Abstract
To accomplish maximal safe resection for insular glioma (IG), we perform awake surgery (AS) for IG. The aim of our study was to identify potential predictors of AS failure for IG. We retrospectively reviewed the records of 24 patients with IGs who underwent resection between January 2014 and April 2024. Their baseline characteristics and clinical outcomes were examined and we analysed the factors associated with AS failure. AS was planned and performed successfully in 13 cases (the AS group). In five cases, AS was planned but failed due to sleepiness or delayed awakening. AS was not planned in six cases. These 11 cases were assigned to the non-AS (NAS) group. The median extent of tumor resection was 87.7% in the AS group vs. 58.0% in the NAS group (p = 0.004). There were no cases with postoperative permanent neurological deficits in both groups. Basal ganglionic involvement, which means the high-intensity area on Fluid Attenuated Inversion Recovery imaging is found in the basal ganglia consisting of the striatum and globus pallidus, was significantly associated with AS failure. AS contributes significantly to the maximal resection of IG. Basal ganglionic involvement is a potential predictor of AS failure for IG.
Similar content being viewed by others
Introduction
The surgical principle for glioma is maximal safe resection. Insular glioma (IG) resection remains challenging because of the complex functional involvement and the intricate vascularization of the insular lobe1,2,3,4,5,6,7. The insular lobe has a variety of functions ranging from sensory processing to representing feelings and emotions, autonomic and motor control, risk prediction and decision-making, self-awareness, and complex social functions like empathy8,9. In terms of anatomy, the insular lobe has a complex shape and a rich vascular supply extending from the internal carotid and middle cerebral arteries. Besides, the basal ganglia, which is related to sleep and wakefulness, is located adjacent to the insular cortex. There is evidence that the extent of tumor resection (EOR) greatly impacts survival in patients with IGs10,11. Therefore, we need a surgical strategy to increase the EOR. To accomplish the maximal safe resection for IG, we use functional magnetic resonance imaging (MRI), tractography, and the Wada test routinely as a preoperative examination, and cortical and subcortical mapping, sensory-evoked potential (SEP), motor-evoked potential (MEP), and cortico-cortical evoked potential (CCEP) as an intraoperative monitoring. In addition, we perform awake surgery (AS) with intraoperative MRI (iMRI) for IG. However, in some cases, there is a relative contraindication to AS because of various factors and risks12,13,14,15. Therefore, the benefits of AS should outweigh its risks and it would be beneficial to know in advance whether AS will be successful to avoid its risk. There have been several studies about the predictors of AS failure for glioma, while no reports have been published regarding the predictors of AS failure for IG. In this study, we aimed to identify potential predictors of AS failure for IG by evaluating the usefulness of AS.
Results
Baseline patient population
Twenty-seven patients underwent surgery for IG over 10 years at Kyoto University Hospital. Of these, 24 who underwent surgical resection were included in the final analysis. AS was planned for 18 patients. AS was successfully performed for 13 (Successful AS group), while five others experienced AS failure due to sleepiness or delayed awakening (Failed AS group). AS was not planned for six patients because of cognitive impairment, low Karnofsky Performance Status (KPS), location on the non-dominant side, or age below 10 years. As a result, 13 and 11 patients were included in the AS and NAS groups, respectively (Fig. 1). The demographic and baseline characteristics of the patients in the two groups are presented in Table 1. The median age was 47.5 years, with 70.1% of patients being male. Based on WHO (World Health Organization) 2021 classification, there were 13 astrocytomas, 4 oligodendrogliomas, 6 glioblastomas, and 1 diffuse paediatric-type high-grade glioma, H3-wildtype and IDH-wildtype. In all cases, iMRI was employed. The NAS group had a higher proportion of patients with basal ganglionic involvement than the AS group (72.7% vs. 23.1%, p = 0.04), while the other baseline characteristics did not differ significantly between the two groups.
Patient flow chart. Awake surgery (AS) was planned and performed successfully in 13 cases, which were assigned to the AS group (the successful AS group). In five cases, AS was planned but failed due to sleepiness or delayed awakening (the failed AS group). AS was not planned in six cases (the unplanned AS group). These 11 cases (failed AS + unplanned AS) were assigned to the NAS group.
Surgical outcomes
The surgical outcomes of the participants in the two groups are presented in Table 2. The median postoperative residual tumor volume was significantly lower in the AS group than in the NAS group (2.2 ml vs. 41.4 ml, p = 0.015), and the EOR was significantly higher in the AS group than in the NAS group (87.7% vs. 58.0%, p = 0.004). There were no statistically significant differences in the additional resection after iMRI, ischemic complication, hemorrhagic complication, and KPS deterioration at discharge between the two groups. The incidence of postoperative transient neurological deficits was 15.4% in the AS group and 18.2% in the NAS group. There were no cases with postoperative permanent neurological deficits in this study.
Awake surgery (AS) failure
There were five cases of AS failure in this analysis (Tables 3 and 4), and they were all due to sleepiness or delayed awakening. The failed AS group had a significantly higher proportion of patients with basal ganglionic involvement than the successful AS group (100% vs. 23.1%, p = 0.007), suggesting that basal ganglionic involvement is a potential predictor of AS failure for IG. The other baseline characteristics did not differ significantly between the two groups (Table 3). The presence of known predictors associated with AS failure and basal ganglionic involvement in each case is summarized in Table 4.
Discussion
In our hospital, AS is performed for IG to accomplish the maximal safe resection in principle. In this study, the authors demonstrated that AS can contribute to the increase in EOR for IG and that basal ganglionic involvement is a potential predictor of AS failure for IG.
A meta-analysis has demonstrated that AS with intraoperative stimulation mapping is associated with more extensive glioma resection and fewer late severe neurologic deficits16. It has also been described that brain mapping during awake craniotomy helps to maximize EOR while preserving neurological function in patients with dominant insular lobe glioma17. In our study, EOR was significantly higher in the AS group than in the NAS group (87.7% vs. 58.0%, p = 0.004; Table 2), suggesting that AS is associated with the increase in EOR for IG and can contribute to the increase in EOR for IG. AS helps us to aggressively resect the tumor close to the internal capsule and language areas by performing cortical and subcortical mapping10,18. The EOR of IG in the AS group in our study was comparable to that reported in previous studies10,11,19,20,21. However, the EOR of IG in the NAS group in our study was relatively low (Table 5). This finding suggests that we should predict and avoid AS failure and strive to increase the EOR in the NAS group, which includes cases of AS failure. In our study, the rate of postoperative transient neurological deficit and postoperative permanent neurological deficit was 15.4% and 0%, respectively, in the AS group. These rates are comparable to those reported in previous studies. Moreover, in the NAS group, the rates of postoperative transient and permanent neurological deficits were also comparable to those in previous studies (Table 5)10,11,19,20,21,22. These results suggest that we prioritized safe IG resection in the NAS group, leading to a decrease in the EOR.
There have been several studies referring to the predictors of AS failure, although the definition of AS failure is different between them. It has been described that preoperative cognitive dysfunction, low KPS, female patients, left-sided surgery, remifentanil administration, asthma, long duration of surgery after cortical mapping, aphasia, IDH1 wild-type tumors, and recurrence (prior operation), 70 years or older, uncontrolled epileptic seizures, previous oncological treatment, hyperperfusion on MRI, and mass effect on midline were predictors of AS failure (Table 6)13,15,23,24,25,26,27. To the best of our knowledge, this is the first report demonstrating that basal ganglionic involvement is a potential predictor of AS failure for IG. It has also been reported that there are several contraindications to AS, including a significant mass effect despite preoperative diuretics and steroids, obesity (Body mass index > 30), obstructive apnea, psychiatric history, emotional instability, juvenility (Age < 10 years), and severe preoperative function impairment12,14. Appropriate patient selection is crucial for ensuring AS success28. In this study, we had five cases of AS failure (Tables 3 and 4). There was a higher incidence of AS failure in this study (5 in 18 cases). The rate of AS failure depends on the definition of AS failure. In the present study, we defined AS failure as cases in which neurological monitoring could not be performed due to sleepiness or delayed awakening during planned AS. Among these five cases, the only common denominator was basal ganglionic involvement, which means the high-intensity area on FLAIR imaging is found in the basal ganglia consisting of the striatum and globus pallidus. Basically, the basal ganglia are involved in motor function, habit formation, and reward or addictive behaviors. In addition, findings based on electrophysiology, neurotoxic lesioning, and the use of transgenic animals have established that the striatum and globus pallidus are key structural elements for the control of sleep and wakefulness. The basal ganglia are strongly connected to the cortex, thalamus, and amygdala, as well as midbrain dopaminergic neurons. They act as a cohesive functional unit in regulating the vigilance state of wakefulness29,30,31. Therefore, our findings are in accordance with these previous reports. In cases with basal ganglionic involvement, modifying the anesthetic technique may represent a viable option.
Nevertheless, our study has some limitations. Being a single-center study with a relatively small sample size, its findings have limited generalizability. Therefore, more multicenter studies with larger samples should be conducted in the future to further investigate our findings.
Conclusions
AS with iMRI contributes significantly to the maximum possible safe resection for IG. It is necessary to discuss and measure predictors of AS failure before surgery. Basal ganglionic involvement could be a good predictor of AS failure for IG.
Methods
Patient population
This study is approved by Ethics Committee of Kyoto University Graduate School of Medicine (approval number: R2088). The requirement for individual informed consent was waived by Ethics Committee of Kyoto University Graduate School of Medicine. The study was conducted in accordance with the Declaration of Helsinki. In this single-center retrospective study, we collected data on IGs for which craniotomy was performed at Kyoto University Hospital between January 2014 and April 2024. When we can monitor neurological findings during planned AS, we define the surgery as successful AS. On the other hand, when we cannot monitor neurological findings due to sleepiness or delayed awakening during planned AS, we define the surgery as failed AS. Patients for whom AS was performed successfully were assigned to the “Awake surgery group (AS group) or Successful AS group” and those for whom AS failed or AS was not planned intentionally based on the previous reports regarding contraindications to AS were assigned to the “Non-awake surgery group (NAS group).”12,14,32. In addition, the authors assigned the patients for whom AS failed to the “Failed AS group” and the patients for whom AS was not planned intentionally to the “Unplanned AS group”.
Preoperative examination
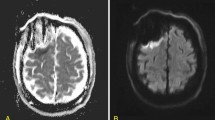
The MRI protocol was a standard T1-weighted MRI with gadolinium contrast, T2-weighted MRI, and Fluid Attenuated Inversion Recovery (FLAIR) MRI in all patients. The high-intensity area on FLAIR imaging that is found in the basal ganglia consisting of the striatum and globus pallidus was defined as basal ganglionic involvement (Fig. 2). In addition, we used functional MRI, tractography, and the Wada test to establish the dominant side and the locations of language-related functions33,34. Preoperative assessments of higher cognitive functions, including language, and motor functions were conducted by speech therapists (STs), physical therapists (PTs), and occupational therapists (OTs).
Awake surgery, intraoperative monitoring, and intraoperative MRI (iMRI)
In principle, we performed the resection of IG through a transsylvian approach. AS was performed as per the Guidelines for Awake Surgery Committee of The Japan Awake Surgery Conference32. Patients were positioned in a semilateral position with the head turned parallel to the floor. After craniotomy, cortical and subcortical language, motor, and sensory mapping were conducted with CCEP, MEP, and SEP. Defining the medial resection boundary is feasible in most cases through identification of the lenticulostriate arteries and functional mapping of the internal capsule10,18,35. Intraoperative assessments of language and motor functions were conducted by STs, PTs, and OTs. The selected tasks were practiced several times preoperatively until the patient was able to respond with confidence. As neuropsychological testing, language tasks, including spontaneous speech, naming (visual naming and naming from verbal descriptions) and counting, were performed. We performed iMRI using High-field 3 Tesla MRI, MAGNETOM Verio Dot (SIEMENS). Intraoperative imaging protocol included T1-weighted MRI with gadolinium contrast and FLAIR. iMRI was conducted based on the surgeon’s assessment that the majority of the targeted lesion had been resected within functional boundaries identified through awake brain mapping. If any residual resectable tumor was detected, additional resection was performed.
Patient outcome measurements
Neurosurgical examinations were performed preoperatively and one day, one week, three months, and six months after surgery. Patients with no clinical improvement at the 6-month follow-up examination were considered to have a permanent neurological deficit. In all cases, MRI was performed one day after surgery and we evaluated ischemic and hemorrhagic complications.
Volumetric analysis
The Brainlab Elements software (Origin server 3.3., Brainlab, Germany, https://www.brainlab.com/) was used to compute volumetric analyses of both preoperative and postoperative tumoral volume by using contrast-enhanced T1-weighted images for glioblastomas and FLAIR images for the other types of tumor. The EOR was calculated as follows: (preoperative tumor volume − postoperative tumor volume)/preoperative tumor volume.
Statistical analysis
Continuous variables were presented as median values with ranges. They were assessed for normality and compared using the Mann–Whitney U. Categorical variables were presented as frequencies and percentages and compared using the χ2 test or Fisher’s exact test. A P-value of < 0.05 was considered statistically significant. Analyses were performed using the statistical software JMP (Version 16.1.0, SAS Institute Inc., Cary, NC, USA, https://www.jmp.com/en/software).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Di Carlo, D. T. et al. Post-operative morbidity ensuing surgery for insular gliomas: a systematic review and meta-analysis. Neurosurg. Rev. 43, 987–997 (2020).
Hervey-Jumper, S. L. & Berger, M. S. Insular glioma surgery: an evolution of thought and practice. J. Neurosurg. 130, 9–16 (2019).
Przybylowski, C. J., Hervey-Jumper, S. L. & Sanai, N. Surgical strategy for insular glioma. J. Neurooncol. 151, 491–497 (2021).
Duffau, H. et al. The insular lobe: physiopathological and surgical considerations. Neurosurgery 47, 801–810 (2000) (discussion 810 – 801).
Pitskhelauri, D. et al. Transsylvian insular glioma surgery: new classification system, clinical outcome in a consecutive series of 79 cases. Oper. Neurosurg. (Hagerstown). 20, 541–548 (2021).
Pallud, J. et al. Surgery of insular diffuse Gliomas-Part 1: transcortical awake resection is safe and independently improves overall survival. Neurosurgery 89, 565–578 (2021).
Singh, A. et al. Insular glioblastoma: surgical challenges, survival outcomes and prognostic factors. Br. J. Neurosurg. 37, 26–34 (2023).
Gogolla, N. The insular cortex. Curr. Biol. 27, R580–R586 (2017).
Uddin, L. Q., Nomi, J. S., Hébert-Seropian, B., Ghaziri, J. & Boucher, O. Structure and function of the human Insula. J. Clin. Neurophysiol. 34, 300–306 (2017).
Sanai, N., Polley, M. Y. & Berger, M. S. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J. Neurosurg. 112, 1–9 (2010).
Skrap, M. et al. Surgery of insular nonenhancing gliomas: volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases. Neurosurgery 70, 1081–1093 (2012) (discussion 1093 – 1084).
Gogos, A. J., Young, J. S., Morshed, R. A., Hervey-Jumper, S. L. & Berger, M. S. Awake glioma surgery: technical evolution and nuances. J. Neurooncol. 147, 515–524 (2020).
Abaziou, T. et al. Incidence and predicting factors of perioperative complications during monitored anesthesia care for awake craniotomy. J. Clin. Anesth. 64, 109811 (2020).
Hervey-Jumper, S. L. et al. Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J. Neurosurg. 123, 325–339 (2015).
Elia, A. et al. A preoperative scoring system to predict Function-Based resection limitation due to insufficient participation during awake surgery. Neurosurgery 93, 678–690 (2023).
De Hamer, W., Robles, P. C., Zwinderman, S. G., Duffau, A. H., Berger, M. S. & H. & Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J. Clin. Oncol. 30, 2559–2565 (2012).
Alimohamadi, M. et al. Application of awake craniotomy and intraoperative brain mapping for surgical resection of insular gliomas of the dominant hemisphere. World Neurosurg. 92, 151–158 (2016).
Moshel, Y. A., Marcus, J. D., Parker, E. C. & Kelly, P. J. Resection of insular gliomas: the importance of lenticulostriate artery position. J. Neurosurg. 109, 825–834 (2008).
Duffau, H. A personal consecutive series of surgically treated 51 cases of insular WHO grade II glioma: advances and limitations. J. Neurosurg. 110, 696–708 (2009).
Eseonu, C. I., ReFaey, K., Garcia, O., Raghuraman, G. & Quinones-Hinojosa, A. Volumetric analysis of extent of resection, survival, and surgical outcomes for insular gliomas. World Neurosurg. 103, 265–274 (2017).
Renfrow, J. J. et al. A review on the surgical management of insular gliomas. Can. J. Neurol. Sci. 50, 1–9 (2023).
Lu, V. M., Goyal, A., Quinones-Hinojosa, A. & Chaichana, K. L. Updated incidence of neurological deficits following insular glioma resection: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 177, 20–26 (2019).
Itoi, C. et al. Predicting sleepiness during an awake craniotomy. Clin. Neurol. Neurosurg. 139, 307–310 (2015).
Lin, H. T. et al. Predictors for delayed awakening in adult glioma patients receiving awake craniotomy under monitored anesthesia care. J. Neurooncol. 165, 361–372 (2023).
Gernsback, J. E., Kolcun, J. P. G., Starke, R. M., Ivan, M. E. & Komotar, R. J. Who needs sleep?? An analysis of patient tolerance in awake craniotomy. World Neurosurg. 118, e842–e848 (2018).
Mizota, T. et al. Factors associated with somnolence during brain function mapping in awake craniotomy. J. Clin. Neurosci. 89, 349–353 (2021).
Takami, H., Khoshnood, N. & Bernstein, M. Preoperative factors associated with adverse events during awake craniotomy: analysis of 609 consecutive cases. J. Neurosurg. 134, 1631–1639 (2021).
Nossek, E. et al. Failed awake craniotomy: a retrospective analysis in 424 patients undergoing craniotomy for brain tumor. J. Neurosurg. 118, 243–249 (2013).
Lazarus, M., Huang, Z. L., Lu, J., Urade, Y. & Chen, J. F. How do the basal ganglia regulate sleep-wake behavior? Trends Neurosci. 35, 723–732 (2012).
Qiu, M. H., Vetrivelan, R., Fuller, P. M. & Lu, J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur. J. Neurosci. 31, 499–507 (2010).
Vetrivelan, R., Qiu, M. H., Chang, C. & Lu, J. Role of basal ganglia in sleep-wake regulation: neural circuitry and clinical significance. Front. Neuroanat. 4, 145 (2010).
Guidelines Committee of the Japan Awake Surgery Conference. Guidelines for Awake Surgery. Neurol Med Chir (Tokyo). 64, 1–27. (2024).
Mikuni, N. & Miyamoto, S. Surgical treatment for glioma: extent of resection applying functional neurosurgery. Neurol. Med. Chir. (Tokyo). 50, 720–726 (2010).
Mikuni, N. et al. Evaluation of adverse effects in intracarotid Propofol injection for Wada test. Neurology 65, 1813–1816 (2005).
Saito, R. et al. Magnetic resonance imaging for preoperative identification of the lenticulostriate arteries in insular glioma surgery. Technical note. J. Neurosurg. 111, 278–281 (2009).
Acknowledgements
We would like to thank Ai Demura (Department of Neurosurgery, Kyoto University Hospital, Kyoto, Japan) for their technical assistance.
Author information
Authors and Affiliations
Contributions
Original draft preparation, S.T.; Review and editing, Y.A.; Data collection, S.T.; All authors reviewed the manuscript, the tables, and the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takada, S., Hattori, E.Y., Sano, N. et al. Potential predictors of awake surgery failure for insular glioma. Sci Rep 15, 17953 (2025). https://doi.org/10.1038/s41598-025-03219-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03219-w