. et al. Concentration gradient of plastic debris larger than 500 μm detected across the Southwest Indian ocean. Sci Rep 15, 22364 (2025). https://doi.org/10.1038/s41598-025-02893-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02893-0