Abstract
The purpose of this study is to analyze FAERS data to identify cases of drug-induced photosensitivity (DIP), examine demographic patterns, determine the drug classes involved, and highlight emerging trends in these reactions. Additionally, we explore potential signal drugs by mining the relevant reported data, aiming to provide insights for safer clinical use of medications. We reviewed the publicly available FAERS database from 2004 to 2023. Using DIP-related search terms such as “photosensitivity reaction,” “polymorphic light eruption,” or et al., we identified reports of DIP. The frequency and trends of these reports were then analyzed. Between 2004 and 2023, the FDA received 17,384,824 reports of adverse reactions, with 20,236 of these linked to DIP. After excluding cases with incomplete data on age, gender, or country of origin, the median patient age was 52 years (IQR = 66). Females comprised 55.71% of the cases (11,274), and 66.96% (12,459) of the reports originated from the United States. The top 45 drugs were responsible for 9,810 cases (48.48%). The three drug classes most commonly associated with DIP in the FAERS database were immunosuppressants, monoclonal antibodies, and antineoplastic agents. A disproportionality analysis of the top drugs revealed several newly identified drugs with signals for photosensitivity, including adalimumab, adapalene, secukinumab, and fingolimod. By analyzing publicly available FAERS data, we identified key themes and trends in DIP reactions. Immunosuppressants and monoclonal antibodies show mild trends in DIP occurrence. Additionally, adalimumab, adapalene, secukinumab, and fingolimod are novel drug signals of DIP.
Similar content being viewed by others
Introduction
Drug-Induced Photosensitivity (DIP) refers to a condition where certain medications, when ingested or applied topically, can make the skin more sensitive to sunlight or artificial ultraviolet (UV) light1. This heightened sensitivity can lead to adverse skin reactions when the skin is exposed to UV radiation, resulting in symptoms such as sunburn, rash, redness, blistering, or other skin changes2. Photosensitivity reactions can be divided into two main categories, photoxic reactions and photoallergic reactions. Photosensitivity reactions induced by drugs have been documented to account for as much as 8% of adverse cutaneous events associated with medications3.
Factors that may cause photosensitive reactions include taking certain medications, such as quinolone, sulfonamide, and tetracycline antibiotics; contact with chemical substances, such as psoralens found in some plants; and having certain diseases, such as systemic lupus erythematosus (SLE) or porphyria. It is widely believed that DIP is significantly underreported due to the challenges of clinical recognition and the lack of documentation in public databases4.
A wide range of drugs has been implicated in DIP, with a total of 393 different compounds reported5,6. The most common categories include cardiovascular agents (e.g., diuretics such as hydrochlorothiazide and furosemide)7, antibiotics (e.g., tetracyclines), nonsteroidal anti-inflammatory drugs (NSAIDs), and certain antihistamines8. Notable examples also include some antipsychotics, chemotherapeutics, and dermatological agents. The evidence supporting photosensitizing effects varies among these drugs, with some exhibiting well-documented potential to induce photosensitivity reactions9. The primary strategy for managing DIP involves discontinuing the offending medication. After cessation, the skin typically begins to heal, and the risk of further reactions decreases. Additionally, protective measures such as avoiding sun exposure, using broad-spectrum sunscreens, and wearing protective clothing can help manage symptoms and prevent exacerbation. Therefore, early identification and prompt intervention in clinical practice are crucial10.
Nonetheless, there is limited summarized data available on exogenous drugs that can lead to the development of DIP. Furthermore, there is a lack of comprehensive analysis based on real-world big data.
Adverse drug reaction (ADR) monitoring is vital for evaluating the post-marketing safety of drugs, providing insights into their real-world use11. Mining data from spontaneous reporting systems enables earlier detection of potential ADRs and reevaluation of known ones. However, limitations inherent in the FDA Adverse Event Reporting System (FAERS) data, which collects adverse drug events from diverse populations, make it difficult to draw definitive conclusions regarding the prevalence, incidence, and causality of ADRs12. Despite these challenges, the FAERS dataset includes a substantial number of drug-related adverse events. To address this, we analyzed FAERS data to evaluate the prevalence of drugs associated with DIP. Our study aimed to identify the top drugs linked to this condition, track the evolution of related reports over time, and compare our findings with existing literature. Additionally, we conducted a disproportionality analysis to identify potential new signal drugs.
Results
Top drugs associated with DIP
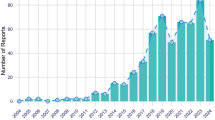
During the study period from 2004 to 2023, the FDA reported a total of 17,384,824 adverse reactions, of which 20,236 were associated with DIP. Excluding patients without age information, the median age of those with DIP was 52 (n = 12,913, interquartile range [IQR] = 66). Excluding patients with missing or unknown gender information, females represented 55.71% (11,274 cases) and males represented 36.24% (7,333 cases), with the remainder categorized as not specified or unknown. Excluding patients without country information, 12,459 out of 19,233 cases (66.96%) were reported from the USA. The top 45 drugs by number of cases were associated with 9,675 cases (47.81%). The primary categories of these drugs include immunosuppressants, monoclonal antibodies, and antineoplastic agents see supplementary table S1.
Pirfenidone was the drug most commonly associated with DIP in the FDA adverse event data, accounting for 4.43% (896/20,236) of all DIP reports during the study period. Details of the top drugs are provided in supplementary table S1.
The highest ranked drug class associated with DIP
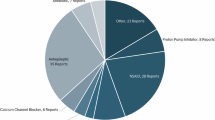
We categorized the top 45 drugs, the result in Table 1, supplementary table S2. The ranking of the main drug classes associated with DIP in cases reported to the FDA were immunosuppressants, monoclonal antibodies, antineoplastic agents(include methotrexate, which functions as both an immunosuppressant and an antineoplastic agent), antibacterials, psychoanaleptics, antiacne preparations, others(antivirals, proton pump inhibitors, NSAIDs-diclofenac, antifungals, biguanides), emollients and protectives, cardiovascular system, interferons and HMG CoA reductase inhibitors.
Among drug categories, immunosuppressants were the most commonly associated class with DIP, accounting for 10.01% (2,026/20,236) of all reported adverse events during the study period. The proportion of immunosuppressant-related photosensitivity cases peaked in 2016, representing 13.97% of the total cases for that year. Pirfenidone was the primary drug involved, with a notable increase to 175 cases in 2016, accounting for 19.53% of the total cases. The second most common drug class identified was monoclonal antibodies, which accounted for 9.60% (1,942/20,236) of all reports. Antineoplastic agents were responsible for 5.21% (1,054/20,236) of all reports.
Perform a linear regression analysis using the year as the independent variable and the number of DIP adverse events in each category as the dependent variable. Linear regression analysis was conducted for each major drug class, associated with DIP, including immunosuppressants, monoclonal antibodies, antibacterials, psychoanaleptics, anti-acne preparations, emollients and protectives, cardiovascular drugs, interferons, HMG-CoA reductase inhibitors, and others such as antivirals (e.g., simeprevir), proton pump inhibitors (e.g., omeprazole), NSAIDs (e.g., diclofenac), antifungals (e.g., terbinafine), and biguanides (e.g., metformin) (see supplementary table S2). The result revealed a slight increasing trend for immunosuppressants and monoclonal antibodies see in Table 1. Specifically, the proportion of DIP cases associated with immunosuppressants increased at an average rate of 0.01% per year (95% confidence interval: 0.00, 0.01), rising from 1.98% in 2004 to 14.92% in 2023. Monoclonal antibodies exhibited an average annual increase of 0.01% (95% confidence interval: 0.00, 0.02), rising from 21.78% in 2004 to 40.70% in 2023. In contrast, psychoanaleptics demonstrated a slight decreasing trend, with an average annual decline of -0.02% (95% confidence interval: -0.02, -0.01), falling from 37.62% in 2004 to 3.29% in 2023. No significant differences were observed in other drug classes year-to-year.
National reporting data
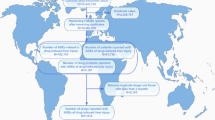
Subsequently, we calculated the proportion of DIP cases reported in the United States for each drug or drug class. Among the top 45 drugs, a total of 20,236 DIP cases were reported, with 12,459 (61.57%) originating from the U.S.. When analyzing the distribution across specific drug classe, we found that cardiovascular drugs had the lowest proportion of DIP reports among all drug classes in the U.S., accounting for only 15.48% (37/239) originating from the U.S.. In contrast, DIP reports for other drug classes ranged from 30 to 98% (see Table 1 and Supplementary Table S3).
Among the top 45 drugs with the highest number of DIP reports, we calculated the percentage of DIP reports occurring in the United States relative to the total DIP reports for each drug (reports without country were excluded). Adapalene accounts for 100%(440/440) of its reported DIP reactions in the United States. Additionally, twelve other drugs have over 90% of their DIP reports originating from the U.S.,including: dupilumab (97.22%,420/432), niraparib (97.38%,371/381), avobenzone/homosalate/octisalate/octocrylene/oxybenzone (99.46%,370/372), rubraca (99.56%,225/226), sumatriptan (99.02%,202/204), lenalidomide (93.53%,130/139), tofacitinib (94.44%,119/126), tecfidera (95.83%,115/120), interferon beta-1a (96.00%,96/100), natalizumab (95.41%,104/109), and apremilast (90.67%,68/75). In contrast, the drugs with the lowest percentage of DIP reports from the U.S. include: omeprazole (5.88%,4/68), amlodipine (13.27%,13/98), metformin (15.63%,10/64), sertraline (18.10%,19/105), and ciprofloxacin (19.47%,44/226). Overall, among the top ten drugs with the highest number of DIP reports, the proportion of cases reported in the United States ranges from 19.47 to 100%.
To gain a more comprehensive understanding of the regional distribution of DIP cases reported to the FDA during the study period, we identified the six countries with the highest number of reported cases and determined the top ten drugs associated with DIP in each of these countries see supplementary table S3 for details).
Signal values associated with DIP
We conducted a disproportionality analysis of the top drugs and identified the drugs with strong signals in Table 2. These drugs showed robust signals across all three monitoring methods, with a lower limit of the 95% confidence interval (CI) greater than 10. The drugs with strong notable signals are as follows: lamotrigine (N = 211, ROR = 360.80, 95% CI: 314.85-413.46), vemurafenib (N = 659, ROR = 68.56, 95% CI: 63.26–74.30), simeprevir (N = 164, ROR = 42.61, 95% CI: 36.41–49.87), voriconazole (N = 410, ROR = 32.57, 95% CI: 29.49–35.98), rubraca (N = 226, ROR = 25.80, 95% CI: 22.59–29.48), pirfenidone (N = 896, ROR = 23.79, 95% CI: 22.23–25.46), sumatriptan (N = 208, ROR = 23.73, 95% CI: 20.66–27.25), niraparib (N = 384, ROR = 18.68, 95% CI: 16.87–20.68), and doxycycline (N = 269, ROR = 18.36, 95% CI: 16.26–20.74). The PRR and IC25 signals for these drugs were also strong (see supplementary table S4 for details).
In addition, we identified some other drugs with mild notable signals for DIP. These drugs are as follows: terbinafine (N = 74, ROR = 8.62, 95% CI: 6.85–10.84), ciprofloxacin (N = 268, ROR = 7.21, 95% CI: 6.39–8.14), amiodarone (N = 127, ROR = 6.58, 95% CI: 5.52–7.84), levofloxacin (N = 138, ROR = 3.61, 95% CI: 3.05–4.27), isotretinoin (N = 152, ROR = 3.55, 95% CI: 3.02–4.16),omeprazole (N = 90, ROR = 3.66, 95% CI: 2.98–4.51), avobenzone/homosalate/octisalate/octocrylene/oxybenzone(N = 378, ROR = 2.80, 95% CI: 2.48–3.17), paroxetine (N = 119, ROR = 2.88, 95% CI: 2.40–3.45), peginterferon alfa-2a (N = 101, ROR = 2.80, 95% CI: 2.30–3.41), adapalene (N = 442, ROR = 2.20, 95% CI: 2.00-2.41), amlodipine (N = 112, ROR = 2.16, 95% CI: 1.79–2.60), methotrexate (N = 259, ROR = 1.97, 95% CI: 1.74–2.23), sertraline (N = 113, ROR = 2.08, 95% CI: 1.73–2.51), dupilumab (N = 435, ROR = 1.83, 95% CI: 1.67–2.02), omalizumab (N = 97, ROR = 1.81, 95% CI: 1.48–2.21), duloxetine (N = 104, ROR = 1.80, 95% CI: 1.48–2.18), venlafaxine (N = 77, ROR = 1.77, 95% CI: 1.42–2.22), rosuvastatin (N = 78, ROR = 1.62, 95% CI: 1.30–2.03), fingolimod (N = 134, ROR = 1.48, 95% CI: 1.25–1.76), secukinumab (N = 186, ROR = 1.31, 95% CI: 1.14–1.52), adalimumab (N = 807, ROR = 1.19, 95% CI: 1.11–1.28), certolizumab pegol (N = 106, ROR = 1.31, 95% CI: 1.09–1.59), tocilizumab (N = 72, ROR = 1.29, 95% CI: 1.02–1.63).
Although etanercept had a relatively high number of reported cases (521), its ROR value was 0.89 (95% CI: 0.82–0.97), with a PRR of 0.89, X2 of 6.76, and an IC025 of −0.29, indicating a negative signal. Similarly, lenalidomide, with 145 cases, had an ROR of 0.39 (95% CI: 0.33–0.46), a PRR of 0.39, X² of 138.43, and an IC025 of −1.58, also showing a negative signal. Tofacitinib, with 126 cases, had an ROR of 0.90 (95% CI: 0.75–1.07), a PRR of 0.90, X2 of 1.42, and an IC025 of −0.41, which similarly indicated a negative signal.
Discussion
Our analysis of publicly available FAERS data has revealed significant patterns and developments in the documentation of DIP reactions. Among these findings, immunosuppressants, monoclonal antibodies, antineoplastic agents, antibacterials, and psychoanaleptics emerge as prominent drug classes frequently reported to DIP. During the study period, females accounted for the majority of reported DIP reactions submitted to the FDA, representing 55.71% of the cases. This finding is consistent with previous literature, which indicates a slightly higher incidence of DIP among females9.
The top-ranking drug classes associated with DIP in cases reported to the FDA were immunosuppressants and monoclonal antibodies. The linear regression analysis for these two categories showed a slight upward trend that was statistically significant, a finding not previously mentioned in the literature. However, this trend may be influenced by factors such as reporting bias, changes in prescribing patterns, or increased awareness rather than a true rise in risk. Given the lack of denominator data in FAERS, it remains unclear whether this pattern reflects an actual increase in incidence per patient or is merely a result of greater drug utilization. To better understand the clinical significance of this trend, further epidemiological studies using structured datasets are necessary to inform clinical decision-making and patient management.
Pre-marketing trials often struggle to detect delayed or rare adverse reactions due to small sample sizes and short durations. However, these limitations can be mitigated by analyzing the FAERS database. In this study, adverse event reports from FAERS were analyzed using the ROR method to identify significant signals, which were then validated with PRR and BCPNN. The ROR method captured nearly all positive signals detected by PRR and BCPNN.
Through disproportionality analysis, we identified several drugs with strong signal values. Consistent with these findings, literature reviews provide substantial evidence supporting the photosensitizing effects of these drugs. For example, lamotrigine, vemurafenib, simeprevir, voriconazole, pirfenidone, and doxycycline are well-documented in the literature as having higher incidences of DIP, corroborating the reliability of our signal identification results. A case report14 published in the Asian Journal of Psychiatry in 2010 described an instance of toxic epidermal necrolysis after sun exposure, likely due to lamotrigine and chlorpromazine. In a 2014 open-label, multicenter safety study15 on vemurafenib, photosensitivity reactions were observed in 31% of participants. Additionally, a Phase III clinical trial16 with a vemurafenib cohort (n = 336) reported an incidence of photosensitivity reactions of 33% for all grades, while a Phase II clinical trial17 (n = 132) reported an incidence rate of 52% for all grades. Simeprevir is a photodynamically active sulfonamide, and numerous studies have reported photosensitivity reactions induced by simeprevir18,19,20,21, including case reports and clinical trials. The drug’s label also mentions photosensitivity as an adverse reaction, most of which are mild (Grade 1); only 2 out of 317 subjects (< 1%) reported Grade 2 photosensitivity reactions. In a review5, sumatriptan was mentioned to cause photosensitivity as an adverse skin reaction. However, no specific literature detailing this was found, although the drug’s label does list photosensitivity as a potential adverse effect. Voriconazole has been associated with photosensitivity in five studies involving 129 cases, although its drug label does not mention photosensitivity22,23,24,25. In 2023, Akkuş E26 reported a case of photodermatitis as a side effect in a patient treated with pirfenidone for idiopathic pulmonary fibrosis (IPF). The drug label for pirfenidone also lists photosensitivity as a potential adverse effect. Similarly, the drug label for doxycycline mentions photosensitivity as an adverse reaction.
Additionally, some of the drugs identified in our signal detection have drug labels that mention photosensitivity as an adverse reaction, even though there are no case reports or clinical trial evidence to support these claims. For example, the drug label for certolizumab pegol mentions photosensitivity. For niraparib, the Japanese Zejula drug label reports photosensitivity-related skin reactions in 5–10% of cases. Similarly, the GSK drug label mentions photosensitivity, while the Chinese drug label does not. However, the latter does note that photosensitivity was observed in post-marketing use, although the causal relationship with the drug remains unclear. No relevant literature was found for Rubraca, although its drug label does mention photosensitivity, and one pharmacovigilance study from the JADER database reports this reaction. Peginterferon alfa-2a is another drug whose drug label lists photosensitivity as a potential adverse effect.
We also identified several novel signals for DIP. Notably, we discovered that adalimumab, an anti-TNF agent, is associated with lupus and other dermatological symptoms, but no prior literature or drug labels have reported DIP as an adverse reaction. Similarly, we found adverse events related to adapalene, a retinoid; however, unlike other drugs in its category, Adapalene has not been previously associated with DIP, and there are no clinical articles supporting this link. We also noted a lack of supporting literature for other drugs such as secukinumab and fingolimod, which similarly have no mention of DIP in their drug labels.
In conclusion, our analysis of FAERS data corroborates previous population studies on DIP. This suggests that FAERS data can be effectively utilized to identify patterns and signals related to DIP reactions. Such insights can aid clinical practitioners in recognizing and addressing the clinical risks associated with these drugs. Early monitoring and vigilance can thus significantly mitigate the risks of adverse drug reactions in clinical settings.
Methods
Data source and collection
The quarterly data extraction document (2004Q1–2023Q4) was obtained by downloading from https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html. The dataset includes demographic and administrative details, initial report case identification numbers, drug and reaction information, patient outcomes, and reporting source information. FAERS data files are available for free download by selecting a year and choosing the desired quarter in ASCII format.
Signal detection method
A query was conducted using the following medical terms: actinic prurigo, hartnup disease, juvenile spring eruption, photosensitivity reaction, polymorphic light eruption, solar dermatitis, sunburn, photodermatosis, injection site photosensitivity reaction, application site photosensitivity reaction, infusion site photosensitivity reaction, actinic elastosis, chronic actinic dermatitis, implant site photosensitivity, administration site photosensitivity reaction, medical device site photosensitivity reaction, vaccination site photosensitivity reaction, and hydroa vacciniforme in the FDA data field. The search results were filtered to include data from 2004 to 2023. Drug names were standardized to their genericname using drugbank, and drug classification was conducted using WHO ATC data from https://www.whocc.no/atc_ddd_index/, supplemented and adjusted manually.
The number of adverse events associated with specific drugs or drug categories across different years was counted to identify the top drugs. The top countries or regions with the highest number of reported adverse events were then identified based on the country of occurrence, and the top 10 drugs in these countries or regions were calculated.
Data mining
Disproportionality analysis, including algorithms such as the reporting odds ratio (ROR), proportional reporting ratio (PRR), and bayesian confidence propagation neural network (BCPNN), is a crucial tool for identifying safety signals13. Due to the advantages of calculating ROR in spontaneous report databases, ROR values were used as the primary signal detection metric in our study. ROR values were calculated for each preferred term (PT). Additionally, we calculated PRR values and IC values using the BCPNN method and compared these results with the signals identified by ROR values. The criteria and equations used in these algorithms are detailed in Table 3.
Data import and extraction were performed using MySQL version 15.0 and Navicat Premium 15, while statistical analyses were conducted with Microsoft Excel 2021.
Data availability
The datasets generated and/or analysed during the current study are available in the FDA website repository, https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
References
Davis, A. E. et al. The phenomenon of phototoxicity and long-term risks of commonly prescribed and structurally diverse drugs. J. Photochem. Photobiol. 19, 100221 (2024).
Pai, S. B. & Anchan, V. N. Phototoxicity and photoallergy—A brief review on pathogenesis, recent advances, and approach to a patient with photosensitivity. IJSA 3, 28–35 (2024).
Blakely, K. M., Drucker, A. M. & Rosen, C. F. Drug-Induced photosensitivity—An update: Culprit drugs, prevention and management. Drug Saf. 42, 827–847 (2019).
Khandpur, S., Porter, R. M., Boulton, S. J. & Anstey, A. Drug-induced photosensitivity: New insights into pathomechanisms and clinical variation through basic and applied science. Br. J. Dermatol. (2016).
Hofmann, G. A. & Weber, B. Drug-induced photosensitivity: Culprit drugs, potential mechanisms and clinical consequences. J. Dtsch. Derma Gesell. 19, 19–29 (2021).
Zuba, E. B., Koronowska, S., Osmola, A. & Jenerowicz, D. Drug-induced photosensitivity. Acta Dermatovenerologica Croatica.
Gómez-Bernal, S. et al. Photosensitivity due to thiazides. Actas Dermo-Sifiliográficas. (Engl. Ed.). 105, 359–366 (2014).
Lugović-Mihić, L. Drug-induced photosensitivity—a continuing diagnostic challenge. acc 277–283 (2017). https://doi.org/10.20471/acc.2017.56.02.11
Alrashidi, A. et al. Systemic drug photosensitivity—culprits, impact and investigation in 122 patients. Photoderm. Photoimm. Photomed. 36, 441–451 (2020).
Kim, W. B. et al. Drug-induced phototoxicity: A systematic review. J. Am. Acad. Dermatol. 79, 1069–1075 (2018).
Sakaeda, T., Tamon, A., Kadoyama, K. & Okuno, Y. Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10, 796–803 (2013).
Fei, W., Shen, J. & Cai, H. Causes of Drug-Induced severe cutaneous adverse reaction epidermal necrolysis (EN): An analysis using FDA adverse event reporting system (FAERS) database. CCID Volume. 16, 2249–2257 (2023).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523 (2004).
Huang, H. T., Chang, C. L. & Tzeng, D. S. Toxic epidermal necrolysis after sun-exposure probably due to lamotrigine and chlorpromazine. Asian J. Psychiatry. 3, 240–242 (2010).
Larkin, J. et al. Vemurafenib in patients with BRAFV600 mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 15, 436–444 (2014).
Chapman, P. B. et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: Final overall survival results of the randomized BRIM-3 study. Ann. Oncol. 28, 2581–2587 (2017).
Sosman, J. A. et al. Survival in BRAF V600–Mutant advanced melanoma treated with Vemurafenib. N. Engl. J. Med. 366, 707–714 (2012).
Borgia, F. et al. Mucocutaneous toxicity during Simeprevir treatment for hepatitis C. A single institution, retrospective case series. Brit J. Clin. Pharm. 83, 1152–1154 (2017).
Bourgeois, S. et al. Pharmacokinetic interactions between Simeprevir and Ledipasvir in Treatment-Naive hepatitis C virus genotype 1-Infected patients without cirrhosis treated with a Simeprevir-Sofosbuvir-Ledipasvir regimen. Antimicrob. Agents Chemother. 61, e01217–e01217 (2017).
Eyre, Z. W., Secrest, A. M. & Woodcock, J. L. Photo-induced drug eruption in a patient on combination Simeprevir/sofosbuvir for hepatitis C. JAAD Case Rep. 2, 224–226 (2016).
Manns, M. et al. Simeprevir with pegylated interferon Alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 384, 414–426 (2014).
Epaulard, O. et al. A multistep Voriconazole-Related phototoxic pathway May lead to skin carcinoma: Results from a French nationwide study. Clin. Infect. Dis. 57, e182–e188 (2013).
Haylett, A. K., Felton, S., Denning, D. W. & Rhodes, L. E. Voriconazole-induced photosensitivity: Photobiological assessment of a case series of 12 patients: Voriconazole-induced photosensitivity. Br. J. Dermatol. 168, 179–185 (2013).
Sheu, J., Hawryluk, E. B., Guo, D., London, W. B. & Huang, J. T. Voriconazole phototoxicity in children: A retrospective review. J. Am. Acad. Dermatol. 72, 314–320 (2015).
Van Hasselt, J. G. C., Van Eijkelenburg, N. K. A., Huitema, A. D. R. & Schellens, J. H. M. Schouten-van Meeteren, A. Y. N. Severe skin toxicity in pediatric oncology patients treated with voriconazole and concomitant methotrexate. Antimicrob. Agents Chemother. 57, 2878–2881 (2013).
Akkuş, E., Çelik, D., Yetkin, Ö. & Lakadamyalı, H. Photodermatit developed with Pirfenidone treatment in patient diagnosed with IPF. JOPIC 1, 76–78 (2023).
Author information
Authors and Affiliations
Contributions
Lele Ge, Xianghua Huang, and Tao Zhong wrote the manuscript; Lele Ge, Tao Zhong designed the research; Xianghua Huang and Zhu Dong performed the research; Xianghua Huang and Zhu Dong acquired and analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ge, L., Huang, X., Dong, Z. et al. Causes of drug-induced photosensitivity: an analysis using FDA adverse event reporting system database. Sci Rep 15, 18102 (2025). https://doi.org/10.1038/s41598-025-03114-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03114-4