Abstract
This study was designed to comprehensively elucidate the mechanism by which human umbilical cord-derived mesenchymal stem cell (hUC-MSC) therapy modulates epithelial cell apoptosis and preserves the integrity of the intestinal barrier. A series of meticulously planned in vivo and in vitro experiments were conducted to evaluate the effects of hUC-MSC treatment on various pathological parameters. Our in vivo findings demonstrated that hUC-MSC therapy significantly attenuated pathological damage, as evidenced by decreased tissue necrosis and inflammation. Furthermore, ultrastructural injury to the intestinal epithelium and mast cell infiltration were notably alleviated. With respect to the systemic immune response, serum levels of key immune factors, including IgG, IgE, mouse mast cell protease (mMCP-1), histamine, interleukin (IL)-6, and tumor necrosis factor (TNF)-α, were observably reduced following hUC-MSC treatment. At the molecular level, in jejunal tissue from food allergy mice treated with hUC-MSCs, the expression of IL-6 and TNF-α mRNA was downregulated, whereas the expression of , interferon (IFN)-γ, T-box transcription factor (T-bet) mRNA was upregulated. In vitro experiments using histamine-induced apoptosis in FHs 74 Int cells revealed that hUC-MSC therapy led to a marked decrease in the expression levels of pro-apoptotic proteins Bax, caspase-3, and cleaved caspase-3. Concurrently, significant upregulation of both the anti-apoptotic protein Bcl-2 and tight junction protein Zonula Occludens-1 (ZO-1) was observed. hUC-MSCs substantially improved cellular viability during histamine challenge by suppressing apoptosis (quantified by Annexin V/7-AAD staining) and reducing reactive oxygen species (ROS) generation in FHs 74 Int cells, demonstrating dual cytoprotective effects against histamine-induced toxicity. Our findings provide compelling evidence that hUC-MSCs mediate anti-apoptotic effects via modulation of the Bax/Bcl-2 pathway in histamine-stimulated intestinal mucosa during allergic responses. These results reveal novel therapeutic potential for hUC-MSCs in maintaining epithelial homeostasis and intestinal barrier function in food allergy-associated enteritis, suggesting promising clinical applications for this disorder.
Similar content being viewed by others
Introduction
Food allergic enteritis represents a spectrum of clinicopathological disorders characterized by multiple pathological processes triggered by diverse allergens. In recent years, the incidence and severity of food allergies in children have risen significantly, driven by environmental factors, genetic predisposition, consumption of processed foods, and other contributing factors1,2. Studies indicate that approximately 50% of patients with IgE-mediated food allergies experience at least one severe allergic reaction during childhood or adolescence3,4. Cow’s milk protein allergy, the leading cause of food allergy in early childhood, can progress to more complex IgE-mediated food allergies, often accompanied by comorbid allergic conditions such as asthma, rhinitis, eczema, and even refractory eosinophilic esophagitis. Unfortunately, safe and effective therapeutic options remain limited5.
The onset of severe allergic reactions is initiated by the specific binding of epitopes to IgE receptors, triggering the rapid and systemic activation of mast cells and the subsequent release of histamine and other inflammatory mediators. This cascade of events induces a series of pathological changes, including disruption and dysfunction of the intestinal mucosal barrier, as well as edema, necrosis, and apoptosis of histopathological cells. Food-allergic enteritis and inflammatory bowel disease share similarities in both pathology and pathogenesis, with their occurrence and development being closely interrelated. The host’s immune function and damage to the intestinal epithelial barrier structure are considered key factors in the pathogenesis of food-allergic enteritis; however, the precise mechanisms remain poorly understood, and further research in this area is limited6,7,8. Histamine plays a pivotal role in the acute phase of food allergies, as its uncontrolled release during allergic reactions initiates a multi-systemic cascade of responses, leading to intestinal mucosal damage and systemic organ dysfunction. Furthermore, histamine promotes the release of various inflammatory factors, triggers inflammatory responses in cardiopulmonary tissues, and induces oxidative stress and apoptosis in these organs. These effects have been corroborated by the robust expression of iNOS and tumor necrosis factor (TNF)-α proteins observed in the affected tissues9.
In recent years, human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) have emerged as a promising therapeutic strategy owing to their immunomodulatory and anti-apoptotic properties. As self-renewing pluripotent cells capable of differentiating into multiple lineages, hUC-MSCs secrete a diverse array of cytokines in response to specific inflammatory microenvironments, thereby promoting local tissue repair and anti-apoptotic responses following injury.
Accumulating evidence suggests that MSC-based therapies alleviate allergic reactions and mitigate pathological changes in the intestines of food-allergic mice by reducing the levels of key immune factors, including IgE, mouse mast cell protease (mMCP-1), TNF-α, and interleukin (IL)−4, in both serum and small intestinal tissues. These therapeutic effects are primarily mediated through the modulation of the T helper type 1 (Th1)/Th2 cell balance. Specifically, hUC-MSCs induce a type 2 immune response, which suppresses the pro-inflammatory immune microenvironment, enhances regulatory T cell populations, and reduces mast cell infiltration into intestinal tissues10,11. However, the existing literature on the anti-apoptotic effects of hUC-MSC therapy in allergic enteritis remains scarce, and the underlying mechanisms are yet to be fully elucidated12,13,14.
In this study, we employed an ovalbumin (OVA)-induced food enteritis mouse model to assess the therapeutic efficacy of hUC-MSCs in mitigating food allergic enteritis. Special emphasis was placed on investigating histamine-induced apoptosis of intestinal epithelial cells, mitochondrial dysfunction, and intestinal barrier injury during the progression of food allergy. To our knowledge, this is the first study to demonstrate the therapeutic potential of hUC-MSCs against apoptosis in both murine models and in vitro experiments of food allergic enteritis. Furthermore, we elucidated the mechanisms by which hUC-MSCs regulate epithelial cell apoptosis and preserve intestinal barrier integrity, thereby providing a foundation for their potential clinical translation.
Results
Characterization of the hUC-MSCs
The hUC-MSCs were characterized according to the minimal criteria established by the International Society for Cellular Therapy (ISCT)15,16. Flow cytometric analysis demonstrated high expression levels of typical mesenchymal stem cell (MSC) surface markers, including CD90, CD73, and CD105, while hematopoietic markers such as CD34, CD45, and HLA-DR were undetectable. Furthermore, the hUC-MSCs exhibited multilineage differentiation potential, as evidenced by their ability to differentiate into adipocytes, osteocytes, and chondrocytes under specific induction conditions (Fig. S1).
Effect of hUC-MSC therapy on allergic reactions in a food allergic enteritis model
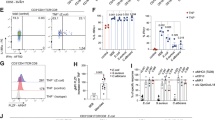
The mouse experiments were conducted according to the experimental protocol (Fig. 1A). Mice induced with OVA exhibited significant allergic reactions, including scratching around the nose and head, labored breathing, immobility, and mortality. Notably, both allergic scores and diarrhea scores were significantly reduced following hUC-MSC therapy (allergic scores: t = 1.222, p = 0.0083; diarrhea scores: t = 1.889, p < 0.0001) (Fig. 1B-C). The incidence of diarrhea (score ≥ 2) after six oral challenges decreased from 88.9% to 44.4% following hUC-MSC treatment (Fig. 1D). The presentation of diarrhea among different groups was also recorded (Fig. 1E). After the sixth oral challenge, rectal temperatures were measured at different time points. Compared to untreated food-allergic mice, a significant increase in temperature was observed at 15 and 30 min post-challenge in hUC-MSC-treated mice (15 min: t = 2.375, p = 0.023; 30 min: t = 2.918, p = 0.01) (Fig. 1F).
(A) Process of allergic sensitization to food allergy mice with OVA: A total of 27 mice were divided into three groups (n = 9 per group), namely the Control group, OVA group, and MSC group. Mice in the OVA and MSC groups were intraperitoneally (i.p.) immunized with 200 µg OVA in 1 mg alum adjuvant dissolved in PBS 200ul on days 0, 5, and 10. On day 15, after tape stripping the skin once, mice in the OVA group underwent six challenges every other day (days 15, 17, 19, 21, 23, and 25). Mice that received hUC-MSCs treatment (1 × 106 cells) were administered on days 19 and 23 in the MSC group. Mice in the control group were i.p. injected and challenged with an equal amount of PBS. Comparison of (B) allergy scores and (C) diarrhea scores among different groups. (D) The occurrence of diarrhea after oral challenges with hUC-MSC therapy. (E) stools of mice in different groups. (F) Rectal temperature–time curve. Each value indicates the mean ± SD. Data are representative of 3 independent experiments. n = 9/group. #p < 0.05 15 min vs. 0 min, **p < 0.01 30 min vs.15 min. (G) Observation in the jejunal tissue by hematoxylin and eosin staining in different groups (magnification, × 200 and × 400). The alterations in tissue cavity morphology, a reduction in goblet cells, and minor nuclear shrinkage with hUC-MSCs therapy. (H) Mast cell filtration by Toluidine blue staining in the jejunal tissue in different groups (magnification, × 200 and × 400). A significant reduction in mast cell infiltration in allergic enteritis mice treated with hUC-MSCs. (I) The representative jejunal ultrastructural imaged by TEM. Red arrows mark key pathological features: swollen mitochondria, dilated ER, damaged epithelium, deformed nuclei, and ruptured membranes. OVA, ovalbumin; hUC-MSC, human umbilical cord-derived mesenchymal stem cell; TEM, Transmission Electron Microscope.
Histology and ultrastructure of intestinal tissues following hUC-MSC therapy
Hematoxylin and eosin (HE) staining, toluidine blue (TB) staining, and transmission electron microscopy (TEM) were employed to assess pathological alterations in jejunal tissues following hUC-MSC treatment for allergic enteritis. HE staining results demonstrated that hUC-MSC therapy attenuated pathological changes in jejunal tissues, including alterations in tissue cavity morphology, a reduction in goblet cells, and minor nuclear shrinkage. Similarly, TB staining revealed a significant reduction in mast cell infiltration in allergic enteritis mice treated with hUC-MSCs (Fig. 1G-H). TEM analysis of jejunal tissues further identified ultrastructural abnormalities, such as swollen mitochondria, dilated endoplasmic reticulum, damaged epithelial cells, nuclear deformation, and membrane rupture. Notably, hUC-MSC treatment significantly ameliorated these ultrastructural injuries (Fig. 1I).
hUC-MSCs alleviated systemic inflammation in mice with allergic enteritis
To evaluate the inflammatory response in OVA-induced mice, serum samples were collected one hour after the final challenge on day 25 and stored at −80 °C until analysis by enzyme-linked immunosorbent assay (ELISA). Notably, significant increases in IgG, IgE, and inflammatory mediators (including mMCP-1, histamine, IL-6, and TNF-α) were observed after six oral challenges, which were markedly attenuated by hUC-MSC treatment (Fig. 2A-F).
Serum concentrations of (A) IgG, (B) IgE, (C) mMCP-1, (D) histamine, (E) IL-6, and (F) TNF-α were quantified by ELISA across all experimental groups. Data are presented as mean ± SD (n = 9 per group). Relative mRNA expression levels of (G) IL-6, (H) TNF-α, (I) IFN-γ, and (J) T-bet in jejunal tissues were assessed by qPCR (n = 4–6 per group). Statistical significance is denoted as *p < 0.05, **p < 0.01, and ***p < 0.001. mMCP-1, mouse mast cell protease-1; IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; T-bet, T-box transcription factor; ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative polymerase chain reaction.
Effects of hUC-MSCs on cytokine mRNA expression in jejunal tissues
In jejunal tissues, the upregulation of IL-6 and TNF-α observed in the OVA group was significantly downregulated following hUC-MSC therapy compared to the Control group. Conversely, the downregulated expressions of Interferon (IFN)-γ and T-box transcription factor (T-bet) in the OVA group were markedly upregulated after hUC-MSC treatment (Fig. 2G-J).
hUC-MSCs enhanced intestinal barrier function in mice with allergic enteritis
The effect of hUC-MSCs on intestinal barrier function was evaluated by assessing the expression of tight junction proteins, including Zonula Occludens (ZO)−1, occludin, and claudin, in the intestinal epithelium. Immunofluorescence analysis demonstrated that hUC-MSC treatment significantly upregulated the expression of these proteins (Fig. 3A). This finding was further corroborated at the protein level through Western blot analysis in mice with allergic enteritis (Fig. 3B).
Effect of hUC-MSCs on intestinal barrier structure of food allergy mice in vivo studies. (A) Immunofluorescence assay and (B) western blotting of ZO-1, occludin, and claudin expression in the jejunal in different groups. This experiment was performed independently three times. Each value represents the means ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. OVA, ovalbumin; hUC-MSC, human umbilical cord-derived mesenchymal stem cell.
hUC-MSCs protected histamine-induced Human small intestinal epithelial cells (FHs 74 Int cells) injury in vitro
FHs 74 Int cells were stimulated with increasing concentrations of histamine (25, 50, 100, 200, and 400 μmol/L) for 24 h, and cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay. The results demonstrated a dose-dependent decrease in cell viability (Fig. 4A). Four experimental groups were established for co-culture over 24 h: (1) Control group (FHs 74 Int cells), (2) Histamine group (FHs 74 Int cells + histamine), (3) Histamine + hUC-MSC group (FHs 74 Int cells + histamine + hUC-MSCs), and (4) hUC-MSC group (FHs 74 Int cells + hUC-MSCs). The supernatant from each group was collected, and the levels of inflammatory cytokines (TNF-α, IL-1β, and IL-6) secreted by FHs 74 Int cells were quantified using ELISA. Compared to the Control group, the Histamine group exhibited significantly elevated levels of TNF-α, IL-1β, and IL-6. Notably, hUC-MSC treatment markedly reduced the levels of these inflammatory cytokines (Fig. 4B-D).
hUC-MSCs released histamine-induced inflammatory injury and apoptosis in the FHs 74 Int cells. (A) The cell viability of FHs 74 Int cells stimulated by different concentration of histamine. FHs 74 Int cells were stimulated with histamine (100umol/L) cocultured in the absence or presence of hUC-MSCs for 24 h. Inflammatory cytokines of (B) TNF-α, (C) IL-1β, and (D) IL-6 in the supernate of cultured cells in different groups with ELISA. (E) Representative flow cytometry plots showing apoptotic cells in different groups (Q4, Annexin V-/7-AAD-), early apoptotic cells (Q3, Annexin V +/7-AAD-), late apoptotic/necrotic cells (Q2, Annexin V +/7-AAD +), and necrotic/damaged cells (Q1, Annexin V-/7-AAD +). (F) The rate of total apoptosis (Q1 + Q2 + Q3). Data are mean ± SD (n = 3); (G) MitoSOX green staining with fluorescence microscopy. Scale bar = 50um. A: Cell viability was measured by Cell Counting Kit-8; E–F: apoptosis was measured by Annexin V-PE apoptosis Detection. * p < 0.05, **p < 0.01, ***p < 0.001, ****p = 0.000.
The results of cellular apoptosis, as assessed by Annexin V/7-AAD staining, revealed that FHs 74 Int cells in the Histamine group exhibited a significant reduction in cell viability compared to the Control group. Notably, treatment with hUC-MSCs significantly restored cell viability, effectively attenuating histamine-induced cellular apoptosis (Fig. 4E-F). Furthermore, MitoSOX Green staining and fluorescence quantification were employed to measure reactive oxygen species (ROS) levels, which are associated with mitochondrial dysfunction and oxidative stress-induced apoptosis. The results revealed that ROS levels in FHs 74 Int cells were markedly elevated in the Histamine group compared to the Control group. Importantly, hUC-MSC treatment substantially reduced ROS levels, indicating a protective role against oxidative damage (Fig. 4G).
Regulation of the Bax/Bcl-2 signaling pathway and intestinal barrier function by hUC-MSCs
To investigate the role of hUC-MSCs in apoptosis regulation, the protein levels of the Bax/Bcl-2 signaling pathway were analyzed using Western blot. Compared to the Control group, histamine treatment significantly increased the expression of pro-apoptotic proteins, including Bax, caspase-3, and cleaved caspase-3, while decreasing the expression of the anti-apoptotic protein Bcl-2 in FHs 74 Int cells. Notably, hUC-MSC treatment reversed these effects by downregulating the expression of pro-apoptotic proteins and upregulating Bcl-2 at the protein level. Furthermore, the impact of hUC-MSCs on intestinal barrier function was assessed by measuring the tight junction protein ZO-1. Compared to the Control group, the protein level of ZO-1 was significantly reduced in the Histamine group. However, hUC-MSC treatment markedly restored ZO-1 expression in vitro. These results suggest that hUC-MSCs mitigate apoptosis by modulating the Bax/Bcl-2 pathway, thereby improving the expression of permeability-related proteins in histamine-induced intestinal epithelial cells (Fig. 5A-B).
Effects of hUC-MSCs on histamine-related signaling pathways in FHs 74 Int cells in vitro (A-B). The expression levels of Bax/Bcl-2 pathway related proteins Bax, Bcl-2, caspase-3, cleaved caspase-3 and level of ZO-1 associated with the intestinal permeability. Cells were treated as above. The density of each band was quantified by ImageJ Software (n = 3, -x ± s). **p < 0.01,*** p < 0.001 vs Control group; #p < 0.05, ##p < 0.01 vs Histamine group.
Discussion
The global prevalence of allergic diseases has been rapidly increasing. During food allergy activation, various cytokines secreted by mast cells, epithelial cells, dendritic cells, basophils, and eosinophils in the skin and mucosa collectively establish an inflammatory microenvironment17. These mediators interact synergistically to initiate a cascade of immune responses, with their release serving as a critical determinant of allergic reaction severity. Mast cells play a particularly crucial role in this process. In IgE/FcεRI-mediated intestinal allergic reactions, mast cells undergo degranulation and proliferation, releasing histamine and other preformed mediators that activate endothelial cells to produce nitric oxide, triggering a signaling cascade. In our study, flow cytometric analysis employing Annexin V-7 AAD staining yielded compelling evidence indicating that histamine treatment significantly increased the apoptosis rate in epithelial cells. This leads to vascular dilation and endothelial barrier dysfunction (e.g., increased vascular permeability), characterized by disrupted cell junctions, enhanced capillary permeability, fluid extravasation, altered intestinal permeability, and microstructural damage18,19,20,21. Food allergic enteritis often affects multiple organ systems, manifesting as vomiting, abdominal pain, diarrhea, and hematochezia7,22,23.
Consistent with previous reports10,11,24, our findings demonstrate that hUC-MSCs effectively attenuate systemic inflammation in food-allergic mice, as shown by significant reductions in key serological markers, including mast cell-derived mediators (histamine and mMCP-1) and allergen-specific immunoglobulins (IgE and IgG). Notably, hUC-MSCs induced a substantial immunomodulatory shift in intestinal tissues, characterized by downregulation of pro-inflammatory cytokines (IL-6 and TNF-α) and upregulation of Th1-associated factors (IFN-γ and T-bet). As a master transcriptional regulator of Th1 cell commitment, T-bet plays a central role in this process by promoting IFN-γ production and restoring immune homeostasis25. Clinically, hUC-MSC administration significantly alleviated allergic symptoms, including diarrhea, and reduced intestinal mucosal pathology, likely through inhibiting mast cell infiltration and activation. These findings collectively demonstrate that hUC-MSCs can simultaneously target multiple pathological pathways in food allergic enteritis, including humoral immunity, T cell polarization, and mast cell-mediated tissue damage.
Our findings demonstrate that hUC-MSCs provide comprehensive protection of intestinal barrier integrity through multifaceted mechanisms. Ultrastructural analysis revealed significant cytoprotective effects, including an reduction in mitochondrial swelling and complete prevention of nuclear membrane rupture. At the molecular level, hUC-MSCs treatment induced substantial upregulation of tight junction proteins, with significant increases in ZO-1, occludin, and claudin expression respectively, while simultaneously reducing necrotic cell death in histlogic tissue. These structural improvements translated to rapid functional recovery, evidenced by core temperature normalization within 30 min post-challenge, The coordinated barrier protection was further reflected in significant attenuation of injury-induced pro-inflammatory cytokine release (IL-6, TNF-α) and mucosal edema reduction. These findings collectively establish hUC-MSCs as a potent therapeutic strategy for preserving intestinal barrier function in allergic colitis through direct epithelial cytoprotection, paracrine modulation of tight junction assembly, and suppression of inflammatory mediator cascades.
Mesenchymal stem cells (MSCs) have emerged as potent immunomodulators and tissue protectors with demonstrated efficacy across multiple allergic disorders, including refractory asthma, contact urticaria, and allergic rhinitis26,27,28,29,30,31. MSCs are capable of secreting the Bcl-2 protein in inflammatory environments, thereby exerting an anti-apoptotic effect. Furthermore, they release fibronectin and periostin, which modulate the migration of airway epithelial cells and promote tissue repair as reported in previous studies32,33,34. Reactive oxygen species (ROS) and apoptosis play pivotal roles in the pathogenesis in cell proliferation and survival, results in macromolecular damage35. In our study, treatment with hUC-MSCs significantly alleviated histamine-induced oxidative stress and mitochondrial dysfunction, as demonstrated by decreased ROS levels and enhanced cell viability. The capacity of hUC-MSCs to scavenge ROS and restore redox balance underscores their antioxidant properties, which are essential for safeguarding cells against oxidative damage.
MSCs exhibit dynamic cytokine secretion profiles (TGF-β, IL-10, IFN-γ) that orchestrate synergistic immunomodulation in inflammatory environments36,37. Our findings demonstrate that hUC-MSCs exert robust anti-apoptotic and anti-inflammatory effects across multiple pathological conditions, including histamine-induced myocarditis, asthma, and intestinal inflammation. In myocarditis models9, MSC treatment significantly downregulated pro-inflammatory cytokines (TNF-α and IL-1β) while modulating apoptotic pathways through reduced expression of caspase-3 and Bax and upregulation of Bcl-2. Similarly, in asthmatic rats, MSC-conditioned medium normalized Bax/Bcl-2 ratios and suppressed caspase-3 activity, leading to marked improvement in airway epithelial pathology38. In our intestinal inflammation models, hUC-MSCs exhibited multimodal protection, probaly including: (1) reduction in apoptosis (Annexin V/7-AAD staining); (2) downregulation of inflammatory mediators (TNF-α, IL-6, IL-1β); (3) inhibition of Bax/Bcl-2 signaling; (4)inhibition of caspase-3/8/9 and NF-κB signaling; and (4) promotion of M2 macrophage polarization39. These coordinated effects preserved epithelial barrier integrity, mitigated mitochondrial dysfunction, and attenuated pathological damage, highlighting the universal therapeutic potential of MSCs in diverse inflammatory conditions through conserved mechanisms of apoptosis regulation and immune modulation.
In conclusion, our study demonstrates that hUC-MSCs exert potent therapeutic effects in food-allergic enteritis through a triad of mechanisms: (1) regulation of apoptosis via the Bax/Bcl-2-caspase-3 axis in intestinal epithelial cells, (2) mitigation of oxidative stress through ROS suppression, and (3) restoration of intestinal barrier integrity via coordinated modulation of inflammatory mediators. Using an OVA-induced murine model, we identified that hUC-MSCs dynamically modulate caspase-3 activation through the Bax/Bcl-2 pathway, thereby orchestrating apoptotic responses in allergen-exposed epithelial cells. Concurrently, hUC-MSCs attenuate histamine-driven inflammatory cascades, preserving tight junction integrity and reducing oxidative damage—a dual-action mechanism that underscores their potential as a multifaceted therapy for food-allergic disorders.
Despite these advances, critical knowledge gaps remain, particularly regarding the identity of key anti-apoptotic factors within the MSC secretome and their temporal secretion profiles. Future research must prioritize the systematic characterization of paracrine signaling dynamics, optimization of delivery strategies (e.g., route, dose, and timing), and preclinical validation of combinatorial approaches with existing immunomodulatory therapies. Additionally, rigorous in vivo studies are needed to delineate context-dependent roles of apoptotic pathways in disease progression and therapy resistance. By addressing these questions, our findings lay the groundwork for translating hUC-MSC-based interventions into clinically viable, standardized treatments for food allergy-related intestinal pathology.
Materials and methods
Animals
All experiments were conducted in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Animal Experiment Committee of the Beijing Center for Physical & Chemical Analysis (Approval No. 210520-SWDWF-005). Female BALB/c mice, aged four weeks and weighing approximately18 ± 2 g, were purchased from SPF (Beijing) Biotechnology Co., Ltd. The mice were housed in microisolator units at the Beijing Center for Physical & Chemical Analysis under controlled environmental conditions, including a 12-h light/dark cycle, a temperature range of 24–26 °C, and a humidity level of 30–50%.
For the food allergic enteritis model, 27 mice were randomly divided into three groups (n = 9 per group). In the OVA-challenged groups, mice received intraperitoneal (i.p.) injections of 200 µg OVA (Cat. No. A5503, Sigma) and 1 mg alum adjuvant (Cat. No. 239186, Sigma) dissolved in 200µL phosphate-buffered saline (PBS) on days 0, 5, and 10. To enhance sensitization, the skin of the mice was tape-stripped once on day 15. Subsequently, the OVA group was subjected to intragastric (i.g.) administration of OVA every other day for a total of six challenges (days 15, 17, 19, 21, 23, and 25)40. The hUC-MSC treatment group received intravenous (i.v.) injections of hUC-MSCs (1 × 106 cells) on days 19 and 23 during the challenge period. The Control group received i.p. injections of an equal volume of phosphate-buffered saline (PBS) and underwent the same challenge protocol.
To evaluate allergic enteritis symptoms in mice during the study period, observations were conducted for up to 60 min following the final challenge. Diarrhea severity was assessed using a modified Likert scale, as previously described41,42. The scoring system was as follows: 0 = normal stools; 1 = few wet and unformed stools; 2 = moderate perianal staining with several wet and unformed stools; 3 = severe watery stools accompanied by significant perianal staining. Allergic responses were evaluated using a 6-point scoring system: 0 = no symptoms; 1 = < 4 episodes of scratching and rubbing around the nose and head; 2 = 4–10 episodes of scratching and rubbing around the nose and head; 3 = > 10 episodes of scratching and rubbing around the nose and head; 4 = hunching and piloerection; 5 = immobility (unresponsive to non-harmful tactile stimulation).
Prior to the final challenge, rectal temperatures were measured in all mice from each experimental group. At the end of the experiment, mice were euthanized via intraperitoneal injection of pentobarbital sodium at a dose of 150 mg/kg body weight.
hUC-MSCs isolation and culture
Fresh umbilical cords were obtained from three healthy human donors (aged 23–28 years) with informed consent at the Sixth Medical Center of PLA General Hospital (Beijing, China) between August and October 2021. Maternal blood samples were screened for infectious disease markers, including HIV I & II, HBV, HCV, and syphilis, and all donors tested negative.
In this study, hUC-MSCs were isolated and cultured. The umbilical cord samples, stripped of arteries and veins, were thoroughly washed with sterile PBS and cut into small fragments (3–5 mm3). These fragments were then placed in T75 culture flasks and incubated at 37 °C in a humidified atmosphere containing 5% CO2. The cells were cultured in MSC basal medium (Cat. No. NC0103 & NC0103.S, YOCON) supplemented with 100 U/mL penicillin and streptomycin. The culture medium was replaced every 7 days initially and then every 3–4 days after two weeks, when the adherent cells reached 80–90% confluence. For subculture, cells were detached using 0.25%Trypsin–EDTA (1 ×) (Cat. No. 25200–056, Gibco) and transferred to new flasks. Cells from passages 4–7 were used for all experiments.
Flow cytometry
Flow cytometric analysis was performed to characterize the surface phenotypes of hUC-MSCs. The harvested hUC-MSCs were washed twice with cold PBS containing 1% fetal bovine serum (FBS) and centrifuged at 300 × g for 5 min. Single-cell suspensions were then incubated with specific antibodies (5µL each) at 4 °C for 30 min. After incubation, the cells were washed again with PBS containing 1% FBS and resuspended in 200µL of PBS for analysis using a FACS Calibur flow cytometer (Beckman Coulter, DxFLEX, USA). Data were analyzed using FlowJo software (version 10, Tree Star, Inc., Ashland, OR, USA). The following antibodies (BioLegend) were used for surface marker analysis: CD45-FITC (Cat. No. 304005), CD34-FITC (Cat. No. 347494), CD73-FITC (Cat. No. 344015), CD105-FITC (Cat. No. 800505), CD90-FITC (Cat. No. 328107), and HLA-DR-FITC (Cat. No. 327005).
Histopathology
HE and TB staining were employed to assess pathological damage and mast cell distribution in the jejunum. For HE staining (Cat. No. C0107 & C0109, Beyotime), jejunal tissues were harvested from euthanized mice and rinsed thoroughly with physiological saline. The tissues were fixed in 4% paraformaldehyde for 12 h, followed by dehydration through a graded ethanol series, clearing in xylene, and embedding in paraffin. Serial sections of 4 µm thickness were prepared, mounted onto glass slides, and deparaffinized. Nuclei were stained with hematoxylin, while cytoplasmic components were counterstained with eosin. The slides were then dehydrated, cleared, air-dried, and coverslipped for microscopic analysis. For TB staining (Cat. No. 3663, Solarbio), rehydrated tissue sections were incubated in a 1 × toluidine blue solution for 2 min. Differentiation was achieved by brief immersion in acid alcohol (30 s), followed by bluing in ammonia water (30 s). Finally, the sections were mounted with resin and examined under a microscope.
Immunohistochemistry, immunofluorescence and ultrastructure of intestinal tissues
Paraffin-embedded tissue sections were deparaffinized by immersion in xylene for 15 min (repeated 2–3 times), rehydrated through a graded ethanol series (100%, 95%, 80%, and 70%) for 5 min each, and rinsed 3 times with PBS (pH 7.4). Antigen retrieval was performed by heating the sections in sodium citrate buffer (pH 6.0) at 100 °C for 30 min, followed by cooling to room temperature and washing with PBS. To block non-specific binding, the sections were incubated with 5% BSA for 30 min at room temperature. Subsequently, the sections were incubated with diluted primary antibodies against occludin (Cat. No. ab216327, Abcam), claudin (Cat. No. ab15098, Abcam), and ZO-1 (Cat. No. ab221547, Abcam) overnight at 4 °C. After washing with PBS, the sections were incubated with fluorophore-conjugated secondary antibodies for 1 h at room temperature in the dark. Nuclear staining was performed using DAPI (1 µg/mL) for 5–10 min. Finally, the sections were mounted with an anti-fade mounting medium, coverslipped, and stored in the dark. Fluorescence images were acquired using a confocal microscope.
MitoSOX Green probes (Cat. No. M36005, Invitrogen) were used to assess intracellular and mitochondrial oxidative stress. Cells were collected and the MitoSOX Green probe was reconstituted in DMSO to prepare a working concentration of 2 µM. After washing with PBS, the cells were incubated with the MitoSOX Green working solution at 37 °C in the dark for 30 min. Excess probe was removed by washing with PBS. The stained cells were mounted with an anti-fade medium and visualized using a fluorescence microscope (IX73, Olympus or Imager D2, Carl Zeiss).
For the analysis of intestinal ultrastructure, small segments of jejunum tissue (0.5 cm2) were promptly fixed in a pre-cooled fixative solution containing 2.5% glutaraldehyde and 1% osmium tetroxide. Subsequently, the samples were dehydrated using a graded series of ethanol solutions, with each step lasting 10 min. A 1:1 mixture of resin (Cat. No. 14300, Biolyst) and the dehydrating agent was gradually infiltrated into the tissue. Following this procedure, the tissue was embedded in resin and polymerized at 60℃ for 24 to 48 h. Ultrathin sections were stained with uranyl acetate and lead citrate and subsequently examined under a transmission electron microscope (TEM) (PANORAMIC MIDI/250, 3DHISTECH, Hungary).
Enzyme-linked immunosorbent assay
The levels of IgG, IgE, mMCP-1, histamine, IL-6, and TNF-α in serum were quantified using commercially available ELISA kits, following the manufacturers’ instructions. The following kits were used:IgG (Cat. No. EK0101, Boster), IgE (Cat. No. 555248 & 550,534, BD Biosciences), mMCP-1 (Cat. No. 446207, BioLegend), histamine (Cat. No. CEA927Ge, USCN), IL-6 (Cat. No. EMC004, Neobioscience), and TNF-α (Cat. No. EMC102a, Neobioscience). Briefly, 96-well plates were pre-coated or coated overnight at 4 °C with the capture antibody. After blocking, 100 µL of serum samples or standards were added to each well and incubated at 37 °C for 90 min. Plates were washed 5 times with wash buffer, followed by the addition of 100 µL/well of biotinylated detection antibody and incubation at 37 °C for 1 h. After another 5 washes, 100 µL/well of streptavidin–horseradish peroxidase (HRP) solution was added and incubated at 37 °C for 30 min. The plates were washed 5 times again, and 100 µL/well of substrate solution was added, followed by a 15-min incubation at 37 °C in the dark. The reaction was terminated by adding stop solution, and the optical density (OD) was measured at 450 nm using a microplate reader. All samples were analyzed in triplicate, and biomarker concentrations were calculated based on standard curves.
Expression of cytokine mRNA
After the 6 th oral challenge, approximately 100 mg of jejunum tissue was collected and flushed with RNA-free PBS. Total RNA was extracted using the RNAprep Pure Tissue Kit (Cat. No. DP451, TIANGEN) according to the manufacturer’s instructions. Reverse transcription was performed using the Exscript RT Reagent Kit (Cat. No. RR036 A, TAKARA) with random primers, followed by real-time PCR amplification. Real-time PCR analysis was conducted to quantify the expression of IL-6, TNF-α, IFN-γ, T-bet and β-actin genes using TB Green Premix Ex Taq II (Tli RNaseH Plus) (TAKARA Bio). The primer pairs used were as follows:
IL-6 forward primer: 5’-CTCCCAACAGACCTGTCTATAC −3’,
reverse primer: 5’-CCATTGCACAACTCTTTTCTCA −3’;
TNF-α forward primer: 5’-CCTGTAGCCCACGTCGTAG −3’,
reverse primer: 5’-AGTAGACAAGGTACAACCC −3’;
IFN-γ forward primer: 5’-CCTGCGGCCTAGCTCTGAG −3’,
reverse primer: 5’- GCCATGAGGAAGAGCTGCA −3’;
T-bet forward primer: 5’-ATTGCCGTGACTGCCTACCAGA −3’,
reverse primer:5’-GGAATTGACAGTTGGGTCCAGG −3’;
β- actin 2 forward primer:5’-AGCCATGTACGTAGCCATCC −3’,
reverse primer: 5’-CTCTCAGCTGTGGTGGTGAA −3’.
Quantitative real-time PCR analysis was conducted using the Mx3000P qPCR system (Stratagene, La Jolla, Calif., USA). The thermal cycling protocol began with an initial denaturation step at 95 °C for 30 s, followed by 40 amplification cycles consisting of denaturation at 95 °C for 5 s and combined annealing and extension at 60 °C for 30 s. Target gene expression levels were normalized to the endogenous reference gene β-actin 2 (ACTB), and quantified using the comparative ΔΔCt method. Data are expressed as fold-changes relative to the mean value of control group samples.
Western blot analysis
Jejunal tissues from mice were homogenized in RIPA lysis buffer (Cat. No. HX1862-1, Huaxingbio) containing complete protease and phosphatase inhibitor cocktails. The lysates were centrifuged at 15,000 × g for 15 min at 4 °C, and the protein-containing supernatants were collected. Protein concentrations were determined using the BCA assay, with 20–40 μg of total protein per sample loaded onto 10% SDS–polyacrylamide gels (Cat. No. Ba1012, Baiqiandu). Following electrophoresis, proteins were transferred onto PVDF membranes (Cat. No. IPVH00010, Millipore) using wet transfer system. Membranes were blocked with 5% non-fat milk (Cat. No. HX1866, Huaxingbio) in TBST for 1 h at room temperature, then incubated overnight at 4 °C with the following primary antibodies from Abcam: anti-Bax (1:1000, Cat. No. ab182733), anti-Bcl-2 (1:1000, Cat. No. ab32124), anti-caspase-3 (1:1000, Cat. No. ab32351), and anti-cleaved caspase-3 (1:1000, Cat. No. ab2302), and anti-ZO-1 (1:1000, Cat. No. ab221547),. After three 5-min washes with TBST (Cat. No. Ba1024, Baiqiandu), membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5000, Cat. No. ab205718, Abcam) for 1 h at room temperature. Protein bands were visualized using enhanced chemiluminescence substrate and imaged with an EPSON scanner (Model No. L1548). Band intensities were quantified using ImageJ software (NIH, Bethesda, MD) and normalized to corresponding loading controls.
Cell culture and evaluation
The human small intestinal epithelial cell line FHs74 Int (Procell Life Science & Technology Co., Ltd., Wuhan, China) was maintained under standard culture conditions at 37 °C in a humidified 5% CO2 atmosphere. For experimental studies, cells were seeded at a density of 2 × 105 cells/mL in RPMI-1640 medium within 24-well Transwell plates (Corning, NY, USA) and allowed to differentiate for 14 days to establish a polarized epithelial monolayer, with culture medium refreshed every 48–72 h.
Cell proliferation was quantitatively assessed using the Cell Counting Kit-8 (CCK-8; Cat. No. 96992, Sigma) following the manufacturer’s instructions. Briefly, after experimental treatments, cells were incubated with CCK-8 reagent (10% v/v in RPMI-1640 medium) for 2 h at 37 °C. Absorbance was measured at 450 nm using a SpectraMax microplate reader (Molecular Devices, San Jose, CA, USA), and cell viability was calculated as a percentage relative to Control groups.
Cellular apoptosis was quantified using an Annexin V-PE/7-AAD apoptosis detection kit (Cat. No. 559763, BD Biosciences). Briefly, cells were harvested by gentle trypsinization (0.25% trypsin–EDTA), washed twice with ice-cold PBS, and centrifuged at 300 × g for 5 min at 4 °C. The cell pellet was resuspended in 1 × Annexin V binding buffer at a density of 5 × 105 cells/mL. For staining, 100 μL of cell suspension was mixed with 5 μL PE-conjugated Annexin V and 5 μL 7-AAD, followed by a 15-min incubation at room temperature in the dark. Flow cytometric analysis was performed immediately using a Beckman DxFLEX flow cytometer (Beckman Coulter, Brea, CA, USA) equipped with 488 nm and 640 nm lasers. Data were acquired and analyzed using NovoExpress software (Version 1.6.1, ACEA Biosciences, San Diego, CA, USA).
Statistical analysis
Data were analyzed by GraphPad Prism v8 (GraphPad Software, San Diego, CA, USA, www.graphpad.com) and SPSS version 21.0 (IBM SPSS, Chicago, USA). Data are expressed as the mean ± standard deviation (SD). For comparison between two groups of parametric variables, a t-test was used, and for multiple group comparisons, variance analysis was adopted. P < 0.05 was considered statistically significant.
Date availability
All data and materials used or analyzed during this study are included in this published article. Futher enquiries can be directed to the corresponding author.
References
Anvari, S. et al. IgE-mediated food allergy. Clin. Rev. Allergy Immunol. 57, 244–260. https://doi.org/10.1007/s12016-018-8710-3 (2019).
Boyce, J. A. et al. Guidelines for the diagnosis and management of food allergy in the united states: Summary of the NIAID-sponsored expert panel report. J. Allergy Clin. Immunol. 126, 1105–1118. https://doi.org/10.1016/j.jaci.2010.10.008 (2010).
Protudjer, J. L. et al. Food-related symptoms and food allergy in swedish children from early life to adolescence. PLoS ONE 11, e0166347. https://doi.org/10.1371/journal.pone.0166347 (2016).
Schussler, E. et al. Workgroup report by the joint task force involving american academy of allergy, asthma & immunology (AAAAI); food allergy, anaphylaxis, dermatology and drug allergy (FADDA) (adverse reactions to foods committee and adverse reactions to drugs, biologicals, and latex committee); and the centers for disease control and prevention botulism clinical treatment guidelines workgroup-allergic reactions to botulinum antitoxin: A systematic review. Clin. Infect. Dis. 66, S65–S72. https://doi.org/10.1093/cid/cix827 (2017).
Li, Z. L. To standardize the diagnosis and management of food allergy related digestive diseases. Chin. J. Pract. Pediatr. 32, 733–735. https://doi.org/10.19538/j.ek2017100603 (2017).
The Editorial Board, Chinese Journal of Pediatrics, the Society of Pediatrics, Chinese Medical Association. Consensus on diagnosis and management of allergic diseases in children. Chin. J. Pediatr. 57, 164–171. https://doi.org/10.3760/cma.j.issn.0578-1310.2019.03.002 (2019).
Savage, J. & Johns, C. B. Food allergy: Epidemiology and natural history. Immunol. Allergy Clin. North Am. 35, 45–59. https://doi.org/10.1016/j.iac.2014.09.004 (2015).
Li, S. et al. Gene expression signatures of circulating human type 1, 2, and 3 innate lymphoid cells. J. Allergy Clin. Immunol. 143, 2321–2325. https://doi.org/10.1016/j.jaci.2019.01.047 (2019).
Hassanen, E. I. et al. The potential mechanism of histamine-inducing cardiopulmonary inflammation and apoptosis in a novel oral model of rat intoxication. Toxicology 484, 153410. https://doi.org/10.1016/j.tox.2022.153410 (2023).
Yan, N. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate the enteropathy of food allergies in mice. Exp. Ther. Med. 16, 4445–4456. https://doi.org/10.3892/etm.2018.6763 (2018).
Lee, D. G. et al. Preventive effects of a human hematopoietic mesenchymal stem cell (hHMSC) therapy in ovalbumin-induced food allergy. Biomedicines 10, 511. https://doi.org/10.3390/biomedicines10020511 (2022).
Guillamat-Prats, R. The role of MSC in wound healing scarring and regeneration. Cells 10, 1729. https://doi.org/10.3390/cells10071729 (2021).
Jo, H. et al. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int. J. Mol. Sci. 22, 2410. https://doi.org/10.3390/ijms22052410 (2021).
Hu, C., Wu, Z. & Li, L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int. J. Biol. Sci. 16, 893–903. https://doi.org/10.7150/ijbs.39725 (2020).
Viswanathan, S. et al. Mesenchymal stem versus stromal cells: International society for cell & gene therapy (ISCT®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy 21, 1019–1024. https://doi.org/10.1016/j.jcyt.2019.08.002 (2019).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8, 315–317. https://doi.org/10.1080/14653240600855905 (2006).
Iweala, O. I., Choudhary, S. K. & Commins, S. P. Food Allergy. Curr. Gastroenterol. Rep. 20, 17. https://doi.org/10.1007/s11894-018-0624-y (2018).
Nguyen, S. M. T. et al. Mechanisms governing anaphylaxis: inflammatory cells, mediators, endothelial gap junctions and beyond. Int. J. Mol. Sci. 22, 7785. https://doi.org/10.3390/ijms22157785 (2021).
Tanno, L. K. et al. Critical view of anaphylaxis epidemiology: open questions and new perspectives. Allergy Asthma Clin. Immunol. 14, 12. https://doi.org/10.1186/s13223-018-0234-0 (2018).
Vanuytsel, T., Tack, J. & Farre, R. The role of intestinal permeability in gastrointestinal disorders and current methods of evaluation. Front. Nutr. 8, 717925. https://doi.org/10.3389/fnut.2021.717925 (2021).
Suber, J. & Iweala, O. I. Strategies for mast cell inhibition in food allergy. Yale J. Biol. Med. 93, 719–731 (2020).
Cianferoni, A. Non-IgE mediated food allergy. Curr. Pediatr. Rev. 16, 95–105. https://doi.org/10.2174/1573396315666191031103714 (2020).
Chen, X. L. & Zheng, C. Z. Effects of adipose-derived stem cells and non-methylated CpG-oligodeoxynucleotides on peripheral blood CD4+CD25+ regulatory T cells in young mice with food allergy. Zhongguo Dang Dai Er Ke Za Zhi 19, 590–595. https://doi.org/10.7499/j.issn.1008-8830.2017.05.022 (2017).
Zhu, X. & Zhu, J. CD4 T helper cell subsets and related human immunological disorders. Int. J. Mol. Sci. 21, 8011. https://doi.org/10.3390/ijms21218011 (2020).
Mohanan, M. M. et al. Role of mesenchymal stem cells and short chain fatty acids in allergy: A prophylactic therapy for future. Immunol. Lett. 260, 1–10. https://doi.org/10.1016/j.imlet.2023.06.002 (2023).
Sun, L. et al. Mesenchymal stem cell-based therapy for allergic rhinitis. Stem Cells Int. 2020, 2367524. https://doi.org/10.1155/2020/2367524 (2020).
Lai, Y. R. & Zheng, C. Z. Immunoregulatory effect of adipose-derived stem cell transplantation in young mouse model of food allergy. Zhongguo Dang Dai Er Ke Za Zhi 18, 656–661. https://doi.org/10.7499/j.issn.1008-8830 (2016).
Faezi, M. et al. The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. J. Chem. Neuroanat. 94, 21–31. https://doi.org/10.1016/j.jchemneu.2018.08.004 (2018).
Liu, B. et al. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology 23, 728–736. https://doi.org/10.1111/nep.13099 (2018).
Kwon, H. M. et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul. Pharmacol. 63, 19–28. https://doi.org/10.1016/j.vph.2014.06.004 (2014).
Terunuma, A. et al. Comparative transcriptomic analysis of human mesenchymal stem cells derived from dental pulp and adipose tissues. J. Stem Cells Regen. Med. 15, 8–11. https://doi.org/10.46582/jsrm.1501003 (2019).
Zhuang, W. Z. et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 28, 28. https://doi.org/10.1186/s12929-021-00725-7 (2021).
Zhang, L. B. & He, M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: A systematic review and meta-analysis. Pulm. Pharmacol. Ther. 54, 39–52. https://doi.org/10.1016/j.pupt.2018.11.007 (2019).
Shin, J. W. et al. mesenchymal stem cells suppress severe asthma by directly regulating Th2 cells and type 2 innate lymphoid cells. Mol. Cells 44, 580–590. https://doi.org/10.14348/molcells.2021.0101 (2021).
Ray, P. D., Huang, B. W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990. https://doi.org/10.1016/j.cellsig.2012.01.008 (2012).
Harrell, C. R. et al. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 8, 467 (2019).
Lee, B. & Kang, I. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res. Ther. 11, 397. https://doi.org/10.1186/s13287-020-01920-3 (2020).
Keyhanmanesh, R. et al. Intra-tracheal delivery of mesenchymal stem cell-conditioned medium ameliorates pathological changes by inhibiting apoptosis in asthmatic rats. Mol. Biol. Rep. 49, 3721–3728. https://doi.org/10.1007/s11033-022-07212-8 (2022).
Yang, J. et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation. Oxidative Stress Apoptosis. PLoS One 10, e0140551. https://doi.org/10.1371/journal.pone.0140551 (2015).
Hong, D. et al. Effect of mesenchymal stem cell therapy in animal models of allergic rhinitis: A systematic review and meta-analysis. Int. Immunopharmacol. 124, 111003. https://doi.org/10.1016/j.intimp.2023.111003 (2023).
Yamamoto, T. et al. Induction of regulatory T cells as a novel mechanism underlying the therapeutic action of Kakkonto, a traditional japanese herbal medicine, in a murine food allergy model. Int. Arch. Allergy Immunol. 169, 146–156. https://doi.org/10.1159/000445433 (2016).
Kanagaratham, C., Sallis, B. F. & Fiebiger, E. Experimental models for studying food allergy. Cell. Mol. Gastroenterol. Hepatol. 6, 356–369. https://doi.org/10.1016/j.jcmgh.2018.05.010 (2018).
Funding
The study was supported by National Key Clinical Specialty Construction program (No.2023(09), Health commission of Shanxi Province).
Author information
Authors and Affiliations
Contributions
Weipeng Liu contributed equally to this work and should be considered co-first authors. Yuan Zhao, Weipeng Liu and Zuo Luan designed the study. Yuan Zhao, Jie Zhou and Fan Zhang performed experiments. Fan Zhang and Yabing Ding analyzed the data. Weipeng Liu, Zhaoyan Wang and Qian Wang confirmed the authenticity of all the raw data.
Corresponding author
Ethics declarations
Competing interests
The data exhibited in this study are available on request from the corresponding author. All authors declare no conflict of interest.
Ethics approval
This study was approved by the Animal Experiment Committee of Beijing Center for Physical & Chemical Analysis (No. 210520-SWDWF-005) and the Human Ethics Committee of Sixth Medical Center of PLA Hospital (No. 2017[02]). Written informed consent was obtained from the parents of hUC-MSC donors to participate in the study.
Consent to participate and publish
All authors approved the final manuscript and agreed to publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Y., Liu, W., Ding, Y. et al. Human umbilical cord-derived mesenchymal stem cells attenate histaminergic effect of intestinal mucosa through bax/bcl-2 pathway in food allergic enteritis. Sci Rep 15, 18442 (2025). https://doi.org/10.1038/s41598-025-03563-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03563-x