Abstract
Two-photon light sheet microscopy offers great potential for a range of biological applications. Still, its practical implementation is impeded by the high cost of laser sources, the complexity of construction, and the challenges associated with adapting to existing microscope setups. Here, we release an open-source design that addresses these limitations by providing detailed building instructions for transforming a brightfield microscope into a versatile one- and two-photon light sheet system. Our design incorporates a specially designed broadband hollow core fiber, enabling the simultaneous utilization of visible laser alongside an expansive pulsed laser source from another setup. This integration allows for uncompromised image resolution and speed. Furthermore, the design reduces the complexity of construction, alignment, and overall cost, thereby significantly enhancing the accessibility of this technology (https://github.com/LJPZebra/OLU).
Similar content being viewed by others
Introduction
Light-sheet fluorescence microscopy (LSFM) has emerged as a cornerstone technique in fields ranging from developmental biology1,2 to cell biology3, organoid research4,5 and neuroscience6,7,8,9.
In LSFM, the sample is illuminated by a thin light sheet orthogonal to the fluorescence detection axis, thus co-aligning fluorescence stimulation and detection plane. This minimizes photodamage and bleaching relative to epifluorescence or confocal modalities. By combining optical sectioning, rapid volumetric imaging, and low photodamage, LSFM has enabled detailed studies of embryogenesis (such as in Drosophila or Danio rerio) and real-time monitoring of neural dynamics in entire brains of small organisms10.
In single-photon LSFM, a continuous-wave (CW) laser in the visible range is conventionally employed. Although such illumination is relatively straightforward, it suffers from scattering and absorption in thicker or more opaque specimens. Compared to single-photon systems, two-photon light sheet microscopes offer an increase in penetration depth and a reduction in the background signal by minimizing photon scattering and absorption in tissues11,12. Furthermore, the infrared source used in two-photon imaging lies outside the visible spectrum of most animals, thus preventing interference with the visual system6,7 during functional imaging.
However, the dissemination of two-photon light-sheet microscopes remains limited due to several factors: (i) they require a dedicated femtosecond laser source, which is costly; (ii) they are difficult to adapt to existing microscopes and thus require a complete custom-made system; and (iii) they are relatively complex to build and challenging to align.
Recent advances in fiber optics are beginning to address these challenges by enabling flexible broadband delivery of laser sources. They allow both visible and pulsed infrared light for one- and two-photon microscopy to be transmitted through the same fiber, with high transmission efficiency and very low pulse dispersion.
Efficient, dispersion-free, and broadband fiber coupling of a two-photon laser source is a long-standing challenge in the field of two-photon microscopy13 and of fiber optics design14. The difficulty arises because standard single-mode optical glass fibers are associated with strong linear and nonlinear pulse dispersion, which reduces the two-photon efficiency. Pre-compensation methods are only efficient at low laser powers and are therefore impractical in the context of fast volumetric two-photon imaging, which requires high photonic fluxes.
The development of single-mode photonic bandgap fibers, in which the light travels through an air-filled hollow core, dramatically reduces dispersion and nonlinear effects and achieves transmission ratios greater than 50%14,15. These fibers have been successfully used for pulsed laser delivery in the context of multiphoton imaging16,17,18,19,20. However, as the light-guiding mechanism is based on creating an optical bandgap, these fibers only allow single-wavelength transmission. They are currently only commercially available for laser wavelengths of 800 nm or 1064 nm (NTK photonics). However, with the rapidly growing collections of genetically encoded actuators and sensors, there is a growing demand for broadband fiber delivery that covers the near-infrared and extends into the visible spectrum. Such capability is essential to fully exploit 2P-LSFM across diverse applications and to facilitate laser source sharing between different setups.
Broadband fiber delivery spanning the visible and the near-infrared spectrum is possible with negative curvature hollow-core photonic crystal fibers (NCF), which do not rely on an optical bandgap to confine the laser light to the fiber’s core21,22,23. The simplest cross-sectional geometry of an NCF is based on a ring of touching or non-touching tubes surrounding the core22. Core diameter, tube diameter, inter-tube distance, and tube wall thickness control the spectral transmission bands, the attenuation level, the quality of higher-order mode suppression, and the sensitivity of the optical properties to bending. Attenuation levels < 0.07 dB/km and bending loss < 0.03 dB/m are reported24. Due to the minimal lattice structure and the reduced light interaction with the cladding structure, these fibers have very high damage thresholds and can even be used for very high laser energy delivery of up to 100 \(\mu \text {J}\) peak power when the core is vacuum pumped to reduce nonlinear effects at these high powers25. Negative curvature fibers are on the verge of being used in several applications such as laser micromachining and laser surgery. A recent study demonstrated the successful use of a custom HC-NCF with a transmission band of 600 - 830 nm at < 0.3 dB/m attenuation in the design of a handheld two-photon microscopy scanner for human skin autofluorescence26. This broadband capability opens the door for experimental setups that require single-photon and two-photon illumination paths without separate hardware. Here, we describe an open-source one- and two-photon light-sheet microscope that capitalizes on the broadband transmission properties of a custom negative curvature hollow-core fiber. We transform a commercial upright electrophysiology microscope into a multiphoton-capable LSFM system by attaching a compact light-sheet forming unit measuring only a few centimeters. The module houses the essential optics for digitally scanned light-sheet generation, enabling the user to align the microscope with minimal manual adjustments. Once assembled, researchers may connect a CW visible laser and an ultrafast near-infrared laser to the fiber input for interchangeable operation in single-photon and two-photon modes. Step-by-step detailed building instructions and blueprints are provided in the open-source online material (https://github.com/LJPZebra/OLU).
We demonstrate the performance of the system by imaging the brain of larval zebrafish expressing the pan-neuronal calcium indicator GCaMP6f. We perform both static anatomical scans and high-speed volumetric recordings of spontaneous neural activity, illustrating the microscope’s capacity for low-phototoxic, high-contrast functional imaging. Overall, this open-source platform has the potential to democratize 2P-LSFM, making advanced imaging techniques more accessible for a broad range of applications in modern biology.
Results
Transformation of a commercial upright microscope into a one- and two-photon light-sheet system
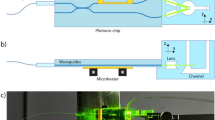
We present a versatile open-source one- and two-photon light-sheet microscope. Step-by-step detailed building instructions and blueprints are provided in the open-source online material (https://github.com/LJPZebra/OLU). The design features a compact module for light sheet formation (Fig. 1) with a small footprint, measuring 6 × 9 × 10 \(\hbox {cm}^3\)27 that can be mounted on standard commercial microscopes. We have successfully integrated this light-sheet unit with an electrophysiology microscope from Scientifica (UK), converting it into a dual-mode one- and two-photon light-sheet system (Fig. 1a, Video S1). The 3D model can be explored interactively at https://a360.co/3C0TOAm and https://a360.co/434IdMt. Complete building instructions and blueprints are available online (https://github.com/LJPZebra/OLU) and in print format.
Our light-sheet unit (Fig. 1c,d) comprises two objectives arranged in a one-to-one telescope configuration. The first objective collimates the laser beam from the fiber, and a galvanometric mirror at the back focal plane of the second objective pivots the beam, thereby sweeping it laterally to generate a digitally scanned light sheet. A key advantage of this light-sheet design is its minimal alignment requirement. During assembly, only two primary adjustments were needed: (1) coarse positioning of the galvanometric mirror at a \(45^{\circ }\) angle (with fine-tuning via the controller offset) to center the laser beam into the second objective (Obj 2), and (2) adjusting the distance between the fiber and the collimating objective (Obj 1) to ensure proper collimation. Once mounted on the microscope stand, no further internal alignment was required to position the light sheet within the focal plane of the imaging objective or to center its waist in the field of view.
The unit is mounted via aluminum brackets to the microscope’s motorized vertical translation stage used for focusing (see Fig. 1b,c). This configuration ensures that the detection objective and the illumination unit move synchronously during focus adjustments, simplifying volumetric scanning. Slow volumetric recordings are achievable via the built-in microscope software controlling the objective stage (see Video S2 and Fig. 1b left). For fast volumetric scanning, two piezo actuators enable high-speed, synchronous motion of the imaging objective and the fiber outlet (see Video S3 and Fig. 1b right).
A key advantage of our dual light-sheet system is the delivery of all laser sources through a single broadband optical fiber. This approach enables sharing a costly femtosecond laser (required for two-photon imaging) with other systems, thereby reducing the overall cost. Moreover, fiber delivery enhances flexibility and simplifies alignment. The entire unit can be manually translated along the z-axis to align the light sheet with the focal plane, and along the x-axis to center the light-sheet waist (see Videos S4 and S5, and Fig. 1e). This alignment procedure is straightforward in one-photon mode, and no additional alignment is needed in two-photon mode because the fiber inherently coaligns the laser beams.
(a) 3D model of the complete setup. (b) Slow scan mode (Left): The unit is mounted on the motorized stage of the microscope that moves the objective, enabling slow z-scans through the sample while keeping the light sheet in the imaging focal plane. Fast scan mode (Right): Synchronized actuation of the piezo positioners, which hold the objective and fiber, enables fast, high-precision z-scans. (c) Step-by-step construction plan of the light-sheet unit. (d) Cross-sectional view of the light-sheet unit, illustrating the galvanometric mirror that scans the laser beam in the sample plane to generate the digitally scanned light sheet. (e) Alignment procedure via mechanical manipulation of the entire light-sheet unit. Left: The unit (LU) is moved vertically using a mechanical z-stage (indicated by the yellow arrow) to align the light sheet with the imaging objective’s focal plane. Right: The LU is moved horizontally using a mechanical x-stage (indicated by the yellow arrow) to position the light-sheet waist at the center of the imaging objective’s field of view. 3D design software: Autodesk Fusion360 v2.0.
Broadband delivery of both CW and femtosecond pulses via a hollow-core negative curvature fiber
Characteristics of the negative curvature hollow core fiber
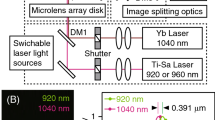
We developed a negative curvature hollow-core (HC-NCF) that exhibits optical characteristics ideally suited for laser delivery in the context of one- and two-photon light-sheet microscopy (Fig. 2). The fiber has an outer diameter of 200 ± 2 µm and a fiber coating diameter of 600 ± 30 µm. Its unique design features an air-filled 30 µm-in-diameter core surrounded by a ring of eight non-contacting cladding tubes, each with an approximate diameter of 10 µm. This hypocycloid negative curvature fiber structure enables the creation of a negative curvature core contour, which is essential for achieving the desired optical properties. Remarkably, this fiber exhibits two broad transmission bands that are well suited for one-, two-, and in principle also three-photon imaging (see Fig. 2a). The first transmission band spans the visible spectrum from 400 to 535 nm (I), while the second transmission band covers the near-infrared range from 700 to 1500 nm (II). Within these spectral bands, the fiber demonstrates low loss, measuring less than 300 dB/km (equivalent to a transmission efficiency of over 93 % for a one-meter-long fiber), and minimal pulse dispersion, with less than 1 dB/(km nm) of the spectral pulse width. Furthermore, this fiber is commercially available as patch-chord cable with standard FC/PC connectors, allowing for convenient disconnection and reconnection to the optical setup without the need for a subsequent realignment on either end. The small core diameter, in comparison to the large infrared wavelength, ensures near single-mode guidance while preserving the Gaussian beam properties of the laser at the fiber output. Our measurements indicate \(\hbox {M}^2\) values of 1.23 and 1.18 at laser wavelengths of 1030 nm and 515 nm, respectively. The near field and far field profiles exhibit high symmetry, with minimal ellipticity of 97.25 %, and 85.57 %, respectively (see Fig. 2c). The mode field diameters at 1/\(e^2\) are 23 ± 1 µm and 26 ± 1 µm respectively, corresponding to a fiber numerical aperture of NA\(\approx\)0.02. In addition, the HC-NCF fibers demonstrate a high damage threshold due to negligible interactions between the light and the fiber material. In our tests, we observed no fiber damage even at peak powers of 40 µJ (1030 nm) and 10 µJ (515 nm) for 400 fs pulses, corresponding to average laser powers of 20 W and 4 W, respectively. These characteristics highlight the fiber’s ability to deliver high-quality pulsed Gaussian beams across a broad spectral range, ensuring high transmission efficiency, and low dispersion, and making it an excellent choice for high-resolution multiphoton microscopy applications.
(a) Attenuation and dispersion spectrum of the hollow-core negative curvature fiber. Two transmission bands (loss < 300 dB/km) are highlighted in yellow. Inset (left): Schematic cross-section of the hypocycloid fiber with eight cladding tubes. Inset (right): Electron micrograph (EM) of the fiber cross-section (scale bar: 10 µm). (b) Schematic of the optical path for coupling the laser source into the fiber and expanding the fiber output’s numerical aperture to match that of the collimation objective (Obj1 in Fig. 1d). (c) Near-field and far-field beam profiles of the fiber output at 515 nm and 1030 nm wavelengths. (d) Measured axial light-sheet profile in the sample plane.
Fiber coupling: optical path and procedure
We achieved laser coupling into the fiber using a femtosecond pulsed Ti:Sapphire laser (MaiTai, Coherent, USA), along with three mirrors (M1-3), a dichroic (D), and a coupling lens (L1, f=40mm, Thorlabs, AC254-040-B-ML, see Fig. 2b). The optical path can be explored interactively at https://a360.co/434IdMt. The fiber was held in place by a differential xyz-translation stage. The continuous blue laser (488 nm, Oxxius, France) was coupled into the same hollow core fiber through the aforementioned dichroic using two additional mirrors (M4-5) and a collimation lens (L2). For efficient optical coupling and suppression of higher laser modes, the width of the laser focus projected onto the fiber input side must match the mode field diameter of the fiber. For the femtosecond laser, we positioned the coupling unit at a distance from the laser source where the slightly diverging laser beam reached the desired beam diameter, necessary to match the mode field diameter in combination with the chosen coupling lens. For the blue laser, we adjusted the beam diameter by selecting the focal distance of the collimation lens with the formula: \(\hbox {f}_{\text {collimation}} = \text {f}_{\text {coupling}} \cdot \frac{\text {NA}_{\text {HC-NCF fiber}}}{\text {NA}_{\text {laser fiber}}} \approx 40 \text {mm} \cdot \frac{0.02}{0.13}\), which boils down to the fact to choose the collimation lens focal distance such that the collimated laser beam diameter matches the diameter of the infrared beam that we previously coupled into the fiber using the chosen coupling lens. At a wavelength of 915 nm, corresponding to the central two-photon absorption peak of GFP and its calcium-sensitive derivative GCaMP, we delivered 100 fs laser pulses through 1,5 m fiber length with 98% power transmission efficiency and minimal pulse dispersion of 28 nm (1 dB/km nm), which we fully precompensated with the Deepsee element of the MaiTai laser source. At 488 nm, the one-photon excitation maximum of GFP and GCaMP, we achieved a transmission efficiency of 75 %.
Two-photon light-sheet microscopy requires not only the delivery of high laser power through the fiber but also precise control of the laser’s polarization state. This is critical because fluorescence emission occurs predominantly perpendicular to the fluorophore’s dipole moment-and thus to the excitation light’s polarization plane28. Consequently, if the laser is polarized perpendicular to the light-sheet plane, fluorescence is emitted mainly orthogonal to the detection objective’s optical axis, resulting in reduced collection efficiency29. To optimize the fluorescence signal, we incorporated a half-wave plate before the fiber coupling unit to carefully adjust the laser polarization. This fine-tuning maximizes fluorescence emission and ensures proper alignment with the detection objective. It is important to note that once the optimal polarization is set, the fiber must remain free of bending or twisting. Cycloid-type negative curvature fibers are not polarization-maintaining24, and mechanical deformation can alter the polarization state through rotation and changes in ellipticity.
Matching the NA of the light-sheet unit
Our compact light-sheet unit relies on an optical fiber to deliver the laser directly into the system. At the fiber outlet, the laser beam is first collimated by an objective and then focused onto the sample by a second objective (see Fig. 1d). This configuration forms a one-to-one telescope that maintains unity magnification, projecting the fiber output directly onto the sample so that the light-sheet waist precisely matches the fiber’s mode field diameter.
In the one-photon implementation described by Migault et al.27, a single-mode optical fiber with a mode field diameter of 2.8–4.1 µm at a 488 nm wavelength was used. This setup provided an ideal light-sheet waist and z-resolution, well-suited for cellular-resolution microscopy.
In contrast, our multiphoton implementation employs a hollow-core fiber with a considerably larger mode field diameter of 23 µm-about five times larger than that of the single-mode fiber. This increase results in a reduced numerical aperture of ≈0.02. To recover the resolution of the one-photon configuration, we introduced an additional lens immediately after the fiber. This lens demagnifies the laser waist by a factor of five-reducing it to less than 5 µm-while simultaneously increasing the beam divergence to match the collimation objective’s numerical aperture (NA 0.1) (see Fig. 2b).
To achieve the targeted demagnification factor (m = 1/5 = 0.2), we positioned a lens with a focal length of f = 2 mm at a distance of \(s = f \cdot (1/m - 1) = 8\,\textrm{mm}\) from the fiber outlet (see Fig. 2b). This placement refocuses the laser to a new position at \(s' = f \cdot [1 + 1/(s/f - 1)] = 2.4\,\textrm{mm}\) beyond the lens, yielding a beam waist of \(w_0' = m \cdot w_0 \). Additionally, the lens scales the laser divergence angle to \(\frac{\text {NA}_{\text {HC-NCF fiber}}}{m}\) to matche the numerical aperture of the collimation objective. Notably, the lens has a numerical aperture of 0.5, ensuring the laser beam is not clipped.
Finally, the compact lens can be directly affixed to the optical fiber outlet using a small lens tube. This add-on, termed the “NA-expander,” allows for seamless interchangeability with a standard single-mode fiber configuration.
Imaging performance
To demonstrate the system’s uncompromised resolution and speed, we recorded brain-wide 3D stacks of an immobilized larval zebrafish at cellular resolution using one- and two-photon imaging modes (Fig. 3g and Video S6). We used transgenic zebrafish larvae expressing GCaMP6f under a pan-neuronal promoter. At six days post-fertilization, the larvae possess a largely transparent body, making them ideal for high-resolution microscopy. The specimens were embedded in low-melting-point agarose within a capillary and oriented so the brain faced the detection objective.
For single-photon imaging, the sample was illuminated with a 488 nm continuous-wave laser light-sheet. In two-photon mode, we employed an ultrafast laser (100 fs pulses at 80 MHz) centered around 915 nm and relayed through the hollow-core fiber. Volumetric scans were achieved by synchronously moving the microscope objective and the light-sheet along the vertical axis using a fast, high-precision scan mode.
We acquired stacks from ventral to dorsal through the entire brain of a 6-day-old larva with 1-µm z-increments (see Video S6 and Fig. 3). Both imaging modes resolved single neurons across the entire brain. Although single-photon imaging produced relatively bright fluorescence signals, it exhibited slightly increased background at deeper layers. In contrast, two-photon imaging offered superior optical sectioning and reduced out-of-focus fluorescence, particularly in highly scattering regions-consistent with previous observations that infrared excitation penetrates deeper into larval zebrafish brains with minimal scattering.
Furthermore, we conducted rapid whole-brain functional imaging of spontaneous neural activity (see Video S7). This approach enabled us to monitor real-time neural dynamics and capture transient events with high temporal fidelity across the entire brain. Delivering several hundred milliwatts of laser power into the sample,a requirement for fast functional two-photon light-sheet imaging,was made possible by the fiber’s high transmission efficiency and damage threshold, further underscoring the versatility of our system. Overall, these findings demonstrate that our compact one- and two-photon light-sheet unit, based on laser delivery via a negative curvature hollowcore fiber, excels in both high-resolution structural imaging and rapid functional imaging. In conclusion, our system offers an integrated solution that combines precision and speed, making it a powerful tool for advanced neurobiological research.
Volumetric imaging of a larval zebrafish brain in one- and two-photon modes. A 6 dpf zebrafish larva expressing pan-neuronal, nucleus-localized GCaMP6f (Elav3-H2B-GCaMP6f) was imaged. Both Sagittal and horizontal sections from the 3D volume are shown for each mode. In the sagittal section, the dashed line indicates the z-position of the horizontal plane shown. The inset highlights single cells resolved in the two-photon mode. In the two-photon mode, the light sheet was partially blocked during recording of ventral layers (dashed rectangle) to prevent it from striking the highly pigmented eyes, which would otherwise absorb excessive high-power infrared light, leading to heating and potential damage. The complete volumetric stack is available in Video S6. Ro rostral, Co caudal, R right, L left.
Discussion
Our results demonstrate that integrating a compact, fiber-coupled light-sheet unit, which employs a negative curvature hollow-core fiber, can transform a standard upright commercial microscope into a dual-mode one- and two-photon LSFM system. This approach overcomes several technical and financial barriers commonly associated with multiphoton microscopy.
First, the system enables sharing a single femtosecond laser source across multiple setups through hollow-core fiber patch cables. Laboratories already equipped with a pulsed ultrafast near-infrared laser for two-photon imaging can extend multiphoton capabilities to an additional microscope without investing in a second expensive laser. Second, fiber-based laser delivery greatly simplifies optical alignment. Because the beam exits the fiber pre-aligned, the unit can be easily translated and rotated to align the light sheet relative to the imaging focal plane without the need to adjust free-space optical elements. Moreover, when incorporating a second laser line, the user only needs to optimize the input coupling. Once initially set up, the system eliminates the need for repeated free-space alignments, reducing maintenance effort.
The broadband transmission of the negative curvature fiber further enhances the system’s flexibility. It supports wavelengths spanning from the visible to the near-infrared spectrum (e.g., 700-1500 nm), enabling the excitation of diverse fluorophores for one- and two-photon imaging, and even facilitating three-photon excitation for deeper tissue penetration when the optical path is appropriately optimized. The same fiber can transmit visible light for single-photon fluorescent excitation, and tunable sources, such as optical parametric oscillators, could be integrated to expand the range of compatible fluorophores and experimental designs. This versatility makes the unit well-suited for advanced applications, including multispectral functional imaging and combined optogenetic photostimulation, where adapting the light sheet wavelength can help reduce cross-talk with the stimulation. In addition, the fiber’s optical properties and broadband transmission spectrum render it ideal for implementation in other microscopy modalities.
Our demonstration of brain-wide calcium imaging in larval zebrafish underscores the system’s capability for rapid, volumetric functional imaging. The compact design and fiber coupling do not compromise microscope resolution or speed. While our validation was performed on zebrafish larvae, the system is readily adaptable to other small model organisms, embryonic samples, organoids, or ex vivo tissues with comparable optical resolution.
Beyond hardware performance, the open-source nature of our design promotes accessibility and further innovation. We provide detailed assembly instructions, mechanical drawings, 3D models, and software for coordinating the galvanometric mirror, objective motion, and camera acquisition. Importantly, by mounting the unit onto the vertical microscope translation stage, the light-sheet unit moves synchronously with the objective, enabling volumetric imaging using standard microscope control software or manual focusing without additional interfaces.
Moreover, the system is compatible with future enhancements. For instance, the fiber’s broadband transmission supports multicolor two-photon imaging30; the digitally scanned light sheet can be paired with line-confocal detection to improve signal-to-noise ratios31; and the fiber’s high damage threshold permits the delivery of high-energy pulses at low repetition rates, thereby increasing imaging speed while reducing phototoxicity12.
In summary, our work provides a unified and flexible framework that brings two-photon light-sheet microscopy closer to a plug-and-play experience. By leveraging a single-fiber approach, we significantly reduce both the cost and complexity of building a two-photon LSFM setup, opening the door to broader adoption of multiphoton light-sheet microscopy, even in laboratories traditionally limited to single-photon modalities.
Methods
Experimental model and subject details
All experiments were conducted on zebrafish nacre mutants aged 6-7 days post-fertilization (dpf). Larvae were reared in Petri dishes filled with E3 solution under a 14/10 hr light/dark cycle at \(28^{\circ }\)C. Fish were provided with powdered nursery food daily from 6 dpf. Calcium imaging experiments were performed in nacre mutant larvae expressing the calcium indicator GCaMP6f. The nearly pan-neuronal promoter elavl3 controlled the expression, and the H2B domain the localization of the sensor in the nucleus Tg(elavl3:H2B-GCaMP6f). The GCaMP6f line was provided by Misha Ahrens and published in Quirin et al.32. The experimental protocols were approved by Le Comitée d’Ethique pour l’Expérimentation Animale Charles Darwin C2EA-05 (02601.01 and #32423-202107121527185 v3).
Fiber coupling procedure
Coupling a laser into our hollow-core fiber is more challenging than coupling into standard fibers due to its low numerical aperture (\(\hbox {NA}_{\text {fiber}}\) = 0.02). To simplify this process, we developed a pre-alignment protocol (detailed instructions are available online at https://github.com/LJPZebra/OLU). Initially, a fiber-coupled visible laser was injected into the hollow-core fiber from the opposite end by connecting it via a fiber-to-fiber connector (Thorlabs, ADAF1) to our FC/PC-connectorized hollow-core fiber. In this configuration, the visible laser traversed the coupling optics in reverse order. The optical components were then pre-aligned so that the visible laser beam was collimated by the coupling lens and centered on the output aperture. Following this initial alignment, the visible laser fiber was disconnected from the hollow-core fiber outlet, a power meter was positioned at the outlet, and the femtosecond laser was activated at low power to prevent fiber damage during subsequent adjustments. Once the pre-alignment yielded sufficient laser transmission for reliable infrared measurements, the coupling was further fine-tuned until a transmission efficiency greater than 90% was achieved.
Characterization of the light-sheet profile
We followed the protocol presented in Wolf et al. 20156 to characterize the light-sheet profile. In brief, we acquired a high-resolution stack of a sample containing sub-diffraction-sized fluorescent beads (FluoSpheres, 505/515, Molecular Probes, USA) with a diameter of 100 nm embedded in 2 % low melting point agarose. The z-spacing between consecutively imaged sections was set at 0.5 \(\mu\)m. For each bead, the fluorescence intensity profile across the light sheet was fitted with a Gaussian function, from which we extracted the half width at 1/\(\hbox {e}^2\). This width, which depends on the bead’s position relative to the light-sheet waist, corresponds to the beam profile of the Gaussian beam generating the light sheet. To determine the light-sheet waist, \(\omega _0\), we fitted the measured bead profile widths as a function of the position x along the light sheet using the Gaussian beam profile equations (Eq. 1 for the one-photon profile and Eq. 2 for the two-photon profile) as described in6.
with the laser wavelength \(\lambda\), the refractive index n, and the waist of the Gaussian beam \(\omega _0\).
Acquisition and processing of functional light-sheet data
Image acquisition, mirror scanning, and objective translation were controlled and synchronized using National Instruments cards (NI 9402 and NI-9263, National Instruments) together with a custom MATLAB program (The MathWorks), which is available open source at https://github.com/LaboJeanPerrin/Lightsheet. Offline image pre-processing and extraction of calcium transients (\(\Delta\)F/F) were performed in MATLAB following the workflow described in9,27. The acquisition parameters for the brain recordings shown in Fig. 3 were as follows: For the one-photon recording, the interlayer interval was 1 µm, the exposure time was 20 ms, the continuous laser power was approximately 1 mW, and the excitation wavelength was 488 nm. For the two-photon recording, the interlayer interval was also 1 µm, the exposure time was 11 ms, the mean laser power in the sample was 710 mW, the pulse duration was 100 fs, the pulse repetition rate was 80 MHz, and the excitation wavelength was 915 nm.
Parts list
Part | Source | Reference | Amount | ||
|---|---|---|---|---|---|
Slow scan | Fast scan | ||||
(a) One-Photon Multicolor System: Parts List | |||||
Laser & Fiber | Continuous laser at 488 nm with fiber coupler | Oxxius | LBX-488-50-CSB-PP | 1 | 1 |
Optical fiber (single mode) | Thorlabs | P1-460-FC-2 | 1 | 1 | |
Optional: Second continuous laser at other wavelength | Oxxius | 1 | 1 | ||
Optional: 50:50 Fiber Optic Couplers | Thorlabs | TW470R5F2 | 1 | 1 | |
Light-Sheet Unit | Collimation & illumination objectives | Zeiss | EC Plan-Neofluar 5x/0.16 M27 (FWD=18.5mm) | 2 | 2 |
Lens tube | Thorlabs | SM05M10 | 1 | 1 | |
FC/PC Fiber Adapter Plate with External SM05 | Thorlabs | SM05FC | 1 | 1 | |
z-piezo nanopositioner + Amplificator | Piezo Jena | PZ 400 OEM SG + E-440-111 | 1 | ||
Galvanometer mirror | Thorlabs | GVS011 | 1 | 1 | |
Power supply for Galvo mirro | Thorlabs | GPS011-EC | 1 | 1 | |
Function generator | Agilent | EDU33211A | 1 | 1 | |
Acquisition board | National Instruments | NI PCIe-6363 | 1 | ||
Microscope | Microscope body | Scientifica | Scientifica SliceScope Pro 6000 | 1 | 1 |
Detection Path | Detection objective (NA 0.95, 25x) | Leica | HC FLUOTAR W VISIR | 1 | 1 |
Objective piezo drive + Driver | Piezo Jena | Mipos 500 SG,Triptics + NV 40/1 CLE | 1 | 1 | |
GFP filter (525 nm) | Thorlabs | MF525-39 | 1 | 1 | |
Notch filter (488 nm) | Thorlabs | NF488-15 | 1 | 1 | |
Camera (sCMOS) | Hamamatsu | C13440 V3 | 1 | 1 | |
Sample Chamber | Capillary + pistons | alphalabs | 5-000-2050 | 1 | 1 |
O-rings | www.oring.fr | 101300 | 1 | 1 | |
Beam Block | Thorlabs | LB2 | 1 | 1 | |
Part | Source | Reference | Amount | ||
|---|---|---|---|---|---|
(b) Two-Photon Upgrade: Parts List | |||||
Laser & Fiber | Femtosecond tunable laser | Spectra-Physics | MaiTai 90077043 | 1 | |
Precompensator | Spectra-Physics | Deep See | 1 | ||
Negative curvature fiber | Glophotonics | PMC-C-9005 B2 | 1 | ||
Laser-to-Fiber Coupling | Motorized Filter Flip Mount with Ø1” Optic Holder, M4 Tap | Thorlabs | MFF101/M | 1 | |
High-Precision Rotation Mount | Thorlabs | PRM1/M | 2 | ||
Half-Wave Plate | Newport | 10RP52-2B | 2 | ||
Polarizing Beamsplitter Cube | Thorlabs | CCM5-PBS202/M | 1 | ||
Beam Trap | Thorlabs | BT610/M | 1 | ||
Protected Silver Mirrors | Thorlabs | PF05-03-P01-Ø1/2” | 5 | ||
Mirror Mount | Thorlabs | POLARIS-K05 - Polaris\(\circledR\)Ø1/2” | 5 | ||
Hex Key Adjusters (4 per pack) | Thorlabs | HKTS-5/64 | 1 | ||
Optical Post | Thorlabs | TR75/M | 5 | ||
Universal Post Holder | Thorlabs | UPH50/M | 5 | ||
3-Axis NanoMax Stage, Differential Drives | Thorlabs | MAX313D/M | 1 | ||
Long, Fixed Mounting Bracket, 56 mm Long | Thorlabs | AMA009 | 1 | ||
FC/PC Fiber Adapter Plate with External SM1 | Thorlabs | SM1FC2 | 1 | ||
SM1-Compatible Flexure Stage Mount | Thorlabs | HCS031 | 1 | ||
Injection lens (40 mm) | Thorlabs | AC254-040-B-ML | 1 | ||
fiber-to-fiber connector (Ceramic) | Thorlabs | ADAF1 | 1 | ||
NA-Expander | NA expander lens (f = 2.00 mm, NA = 0.50, ARC: 600 - 1050 nm) | Thorlabs | C151TMD-B | 1 | |
Adapters for M6 x 0.5 Threaded Aspheres | Thorlabs | S05TM06 | 1 | ||
Accessories | IR Detector Card, 700 - 1400 nm | Thorlabs | VRC5 | 1 | |
Power meter | Thorlabs | PM130D | 1 | ||
Fiber Inspection Scope | Thorlabs | FS201 | 1 | ||
Cable Continuity Tester | Fluke Networks | VisiFault | 1 | ||
Laser safety glasses | Thorlabs | LG3 | 1 | ||
Light-Sheet Unit | Collimation & illumination objectives | Olympus | LMPLN5xIR/0.10 | 2 | |
RMS to M27 Adapter | Thorlabs | RMSA3 | 2 | ||
Detection Path | Multiphoton short-pass emission filter | Semrock | FF01-750/SP-25 | 1 | |
Data Availability
All data generated or analyzed during this study are included in this published article, its supplementary information files, and the online resources (https://github.com/LJPZebra/OLU).
References
Tomer, R., Khairy, K., Amat, F. & Keller, P. J. Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy. Nat. Methods 9(7), 755–763. https://doi.org/10.1038/nmeth.2062 (2012).
Reeves, G. T. et al. Dorsal-ventral gene expression in the drosophila embryo reflects the dynamics and precision of the dorsal nuclear gradient. Dev. Cell 22(3), 544–557. https://doi.org/10.1016/j.devcel.2011.12.007 (2012).
Gao, L., Shao, L., Chen, B.-C. & Betzig, E. 3D live fluorescence imaging of cellular dynamics using Bessel beam plane illumination microscopy. Nat. Protoc. 9(5), 1083–1101. https://doi.org/10.1038/nprot.2014.087 (2014).
Andilla, J. et al. Imaging tissue-mimic with light sheet microscopy: A comparative guideline. Sci. Rep. 7(1), 44939. https://doi.org/10.1038/srep44939 (2017).
Richards, D. J. et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 4(4), 446–462. https://doi.org/10.1038/s41551-020-0539-4 (2020).
Wolf, S. et al. Whole-brain functional imaging with two-photon light-sheet microscopy. Nat. Methods 12(5), 379–380. https://doi.org/10.1038/nmeth.3371 (2015).
Wolf, S. et al. Sensorimotor computation underlying phototaxis in zebrafish. Nat. Commun. 8(1), 651. https://doi.org/10.1038/s41467-017-00310-3 (2017).
Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M. & Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10(5), 413–420. https://doi.org/10.1038/nmeth.2434 (2013).
Panier, T. et al. Fast functional imaging of multiple brain regions in intact zebrafish larvae using Selective Plane Illumination Microscopy. Front. Neural Circuits 7, 65. https://doi.org/10.3389/fncir.2013.00065 (2013).
Keller, P. J. & Ahrens, M. B. Visualizing whole-brain activity and development at the single-cell level using light-sheet microscopy. Neuron 85(3), 462–483. https://doi.org/10.1016/j.neuron.2014.12.039 (2015).
Truong, T. V., Supatto, W., Koos, D. S., Choi, J. M. & Fraser, S. E. Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nat. Methods 8(9), 757–760. https://doi.org/10.1038/nmeth.1652 (2011).
Maioli, V. et al. Fast in vivo multiphoton light-sheet microscopy with optimal pulse frequency. Biomed. Opt. Express 11(10), 6012. https://doi.org/10.1364/boe.400113 (2020).
Helmchen, F., Denk, W. & Kerr, J. N. D. Miniaturization of two-photon microscopy for imaging in freely moving animals. Cold Spring Harbor Protoc. 2013(10), 904–913. https://doi.org/10.1101/pdb.top078147 (2013).
Wang, Y. et al. Hollow-core photonic crystal fibre for high power laser beam delivery. High Power Laser Sci. Eng. 1(1), 17–28. https://doi.org/10.1017/hpl.2013.3 (2013).
Cregan, R. F. et al. Single-mode photonic band gap guidance of light in air. Science 285(5433), 1537–1539. https://doi.org/10.1126/science.285.5433.1537 (1999).
Tai, S.-P. et al. Two-photon fluorescence microscope with a hollow-core photonic crystal fiber. Opt. Express 12(25), 6122. https://doi.org/10.1364/opex.12.006122 (2004).
Flusberg, B. A., Jung, J. C., Cocker, E. D., Anderson, E. P. & Schnitzer, M. J. In vivo brain imaging using a portable 3.9? Gram two-photon fluorescence microendoscope. Opt. Lett. 30(17), 2272–2274. https://doi.org/10.1364/ol.30.002272 (2005).
Engelbrecht, C. J., Johnston, R. S., Seibel, E. J. & Helmchen, F. Ultra-compact fiber-optic two-photon microscope for functional fluorescence imaging in vivo. Opt. Express 16(8), 5556–5564. https://doi.org/10.1364/oe.16.005556 (2008).
Piyawattanametha, W. et al. In vivo brain imaging using a portable 2.9 g two-photon microscope based on a microelectromechanical systems scanning mirror. Opt. Lett. 34(15), 2309–2311. https://doi.org/10.1364/ol.34.002309 (2009).
Choi, H. & So, P. T. C. Improving femtosecond laser pulse delivery through a hollow core photonic crystal fiber for temporally focused two-photon endomicroscopy. Sci. Rep. 4, 6626. https://doi.org/10.1038/srep06626 (2014).
Fei, Yu., Mengrong, X. & Knight, J. C. Experimental study of low-loss single-mode performance in anti-resonant hollow-core fibers. Opt. Express 24(12), 12969–12975. https://doi.org/10.1364/oe.24.012969 (2016).
Pryamikov, A. D. et al. Demonstration of a waveguide regime for a silica hollow–core microstructured optical fiber with a negative curvature of the core boundary in the spectral region \(>\) 3.5 \(\mu\)m. Opt. Express 19(2), 1441–1448. https://doi.org/10.1364/oe.19.001441 (2011).
Fei, Yu., Wadsworth, W. J. & Knight, J. C. Low loss silica hollow core fibers for 3–4 \(\mu\)m spectral region. Opt. Express 20(10), 11153–11158. https://doi.org/10.1364/oe.20.011153 (2012).
Debord, B. et al. Ultralow transmission loss in inhibited-coupling guiding hollow fibers. Optica4(2), 209–217 (2017). https://doi.org/10.1364/optica.4.000209. Rc fiber core inner radius \(\delta\) inter-tube gap t thickness of capillaries tube wall.
Li, F. et al. High energy femtosecond laser micromachining with hollow core photonic crystal fiber delivery. Optik 163093 (2019).
Sherlock, B. et al. Tunable fibre-coupled multiphoton microscopy with a negative curvature fibre. J. Biophotonics 9(7), 715–720. https://doi.org/10.1002/jbio.201500290 (2016).
Migault, G. et al. Whole-brain calcium imaging during physiological vestibular stimulation in larval zebrafish. Curr. Biol. 28(23), 3723–3735. https://doi.org/10.1016/j.cub.2018.10.017 (2018).
Lazar, J., Bondar, A., Timr, S. & Firestein, S. J. Two-photon polarization microscopy reveals protein structure and function. Nat. Methods 8(8), 684–690 (2011).
de Vito, G. et al. Effects of excitation light polarization on fluorescence emission in two-photon light-sheet microscopy. Biomed. Opt. Express 11(8), 4651. https://doi.org/10.1364/boe.396388 (2020).
Mahou, P., Vermot, J., Beaurepaire, E. & Supatto, W. Multicolor two-photon light-sheet microscopy. Nat. Methods 11(6), 600–601. https://doi.org/10.1038/nmeth.2963 (2014).
Baumgart, E. & Kubitscheck, U. Scanned light sheet microscopy with confocal slit detection. Opt. Express 20(19), 21805–21814 (2012).
Quirin, S. et al. Calcium imaging of neural circuits with extended depth-of-field light-sheet microscopy. Opt. Lett. 41(5), 855–858. https://doi.org/10.1364/ol.41.000855 (2016).
Acknowledgements
The authors thank the IBPS fish facility staff for maintaining the fish. We thank Misha Ahrens for providing transgenic fish lines. We are grateful to Carounagarane Dore for his contribution to the design of the experimental setup. We thank Loic Royer for his valuable feedback and discussions related to the mansucript and the online material. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research innovation program, grant agreement number 715980. Furthermore, it was supported by grants from Région Ile-de-France, specifically by DIM cerveau et pensée and DIM-ELICIT. Moreover, the project received partial funding from the CNRS and Sorbonne Université. GM had a Ph.D. fellowship from the Doctoral School in Physics, Ile de France (EDPIF), HT had a Ph.D. fellowship from Ile de France (ARDoC 17012950).
Author information
Authors and Affiliations
Contributions
AH, TP, GM, HT, GD, and VB designed the project, performed research, analyzed data, and wrote the manuscript. BB developed and characterized the broadband optical fiber.
Corresponding author
Ethics declarations
Competing interests
BB is affiliated with GLO Photonics, which produced the optical fiber and performed the spectral characterization of this fiber, as shown in Fig. 1c. All other authors have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information 2.
Supplementary Information 3.
Supplementary Information 4.
Supplementary Information 5.
Supplementary Information 6.
Supplementary Information 7.
Supplementary Information 8.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hubert, A., Panier, T., Migault, G. et al. A versatile and open source design for one and two photon light sheet microscopy. Sci Rep 15, 23760 (2025). https://doi.org/10.1038/s41598-025-03107-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03107-3