Abstract
The relationship between luminal stenosis severity and plaque vulnerability in chronic stable angina (CSA) is not well studied. This study aimed to investigate the link between stenosis severity, plaque characteristics, and thrombus prevalence in CSA patients using optical coherence tomography. The 174 lesions (146 patients) with CSA divided into three groups according to the percentage of area stenosis (AS); group A (AS < 50%), group B (50% ≤ AS < 70%), and group C (AS ≥ 70%). Twenty-four lesions of group A, 51 lesions of group B, and 99 lesions from group C were studied. The prevalence of thrombus was 19.0% and it was significantly different among the three groups (none in group A vs. 17.6% in group B vs. 24.2% in group C; P = 0.024). The more severe the luminal narrowing, the more thrombus occurred, with a higher prevalence at the minimal lumen area site. Additionally greater the area stenosis, the higher the prevalence of lipid-rich plaque, thin cap plaque, macrophage infiltration, and ruptured plaque. In the multivariate analysis, thrombus was independently associated with ruptured plaque (OR = 20.96, CI 8.40–57.69, P < 0.001), macrophage infiltration (OR = 3.77, CI 1.53–10.72, P = 0.007), lipid angle (OR = 1.01, CI 1.00–1.01, P = 0.004), and area stenosis (OR = 1.05, CI 1.02–1.10, P = 0.002). In patients with CSA, the prevalence of thrombus, as assessed by OCT, is not uncommon. Plaque vulnerability, including thrombus occurrence, increased with the severity of area stenosis and was more prevalent at the minimal lumen site exposed to high shear stress.
Similar content being viewed by others
Introduction
Chronic stable angina (CSA) is a major clinical manifestation of coronary artery disease, characterized by predictable chest pain due to fixed atherosclerotic narrowing. While the degree of luminal stenosis is traditionally considered the key determinant of ischemia, recent advances in intracoronary imaging, particularly optical coherence tomography (OCT), have highlighted the importance of plaque characteristics, such as lipid content, macrophage infiltration, and fibrous cap status. However, the detailed relationship between stenosis severity and plaque features, including the presence of thrombus, in patients with CSA has not been systematically investigated. Such investigation is important to better understand lesion behavior, refine risk stratification, and guide treatment decisions. The understanding of the pathophysiology of coronary atherosclerosis has undergone significant paradigm shifts over the past decades, particularly in terms of plaque formation, growth, and destabilization1,2,3. Atherothrombosis is one of the pathognomonic findings of acute coronary syndrome but not in CSA. The relationship between the severity of luminal stenosis and plaque characteristics with thrombus formation has not been systematically studied in patients of CSA. Furthermore, there was limited data on the relationship between the prevalence of thrombus and degree of luminal narrowing in living CSA patients. The increase in luminal narrowing severity causes local flow disturbance and increase in endothelial shear stress in stenotic lesion lead to endothelial damage, activate platelets and coagulation factors and thrombus formation4,5.
OCT images provide highly sensitive and specific characterization of various of atherosclerotic plaques including types including identify thrombus and its characteristics6,7. We hypothesized that the plaque characteristics and thrombus formation might be associated with the degree of luminal narrowing even in CSA patients with stable plaques. Therefore, the aim of this study was to evaluate the associations between OCT-derived area stenosis and plaque characteristics, including thrombus prevalence, in patients with CSA. This study seeks to provide novel insights into the morphological features of high-grade lesions in stable patients and their potential clinical implications.
Results
Baseline characteristics are summarized in Table 1. There were 14 patients (9.6%) with AS \(<\) 50%, 38 patients (26.0%) with 50% \(\le\) AS \(<\) 70%, and 94 patients (64.4%) with AS \(\ge\) 70%. The mean age was 61.01 ± 9.6 years, and 86.3% were men. There were no significant differences among the groups in age, gender, and common cardiovascular risk factors, except the triglyceride level (P = 0.010).
OCT findings
The OCT characteristics of the study patients, according to the AS, are reported in Table 2 and Fig. 1. The frequency of fibrous plaque, lipid-rich plaque, and fibro-calcific plaque was 13.8% vs. 58.6% vs. 27.6%, respectively. As area stenosis increased, fibrous plaques and fibro-calcific plaques decreased and lipid-rich plaques increased. OCT-defined macrophage infiltration (33.3% in Group A, 51.0% in Group B, 71.7% in Group C; P = 0.001) and maximal lipid angle (148.9 ± 79.5° in Group A, 189.9 ± 71.0° in Group B, 209.0 ± 66.0° in Group C; P = 0.001) were increased according to AS severity. The thin cap fibroatheroma of less than 65 µm tended to increase as AS worsened but is not statistically insignificant (P = 0.414). The thin cap calcified plaque was identified 70 of 174 lesions and significantly increased according to AS severity (P = 0.002). As AS increased, lumen irregularity was increased (8.3% in Group A, 23.5% in Group B, 37.4% in Group C; P = 0.011). Thirty-three lesions (19.0%) presented a ruptured plaque; 0 case in Group A, 8 cases belonged to Group B and 25 cases to the cohort with Group C (P = 0.014), and they were most present in part with the most stenotic lesion, MLA site. The more severe the AS, the higher the thrombus prevalence (none in Group A, 17.6% in Group B, 24.2% in Group C; P = 0.024) (Fig. 2). In particular, 69.7% of thrombus has been observed with lumen irregularity, and 45.5% of thrombus has been observed with rupture plaque. Thrombus was mainly located at the site with the most stenotic lesions, MLA site. The area and diameter of the thrombus were numerically larger as the AS was severe.
Indipendent predictors of presence of thrombus and ruptured plaque
At the multivariable analysis, the predictors of the presence of thrombus in these coronary lesions were represented by age (OR = 0.96, CI 0.92–1.00, P = 0.043), macrophage infiltration (OR = 3.77, CI 1.53–10.72, P = 0.007), ruptured plaque (OR = 20.96, CI 8.40–57.69, P < 0.001), lipid angle (OR = 1.01, CI 1.00–1.01, P = 0.004), minimal lumen area (OR = 0.62, CI 0.42–0.86, P = 0.010), and the AS (OR = 1.05, CI 1.02–1.10, P = 0.002) (Table 3). The predictors of the presence of ruptured plaque were represented by lipid-rich plaque (OR = 2.61, CI 1.23–5.90, P = 0.016), macrophage infiltration (OR = 4.72, CI 2.04–12.39, P = 0.001), lipid angle (OR = 1.01, CI 1.00–1.01, P = 0.003), minimal lumen area (OR = 0.70, CI 0.51–0.93, P = 0.020), and the AS (OR = 1.07, CI 1.04–1.11, P < 0.001) (Table 3).
Discussion
The current study evaluated the association between the severity of area stenosis and plaque characteristics with thrombus prevalence in patients with CSA as assessed by OCT. The main findings are as follows. First, although microthrombus in size, thrombus was present in 19% of the lesions in CSA patients. Second, there was no thrombus when the AS was less than 50%. With AS \(\ge\) 50% showed thrombus occurrence, especially, thrombus prevalence increased as the AS was severe. Third, more severe AS, the more lipid-rich plaque, macrophage infiltration and plaque rupture. Fourth, thrombus and plaque rupture were mainly observed in the most stenotic, MLA site. Fifth, macrophage infiltration, lipid angle, ruptured plaque, MLA, and area stenosis were the independent predictors of thrombus presence.
Although several attempts have been made to characterize the different features of the unstable coronary plaque through OCT, there are few data about the patients with CSA. In a previous OCT study about CSA, intermediate stenosis (MLA = 3.5 ± 1.5 mm2), thrombus was found in 10% and plaque rupture in 16.7%8. In another CSA OCT study, when the MLA was 3.2 mm2 (median, 2.4–4.7mm2), thrombus was found in 9.9% and ruptured plaque was found in 9.0%9. When considering MLA, these results are comparable to Group B results of the present study, we found 17.6% of thrombus and 15.7% of ruptured plaque. However, these studies compared OCT findings of CSA and acute coronary syndrome (ACS), and did not observe OCT findings according to stenosis severity in CSA like this study.
This study showed that characteristics suggesting plaque vulnerability (lipid-rich plaque, macrophage infiltration, lipid angle, thin cap plaque, ruptured plaque, and thrombus prevalence) increased as luminal stenosis worsened, even in CSA. In particular, thrombus and ruptured plaques were most frequently found at the MLA site with the greatest shear stress. A previous study showed that the coronary stenosis severity related to shear stress might have caused coronary up-regulation of platelet, and monocyte activation in human coronary arteries. A hemodynamically significant coronary lesion creates a range of fluid dynamic forces around it, including wall shear stress, which is the tangential force exerted by blood flow on the vessel wall. High wall shear stress can activate matrix metalloproteinases in plaques, leading to features of plaque vulnerability. These features include the growth of the plaque’s necrotic core and calcium deposits, reduction of fibrous and fibrofatty tissues, greater expansive remodeling, and increased plaque strain. High-risk plaques, like thin-cap fibroatheromas, often occur in regions of high wall shear stress, particularly in the proximal segments of lesions10. This is associated with the severity of intracoronary stenosis and calculated shear stress, and it happens even with the simultaneous administration of aspirin and clopidogrel11. An animal study using electron microscopy demonstrated that a 40–60% decrease in luminal diameter of the left anterior descending coronary arteries led to endothelial denudation, platelet deposition, and thrombus formation regions proximal to the site of maximal constriction12. This phenomenon is not an exception even if there is temporary luminal stenosis caused by coronary artery spasm. Our previous study showed that plaque erosion with thrombus at spasm sites occurred in more than one-fourth of patients13. Thrombus and plaque erosion were more frequently observed at spasm segments compared to nonspasm segments, as assessed by OCT in patients with suspected vasospastic angina14. The severity of coronary stenoses as well as vulnerable plaques are known risk factors for acute coronary syndrome. In a previous study, the most ST-segment-elevation myocardial infarction occurred at the site of severe coronary stenosis and the diameter stenosis, with diameter stenosis severity being less than 50% in a minority of cases15. In addition, many studies have demonstrated the prognostic impact of coronary stenosis severity by imaging or physiology and plaque characteristics10,16,17,18. In a study, high-risk plaque characteristics were identified using coronary computed tomography angiography and included a minimum lumen area of less than 4 mm2, a plaque burden of 70% or more, low attenuating plaque, positive remodeling, napkin-ring sign, or spotty calcification. Both fractional flow reserve and the number of high-risk plaque characteristics were significantly associated with the estimated risk of vessel-oriented composite outcome (P = 0.008 and P = 0.023, respectively). Among patients with fractional flow reserve greater than 0.80, lesions with three or more high-risk plaque characteristics had a significantly higher risk of vessel-oriented composite outcome compared to those with fewer than three high-risk plaque characteristics (15.0% vs. 4.3%; hazard ratio: 3.96; 95% confidence interval: 1.45–10.83; P = 0.007).
Our findings highlight an important clinical observation. Although some high-grade stenosis lesions exhibited features of plaque vulnerability, including lipid-rich plaques, macrophage infiltration, or even rupture, none of the patients progressed to ACS. This can be explained by the fact that plaque vulnerability alone is insufficient to precipitate an acute event; additional factors, such as systemic inflammation, hypercoagulability, local hemodynamic shear stress, and the formation of an occlusive thrombus, are required to trigger plaque destabilization and clinical manifestation. This aligns with previous concepts proposing that plaque vulnerability exists on a spectrum, and not all vulnerable plaques progress to clinical events. In the PROSPECT trial, of 595 thin-cap fibroatheromas identified on radiofrequency intravascular ultrasonography, only 26 were sites of recurrent events at a median follow-up of 3.4 years19. This suggests that among the many vulnerable plaques, only a very small number of cases will actually develop an event. Many such plaques may remain clinically silent or heal without causing an ACS, particularly in patients without systemic or local prothrombotic conditions. In our study, the absence of elevated high-sensitivity cardiac troponin across all groups confirmed the lack of ongoing myocardial injury, supporting the notion that these high-grade, vulnerable plaques remained stable without progressing to acute events.
Furthermore, a previous study demonstrate the role of residual thrombus in the process of rethrombosis, finding that a residual thrombus is a highly thrombogenic surface that may significantly contribute to reocclusion even in the presence of heparinized blood20. Although CSA patients have fewer thrombotic events compared to ACS patients, it is considered necessary to take antithrombotics for primary prevention in patients with luminal stenosis. Future clinical larger studies are required to investigate whether the presence of thrombus by OCT is associated with a poor prognosis in CSA patients.
This study has several limitations. First, it was a retrospective analysis based on data from a combined multicenter registry, which means that selection bias cannot be entirely ruled out. Second, biomarkers and blood factors assessment are required to explain whether the peculiar features of the coronary plaque are associated with different mechanisms of thrombus. Third, clinical link is unclear because of the small thrombotic size.
Using OCT, the present study revealed that the prevalence of thrombus in patients with CSA is not uncommon, and the prevalence of thrombus and plaque vulnerability increased according to severity of AS. The AS severity was the independent predictor of thrombus formation in a patient with CSA. Therefore, the CSA patients with more than half of AS may be needed active treatment of antithrombotics. This approach could potentially improve patient outcomes by preventing thrombotic events in high-risk CSA patients.
Methods
Study population
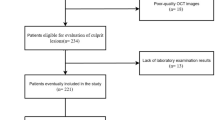
The study population was retrospectively enrolled, 146 patients, from four University Hospital cardiovascular centers (Ulsan University Hospital, Pusan National University Hospital, Keimyung University Dongsan Medical Center, Seoul National University Hospital) in South Korea from January 2013 to June 2015, for outpatient coronary angiography to investigate symptoms suggestive of SA and more than 30% of visual estimated stenotic lesion was targeted to evaluate by OCT. The lesions were divided into three groups according to OCT-derived percentage of area stenosis (AS) degree: lesions with AS < 50% (mild, Group A), 50% \(\le\) AS < 70% (intermediate, Group B), and AS \(\ge\) 70% (severe, Group C). These thresholds were selected because they are clinically meaningful in assessing lesion severity and reflect commonly used cutoffs in prior studies. Specifically, < 50% stenosis is typically regarded as mild and hemodynamically insignificant, 50–70% as intermediate, and ≥ 70% as severe, often associated with ischemic risk and the potential need for revascularization, and we adopted these thresholds to facilitate clinically relevant comparisons in our analysis21,22. To clarify, the Canadian Cardiovascular Society angina classification was used solely to categorize the severity of stable angina symptoms and does not include patients with acute ischemic events or unstable clinical conditions. High‑sensitivity cardiac troponin‑T was measured prior to angiography in all patients, and values ≥ the 99th‑percentile upper reference limit led to exclusion. Criteria of the exclusion were defined as follows: acute coronary syndrome including myocardial infarction or unstable angina in the previous 6-month, cardiogenic shock, left main lesions, reference vessel diameters of less than 2.0 mm, chronic renal failure (serum creatinine ≥ 2.0 mg/dl). The primary endpoints of this study were to investigate the relationship between the severity of area stenosis and plaque characteristics with thrombus prevalence in patients with CSA, as assessed by OCT. The study protocol was approved by the Institutional Review Boards of Ulsan University Hospital, Pusan National University Hospital, Keimyung University Dongsan Medical Center, and Seoul National University Hospital. Written informed consent was obtained from all participants at the time of study enrollment. All methods were performed in accordance with the relevant guidelines and regulations.
OCT image acquisition
OCT was performed on the target lesion after administration of 200 mg of coronary nitroglycerin, and images were acquired using a commercially available frequency-domain OCT system and optic catheter (C7XR and Dragon Fly catheter, LightLab Imaging/St. Jude Medical, Westford, Massachusetts). All patients received aspirin, P2Y12 inhibitor, and unfractionated heparin (100 IU/kg) before the catheterization procedure. The OCT imaging catheter was advanced distal to the lesion, and automated pullback was performed while injecting contrast media through the guiding catheter to clear blood from the imaging field. Predilation with balloon angioplasty was not permitted before OCT imaging. All OCT images were de-identified, digitally stored, and sent to Imaging core lab of Cardiovascular Research Foundation in Dong-A University Hospital, South Korea, where they were analyzed by one independent investigator blinded to clinical and angiographic data, using an offline review work station (Ilumien Optis, St. Jude Medical).
OCT image analysis
Thrombus was defined as an irregular mass attached to the luminal surface or floating within the lumen. Thrombus was classified into two types: (1) white thrombus, characterized by homogeneous backscattering with low signal attenuation, and (2) red thrombus, characterized by high backscattering with high signal attenuation8. In this study, lumen irregularity was defined as the presence of an irregular luminal surface contour, characterized by surface roughness or protrusions, without evidence of thrombus or plaque rupture, as assessed by OCT13. Tissue characteristics of underlying plaque were defined using previously established criteria7,8. Plaques were classified as: (1) fibrous (homogeneous, high-backscattering region), (2) lipid (low-signal region with diffuse border), or (3) fibro-calcific. Lipid was defined as a signal-poor region with a poorly defined or diffuse border, and the degree of lipid arc was measured in lipid plaques. Lipid rich plaque was defined as a plaque with a maximal lipid arc > 180°. Macrophage infiltration was defined as signal-rich, distinct or confluent punctuated regions that exceeded the intensity of background speckle noise. Calcification was defined as a signal-poor or heterogeneous region with a sharply delineated border. Multi-layered plaque was identified as a superficial layer that had a different optical intensity and a clear demarcation from underlying plaque. When the fibrous cap thickness was less than 65 µm, it was defined as a thin cap. Moreover, plaque rupture was defined as the presence of fibrous cap discontinuity leading to a communication between the inner (necrotic) core of the plaque and the lumen. Reference lumen area was defined as the mean of the largest lumen area proximal and distal to the luminal narrowing. The lesion segment was divided by the minimal lumen area (MLA) site. Using these criteria, the target lesions were divided into proximal, MLA site, and distal segments. Percent AS was calculated as the percentage decrease of lumen area at the narrowest frame using the following formula: (1 − [mean reference lumen area − minimal lumen area]/mean reference lumen area) × 100.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median, depending on whether they follow a normal or non-normal distribution, and were compared using Student’ t-test and Mann–Whitney U test, respectively. Categorical variables were compared using the Chi-square test or Fisher’s exact test. Logistic regression analysis was conducted to estimate odds ratio and 95% confidence intervals for the presence of thrombus. In a multivariable logistic regression model, variables that reached statistical significance (p < 0.05) in a univariate logistic regression analysis were tested for their independent association with thrombus. All probability values were two-sided, and P values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Data availability
The data generated in this study is available from the corresponding author upon reasonable request.
References
Libby, P. Mechanisms of acute coronary syndromes. N Engl. J. Med. 369, 883–884. https://doi.org/10.1056/NEJMc1307806 (2013).
Crea, F. & Liuzzo, G. Pathogenesis of acute coronary syndromes. J. Am. Coll. Cardiol. 61, 1–11. https://doi.org/10.1016/j.jacc.2012.07.064 (2013).
Finn, A. V., Nakano, M., Narula, J., Kolodgie, F. D. & Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 30, 1282–1292. https://doi.org/10.1161/ATVBAHA.108.179739 (2010).
Chiu, J. J. & Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387. https://doi.org/10.1152/physrev.00047.2009 (2011).
Chatzizisis, Y. S. et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49, 2379–2393. https://doi.org/10.1016/j.jacc.2007.02.059 (2007).
Tearney, G. J. et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the international working group for intravascular optical coherence tomography standardization and validation. J. Am. Coll. Cardiol. 59, 1058–1072. https://doi.org/10.1016/j.jacc.2011.09.079 (2012).
Prati, F. et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 31, 401–415. https://doi.org/10.1093/eurheartj/ehp433 (2010).
Di Vito, L. et al. Comparison between intermediate and severe coronary stenoses and clinical outcomes of an OCT-guided PCI strategy. J. Cardiovasc. Med. (Hagerstown) 17, 361–367. https://doi.org/10.2459/JCM.0000000000000280 (2016).
Yamamoto, M. H. et al. Optical coherence tomography assessment of morphological characteristics in suspected coronary artery disease, but angiographically nonobstructive lesions. Cardiovasc. Revasc. Med 20, 475–479. https://doi.org/10.1016/j.carrev.2018.07.011 (2019).
Kumar, A. et al. High coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J. Am. Coll. Cardiol. 72, 1926–1935. https://doi.org/10.1016/j.jacc.2018.07.075 (2018).
Yong, A. S. et al. Intracoronary shear-related up-regulation of platelet P-selectin and platelet-monocyte aggregation despite the use of aspirin and clopidogrel. Blood 117, 11–20. https://doi.org/10.1182/blood-2010-04-278812 (2011).
Gertz, S. D., Uretsky, G., Wajnberg, R. S., Navot, N. & Gotsman, M. S. Endothelial cell damage and thrombus formation after partial arterial constriction: Relevance to the role of coronary artery spasm in the pathogenesis of myocardial infarction. Circulation 63, 476–486. https://doi.org/10.1161/01.cir.63.3.476 (1981).
Shin, E. S. et al. OCT-defined morphological characteristics of coronary artery spasm sites in vasospastic angina. JACC Cardiovasc. Imag. 8, 1059–1067. https://doi.org/10.1016/j.jcmg.2015.03.010 (2015).
Shin, E. S. et al. Thrombus and plaque erosion characterized by optical coherence tomography in patients with vasospastic angina. Rev. Esp. Cardiol. (Engl Ed) 70, 459–466. https://doi.org/10.1016/j.rec.2016.11.003 (2017).
Manoharan, G. et al. Severity of coronary arterial stenoses responsible for acute coronary syndromes. Am. J. Cardiol. 103, 1183–1188. https://doi.org/10.1016/j.amjcard.2008.12.047 (2009).
Lee, J. M. et al. Prognostic implications of plaque characteristics and stenosis severity in patients with coronary artery disease. J. Am. Coll. Cardiol. 73, 2413–2424. https://doi.org/10.1016/j.jacc.2019.02.060 (2019).
Lee, J. M. et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC Cardiovasc. Imag. 12, 1032–1043. https://doi.org/10.1016/j.jcmg.2018.01.023 (2019).
Park, J. et al. Relevance of anatomical, plaque, and hemodynamic characteristics of non-obstructive coronary lesions in the prediction of risk for acute coronary syndrome. Eur. Radiol. 29, 6119–6128. https://doi.org/10.1007/s00330-019-06221-9 (2019).
Stone, G. W. et al. (2011) A prospective natural-history study of coronary atherosclerosis. N Engl. J. Med. 364(3), 226–235 (2011).
Badimon, L., Chesebro, J. H. & Badimon, J. J. Thrombus formation on ruptured atherosclerotic plaques and rethrombosis on evolving thrombi. Circulation 86, III74–85. https://www.ncbi.nlm.nih.gov/pubmed/1424053 (1992).
Neglia, D. et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ. Cardiovasc. Imag. 8(3), e002179 (2015).
Lee, J. M. et al. Prognostic implications of plaque characteristics and stenosis severity in patients with coronary artery disease. J. Am. Coll. Cardiol. 73, 2413–2424 (2019).
Acknowledgements
None.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Study concept and design: ES Shin and BK Koo; Acquisition of data: ES Shin, JH Oh, CW Nam, BK Koo; Analysis and interpretation of data: ES Shin; Drafting of the manuscript: ES Shin; Critical revision of the manuscript for important intellectual content: ES Shin, JH Oh, CW Nam and BK Koo.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shin, ES., Oh, JH., Nam, CW. et al. Plaque vulnerability and thrombus formation according to area stenosis in chronic stable angina. Sci Rep 15, 22831 (2025). https://doi.org/10.1038/s41598-025-04190-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04190-2