Abstract
The prevalence and mortality rates of acute kidney injury (AKI) remain high, with ischemia-reperfusion (I/R) being a major cause in clinical settings. Dendritic cells (DCs) play a crucial role in inducing the infiltration of inflammatory cells into the kidneys during I/R, leading to persistent kidney damage. However, immature DCs (imDCs) maintain self-tolerance under homeostatic conditions. Therefore, targeting the immunomodulatory duality of DCs to prevent I/R-AKI is of significant importance. In this study, we found Pre-treatment of bone marrow-derived dendritic cells (BMDCs) with the EP4 receptor agonist L-902,688 induced the generation of EP4highCCR7high DC (LDC), However, there was a decrease in its maturation markers (CD80, CD86, and MHCII). Additionally, treatment with the EP4 receptor antagonist GW 627,368 resulted in reduced CCR7 expression on DCs without significantly affecting DC maturation. Furthermore, levels of pro-inflammatory cytokines decreased in the supernatant of LDCs while secretion of the anti-inflammatory cytokine IL-10 surged, indicating that LDCs possess stronger immune tolerance. Subsequent co-culturing mouse renal tubular epithelial cells TCMK-1 with LDCs did not impair TCMK-1 cells viability but rather enhanced cell migration rates. Following hypoxia-reoxygenation (H/R) treatment, TCMK-1 cells co-cultured with LDCs exhibited reduced intracellular ROS levels, improved oxidative stress response, reduced apoptosis, and preserved migratory capacity. In addition, our in vivo pharmacological intervention experiments manifested that the preemptive activation of the EP4 receptor conferred remarkable renal protection by inhibiting renal DC maturation. Collectively, our findings further investigate the involvement of PGE2-EP4 signaling in the regulation of DC immune function, emphasizing the potential benefits of targeting the PGE2-EP4-DC axis for preventing I/R-AKI.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a critical clinical condition characterized by a rapid decline in glomerular filtration rate, an increase in serum creatinine levels, decreased urine output, and the accumulation of metabolic waste and toxic compounds1. Approximately 2 million individuals succumb to AKI annually worldwide, with a 90-day mortality rate of approximately 28%, posing a significant threat to patient health2. Common complications of AKI include disturbances in acid-base equilibrium or electrolyte levels, as well as impairment of the immune system. Furthermore, AKI has the potential to advance to chronic kidney disease or end-stage renal disease3. Despite the widespread attention that AKI has garnered, effective prevention and treatment methods remain lacking4. Therefore, it is imperative to further explore therapeutic or preventive targets for AKI.
Ischemia-reperfusion injury (IRI) is the predominant etiology of AKI, frequently arising from diverse clinical scenarios such as hypovolemic shock, vascular or cardiac surgery, myocardial infarction, and kidney transplantation5. Ischemia results in inadequate oxygen delivery, leading to ATP depletion in the renal outer medulla and triggering the production of reactive oxygen species (ROS), which instigates damage and apoptosis of renal tubular epithelial cells6. Following reperfusion, renal function does not ameliorate; instead, kidney injury is exacerbated by heightened levels of ROS, inflammatory mediators, and chemokines7.
Dendritic cells (DCs) are pivotal in bridging the gap between innate and adaptive immunity, serving as a critical link in the immune response. Additionally, DCs can recruit inflammatory cells to infiltrate the kidney during I/R8, leading to sustained renal damage. Immature DCs (imDCs) recognize necrotic inflammation and pathogens in AKI through pattern recognition receptors (PRRs)9, subsequently engulfing pathogenic antigens and upregulating the expression of MHC class II, co-stimulatory molecules, and CC chemokine receptor 7 (CCR7), which triggers their maturation. At this stage, kidney DCs undergo a transition from antigen phagocytosis to antigen presentation, under the influence of CCR7 migrating to secondary lymphoid tissues rich in T cells10, driving differentiation of naive T cells into cytotoxic CD8+ T cells or naive CD4+ T cells11. However, by inhibiting DC maturation, it is possible to induce activation of CD4+ T cells towards secretion of regulatory T cells (Tregs), thereby suppressing systemic adaptive immune responses and promoting immune tolerance10,12, ultimately mitigating tubular epithelial cell apoptosis and preventing or alleviating acute kidney injury caused by I/R13.
The existing literature has documented the pivotal role of prostaglandin E2 (PGE2) in the maturation of DCs14. This lipid mediator, a metabolite of arachidonic acid via cyclooxygenase (COX), is capable of upregulating surface maturation markers on DCs and enhancing CCR7 expression through its EP4 receptor, thereby facilitating the migration of DCs to draining lymph nodes15. However, recent studies have suggested that PGE2 promotes the differentiation of mature monocyte-derived dendritic cells (moDCs) towards a Th2/Tregs response in T cells, with EP4 mediating the upregulation of CCR7, IL-10, and Treg differentiation capabilities16. Therefore, further exploration of the immunological effects of the PGE2-EP4 pathway on DCs is crucial. While PGE2 is commonly recognized as an active inflammatory mediator promoting local vasodilation and recruitment and activation of inflammatory cells during early inflammation, it also possesses the capacity to limit nonspecific inflammation by inducing IL-10 and suppressing various pro-inflammatory cytokines17. Consequently, there remains ongoing debate regarding the damaging versus protective effects of the PGE2-EP4 pathway in I/R18,19,20,21.
Therefore, this study primarily investigates the role of the PGE2-EP4 pathway in modulating the immune function of DCs in I/R-AKI. We utilized the EP4 selective agonist L-902,688 to pretreat in vitro cultured mouse bone marrow-derived DCs (hereafter referred to as LDCs), which exhibited upregulated CCR7 expression on their surface, while their co-stimulatory molecules and MHC class II expression were suppressed. Conversely, bone marrow-derived DCs exposed to the EP4 selective antagonist GW 627,368 (hereafter referred to as GWDCs) displayed downregulated CCR7 levels, yet their maturation status remained unaffected. Notably, LDCs secreted lower levels of pro-inflammatory cytokines, including IL-1β, IL-6, IL-17 A, TNF-α, and IFN-γ, while producing higher amounts of the anti-inflammatory cytokine IL-10, thus enhancing DC tolerance. Subsequently, when co-cultured with mouse renal tubular epithelial cells TCMK-1, all co-culture groups exhibited no apoptosis in TCMK-1 cells and a marked increase in migration rates observed in TCMK-1 cells co-cultured with LDCs. Finally, upon subjecting the co-cultured TCMK-1 cells to hypoxia-reoxygenation(H/R) treatment, the apoptosis rate in the LDC-co-culture-H/R group significantly decreased while maintaining a certain degree of cell migration. We observed reduced ROS generation in the LDC-co-culture-H/R group which mitigated oxidative stress following H/R and alleviated cell damage. Furthermore, our in vivo experiments showed that the preemptive activation of the EP4 receptor conferred significant renal protection by inhibiting renal DC maturation. In conclusion, our findings suggest that EP4 receptor agonists/antagonists may offer a novel direction for pharmacological intervention in IR-AKI by modulating DC immune responses.
Materials and methods
Experimental animals
Healthy male C57BL/6 mice (6–8 weeks old) of SPF-grade were procured from the Animal Experimental Center of Ningxia Medical University. All experimental procedures were conducted in accordance with ethical standards for animal research.
Isolation and culture of bone marrow-derived dendritic cells
Procedures for bone marrow-derived dendritic cells (BMDCs) were conducted in accordance with our established protocol22. Firstly, mice were administered an intraperitoneal injection of 1% sodium pentobarbital solution and sacrificed by cervical dislocation under anesthesia. Then, BMDCs were isolated from mouse femurs, seeded in 6-well plates, and cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% FBS (VivaCell, China) and 1% penicillin-streptomycin (Servicebio, China). Additionally, 10 ng/mL recombinant mouse GM-CSF (rmGM-CSF) (PeproTech, USA) and 10 ng/mL recombinant mouse IL-4 (rmIL-4) (PeproTech, USA) were added to the medium. The cells were then cultured until day 7. On day 3, half of the old medium was exchanged with fresh medium containing rmGM-CSF and rmIL-4. On day 5, various drug treatments were administered to generate distinct populations of CD11c + DCs by day 7. The specific treatment groups were as follows: (1) Immature DC (imDC): cultured with only rmGM-CSF and rmIL-4; (2) Mature DC (mDC): cultured with rmGM-CSF, rmIL-4, and 1 µg/mL lipopolysaccharide (LPS) (Sigma, Germany); (3) DC pretreated with the EP4 agonist L-902,688 (LDC) (Glpbio, USA): DCs were pretreated with the agonist for 1 h, followed by replacement with medium containing rmGM-CSF, rmIL-4, and 1 µg/mL LPS; 4)DC pretreated with the EP4 antagonist GW 627,368 (GWDC) (Glpbio, USA): DCs were pretreated with the antagonist for 1 h, followed by replacement with medium containing rmGM-CSF, rmIL-4, and 1 µg/mL LPS (Supplemental Fig. 1A).
Real-time quantitative PCR
In accordance with the manufacturer’s instructions, total RNA was extracted from different groups of DC using the Quick RNA Extraction Kit for Cells/Tissues (NCM Biotech, China). Subsequently, cDNA was synthesized using the PrimeScript™ RT Master Mix Kit (Takara, Japan). Further analysis was conducted using TB Green® Premix Ex Taq™ II (Takara, Japan) for RT-PCR. Finally, the transcription levels of key genes were analyzed utilizing the 2-ΔΔCt method. The primer sequences employed for RT-PCR are provided in Table 1.
Western blot
Protein samples from the cells were extracted and prepared using the Total Protein Extraction Kit (KeyGEN BiOTECH, China). After determining the protein concentration using the BCA method, total cellular proteins were separated using SDS-PAGE and subsequently transferred onto a PVDF membrane. The expression levels of various proteins in the cells were assessed using antibodies against EP4 (Proteintech, China), CCR7 (Abcam, UK), HIF-1α (Abcam, UK), β-actin (Proteintech, China), and GAPDH (Servicebio, China). Grayscale values of resulting bands were analyzed using ImageJ software.
Renal IRI and treatment
Experimental mice were randomly allocated into four groups: Sham, I/R, L-902,688 + I/R, and GW 627,368 + I/R. Following anesthesia with pentobarbital, a laparotomy was performed to expose the renal pedicles. Surgical procedures varied according to group specifications: (1) Sham group underwent abdominal incision without vascular occlusion; (2) I/R group received bilateral renal pedicle clamping for 35 min followed by vascular clamp release for reperfusion; (3) L-902,688 + I/R group received intraperitoneal administration of EP4 receptor agonist L-902,688 (1 µg/g) 1.5 h prior to I/R induction, followed by identical surgical procedures as the I/R group; (4) GW 627,368 + I/R group received intraperitoneal injection of EP4 receptor antagonist GW 627,368 (1 µg/g) 1.5 h prior to I/R induction, followed by identical surgical procedures as the I/R group. Renal tissue samples were collected 24 h post-reperfusion for further analysis.
Renal histological evaluation
Collected kidney specimens were sectioned and processed for hematoxylin-eosin (H&E) staining. Tubular injury severity was assessed through systematic quantification of pathological features including tubular necrosis, hydropic degeneration, hemorrhage, and tubular dilatation as follows: grade 0, none; grade 1, 1–10%; grade 2, 11–25%; grade 3, 26–45%; grade 4, 46–75%; and grade 5, 75%.
Flow cytometry
-
(1)
BMDCs: Following the centrifugation and isolation of DCs from each experimental group, the cells were washed with PBS. Then, dead cells were labeled using Fixable Viability Stain 510 (BD Biosciences, USA), followed by staining with anti-mouse antibodies (eBioscience, USA) including PE-CD11c, MHCII-FITC, CD80-APC, and CD86-PE-Cyanine5.5 at 4 °C.
-
(2)
Kidney specimens: Following tissue dissociation and erythrocyte lysis, single-cell suspensions were immunophenotyped using the following anti-mouse monoclonal antibodies (eBioscience, USA) at 4 °C: CD45- PE-cy5.5, F4/80- eflour V450, CD11c-PE, CD80- APC and MHCII- FITC.
The cell data were then acquired using the BD Celesta flow cytometer and analyzed utilizing FlowJo software.
ELISA
In accordance with the guidelines offered by the manufacturer, the concentrations of IL-1β, IL-6, IL-10, IL-17 A, TNF-α, and IFN-γ in the supernatants of DCs from diverse experimental groups were quantified by multiple ELISA kits (ABclonal, China); and the kidney injury molecule-1 (KIM-1) was detected in renal tissue lysates with an ELISA kit (MEIMIAN, China).
Cell culture
Mouse renal tubular epithelial cells TCMK-1 (Servicebio, China) were cultured in high-glucose DMEM (Servicebio, China) supplemented with 10% FBS and 1% penicillin/streptomycin in a 37℃ incubator with 5% CO2. The Cells were subcultured upon reaching more than 80% confluence.
Establishing a cellular hypoxia-reoxygenation model
A suspension of TCMK-1 cells was prepared at a concentration of 2 × 104 cells/mL and 100 µL was seeded into each well of a 96-well plate. After a 24-hour incubation period, the previous medium was substituted with varying concentrations of CoCl2 (Sigma, Germany) (200, 400, 600, 800, 1000, 1200, 1400, 1600 µM), followed by an additional incubation at 37 °C for another 24 h. The optimal hypoxic concentration was determined based on Cell Counting Kit-8 (CCK-8) (Glpbio, USA) results and HIF-1α protein expression levels measured by Western blot analysis. Following hypoxic treatment, reoxygenation was carried out using high-glucose DMEM at 37 °C for 2, 4, 6, 8, 10, and 12 h, the optimal reoxygenation time being determined from CCK-8 results.
Co-culture and grouping
The co-culture experimental model, as illustrated in Supplemental Fig. 1B, involved the non-contact co-culturing of TCMK-1 cells and various groups of DCs using Transwell inserts at a cell ratio of 1:4. Briefly, adherent TCMK-1 cells were seeded into a 6-well plate while pre-cultured DCs from each group were collected after 7 days and seeded into the Transwell inserts, which were then placed back into a 6-well plate. The entire co-culture setup was incubated for 24 h. Subsequently, the inserts were removed, and the adherent cells in the 6-well plate were subjected to H/R treatment as described above. The co-cultured cells are divided into the ordinary co-culture group and the H/R group after co-culture, as detailed below:
The ordinary co-culture group included: (1) Control group: TCMK-1; (2) imDC co-culture group: TCMK-1 + imDC; (3) mDC co-culture group: TCMK-1 + mDC; (4) LDC co-culture group: TCMK-1 + LDC; (5) GWDC co-culture group: TCMK-1 + GWDC.
The H/R group after co-culture included: (1) Control group: TCMK-1; (2) H/R group: TCMK-1 + H/R; (3) imDC co-culture + H/R group: TCMK-1 + imDC + H/R; (4) mDC co-culture + H/R group: TCMK-1 + mDC + H/R; (5) LDC co-culture + H/R group: TCMK-1 + LDC + H/R; (6) GWDC co-culture + H/R group: TCMK-1 + GWDC + H/R.
Cell apoptosis analysis
Cell apoptosis in different co-culture groups was assessed by staining with Hoechst 33,342 solution (Biosharp, China). Specifically, a concentration of 10 µg/mL Hoechst 33,342 was applied to the cells in a 6-well plate and incubated at room temperature for 10 min. After two PBS washes, fluorescent images were acquired using a fluorescence microscope (Olympus, Japan). Cells exhibiting blue fluorescent nuclei indicative of fragmented and/or condensed chromatin were classified as apoptotic cells. Quantitative analysis was conducted using ImageJ software.
Wound healing assay
The wound healing assay was utilized to evaluate cell migration in various co-culture groups. Following co-culture, a wound area was induced in the lower chamber cells through scraping with a 10 µL pipette tip, followed by two washes with PBS and time marking as 0 h. Subsequently, serum-free DMEM was introduced for continued culture for 24 h or CoCl2 hypoxic culture medium for H/R treatment. Microscopic images of cell migration were captured at 0 h, 24 h, or after H/R treatment. The wound area was quantified using ImageJ software.
Measurement of reactive oxygen species generation
The levels of reactive oxygen species (ROS) in the co-culture groups were assessed using a ROS detection kit (Servicebio, China). Following the manufacturer’s protocol, 1000 µL/well of DCFH-DA working solution was added to each group and incubated in the dark for 30 min. Subsequently, the working solution was aspirated, and the cells were washed twice with PBS. Fluorescent images were captured using a fluorescence microscope, and quantitative analysis was conducted utilizing ImageJ software.
Data analysis
Data analysis was performed using GraphPad Prism 9 software. The data from three independent experiments were presented as mean ± SD. The t test was employed for comparisons between two groups, while one-way analysis of variance(ANOVA) was utilized for comparisons among three or more groups (the Tukey test was adopted for equal variance, and the Dunnett test was applied for unequal variance). A p-value of < 0.05 was considered to indicate statistical significance.
Results
Identification of bone marrow-derived dendritic cells
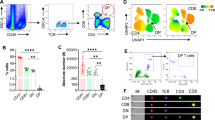
In order to facilitate the subsequent co-culture of DCs with TCMK-1 cells, we initially cultured and identified CD11c+DCs. On the seventh day of isolating and culturing BMDCs, 1 × 107 cells were collected for flow cytometry analysis. The results revealed that approximately 60% of the BMDCs cultured for seven days expressed CD11c+DC markers (Fig. 1A), with a dead cell rate of about 15%, resulting in an indirect viability rate exceeding 85% (Fig. 1B). This confirms the successful induction of CD11c+DCs.
(A) Percentage of CD11c + cells in BMDCs cultured for up to 7 days. (B) Percentage of cell death in BMDCs cultured for up to 7 days. (C–F) The impact of different concentrations of EP4 agonists/antagonists on the expression of EP4 mRNA and CCR7 mRNA in DCs. (G,H) The effect of 30 µM EP4 agonists/antagonists on the expression of EP4 and CCR7 proteins in DCs. The membrane image is acquired by excising specific sections of the same membrane to eliminate irrelevant components. Data are presented as mean ± SD. n ≥ 3. ns p > 0 0.05; *p < 0.05; **p < 0 0.01; ***p < 0 0.001.
Effects of the PGE2/EP4 signaling pathway on CCR7 levels in DCs
Compared to the imDC group, the mDC group demonstrated a significant increase in EP4 mRNA levels (p < 0.05), along with a notable elevation in CCR7 mRNA expression (p < 0.01). Furthermore, treatment with the 1 µM EP4 agonist L-902,688 resulted in a reduction of both EP4 mRNA and CCR7 mRNA levels, although this change was not statistically significant (p > 0.05). Interestingly, 30 µM agonist promoted EP4 mRNA overexpression in DCS; however, the difference was not statistically significant (p > 0.05) (Fig. 1C), but it significantly increased CCR7 mRNA expression (p < 0.01) (Fig. 1D). Additionally, as shown in Fig. 1E and F, the levels of EP4 mRNA and CCR7 mRNA exhibited a gradual decrease with increasing concentrations of the EP4 antagonist GW 627,368 when compared to the mDC group. At a concentration of 30 µM GW 627,368 demonstrated a significant ability to inhibit EP4 mRNA expression (p < 0.01) and downregulated CCR7 mRNA levels (p > 0.05). Furthermore, at the protein level, 30 µM agonist promoted elevated levels of EP4 and CCR7 expression (p < 0.05)(Fig. 1G and H). Then 30 µM GW 627,368 could markedly reduce CCR7 (p < 0.05)(Fig. 1H), while down-regulating EP4 expression (p > 0.05) (Fig. 1G). This evidence suggests that the expression of CCR7 in DCs may be modulated via the PGE2/EP4 pathway. Subsequently, we utilized 30 µM L-902,688 to stimulate EP4highCCR7high DCs (LDC) and 30 µM GW 627,368 to induce EP4lowCCR7low DCs (GWDC).
Effects of the PGE2/EP4 signaling pathway on the maturity of DCs
Upon further examination, we investigated the expression of co-stimulatory molecules on the surface of DCs across various treatment groups. Consistent with our previous research22, it was observed that compared to imDCs, mDCs exhibited markedly elevated levels of co-stimulatory molecules CD80, CD86, and MHCII following LPS stimulation (p < 0.001 or p > 0.05). Interestingly, a significant reduction in both CD80 and CD86 levels was noted in LDCs compared to mDCs (p < 0.05), indicating a lower maturity level for LDCs. Conversely, the expression levels of CD80, CD86, and MHCII in GWDCs were comparable to those in mDCs (p > 0.05), suggesting that the maturity of GWDCs did not significantly differ from that of mDCs (Figs. 2A-C).
Effects of the PGE2/EP4 signaling pathway on the secretion of inflammatory factors by DCs
The production of proinflammatory cytokines IL-1βand IL-6 in the supernatant of mDC, LDC, and GDC was significantly higher than that of imDC (p < 0.0001) (Fig. 3A and B). Nevertheless, among these four groups, the secretion of IL-1β in LDC was moderate and significantly lower than that in mDC (p < 0.001) (Fig. 3A), while the ability of LDC to secrete IL-6 was weaker than that in mDC (p < 0.05) (Fig. 3B). Next, as depicted in Fig. 3C, the expression of the proinflammatory factor TNF-α in mDC was significantly higher than that in the other three groups (p < 0.0001); however, the expression of TNF-αin LDC was significantly higher than that in imDC (p < 0.01), but significantly lower than that in GDC (p < 0.001), and the expression of TNF-α in imDC was the lowest. Nevertheless, in contrast to imDC, the expression of the pro-inflammatory factor IL-17 A in mDC was elevated (p < 0.01) and significantly higher than that in LDC (p < 0.001), while the IL-17 A content in LDC was lower than that in imDC, yet the difference was not statistically significant (Fig. 3D). Intriguingly, among the four groups, mDC had the most potent ability to secrete the proinflammatory factor IFN-γ, which was significantly higher than imDC and LDC(p < 0.05), although the IFN-γ content in LDC was higher than imDC, but the difference was not statistically significant (Fig. 3E). Finally, strikingly, as presented in Fig. 3F, the expression of the anti-inflammatory factor IL-10 in imdcs was lower than that in mDC (p < 0.05) and significantly lower than that in LDC and GDC (p < 0.01), whereas LDC had the greatest ability to secrete IL-10.
Establishment of hypoxia-reoxygenation cell model
Compared to the control group, the viability of TCMK-1 cells exhibited a modest enhancement when exposed to a CoCl2 concentration of 200 µM (p < 0.05). However, with increasing concentrations of CoCl2, there was a gradual decrease in cell viability (p < 0.0001) (Supplemental Fig. 2A). Subsequently, TCMK-1 cells were treated with CoCl2 concentrations (600–1200 µM) resulting in cell viability between 30% and 70%, to assess the potential promotion of HIF-1α expression by CoCl2 in TCMK-1 cells. Supplemental Fig. 2B demonstrates that at a CoCl2 concentration of 600 µM, there was a peak in the expression of HIF-1α protein compared to the control group (p < 0.0001). Nevertheless, as the CoCl2 concentration further increased, the expression of HIF-1α protein gradually decreased (p < 0.01 or p < 0.05). Therefore, for subsequent experiments, we utilized a concentration of 600 µM CoCl2 to induce hypoxia in TCMK-1 cells.
Then, Supplemental Fig. 2C demonstrates a significant decrease in cell viability at 2 h of reoxygenation compared to 0 h (p < 0.05). As the duration of reoxygenation increased, there was a corresponding increase in cell viability, reaching its peak at 12 h (p < 0.05). Therefore, for subsequent experiments, we employed a protocol involving the induction of TCMK-1 cells with 600 µM CoCl2 for 24 h under hypoxic conditions followed by 2 h of reoxygenation to establish the H/R model.
Effects of different DC groups on the apoptosis and migration of TCMK-1 cells
Firstly, Hoechst 33,342 staining revealed that the apoptosis rate of TCMK-1 cells co-cultured with various DC groups did not show a significant difference compared to the control group (p > 0.05) (Supplemental Fig. 3A and 3 C). Subsequently, in the wound healing assay, the migration rate of cells in the imDC co-culture group showed a slight increase compared to the control group (p > 0.05), while the migration rate in the LDC co-culture group was significantly enhanced (p < 0.05). In contrast, both mDC and GWDC co-culture groups showed slightly lower levels of healing compared to the control group (p > 0.05), but were significantly lower than those in the imDC and LDC co-culture groups (p < 0.05 or p < 0.01) (Supplemental Fig. 3B and 3D). This indicates that imDCs and LDCs promote migratory activity of TCMK-1 cells, while mDCs and GWDCs have an inhibitory effect on cell migration.
Effects of different DC groups on alleviating H/R-induced damage to TCMK-1
At the outset, the Hoechst 33,342 staining method revealed a marked increase in apoptosis rate within the H/R group compared to the Control group (p < 0.0001). In both the imDC co-culture + H/R group and LDC co-culture + H/R group, there was a notable decrease in apoptosis rate compared to the H/R group (p < 0.01). However, both the mDC co-culture + H/R group and GWDC co-culture + H/R group exhibited only a slight reduction in apoptosis rates relative to the H/R group, which did not reach statistical significance (Fig. 4A and C).
(A) Hoechst 33,342 cell staining was performed at a magnification of ×200, with a scale bar of 100 μm. (B) Images of cell scratch healing were captured at a magnification of ×40, with a scale bar of 500 μm. (C) The apoptosis rates of cells from different groups were evaluated following co-culture. (D) The migration rates of cells from different groups were assessed after co-culture. Data are presented as mean ± SD. n = 3. ns p > 0.05; *p < 0.05; **p < 0.01; ****p < 0.0001.
Subsequently, the wound healing assay demonstrated that the cell migration rate in the Control group reached approximately 25%, while the healing capacity of the H/R group was significantly impaired (p < 0.0001). Compared to the H/R group, the imDC co-culture + H/R group showed an increase in cell migration rate, albeit without statistical significance, whereas the LDC co-culture + H/R group exhibited a significant enhancement in healing (p < 0.05). In addition, in contrast to the mDC co-culture + H/R group, the cell migration rate of the LDC co-culture + H/R group was significantly elevated (p < 0.05). Interestingly, both the mDC co-culture + H/R group and GWDC co-culture + H/R group displayed a slight increase in cell migration rate compared to the H/R group. Nevertheless, this increase was not statistically significant (Fig. 4B and D).
Regulation of ROS levels in TCMK-1 by different DC groups under H/R conditions
Compared to the Control group, the relative fluorescence intensity of ROS in the H/R group exhibited a significant elevation (p < 0.0001). The ROS levels in both the imDC co-culture + H/R group and LDC co-culture + H/R group were markedly reduced compared to the H/R group (p < 0.001), with both groups demonstrating comparable abilities to downregulate ROS levels. Surprisingly, compared to the H/R group, there was also an improvement in ROS levels in both the mDC co-culture + H/R group and GWDC co-culture + H/R group (p > 0.05 or p < 0.05) (Figs. 5A-B).
(A) The impact of different groups of DCs on the relative fluorescence intensity of ROS in TCMK-1 cells following H/R exposure was observed at a magnification of ×200, with a scale bar of 200 μm. (B) Relative fluorescence intensity of ROS. Data are presented as mean ± SD. n = 3. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
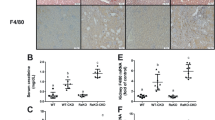
Protective role of PGE2-EP4 signaling in renal IRI
To investigate the in vivo role of the PGE2-EP4 pathway in renal IRI, we evaluated the effects of EP4 agonist/antagonist interventions using histopathological H&E staining and renal KIM-1 quantification. Compared to the Sham group, the I/R group displayed severe tubular injury (p < 0.001), characterized by tubular epithelial necrosis, nuclear pyknosis, cytoplasmic disintegration, and interstitial hemorrhage, accompanied by markedly elevated renal KIM-1 levels (p < 0.0001)(Fig. 6A-C). However, compared with the I/R group, pretreatment with the EP4 agonist L-902,688 (L-902688 + I/R group) significantly improved the renal tubular injury after I/R (p < 0.05), achieving injury scores comparable to Sham controls(p > 0.05) (Fig. 6A-C). In contrast, the GW 627,368 + I/R group exacerbated tubular injury scores and KIM-1 elevation versus Sham (p < 0.01), though slight mitigation was observed relative to the I/R group (p > 0.05) (Fig. 6A-C).These findings demonstrate that pharmacological activation of the PGE2-EP4 axis confers substantial protection against renal IRI.
Activation of PGE2-EP4 signaling pathway alleviates renal IRI. (A) H&E staining of renal pathological section. Original magnification, ×200. Scale bar, 100 μm. (B) Renal tubular injury score. (C) Kidney Kim-1 level. Data are presented as mean ± SD, n = 6. ns p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Modulation of DC maturation by PGE2-EP4 signaling in renal IRI
We subsequently investigated the regulatory effects of PGE2-EP4 signaling on DC maturation kinetics using flow cytometry. Initial analysis revealed comparable frequencies of macrophage-depleted leukocytes (CD45+F4/80−) and conventional DCs (CD45+F4/80−CD11c+) across experimental groups versus Sham controls (p > 0.05) (Supplemental Figs. 4 A-B). Notably, neither EP4 agonist L-902,688 nor antagonist GW 627,368 pretreatment altered these baseline populations compared to the I/R group (p > 0.05)(Supplemental Figs. 4 A-B).
Critical evaluation of DC maturation markers demonstrated marked elevation in CD80+ (p < 0.01) and MHCII+ (p < 0.0001) expression within the CD45+F4/80−CD11c+ population in I/R versus Sham groups (Fig. 7A,B). Pharmacological EP4 activation (L-902.688 + I/R groups) significantly attenuated I/R-induced DC maturation, reducing CD80+ (p < 0.05) and MHCII+ (p < 0.01) expression to near-baseline levels. Conversely, EP4 antagonism (GW 627,368 + I/R groups) maintained elevated CD80+/MHCII+ DC proportions comparable to the I/R group (p > 0.05) (Fig. 7A,B).
Effect of PGE2-EP4 signaling pathway on renal DCs in IRI. (A) The proportion of CD45+F4/80−CD11c+CD80+ DC in macrophage-depleted leukocytes (CD45+F4/80−). (B) The proportion of CD45+F4/80−CD11c+MHCII+ DC in macrophage-depleted leukocytes (CD45+F4/80−). Data are presented as mean ± SD, n = 6. ns p > 0.05; *p < 0.05; **p < 0.01; ****p < 0.0001.
These data establish that selective EP4 receptor activation suppresses ischemia-induced DC maturation in kidney.
Discussion
AKI seriously jeopardizes human health worldwide due to high prevalence and mortality rates and unaffordable healthcare costs23. To date, there is no alternative to dialysis to prevent or treat AKI24. Renal IRI is one of the major causes of AKI25, and its pathophysiological mechanisms are very complex, involving both innate and adaptive immunity. Many factors (e.g., renal vascular endothelial dysfunction, chemokines, damage-associated molecular patterns, hypoxia-inducible factors (HIFs), cytokines, and toll-like receptors) lead to the activation and recruitment of immune cells, especially DCs, to the damaged kidney26. While DCs can induce both tolerance and immune activation, DCs of different maturity levels will play different roles17. Our previous study found that delayed ischemic preconditioning (DIPC) could reduce the maturity of renal DCs and increase Tregs infiltration, thus attenuating the inflammatory response induced by renal IRI and protecting renal function27. However, DIPC requires early clamping of the renal hilum, a limitation that makes it difficult to be applied on a large scale in the clinic. Therefore, regulating DCs maturation by drugs could be a better strategy.
PGE2 promotes DCs maturation and migration to initiate naive T cells, as has been widely reported28,29,30. However, PGE2 is also a key inducer of the suppressor IL-1015. Recently, it was reported that although PGE2 promotes an increase in CCR7 on the surface of DCs and aids DCs migration to draining lymph nodes, it inhibits the ability of DCs to attract naive, memory, and effector T cells, enhances regulatory T-cell differentiation31, and acts predominantly through its EP4 receptor32. However, the specific regulatory immune effects of the PGE2-EP4 pathway on DCs are not known, as well as it is unclear whether the modulation of DCs immune function via the PGE2-EP4 pathway is followed by a positive effect on I/R-AKI. Here, we found that surface CCR7 was upregulated in DCs (LDC) pretreated with the EP4 receptor agonist L-902,688 receiving PGE2, but surface maturation markers CD80, CD86, and MHCII were not increased. However, EP4 antagonist GW 627,368 pretreated DCs (GWDC) surface CCR7 was down-regulated, and maturation markers were expressed to a comparable extent as mDC. In the healthy state of the kidney, DCs are often immature, constantly sensing pathogen- or inflammation-related signals and keeping peripheral T cells in a resting state26. Kidney DCs (KDCs) harvest self-antigens from renal tubules and glomeruli and subsequently migrate to renal lymph nodes, and this migratory capacity is primarily regulated by the expression of CCR733. Within these lymph nodes, KDCs maintain autoimmune tolerance, immune system function, and tissue homeostasis by inducing Tregs activation, suppressing T cell activation, proliferation, and effector capacity, and regulating autoreactive T cells34. When an inflammatory response occurs, DCs can recognize inflammation-related signals through pattern recognition receptors (PRRs), and then begin to mature and migrate toward the site of damage35. After renal IRI, dilated renal lymphatics express high levels of CCL21, which stimulates the recruitment of more CCR7+DCs to the renal draining lymph nodes, exacerbating renal inflammation and fibrosis36. Our previous study found that ischemic preconditioning protects renal function after IRI, reduces IL-17 levels, decreases renal CCR7 expression, and prevents DC maturation27. However, the use of an EP4 antagonist to reduce EP4 and CCR7 expression in vitro in this experiment did not inhibit DC maturation. Interestingly, treatment of BMDCs with EP4 agonists in vitro resulted in a decrease in DC surface maturation markers (CD80, CD86, and MHCII) despite CCR7 overexpression. These results suggest that EP4 agonists amplify the induction of CCR7 by PGE2, conferring a greater migratory capacity to DCs but decreasing DC maturation. In addition, we found in vivo that the intraperitoneal administration of the EP4 agonist 1.5 h before renal I/R surgery effectively suppressed the DC maturation induced by IRI in the kidney and preserved renal function.
This seems to be different from a previous article, which showed that PGE2-EP2/EP4 signaling enhances the production of Ccl22 and Ccl17 by stimulating mregdc (mature DC enriched with immunomodulatory molecules) and promotes Tregs infiltration37. This difference may be caused by the fact that they adopt different DC maturation markers (CD40, CCR7, and IL-12b) than we do. In the past, it was widely recognized that PGE2 is critical for DC maturation, inducing the dissolution of actin-rich attachment structures (called pedunculated plasmodesmata), which is an important step for DC maturation and acquisition of a highly migratory phenotype14. For example, the efficiency of PGE2 to promote DC migration is often considered the gold standard for facilitating DC migration in clinical trials of DC vaccines28. Live BCG vaccines are required to activate DC migration through the COX-PGE2-EP2/4 pathway38. It is through the EP4 receptor that CCR7 is upregulated to promote DC migration to draining lymph nodes16, which is consistent with our results. Although PGE2 supports DC differentiation, maturation, and migration, it inhibits their ability to attract naive, memory, and effector T cells39. Recent studies have shown that PGE2 dysfunctions cDC1 in tumors by downregulating IRF8, limiting cDC1-dependent anticancer CD8+ T cell responses31.
To investigate the effect of the PGE2-EP4 pathway on the secretion of inflammatory factors by DCs, ELISA was employed to detect the levels of inflammatory factors in the supernatant of different groups of DC. It was revealed that among the four groups of DCs, the expression of pro-inflammatory factors IL-1β, IL-6, TNF-α, and IFN-γ in the supernatant of imDC was the lowest. Nevertheless, mDC exhibited the strongest capacity to secrete pro-inflammatory factors. This is attributed to the fact that when imDCs are stimulated by the external environment, they differentiate into mDCs and migrate to the draining lymph nodes with captured antigens, secreting a large quantity of pro-inflammatory factors to induce effector T cells and promote the adaptive immune response40. We discovered that although the expression of IL-1β, IL-6, and TNF-α in LDC was higher than that in imDC, it was also significantly lower than that in mDC, while the contents of IL-17 A and IFN-γ in the supernatant of LDC were similar to those of imDC. Furthermore, LDC demonstrated the strongest ability to secrete IL-10. Then, the expression of costimulatory molecules on the surface of LDC lies between that of imDC and mDC. Hence, we contend that LDC is a sort of “semi-mature” DC, which can also be denominated as tolerogenic DC41. Tolerogenic dendritic cells are typically regarded as homeostatic semi-mature DCs with the capacity to re-establish immune tolerance. While expressing low levels of T cells and costimulatory molecules, tolerogenic markers are overexpressed and tolerogenic cytokines such as TGF-b, IL-10, and PDL-1 are released to induce Treg differentiation and further mediate the tolerogenic immune response42,43. Upon activation by LPS, imDCs differentiate into mDCs and secrete large quantities of proinflammatory cytokines IL-1β, IL-6, TNF-α, IFN-γ and IL-17 A. Although the secretion of the anti-inflammatory factor IL-10 is augmented, mDCs often exert a pro-inflammatory effect44,45. Nevertheless, activation of the PGE2-EP4 pathway prior to the addition of LPS was capable of reducing the production of proinflammatory factors and promoting the secretion of the anti-inflammatory factor IL-10, which is in accordance with reports17,46. Additionally, PGE2 is a key inducer of IL-1015. The PGE2-EP4 axis was also reported to facilitate the upregulation of CCR7, IL-10, and the differentiation capacity of Treg in human cDC2s16. It has been discovered that in the low-dose LPS-induced liver injury model, H-LF41 pretreatment can alleviate liver injury and inflammation via the PGE2-EP4 pathway and IL-1046. Consequently, we draw the conclusion that the activation of the PGE2-EP4 pathway is capable of enhancing DC tolerance, facilitating the TH2 response, and reducing cell apoptosis.
Then we co-cultured different groups of DC with TCMK-1 and found that none of the DC receiving different treatments affected TCMK-1 activity, and LDC also increased TCMK-1 migration rate. In addition, we found that LDC ameliorated H/R apoptosis and prevented further reduction of cell migration rate. This may be because LDC could reduce ROS levels in TCMK-1 cells after H/R, which attenuated H/R induced cell injury. In vitro cellular H/R is commonly used to mimic in vivo I/R, and CoCl2, which allows stable expression of HIF-1α for several hours under normoxic conditions, is one of the most commonly used hypoxic drugs47. The principle is that Co2 + replaces Fe2 + in the hemoglobin porphyrin ring, leading to cellular hypoxia48. In this study, we used different concentrations of CoCl2 to treat TCMK-1 cells to simulate a hypoxic environment. Then, based on HIF-1α abundance, we determined the optimal CoCl2 concentration. Finally, the CoCl2 hypoxic culture medium was replaced with DMEM complete medium and cultured at 37 °C in 5% CO2 for 2 h to successfully establish an in vitro H/R cell model. Previous studies have shown that different cells have different tolerances to CoCl2. The cellular activity of hippocampal neuronal cells under 150 µM CoCl2 treatment was approximately 50% of that of the control group49, whereas the CoCl2 concentration required to inhibit 50% of the cell viability of cardiomyoblasts was 800 µM50. Despite the different CoCl2 concentrations used, they all achieved the common goal of inducing cellular hypoxia.
PGE2 is a lipid mediator of the eicosanoid family of oxygenated arachidonic acids and is therefore a potent modulator of immune responses in both autocrine and paracrine ways. During inflammation, arachidonic acid (AA) is released from membrane phospholipids catalyzed by phospholipase A2 (PLA2) to produce PGE2. AAs are then oxidized by cyclooxygenase (COX) to form prostaglandin H2 (PGH2), which is then converted to PGE2 by the enzyme terminal PGE2 synthase (PGES). PGE2 is predominantly mediated through the four seven-transmembrane structural domains of the G protein-coupled receptors EP1-EP430,51 and is often considered a key mediator of fever, nociceptive sensitization, arteriolar dilatation, or inflammation52, and its role in I/R has attracted much attention in recent years. It has been reported that netrin-1 inhibits COX-2 expression, PGE2, and thromboxane production, reduces neutrophil infiltration in the kidney, and ameliorates renal IRI in mice21. PGE2 reduces the expression of proximal tubular organic anion transport proteins Oat1 and Oat3, aggravating renal injury53. However, more researchers believe that PGE2 plays a beneficial role in I/R-AKI. For example, up-regulation of monocytes and macrophages express the transcription factor (MAFB) through the COX-2/PGE2/EP4 pathway promotes the conversion of inflammatory lipid mediators to specific pro-inflammatory abrogating mediators54. Inhibition of the arginine-metabolizing enzyme arginase 2 and activation of PGE2 can effectively increase renal blood flow18. Prophylactic administration of the 15-PGDH antagonist SW033,291 significantly increased PGE2 levels in renal tissues, induced high levels of renal EP4 receptors and cAMP, and increased renal blood flow and renal small-artery area after AKI20. These studies are consistent with our results that activation of the PGE2-EP4 pathway improves I/R-AKI. We found that TCMK-1 cells co-cultured with LDC effectively ameliorated high levels of ROS induced by H/R to the extent that it promoted cell migration and reduced apoptosis after H/R. In addition, EP4 agonist pretreatment reduced the pro-inflammatory factors IL-1β, IL-6, IL-17 A, TNF-α, and IFN-γ secreted by DCs, and surged the expression of the anti-inflammatory factor IL-10, which promotes TH2 response. This may be one of the reasons for the attenuation of TCMK-1 damage brought about by H/R after co-culture.
In summary, our data suggest that activation of the PGE2-EP4 pathway using EP4 agonists upregulates CCR7, inhibits the expression of DC maturation markers CD80, CD86, and MHCII, suppresses the production of pro-inflammatory factors IL-1β, IL-6, IL-17 A, TNF-α, and IFN-γ in dendritic cells, and promotes the secretion of the anti-inflammatory factor IL-10, which biases the T cell response to favor TH2 direction of differentiation and enhance DC tolerance. The cell activity of TCMK-1 is not affected after co-culture with LDC, but instead promotes cell migration rate. After receiving H/R treatment, the LDC-co-culture-H/R group had lower ROS levels and improved oxidative stress, thus reducing apoptosis and protecting cell migration function. In addition, our in vivo pharmacological intervention experiments manifested that the preemptive activation of the EP4 receptor conferred remarkable renal protection by inhibiting renal DC maturation. This study provides a new perspective on the prevention of I/R-AKI in the direction of drug modulation of DC immune effects. However, the different sources of DC, different agonist/antagonist choices, and different drug concentrations may lead to some differences between the experimental results and others. Therefore, further exploration of the immune mechanism of PGE2-EP4-DC axis and I/R-AKI may have a profound impact on clinical application.
Data availability
The data supporting these findings are available upon request from the corresponding author.
References
Zuk, A. & Bonventre, J. V. Acute kidney injury. Annu. Rev. Med. 67, 293–307. https://doi.org/10.1146/annurev-med-050214-013407 (2016).
Zeng, C. et al. Renal-Clearable probe with water solubility and photostability for Biomarker-Activatable detection of acute kidney injuries via NIR-II fluorescence and optoacoustic imaging. ACS Appl. Mater. Interfaces. 15, 17664–17674. https://doi.org/10.1021/acsami.3c00956 (2023).
Kwiatkowska, E., Kwiatkowski, S., Dziedziejko, V., Tomasiewicz, I. & Domanski, L. Renal microcirculation injury as the main cause of ischemic acute kidney injury development. Biology 12. https://doi.org/10.3390/biology12020327 (2023).
Levey, A. S. & Defining, A. K. D. The spectrum of AKI, AKD, and CKD. Nephron 146, 302–305. https://doi.org/10.1159/000516647 (2022).
Rajendran, G. et al. Inhibition of endothelial PHD2 suppresses Post-Ischemic kidney inflammation through Hypoxia-Inducible Factor-1. J. Am. Soc. Nephrology: JASN. 31, 501–516. https://doi.org/10.1681/ASN.2019050523 (2020).
Kinsey, G. R., Li, L. & Okusa, M. D. Inflammation in acute kidney injury. Nephron. Exp. Nephrol. 109, e102–e107. https://doi.org/10.1159/000142934 (2008).
Chen, L. et al. Trans-cinnamaldehyde attenuates renal ischemia/reperfusion injury through suppressing inflammation via JNK/p38 MAPK signaling pathway. Int. Immunopharmacol. 118, 110088. https://doi.org/10.1016/j.intimp.2023.110088 (2023).
Cuenca-Escalona, J. et al. EP2/EP4 targeting prevents tumor-derived PGE2-mediated immunosuppression in cDC2s. J. Leukoc. Biol. 08, qiae213. https://doi.org/10.1093/jleuko/qiae164 (2024).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820. https://doi.org/10.1016/j.cell.2010.01.022 (2010).
Liu, J., Zhang, X., Cheng, Y. & Cao, X. Dendritic cell migration in inflammation and immunity. Cell Mol. Immunol. 18, 2461–2471. https://doi.org/10.1038/s41423-021-00726-4 (2021).
Li, N. et al. IRF8-Dependent type I conventional dendritic cells (cDC1s) control Post-Ischemic inflammation and mildly protect against Post-Ischemic acute kidney injury and disease. Front. Immunol. 12, 685559. https://doi.org/10.3389/fimmu.2021.685559 (2021).
Singh, P., Hoggatt, J., Hu, P., Speth, J. M. & Fukuda, S. Blockade of prostaglandin E2 signaling through EP1 and EP3 receptors attenuates Flt3L-dependent dendritic cell development from hematopoietic progenitor cells. Blood 119, 1671–1682. https://doi.org/10.1182/blood-2011-03-342428 (2012).
Zheng, L., Gao, W., Hu, C., Yang, C. & Rong, R. Immune cells in ischemic acute kidney injury. Curr. Protein Pept. Sci. 20, 770–776. https://doi.org/10.2174/1389203720666190507102529 (2019).
Vleeshouwers, W. et al. Characterization of the signaling modalities of prostaglandin E2 receptors EP2 and EP4 reveals crosstalk and a role for microtubules. Front. Immunol. 11, 613286. https://doi.org/10.3389/fimmu.2020.613286 (2020).
Florez-Grau, G. et al. Up-regulation of EP(2) and EP(3) receptors in human tolerogenic dendritic cells boosts the immunosuppressive activity of PGE(2). J. Leukoc. Biol. 102, 881–895. https://doi.org/10.1189/jlb.2A1216-526R (2017).
Cuenca-Escalona, J., Florez-Grau, G., van den Dries, K., Cambi, A. & de Vries, I. J. M. PGE2-EP4 signaling steers cDC2 maturation toward the induction of suppressive T-cell responses. Eur. J. Immunol. 54, e2350770. https://doi.org/10.1002/eji.202350770 (2024).
Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 188, 21–28. https://doi.org/10.4049/jimmunol.1101029 (2012).
Xiong, M. et al. Tubular Elabela-APJ axis attenuates ischemia-reperfusion induced acute kidney injury and the following AKI-CKD transition by protecting renal microcirculation. Theranostics 13, 3387–3401. https://doi.org/10.7150/thno.84308 (2023).
Pan, B. et al. Indoleamine-2,3-Dioxygenase activates Wnt/beta-Catenin inducing kidney fibrosis after acute kidney injury. Gerontology 67, 611–619. https://doi.org/10.1159/000515041 (2021).
Kim, H. J. et al. Inhibition of 15-PGDH prevents ischemic renal injury by the PGE(2)/EP(4) signaling pathway mediating vasodilation, increased renal blood flow, and increased adenosine/A(2A) receptors. Am. J. Physiol. Renal. Physiol. 319, F1054–F1066. https://doi.org/10.1152/ajprenal.00103.2020 (2020).
Ranganathan, P. V., Jayakumar, C., Mohamed, R., Dong, Z. & Ramesh, G. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int. 83, 1087–1098. https://doi.org/10.1038/ki.2012.423 (2013).
Wang, P. & Li, C. Tolerogenic CD11c(+)dendritic cells regulate CD4(+)Tregs in replacing delayed ischemic preconditioning to alleviate ischemia-reperfusion acute kidney injury. FASEB J. 38, e23575. https://doi.org/10.1096/fj.202302299RR (2024).
Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14, 607–625. https://doi.org/10.1038/s41581-018-0052-0 (2018).
Pickkers, P. et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. 47, 835–850. https://doi.org/10.1007/s00134-021-06454-7 (2021).
Pabla, N. & Bajwa, A. Role of mitochondrial therapy for Ischemic-Reperfusion injury and acute kidney injury. Nephron 146, 253–258. https://doi.org/10.1159/000520698 (2022).
Lv, D. et al. Advances in Understanding of dendritic cell in the pathogenesis of acute kidney injury. Front. Immunol. 15. https://doi.org/10.3389/fimmu.2024.1294807 (2024).
Zhang, T. et al. Delayed ischemic preconditioning attenuated renal Ischemia-Reperfusion injury by inhibiting dendritic cell maturation. Cell. Physiol. Biochemistry: Int. J. Experimental Cell. Physiol. Biochem. Pharmacol. 46, 1807–1820. https://doi.org/10.1159/000489366 (2018).
Zhang, R., Tang, L., Wang, Y., Li, Q. & Yang, L. alpha-d-Glucose-1,6-Biphosphate induces dendritic cell homing to enhance the antitumor effect of neoantigen vaccines. J. Immunol. 211, 932–943. https://doi.org/10.4049/jimmunol.2200687 (2023).
Zandvakili, R. et al. Vaccination with celecoxib-treated dendritic cells improved cellular immune responses in an animal breast cancer model. Adv. Med. Sci. 68, 157–168. https://doi.org/10.1016/j.advms.2023.03.002 (2023).
Legler, D. F., Krause, P., Scandella, E., Singer, E. & Groettrup, M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 176, 966–973. https://doi.org/10.4049/jimmunol.176.2.966 (2006).
Bayerl, F. et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 56, 1341–1358. https://doi.org/10.1016/j.immuni.2023.05.011 (2023).
Cuenca-Escalona, J., Florez-Grau, G., van den Dries, K., Cambi, A. & de Vries, I. J. M. PGE2-EP4 signaling steers cDC2 maturation toward the induction of suppressive T-cell responses. Eur. J. Immunol. 54 (3), e2350770. https://doi.org/10.1002/eji.202350770 (2023).
Wen, Y. et al. C-C motif chemokine receptor 7 exacerbates hypertension through effects on T lymphocyte trafficking. Hypertension 75, 869–876. https://doi.org/10.1161/HYPERTENSIONAHA.119.14148 (2020).
Kurts, C., Ginhoux, F. & Panzer, U. Kidney dendritic cells: fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol. 16, 391–407. https://doi.org/10.1038/s41581-020-0272-y (2020).
Nutt, S. L. & Chopin, M. Transcriptional networks driving dendritic cell differentiation and function. Immunity 52, 942–956. https://doi.org/10.1016/j.immuni.2020.05.005 (2020).
Pei, G. et al. Lymphangiogenesis in kidney and lymph node mediates renal inflammation and fibrosis. Sci. Adv. 5, eaaw5075. https://doi.org/10.1126/sciadv.aaw5075 (2019).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262. https://doi.org/10.1038/s41586-020-2134-y (2020).
Krmeska, V., Aggio, J. B., Nylen, S., Wowk, P. F. & Rothfuchs, A. G. Cyclooxygenase-Derived prostaglandin E(2) drives IL-1-Independent Mycobacterium bovis Bacille Calmette-Guerin-Triggered skin dendritic cell migration to draining lymph node. J. Immunol. 208, 2549–2557. https://doi.org/10.4049/jimmunol.2100981 (2022).
De Keijzer, S., Meddens, M. B., Torensma, R. & Cambi, A. The multiple faces of prostaglandin E2 G-protein coupled receptor signaling during the dendritic cell life cycle. Int. J. Mol. Sci. 14, 6542–6555. https://doi.org/10.3390/ijms14046542 (2013).
Hayen, S. M., Knulst, A. C., Garssen, J., Otten, H. G. & Willemsen, L. E. M. Fructo-Oligosaccharides modify human DC maturation and Peanut-Induced autologous T-Cell response of allergic patients in vitro. Front. Immunol. 11, 600125. https://doi.org/10.3389/fimmu.2020.600125 (2020).
Xie, Z. X. et al. Role of the immunogenic and tolerogenic subsets of dendritic cells in multiple sclerosis. Mediat. inflamm. 2015, 513295. https://doi.org/10.1155/2015/513295 (2015).
Sim, W. J., Ahl, P. J. & Connolly, J. E. Metabolism is central to tolerogenic dendritic cell function. Mediat. inflamm. 2016, 2636701. https://doi.org/10.1155/2016/2636701 (2016).
Marin, E., Cuturi, M. C. & Moreau, A. Tolerogenic dendritic cells in solid organ transplantation: where do we stand?? Front. Immunol. 9, 274. https://doi.org/10.3389/fimmu.2018.00274 (2018).
Xia, C. Q., Peng, R., Annamalai, M. & Clare-Salzler, M. J. Dendritic cells post-maturation are reprogrammed with heightened IFN-gamma and IL-10. Biochem. Biophys. Res. Commun. 352, 960–965. https://doi.org/10.1016/j.bbrc.2006.11.136 (2007).
Loison, E. & Gougeon, M. L. Thimerosal compromises human dendritic cell maturation, IL-12 production, chemokine release, and T-helper polarization. Hum. Vaccines Immunotherapeutics. 10, 2328–2335. https://doi.org/10.4161/hv.29520 (2014).
Jin, P. et al. Lactobacillus fermentum ZYL0401 attenuates Lipopolysaccharide-Induced hepatic TNF-alpha expression and liver injury via an IL-10- and PGE2-EP4-Dependent mechanism. PloS One. 10, e0126520. https://doi.org/10.1371/journal.pone.0126520 (2015).
Munoz-Sanchez, J. & Chanez-Cardenas, M. E. The use of Cobalt chloride as a chemical hypoxia model. J. Appl. Toxicology: JAT. 39, 556–570. https://doi.org/10.1002/jat.3749 (2019).
Chen, C., Mao,, W. F. & Wu, Y. F. The effects of Hypoxia-Reoxygenation in mouse digital flexor Tendon-Derived cells. Oxidative Med. Cell. Longev. 2020 (7305392). https://doi.org/10.1155/2020/7305392 (2020).
Liao, X. et al. Imperatorin exerts antioxidant effects in vascular dementia via the Nrf2 signaling pathway. Sci. Rep. 13, 5595. https://doi.org/10.1038/s41598-022-21298-x (2023).
Wang, H. et al. Protective effects of Safranal on hypoxia/reoxygenation-induced injury in H9c2 cardiac myoblasts via the PI3K/AKT/GSK3beta signaling pathway. Experimental Therapeutic Med. 22, 1400. https://doi.org/10.3892/etm.2021.10836 (2021).
Cheng, H., Huang, H., Guo, Z., Chang, Y. & Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 11, 8836–8854. https://doi.org/10.7150/thno.63396 (2021).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000. https://doi.org/10.1161/ATVBAHA.110.207449 (2011).
Schneider, R. et al. Oat1/3 restoration protects against renal damage after ischemic AKI. Am. J. Physiol. Renal. Physiol. 308, F198–F208. https://doi.org/10.1152/ajprenal.00160.2014 (2015).
Kanai, M. et al. MAFB in macrophages regulates prostaglandin E2-Mediated lipid mediator class switch through ALOX15 in ischemic acute kidney injury. J. Immunol. 213, 1212–1224. https://doi.org/10.4049/jimmunol.2300844 (2024).
Funding
This work was supported by grants from the the Natural Science Foundation of Ningxia Province (2023AAC03530), and National Natural Science Foundation of China (82460143).
Author information
Authors and Affiliations
Contributions
C.Y.L., T.Z, P.P.W. carried out the experimental design; C.Y.L., C.P.Y., J.X. , P.P.W. completed the main experiments; A.J.W., J.J.Z., W.L.S., T.P. performed experimental assistance; C.P.Y., Y.Z., Q.M. carried out the data analysis and pictures drawing; C.Y.L. wrote the main manuscript text; T.Z., J.X., C.P.Y., A.J.W. reviewed and edited the manuscript; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study complied with the ARRIVE guidelines, and the experimental protocol received approval from the Medical Research Ethics Committee of Ningxia Medical University General Hospital, with approval number: KYLL-2022-0794.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, C., Yang, C., Wang, P. et al. Prostaglandin E2 receptor EP4 activation induces tolerogenic dendritic cells to mitigate ischemic acute kidney injury. Sci Rep 15, 19170 (2025). https://doi.org/10.1038/s41598-025-03085-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03085-6
Keywords
This article is cited by
-
Combining spatial and single-cell transcriptome data to analyze tertiary lymphoid structures in clear cell renal cell carcinoma reveals prognostic biomarkers
Journal of Translational Medicine (2026)