Abstract
This multicenter, retrospective study evaluated the impact of pelvic lymph node dissection (PLND) on survival outcomes in non-muscle invasive bladder cancer (NMIBC) patients undergoing radical cystectomy (RC) and identified factors associated with upstaging. A total of 544 NMIBC patients who underwent RC with or without PLND between 2019 and 2024 were analyzed. Survival outcomes, including cancer-specific survival (CSS) and recurrence-free survival (RFS), were compared using Kaplan-Meier analysis and Cox proportional hazards models, while factors associated with upstaging were examined through logistic regression. Of the 544 patients, 509 (93.6%) were staged as cT1, and 412 (75.7%) underwent PLND. Upstaging occurred in 193 patients (35.5%), with pathological stages distributed as pT1 (50.0%), pT2 (20.8%), pT3 (11.2%), and pT4 (3.5%). Among patients who underwent PLND, 29 (7.0%) had positive lymph nodes. PLND was associated with improved RFS (5-year: 84.3% vs. 71.5%; adjusted hazard ratio [HR] = 0.33, 95% confidence interval [CI]: 0.20–0.56, p < 0.001) but did not significantly impact CSS (5-year: 86.5% vs. 81.6%; adjusted HR = 0.57, 95% CI: 0.32–1.02, p = 0.06). Lymph node positivity was linked to the worst prognosis. cT1 tumors, histological subtypes, and PLND were significant predictors of upstaging. In patients with cT1 tumors or histological subtypes, repeat transurethral resection is recommended to obtain more precise staging, which may inform further therapeutic decisions. While PLND is not routinely recommended for all NMIBC patients, it may be considered in those with high-risk features, particularly cT1 tumors or histological subtypes.
Similar content being viewed by others
Introduction
Non-muscle invasive bladder cancer (NMIBC) accounts for approximately 75% of all newly diagnosed bladder cancer cases1. Despite its relatively favorable prognosis compared to muscle-invasive bladder cancer (MIBC), NMIBC presents significant challenges due to its high recurrence and progression rates. Intravesical Bacillus Calmette-Guérin (BCG) therapy has been shown to reduce recurrence rates by approximately 30% compared to transurethral resection (TUR) alone, and also delays disease progression2. However, up to 30% of patients with clinical T1 stage disease ultimately experience disease progression to muscle-invasive stages, necessitating radical cystectomy (RC)3.
Bilateral pelvic lymph node dissection (PLND) during RC has long been considered a standard practice for patients with MIBC, providing essential information on lymph node involvement (pN stage) and offering potential therapeutic benefits through the removal of micrometastases1. Landmark studies have shown that extending the PLND template increases nodal-positivity detection by approximately 33% compared to limited dissection and doubles the detection rate of occult metastases (26% vs. 13%), translating into a 10–20% absolute improvement in 5-year recurrence-free survival (RFS) among patients with pT2–pT3 MIBC4,5. However, the role of PLND in NMIBC remains controversial. A retrospective cohort study suggested that adequate PLND, defined by the authors as removal of at least 10 lymph nodes, may improve overall survival (OS)6, although this was not supported by Lin et al., who found no RFS benefit7. Another study reported that this potential survival benefit was particularly evident in patients with cT1 disease8. In contrast, recent prospective data have shown no clear oncologic advantage and have highlighted increased postoperative morbidity9. The role of PLND and the clinical significance of lymph node yield in NMIBC remain to be clarified.
In this multicenter study, we aim to evaluate the impact of PLND on cancer-specific survival (CSS) and RFS, as well as to identify key factors associated with upstaging in NMIBC patients undergoing RC.
Materials and methods
Ethics statement
This multicenter study was approved by the Medical Ethics Committee of the Second Hospital of Tianjin Medical University (Approval No. Kesh[2024] No. 066). Data-sharing agreements were established with all participating institutions prior to the initiation of the study. All data were de-identified before analysis, and strict protocols for patient confidentiality and data protection were followed throughout the study. As a retrospective analysis, written informed consent was waived by the Medical Ethics Committee of the Second Hospital of Tianjin Medical University. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Study population
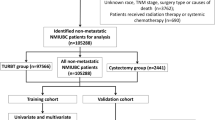
This multicenter retrospective study included 544 patients from three participating centers. Inclusion criteria were: (1) a clinical diagnosis of NMIBC (cTa, Tis, or cT1) based on TUR pathology, with confirmation of the presence of muscularis propria in the specimen; (2) available re-TUR results when indicated, with clinical staging determined by the highest pathological stage; (3) preoperative imaging excluding muscle-invasive or metastatic disease; and (4) subsequent treatment with RC. Exclusion criteria were: (1) prior neoadjuvant therapy; (2) non-urothelial carcinoma; (3) metastatic disease; (4) failure to undergo RC or RC performed more than 3 months after TUR; and (5) incomplete medical records. The choice of surgical approach (laparoscopic or robotic) was made based on patient and surgeon preferences. PLND was performed in cases with radiologically suspicious nodes on preoperative imaging or when palpable nodal abnormalities were encountered intraoperatively. In the absence of such findings, the decision to proceed with lymph node dissection was based on the operating surgeon’s discretion, taking into account institutional practices, imaging results, and patient-specific risk factors. Adjuvant chemotherapy with a platinum-based regimen was administered to patients with pT ≥ 3 or pN + disease.
Clinicopathologic data and survival outcomes
Clinicopathologic data, oncological follow-up, and survival status, including the underlying cause of death, were collected. All surgical specimens were processed according to standard pathological procedures. Tumor staging was performed based on the 2017 American Joint Committee on Cancer (AJCC) TNM staging system10. Tumor grading followed the 2022 World Health Organization (WHO) grading system11. Histological subtypes were defined as UC exhibiting any variant differentiation (e.g., squamous, glandular, micropapillary) according to the 2022 WHO classification11. Histological subtypes were determined by the TUR pathology.
CSS was defined as the time from RC to either cancer-specific death or the date of the last follow-up. The cause of death was determined by the attending physician based on medical chart review and death certificates12. RFS was defined as the time from RC to the first occurrence of recurrence (locoregional or distant) or the date of the last follow-up. Upstaging was defined as the transition from clinical Ta/Tis/T1 to pathological T ≥ 2 or N+.
The primary endpoint was CSS, comparing patients who underwent RC with PLND versus those who did not (no-PLND). The secondary endpoints included RFS, the upstaging rate, and factors associated with upstaging prior to RC.
Follow-up protocol
Patients were followed according to institutional protocols. Typically, postoperative surveillance included physical examination, laboratory tests, and radiographic evaluation (chest and abdominal CT scans). Surveillance was conducted at 3- to 4-month intervals during the first year, semiannually for the next 4 years, and annually thereafter.
Statistical analyses
The clinicopathological characteristics were compared using the chi-squared or Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. Kaplan-Meier curves were used to estimate survival outcomes for CSS and RFS, with differences between groups compared using the log-rank test. Cox proportional hazards models were employed to evaluate associations between clinicopathological variables and survival outcomes. Logistic regression was utilized to identify factors associated with upstaging prior to RC. Variables with statistical significance in univariate analysis were included in the multivariable models. In addition, gender was included in the multivariable logistic regression model for upstaging due to its borderline significance in univariate analysis. All statistical analyses were performed using R version 4.2.2. A two-sided p-value of less than 0.05 was considered statistically significant.
Results
Clinical and pathological characteristics
The clinical and pathological characteristics of the 544 patients included in the study are summarized in Table 1. The median age was 69 years (interquartile range [IQR]: 63–75), with 82% of patients being male, and 93.6% classified as cT1 stage. The median number of days from TUR to RC was 38 days (IQR: 26–55). A total of 75.7% of patients underwent PLND. There were no significant differences in baseline characteristics between the PLND and no-PLND groups prior to RC (p > 0.05). The pathological T-stages were as follows: pT1 in 50.0%, pT2 in 20.8%, pT3 in 11.2%, and pT4 in 3.5%. A significant difference was observed between the PLND and no-PLND groups in terms of pT stage (p = 0.017). In the PLND group, the median number of lymph nodes dissected was 14 (IQR: 8–21), with 7.0% of patients having positive lymph nodes (pN+). (Table 1).
Predictors of upstaging
A total of 193 patients (35.5%) experienced upstaging. Multivariable logistic regression analysis revealed that cT1 stage (OR: 2.74, 95% CI: 1.03–7.26, p = 0.026), the presence of histological subtypes (OR: 1.90, 95% CI: 1.23–2.93, p = 0.004), and PLND (OR: 1.67, 95% CI: 1.07–2.61, p = 0.021) were significantly associated with upstaging to pT ≥ 2 or pN + status at the time of RC (Table 2). Model calibration was assessed using a calibration plot, which demonstrated good agreement between predicted and observed probabilities (Fig. S1).
Predictors of cancer-specific survival and recurrence-free survival
The median follow-up time was 32.2 months (range: 1.4–64.2). A total of 37 patients (9.0%) in the PLND group and 17 patients (12.8%) in the no-PLND group died from bladder cancer (p = 0.241), while recurrence occurred in 44 patients (10.7%) and 23 patients (17.4%), respectively (p = 0.048). Patterns of recurrence are detailed in Table S1. Kaplan-Meier curves showed that the 5-year CSS rates for the PLND and no-PLND groups were 86.5% and 81.6%, respectively (adjusted HR = 0.57, 95% CI: 0.32–1.02, p = 0.06), while the 5-year RFS rates were 84.3% versus 71.5% (adjusted HR = 0.33, 95% CI: 0.20–0.56, p < 0.001) (Fig. 1A and B). The pNx stage exhibited CSS and RFS outcomes slightly worse than pN0, while pN + patients had the worst CSS and RFS (Fig. 1C and D). Similarly, pT2 patients had CSS and RFS rates comparable to those with pT ≤ 1, whereas patients with pT ≥ 3 had the worst CSS and RFS outcomes (Fig. 1E and F).
In univariate Cox regression analysis, pathological T-stage and N-stage were significantly associated with CSS (Table 3). Multivariable analysis demonstrated that pN0 (HR: 0.47, 95% CI: 0.25–0.86, p = 0.015) was associated with better CSS, while pN+ (HR: 2.82, 95% CI: 1.04–7.62, p = 0.041) was associated with poorer CSS (Table 3).
For RFS, univariate Cox regression analysis identified the presence of histological subtypes, pathological T-stage, and N-stage as significant predictors (Table 4). Multivariable analysis showed that compared to pT ≤ 1, pT2 (HR: 2.08, 95% CI: 1.02–4.25, p = 0.044) and pT ≥ 3 (HR: 9.37, 95% CI: 4.85–18.10, p < 0.001) were associated with poorer RFS (Table 4).
In the PLND group, the number of lymph nodes dissected (greater than 10 versus 10 or fewer) was not significantly associated with either CSS (HR: 0.98, 95% CI: 0.51–1.90, p = 0.954) or RFS (HR: 1.23, 95% CI: 0.65–2.33, p = 0.517).
Sensitivity analyses
Sensitivity analyses restricted to patients with histological subtypes (n = 119) showed findings consistent with the overall cohort, with PLND significantly improving RFS and no significant impact on CSS (Table S2 and Fig. S2).
Discussion
In this multicenter study, we evaluated the impact of PLND on cancer-specific survival and recurrence-free survival in patients with NMIBC undergoing RC. Our findings showed that, although PLND did not significantly improve 5- year CSS (86.5% vs. 81.6%), it was associated with a significant improvement in 5- year RFS (84.3% vs. 71.5%) when compared to the non-PLND group. Further analysis revealed that patients with positive lymph nodes (pN+) had the worst CSS and RFS, whereas those with pN0 had significantly better survival, underscoring the critical role of lymph node status in prognostic assessment of bladder cancer13. Furthermore, PLND plays a critical role in accurately assessing lymph node status, which is essential for prognosis and guiding postoperative treatment.
The incidence of lymph node metastasis in MIBC during RC ranges from 24–43%14–16, and PLND is considered a standard procedure in MIBC to provide more accurate pN staging and improve prognosis1. However, the lymph node metastasis rate in NMIBC is also non-negligible, with previous studies reporting a range of 6–19%17. In our study, the lymph node metastasis rate was 7%, which is consistent with prior reports. The prognosis of NMIBC with positive lymph nodes is significantly worse18, highlighting the potential necessity of PLND in this patient population. However, the role of PLND in NMIBC prognosis remains under-researched, and results from existing studies are inconsistent. Lyu et al.9 reported a prospective randomized controlled trial of 101 NMIBC patients, finding no survival benefit of PLND but an increased incidence of complications. In contrast, Kitamura et al.19 suggested that PLND might offer survival benefits by removing undetected micrometastases. Similarly, Moldovan et al.8, based on an NCDB cohort of 9,399 NMIBC patients, and Tang et al.20, using a SEER analysis of 1,701 patients, both found that PLND improved OS in T1 but not in Ta/Tis disease.
One potential reason for these differing results could be the number of lymph nodes removed. Khanna et al.21 found that removing more than 10 lymph nodes was associated with improved RFS, and removing more than 20 lymph nodes correlated with improved CSS and OS in NMIBC patients. Similarly, Lenis et al.6 demonstrated that removal of ≥ 10 lymph nodes significantly improved OS in NMIBC patients. However, conflicting findings have been reported. A study spanning over 20 years found no difference in RFS between patients with ≥ 10 or < 10 lymph nodes removed7. In our study, the median number of lymph nodes removed in the PLND group was 14 (IQR: 8–21), and no significant correlation was found between the number of lymph nodes removed and either CSS or RFS. Some researchers argue that adherence to meticulous dissection techniques within an extended template is more critical for achieving optimal oncologic outcomes than the total number of lymph nodes removed22.
Another factor contributing to the variation in results could be population heterogeneity. Previous studies have reported upstaging rates of 26–78% in NMIBC patients undergoing RC17,23,24. In our study, 193 patients (35.5%) experienced upstaging, defined as the progression from NMIBC to pT ≥ 2 or pN+. In contrast, in the randomized study by Lyu et al.9, patients found to have MIBC at RC were excluded, and no lymph node metastases were detected in the PLND group, resulting in a more selected and lower-risk NMIBC cohort. It is well established that MIBC differs significantly from NMIBC in terms of RFS and CSS17. Therefore, the varying proportions of pT stages in different studies could potentially explain the divergent outcomes. Our study found that both cT1 stage and the presence of histological subtypes were significantly associated with upstaging. These findings suggest that NMIBC patients with histological subtypes may be at a higher risk of understaging during TUR and, in accordance with current guidelines25, should be managed similarly to cT1 patients, including consideration for repeat TUR.
This study has several limitations. First, as a retrospective study, it is subject to inherent selection bias, particularly in the decision-making process for PLND, which was based on preoperative imaging findings, intraoperative assessment, and surgeon discretion rather than a standardized protocol. Second, although the study included a large multicenter cohort, variations in the quality of TUR and RC procedures across centers may have introduced confounding factors. Third, the absence of a central pathology review could have led to inter-institutional variability in pathological assessments, potentially affecting the consistency and accuracy of staging. This limitation reflects the real-world practice across different centers but may reduce uniformity. Fourth, molecular subtyping was not performed in this cohort, which may limit more refined risk stratification and prognostic evaluation. Fifth, while this study provides important real-world evidence, prospective validation in a randomized controlled setting would be valuable to confirm these findings and minimize potential biases. Finally, the potential role of sentinel lymph node mapping26, as a less invasive alternative to standard or extensive PLND, warrants further investigation in future studies.
Conclusions
In this multicenter cohort, PLND was significantly associated with improved RFS (HR 0.33, 95% CI 0.20–0.56, p < 0.001), but had no significant impact on CSS (HR 0.57, 95% CI 0.32–1.02, p = 0.06) in NMIBC patients undergoing RC. pN + disease was identified as an independent poor prognostic factor, highlighting the importance of accurate lymph node staging. Both cT1 stage and histological subtypes were significantly associated with upstaging, suggesting that repeat TUR may benefit selected patients. These findings indicate that PLND should be selectively considered based on patient risk profiles, particularly in those with high-risk features such as cT1 stage or histological subtypes.
Data availability
De-identified raw data are available upon reasonable request from the corresponding author.
Change history
25 June 2025
The original online version of this Article was revised: The Funding section in the original version of this Article was incomplete. It now reads: “This study was supported by the Tianjin Municipal Health Industry Key Project fund (grant no. TJWJ2022XK014), the Scientific Research Project fund of Tianjin Municipal Education Commission (grant no. 2022ZD069), and the National Natural Science Foundation of China (grant no. 82403319).”
References
Alfred Witjes, J. et al. European association of urology guidelines on Muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur. Urol. 85, 17–31. https://doi.org/10.1016/j.eururo.2023.08.016 (2024).
Babjuk, M. et al. European association of urology guidelines on Non-muscle-invasive bladder Cancer (Ta, T1, and carcinoma in Situ). Eur. Urol. 81, 75–94. https://doi.org/10.1016/j.eururo.2021.08.010 (2022).
Shahin, O., Thalmann, G. N., Rentsch, C., Mazzucchelli, L. & Studer, U. E. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade 3 bladder cancer: recurrence, progression and survival. J. Urol. 169, 96–100. https://doi.org/10.1016/s0022-5347(05)64044-x (2003). discussion 100.
Leissner, J. et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J. Urol. 171, 139–144. https://doi.org/10.1097/01.ju.0000102302.26806.fb (2004).
Dhar, N. B. et al. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J. Urol. 179, 873–878. https://doi.org/10.1016/j.juro.2007.10.076 (2008). discussion 878.
Lenis, A. T. et al. Predictors of adequate lymph node dissection in patients with non-muscle invasive bladder cancer undergoing radical cystectomy and effect on survival. Urol. Oncol. 38 (796 e797-796 e714). https://doi.org/10.1016/j.urolonc.2020.04.027 (2020).
Lin, J., Deibert, C. M., Holder, D., Benson, M. C. & McKiernan, J. M. The role of pelvic lymphadenectomy in non-muscle invasive bladder cancer. Can. J. Urol. 21, 7108–7113 (2014).
Moldovan, M. et al. Oncological and survival outcomes of pelvic lymph node dissection in patients with nonmuscle invasive bladder Cancer undergoing radical cystectomy using the National Cancer database. Clin. Genitourin. Cancer. 22, 102197. https://doi.org/10.1016/j.clgc.2024.102197 (2024).
Lyu, Q., Yang, X., Cao, Q. & Zhuang, J. Unveiling the necessity: should (very) high-risk NMIBC patients undergoing RC opt for pelvic lymph node dissection?—a prospective cohort study. J. Urol. 211 (5S), e625. https://doi.org/10.1097/01.JU.0001008848.77629.6f (2024).
O’Sullivan, B. et al. The TNM classification of malignant tumours-towards common Understanding and reasonable expectations. Lancet Oncol. 18, 849–851. https://doi.org/10.1016/s1470-2045(17)30438-2 (2017).
Netto, G. J. et al. The 2022 world health organization classification of tumors of the urinary system and male genital Organs-Part B: prostate and urinary tract tumors. Eur. Urol. 82, 469–482. https://doi.org/10.1016/j.eururo.2022.07.002 (2022).
Rink, M. et al. Death certificates are valid for the determination of cause of death in patients with upper and lower tract urothelial carcinoma. Eur. Urol. 61, 854–855. https://doi.org/10.1016/j.eururo.2011.12.055 (2012).
Cattaneo, F., Motterle, G., Zattoni, F. & Morlacco, A. Dal Moro, F. The role of lymph node dissection in the treatment of bladder Cancer. Front. Surg. 5, 62. https://doi.org/10.3389/fsurg.2018.00062 (2018).
Liu, S., Chen, X. & Lin, T. Lymphatic metastasis of bladder cancer: molecular mechanisms, diagnosis and targeted therapy. Cancer Lett. 505, 13–23. https://doi.org/10.1016/j.canlet.2021.02.010 (2021).
Liedberg, F., Chebil, G., Davidsson, T., Gudjonsson, S. & Månsson, W. Intraoperative Sentinel node detection improves nodal staging in invasive bladder cancer. J. Urol. 175, 84–88. https://doi.org/10.1016/s0022-5347(05)00066-2 (2006). discussion 88–89.
Abol-Enein, H., El-Baz, M., El-Hameed, A., Abdel-Latif, M. A., Ghoneim, M. A. & M. & Lymph node involvement in patients with bladder cancer treated with radical cystectomy: a patho-anatomical study–a single center experience. J. Urol. 172, 1818–1821. https://doi.org/10.1097/01.ju.0000140457.83695.a7 (2004).
Fritsche, H. M. et al. Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. Eur. Urol. 57, 300–309. https://doi.org/10.1016/j.eururo.2009.09.024 (2010).
May, M. et al. Pathological upstaging detected in radical cystectomy procedures is associated with a significantly worse tumour-specific survival rate for patients with clinical T1 urothelial carcinoma of the urinary bladder. Scand. J. Urol. Nephrol. 45, 251–257. https://doi.org/10.3109/00365599.2011.562235 (2011).
Kitamura, H., Masumori, N. & Tsukamoto, T. Role of lymph node dissection in management of bladder cancer. Int. J. Clin. Oncol. 16, 179–185. https://doi.org/10.1007/s10147-011-0235-1 (2011).
Tang, Y., Wu, K. & Li, X. Contemporary use trends and effect on survival of pelvic lymph node dissection for non-muscle-invasive bladder cancer. Front. Surg. 9, 961430. https://doi.org/10.3389/fsurg.2022.961430 (2022).
Khanna, A. et al. Role of lymphadenectomy during radical cystectomy for Nonmuscle-Invasive bladder cancer: results from a Multi-Institutional experience. J. Urol. 207, 551–558. https://doi.org/10.1097/ju.0000000000002266 (2022).
Dorin, R. P. et al. Lymph node dissection technique is more important than lymph node count in identifying nodal metastases in radical cystectomy patients: a comparative mapping study. Eur. Urol. 60, 946–952. https://doi.org/10.1016/j.eururo.2011.07.012 (2011).
Denzinger, S. et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur. Urol. 53, 146–152. https://doi.org/10.1016/j.eururo.2007.06.030 (2008).
Cheng, L. et al. Grading and staging of bladder carcinoma in transurethral resection specimens. Correlation with 105 matched cystectomy specimens. Am. J. Clin. Pathol. 113, 275–279. https://doi.org/10.1309/94b6-8vfb-mn9j-1nf5 (2000).
Flaig, T. W. et al. NCCN Guidelines® insights: bladder cancer, version 3.2024. J. Natl. Compr. Canc Netw. 22, 216–225. https://doi.org/10.6004/jnccn.2024.0024 (2024).
van Gennep, E. J. et al. Prospective clinical study of Sentinel node detection in bladder cancer using a hybrid tracer - Towards replacement of pelvic lymph node dissection in cases with Sentinel node visualization on SPECT/CT? Eur. J. Nucl. Med. Mol. Imaging. https://doi.org/10.1007/s00259-025-07240-z (2025).
Acknowledgements
We would like to thank Associate Professor Changping Li of the School of Public Health, Tianjin Medical University for her contribution to the statistical aspects of the article.
Funding
This study was supported by the Tianjin Municipal Health Industry Key Project fund (grant no. TJWJ2022XK014), the Scientific Research Project fund of Tianjin Municipal Education Commission (grant no. 2022ZD069), and the National Natural Science Foundation of China (grant no. 82403319).
Author information
Authors and Affiliations
Contributions
H.H., Z.L.W., and Y.K.Q.: Conception and design of the study, data acquisition, manuscript writing; S.W.H., K.P.J., J.M.C., J.F.D., W.C., S.L., and J.W.H.: Data acquisition, analysis, manuscript writing; J.N.G. and H.T.C.: Data acquisition, manuscript editing; C.S., Z.Z., and X.S.L.: Study support, manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, S., Jia, K., Cui, J. et al. Impact of pelvic lymph node dissection on survival outcomes in non-muscle invasive bladder cancer: a multicenter retrospective study. Sci Rep 15, 18905 (2025). https://doi.org/10.1038/s41598-025-03916-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03916-6