Abstract
Yarrow (Achillea millefolium L.) is an important medicinal and aromatic plant the active biologically compounds in particular essential oil is used as raw material in the pharmaceutical, food, and cosmetics industries. The impacts of the foliar spraying of chitosan (control and 2.5 g/L chitosan), the use of soil-based biochar (control and soil application at 2.0 kg/m2) under three moisture levels (irrigation at 80–85%, 60–65%, and 40–45% F.C.) on the agronomic traits and essential oil of yarrow were investigated. Results indicated that the deficit irrigation meaningfully decreased the yields of biological and flower, and essential oil yield, however, the contents of proline and essential oil under reduced irrigation significantly increased. According to essential oils analysis by GC-FID and GC/MS, the major constituents were α-pinene, 1,8-cineole, borneol, β-bisabolene, and caryophyllene oxide. A considerable increase and decrease were observed respectively in the monoterpenes and sesquiterpenes contents under reduced irrigation. The utilization of biochar along chitosan maintained higher secondary metabolites in particular α–pinene, β-myrcene, borneol, and 1,8-cineole under deficit irrigation. Generally, it appears that using the foliar spraying of chitosan along the soil application of biochar can be effective in improving the qualitative and quantitative features of the essential oil of A. millefolium in arid and semiarid climates.
Similar content being viewed by others
Introduction
Yarrow (Achillea millefolium L.) is a perennial herb belonging to the Asteraceae family that grows wildly in Asia, Africa, Europe, and America. Nowadays, the essential oil from the leaves and inflorescences of yarrow as an important therapeutic ingredients is widely used in traditional and modern medicine, food, cosmetic, and pharmaceutical industries1. The yarrow essential oil is used as an analgesic, antioxidant, anti-inflammatory, and stomach protector, as well as in the production of skincare products and aromatherapy2,3. A. millefolium has a significant position in the pharmaceutical and cosmetic industries, and conducting agronomic research will greatly contribute to expanding and improving the economic justification for cultivating this herb1,3. The major components of essential oil from the yarrow flowers due to the existence of several chemotypes are very complex. The results of previous investigations have indicated that α/β-pinene, borneol, camphor, 1,8-cineol, myrcene, α/β-thujone, bornyl acetate, sabinene, and spathulenol were the main constituents of the yarrow essential oil1,2,4,5.
The growth, physiology, and biochemistry responses, as well as the biosynthesis of secondary metabolites in particular essential oils in the medicinal and aromatic plants vary under the influence of genetic1,3, environmental factors such as climate, edaphic, and agronomic practices, biotic and abiotic stresses6,7,8, and interaction effects of them. Among environmental stresses, water deficit stress being one of the major abiotic factor affecting the quantity and quality essential oil in the medicinal and aromatic crops9. Generally, water deficit stress that requires immediate attention, as the world faces water supply constraints in agricultural areas to achieve higher agricultural productivity. An imbalance between precipitation and evapotranspiration in a specific area leads to drought stress10. One of the impacts of drought and water deficit stress is the induction of oxidative damage due to the production of reactive oxygen species, which leads to the oxidation of lipids, proteins, nucleic acids, and carbohydrates, and disrupts physiological processes such as glycolysis and photosynthesis6,8. Plants have various defense mechanisms to cope with the detrimental impacts of water deficit stress including antioxidant enzymes (catalase, ascorbate peroxidase, superoxide dismutase, etc.) and non-enzymatic antioxidant compounds (phenols, carotenoids, flavonoids, tocopherols, ascorbate, etc.).
Some biological or organic compounds can act as stimulants for modifying secondary metabolites and, consequently, the therapeutic impacts of the medicinal and aromatic plants. In recent years, the application of signaling compounds as elicitors has evolved as an effective strategy for producing desired secondary metabolites in the medicinal and aromatic plants11,12. One of the elicitors is chitosan that a natural polysaccharide obtained through the acetylation reaction of chitin extracted from the exoskeletons of crustaceans such as shrimp and crabs or from the cell wall of certain fungi13. Chitosan is a non-toxic, biocompatible, and biodegradable compound, which has led to its widespread application in agriculture. Among the beneficial impacts of chitosan is its ability to improve plant tolerance to biotic and abiotic stresses11,12,14. In general, chitosan stands out as a versatile and eco-friendly bio-stimulant with immense potential to revolutionize agriculture. Its ability to enhance disease resistance, improve growth, and mitigate abiotic stress makes it a valuable tool for sustainable crop production. Moreover, the foliar application of chitosan can cause improvement in photosynthesis, respiration and plant metabolisms, plant growth promotion, and nutritional values. These positive effects cause sustainability in the production of biomass and quantity yield, as well as improving the production quality in horticultural and agronomic crops in particular under water deficit stress10,11,15.
Another approach to cope with water deficit stress caused by evaporation and moisture depletion is the application of amendments to the soil. The use of natural materials to enhance fertility, improve the physical and chemical structure of the soils, and increase the water retention capacity of the soil has gained attention from researchers, among which biochar can be mentioned16. Biochar, or biomass charcoal, is added as a soil amendment for carbon storage in agricultural soils. Biochar is charcoal produced through the pyrolysis process from plant biomass and agricultural waste such as straw, wheat, corn, bran, rice, and sugarcane bagasse. Previous investigations have attempted to convert food waste into biochar and use it in the soil, and positive results have been suggested for soil fertility, crop productivity, and mitigation of environmental stress impacts17,18. Biochar is a rich source of many important nutrient elements such as nitrogen, phosphorus, potassium, and micronutrients16,19. Notably, biochar has the potential to enhance soil physicochemical properties, improving moisture retention and agricultural productivity. Additionally, biochar can improve plant performance and preserve the environment, with significant implications for the alleviation of drought and sustainable crop production in arid and semiarid climates19. Biochar also can alleviate environmental stresses especially salt and drought damage stress to plants through adsorption20. Furthermore, investigations have been done on the positive effect of the use of biochar in reducing the negative influence of water deficit stress on some characterization of growth and yield as well as essential oil compounds of the medicinal and aromatic plants such as borage, marjoram, and quinoa21,22,23. Alharbi and Alaklabi24 reported that the utilization of biochar along exogenous application of jasmonic acid improved salt tolerance in wheat by boosting photosynthetic attributes, mineral uptake from the soil, and enhances in osmolytes and secondary metabolites.
Despite some credible studies on soil amendment of biochar in some herbs and the foliar application of chitosan, the impacts of the biochar utilization along the foliar-spraying of chitosan and their synergist effects on the growth and essential oil of yarrow (A. millefolium) have not been well examined yet. Therefore, the aims of this experiment were: (i) to study the impact of the foliar application of chitosan and the soil utilization of biochar on the yield and biochemistry characteristics of yarrow under reduced irrigation; (ii) to assess the chemical constituents, content, and yield of essential oil from yarrow under experimental treatments.
Results
Flower and biological yield
According to a combined analysis of variance for two experimental years, the flower and biological yields of yarrow in this study was significantly (p ≤ 0.05) affected by the interaction of the experimental factors i.e. biochar × chitosan × soil moisture levels (Table 1). The effect simple of deficit irrigation treatment significantly decreased the flower and biological yields of yarrow (Fig. 1.). Indeed, water deficit stress limited the growth and yield parameters of yarrow like other agronomic and horticultural crops. However, the soil amendment of biochar along the foliar-spraying of chitosan had an increasing impact on the yield of this plant under varying soil moisture levels, both with and without water deficit stress. The highest values the flower (1323.3 kg/h) and biological (9197.7 kg/h) yields obtained from the plants treated by biochar and chitosan in optimum irrigation condition (Table 1), whereas, the lowest the flower yield (609.2 kg/h) and biological yield (4135.9 kg/h) were associated with the treatment of the interaction of non-application of biochar and chitosan under severe water deficit stress or deficit irrigation (Table 1).
Biochemical characteristics
In this study, the interaction impact of triple of the experimental factors (p ≤ 0.01) on the proline content, as an important physiological trait of the yarrow plants was significant (Table 1). The application of biochar and chitosan, especially under water deficit conditions, increased the proline content (Fig. 2). The highest value of this trait (49.07 µmol/g fresh weight) was associated with the treatment of biochar and chitosan applications under deficit irrigation based on 40% (severe water deficit stress), while the lowest value of the proline content (14.40 µmol/g fresh weight) was related to the treatment without the applications of biochar and chitosan under optimum irrigation (non-stress) (Table 1).
For the ascorbate peroxidase and catalase enzymes, there were significant differences (p ≤ 0.01) between the experimental treatment in particular triple interaction effects of the experimental factors (Table 1). The results indicated that increasing the intensity of drought stress led to an increase in the activity of these enzymes (Fig. 2). The maximum activities of the ascorbate peroxidase and catalase enzymes in the yarrow plants were obtained from the treatment of the interaction of the applications of biochar and chitosan under severe water deficit or irrigation at 40% F.C., while the lowest enzyme activities belonged to the treatment of no biochar application × no chitosan application × optimum irrigation (Table 1).
Triple effects of the experimental factors had significantly impacts (p ≤ 0.01) on the activity of the peroxidase enzyme in the yarrow plants (Table 1). The maximum activity of this enzyme was observed in the treatment of non-biochar application under severe water deficit stress (Table 1). Meanwhile, the lowest enzyme activity was recorded in the treatment without biochar and chitosan under normal irrigation and non-stress (Table 1).
The interaction effect of the experimental factors on the DPPH antioxidant capacity of the yarrow plants was significant (p ≤ 0.01) (Table 1). Enhancing the intensity of water deficit stress and the application of biochar and chitosan led to an increase in DPPH antioxidant capacity (Fig. 3). The highest value of this feature (65.38%) was associated with the soil amendment of biochar and the foliar-spraying of chitosan under water deficit stress or irrigation at 40% F.C., whereas, the lowest value was associated with non-applications of chitosan and biochar under normal moisture condition.
Generally, the activity of antioxidant enzymes and the antioxidant capacity of the plant extract measured by the DPPH method increased under deficit water stress conditions compared to the non-stress or full irrigation treatment.
Essential oil content and yield
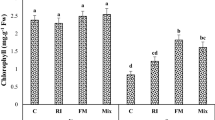
The essential oil content of yarrow was significantly affected by the interaction of the soil application of biochar × foliar-spraying of chitosan under varying soil moisture levels at the 1% level (Table 1). The results showed that the essential oil content of this medicinal plant experienced a significant increase with the soil application of biochar and the spraying of chitosan under the reduction of available water (Fig. 4a). The lowest essential oil content (0.29%) was recorded in the control (no biochar and no chitosan application) under optimum irrigation, while the highest essential oil content (0.44%) was obtained from the biochar and chitosan applications under severe stress or irrigation at 40% F.C. (Table 1).
As shown in Table 1, triple interactive had significant effects on the essential oil yield (p ≤ 0.05). The maximum essential oil yield (4.39 L/ha) was obtained from the soil amendment of biochar and the foliar application of chitosan under optimum irrigation, while the minimum essential oil yield (2.46 L/ha) was recorded in the non-applications of biochar and chitosan under water deficit condition (Table 1). In this study, the increased intensity of drought stress due to reduced irrigation water resulted in an increased percent of the essential oil in yarrow (Fig. 4b).
Essential oil profile
In this study, 26 compounds were detected in the essential oil of the medicinal plant yarrow across the investigated treatments. The major compounds included α-pinene, β-pinene, and β-myrcene belongs to the group of hydrocarbon monoterpenes, and 1,8-cineole, camphor, bornyl acetate, borneol, and terpinen-4-ol are part of the oxygenated monoterpenes, along with caryophyllene oxide, β-bisabolene, and nerolidol which belong to the group of sesquiterpenes (Table 2). As shown in Figs. 5, 6 and 7, the moderate and severe water deficit stress induced by irrigation based on 60 and 40% of F.C. in the yarrow plants led to an increase in hydrocarbon and oxygenated monoterpenes and a decrease in hydrocarbon and oxygenated sesquiterpenes.
Based on our finding, the foliar-spraying to the yarrow plants with chitosan and soil-applying of biochar under varying soil moisture levels increased the hydrocarbon and oxygenated monoterpenes and sesquiterpenes in the essential oils of yarrow. In general, the triple interactive impact of biochar × chitosan × soil moisture levels was significant on the amounts of the major compounds belonging to monoterpenes i.e. α-pinene, β-pinene, β-myrcene, camphor, bornyl acetate, borneol, 1,8-cineole, and terpinen-4-ol and the main of sesquiterpenes like β-bisabolene, nerolidol, and caryophyllene oxide present in the yarrow essential oil (Table 2). According to our results, with the increase in water deficit stress intensity and the applications of chitosan and biochar, the amounts of these constituents improved (Table 2). The highest percent of monoterpenes e.g. α-pinene (13.39%), β-pinene (3.60%), β-myrcene (5.73%), camphor (4.51%), bornyl acetate (7.02%), borneol (14.79%), 1,8-cineole (15.34%), and terpinen-4-ol (4.18) were obtained from the soil amendment of biochar and the foliar-spraying of chitosan under deficit irrigation or severe water deficit stress, while the treatment of non-applications of biochar and chitosan under optimum irrigation recorded the lowest values of these compositions (Table 2). However, the maximum concentrations of β-bisabolene (13.12%), nerolidol (8.5%) and caryophyllene oxide (13.11%) were achieved from non-applications of biochar and chitosan under optimum irrigation, whereas, the minimum amounts of β-bisabolene, nerolidol, and caryophyllene oxide were detected in the treatment of non-application of biochar and the foliar application of chitosan under deficit irrigation conditions (1.37, 1.00, and 1.36%, respectively) (Table 2).
Discussion
In the present work, the reduction in the dry flower and biological yields under water deficit stress condition was likely due to the fact that as drought stress intensity increased, photosynthesis decreased because of reduced chlorophyll, increased protease activity, and stomatal closure. Indeed, water shortage limited morphological features and growth parameters of crops. Similar to the results of the present research, found that the biomass production of yarrow significantly decreased under increasing irrigation intervals. Decrease in the characteristics of the plant growth and its correlation with quantity yield in other medicinal and aromatic species under water deficit conditions has been reported in some investigations6,8,11,12,14,25,26. Consequently, the allocation of photosynthetic materials to plant organs especially reproductive organs like flowers, decreased, leading to increased flower drop, abortion, and drying6,14,26. In this investigation, the treatments containing biochar, particularly under water deficit stress conditions, likely enhanced plant performance due to the positive impacts of biochar on nutrient retention and mobility and transformation of micronutrients, improvement of soil pH, improvement of soil physical properties including water retention, and increased population and activity of beneficial soil microbes like mycorrhizal fungal18,21,22,23,27. In line with these results, Yang, Akhtar21 and Seham, El-Din23 also reported increased yields of quinoa and borage under the biochar application and water deficit condition.
In addition in present research, the foliar-spraying of chitosan was able to improve the growth and development of plants by enhancing the accessibility and absorb of indispensable nutrients and water28,29. Moreover, the increase in yarrow yield under the use of chitosan compared to the non-application treatment, particularly under water deficit stress, was likely due to increased activity of enzymes involved in nitrogen metabolism such as nitrate reductase, glutamine synthetase, and protease, improved photosynthesis rate, increased chlorophyll content, adequate supply of essential nutrients, reduced water evaporation from the plant, which finally they were able to improve plant water status11,12,25.
Additionally, deficit irrigation treatment meaningfully decreased the yields of biological, flower, and essential oil, however, enzymatic activity of catalase, peroxidase, and ascorbate peroxidase, and antioxidant activity and as well as the contents of proline and essential oil under reduced irrigation significantly increased. Indeed, the increased synthesis and accumulation of low molecular weight compounds known as osmolytes, such as proline, are also among the defense mechanisms involved in plant cells that reduce osmotic stress6,30,31. The reduction of protein content in plants under water deficit stress is associated with increased activity of protein-degrading enzymes and the accumulation of free amino acids such as proline6,26,32. According to our finding, the soil application of biochar and the foliar-spraying of chitosan mitigated the negative impacts of water deficit stress caused by irrigation at 60% and 40% of F.C. Also, the increase in proline content in the yarrow plants under reduced irrigation and increased water deficit stress intensity from 60% F.C. (moderate stress) to 40% F.C. (severe stress) was likely due to the increased expression of the genes for the enzymes delta-proline-5-carboxylate synthetase (P5C) and delta-proline-5-carboxylate reductase (P5cR) under water deficit stress conditions, which are involved in proline production from the glutamic acid pathway, as noted by Liang, Zhang33. In line with our results, an increase in proline in Lallemantia royleana (Benth.) under drought stress was reported34. The increase in proline content in yarrow with the applications of biochar and chitosan under water deficit stress conditions can be related to increasing expression of the delta-1-proline-5-carboxylate synthetase (P5CS) gene influenced by the application of chitosan and biochar, according to the findings of Zhang, Qiu35.
Hafez, Attia16 and Alavi Samany, Ghasemi Pirbalouti14 reported an increase in the proline content in barley and hyssop under the foliar-spray application of chitosan and deficit water condition, and Zemanova, Břendová36 reported an increase in the concentration of proline in spinach with the biochar application. Mehmood, Ahmed37 also reported that the activity of antioxidant enzymes and gene expression levels in soybean triggered by salinity stress, while the soil utilization of biochar significantly further boosted the expression profile of four genes encoding antioxidant enzyme and two salt-tolerant conferring genes.
Interestingly, the soil application of biochar and the foliar-spraying of chitosan had synergic impacts on the yarrow performances and they were able alleviated the negative influences of water deficit stress on the dry flower and biological yields of yarrow. Probably, this synergic positive effect of the applications of biochar and chitosan can be related to boosting photosynthetic attributes, mineral uptake, increases in osmolytes and secondary metabolites24.
DPPH is a stable free radical that has an unpaired electron on one of its nitrogen bridge atoms. The inhibition of the DPPH radical forms the basis for assessing antioxidant capacity, which is used to measure antioxidant activity in plants and indicates the plants’ ability to cope with stress38. Water deficit stress leads to increased production of reactive oxygen species in cells, lipid peroxidation of membranes, and increased ion leakage, which enhances the activity of the plant’s antioxidant system, thereby controlling the excess production of reactive oxygen species under stress and protecting itself against the harmful impacts of stress6,31. In accordance with these results, Arabsalehi, Rahimmalek39 also reported an increase in antioxidant activity in Ducrosia anethifolia Boiss. under drought stress conditions. According to our results, the activities of antioxidant enzymes including catalase, peroxidase, and ascorbate peroxidase and also the DPPH antioxidant capacity in the yarrow plants under the soil application of biochar, especially under water deficit stress, were likely due to increased expression of antioxidant genes in response to stress40. Alharbi, Khan41 reported that the soil application of biochar along exogenously applied β-sitosterol on the thymus (Thymus vulgaris L.) plants significantly decreased the electrolytic leakage of cells in heat stress condition. They claim that the reactive oxygen species was decreased, however, the synthesis of antioxidants enhanced with the treatment of biochar and β-sitosterol. Moreover, they found that the synergistic application of biochar and β-sitosterol led to an upregulation in the synthesis of antioxidant enzymes e.g. 15.29% in SOD, 73.28% in APOX, and 14.67% in POD compared to the control41.
In other respects, the increase in the activities of the antioxidant enzymes (catalase, peroxidase, and ascorbate peroxidase) and DPPH antioxidant capacity in yarrow under the foliar-spraying of chitosan can be related to increasing antioxidant activities in stressed plants by chitosan as strong stimulant, which this increase is induced through the positive signaling regulation of defense-related genes, including antioxidant genes in the plant11,42. In addition, the reduction of reactive oxygen species accumulation through increased activity of antioxidant enzymes, stimulation of physiological processes, improved growth, and increased carbon dioxide fixation was influenced by the foliar application of chitosan11,12,25.
Complex processes are activated in plants in response to environmental stresses, which include hormonal modulation, measurement and signaling due to transcription factors, and the production of secondary metabolites6,39. Among these factors, secondary metabolites like essential oils are significant because these compounds play crucial roles in regulating plant environmental interactions and subsequent adaptive responses11,32. Water shortage affects the quantity and quality of essential oils by influencing the expression of genes or the activity of enzymes involved in the biosynthesis pathways of secondary metabolites8,14. The increase in the percent of essential oil in yarrow in this study under biochar application, particularly under water deficit stress, was likely due to the fact that essential oils are often terpenoid compounds that require isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) as building blocks, along with a fundamental need for NADPH and ATP.
In addition, according to results of this research, the increased percent of essential oil in yarrow under chitosan elicitor spray, particularly under water deficit stress can be related to chitosan’s role in activating new genes and various biosynthetic pathways for essential oil production, as well as increasing the number of essential oil-secreting glands and the biosynthesis of monoterpenes30. Previous literature illustrated that the essential oil contents of French lavender (Lavandula dentata L.) in the plants treated by the foliar application of chitosan under water deficiency was higher than treatment of water deficiency without chitosan43. Consistent with these results, Ghasemi Pirbalouti, Malekpoor11 also reported increased the content and yield of essential oil of two species of basil under chitosan application and both stress and non-stress conditions. In the present study, the reduction in essential oil yield under moderate and severe water deficit stress compared to optimum irrigation was likely due to the decrease in available soil water and the detrimental impacts of drought stress on the growth and dry matter yield of yarrow. Given that the essential oil yield is a product of the percent of the essential oil and the plant’s dry matter yield, the lower essential oil yield in water deficit stress treatments that produced a lower percent of the essential oil is likely due to lower dry matter yield under stress conditions.
In general, the effect interaction of the foliar-spraying of chitosan and the soil application of biochar under deficit water stress on the essential oil content may be due to its impact on enzyme activity and metabolism of essential oil output. The increase in essential oil yield under the biochar and chitosan applications in both stress and non-stress conditions was also due to the increased percent of essential oil and the yield of yarrow influenced by these two stimulants.
In line with our finding, Farhadi, Babaei1, 28 compounds were also identified in the essential oil of yarrow, with 1,8-cineole, camphor, chamazulene, and α-eudesmol being the most significant compounds found. In previous investigation by Alvarenga, Pacheco2, 1,8-cineole, borneol, β-caryophyllene, and sabinene accounted for the highest proportion of the essential oil compounds of this species of yarrow. In another study5, the major constituents of the essential oil from A. millefolium cultivated in Saudi were monoterpenes, e.g., myrcene, 1,8-cineole, camphor, α-thujone, and β-thujone. Reduction in the primary metabolism of the plants during environmental stresses can result in the accumulating of specific products, which can be shifted toward the biosynthesis and accumulation of secondary metabolites like essential oils30,44. The soil amendment of biochar may effectively decrease the harmful impacts of environmental stresses on the crops by improving the soil cation exchange capacity, soluble, and exchangeable K+, N use efficiency, and fertilities, decreasing soil electrical conductivity and water evaporation. The results of previous investigation by Eghlima, Mohammadi44 have indicated that biosynthesis and accumulation of monoterpenes like carvacrol, as the dominate compound in the essential oil of Satureja species, in response to environmental stresses like salt and drought. They realized that the percent of carvacrol in salinity stress conditions was higher than control and also stated that the biochar application effectively reduced the damage caused by salinity stress and the utilization of 3% biochar could have the positive impacts on the essential oil content, carvacrol percent, and SPAD index of Satureja khuzistanica Jamzad. under salinity stress. Results of a previous research by Abd-Rabbu, Wahba43 showed that the amounts of hydrocarbons monoterpenes and oxygenated monoterpenes produced from the water deficit stressed plants under 6 m/L chitosan application was higher that the stressed plants without chitosan application. The impact of water deficit stress on the quality and quantity of the essential oil compounds in medicinal plants is likely due to changes in the expression of genes or the activity of enzymes involved in the biosynthesis of monoterpenes and sesquiterpenes or may relate to the way the plant neutralizes water deficit stress45. Under such stress conditions, the plant may increase the amount of monoterpenes to counteract oxidative damage caused by the accumulation of reactive oxygen species2. Ghassemi and Raei46 reported that the enhancing all of the essential oil compositions of the garlic bulbs and stimulate the biosynthesis of some constituents by the utilization of biochar and polyamine (putrescine) can be related with up/down regulation of some enzymes and proteins in metabolism of plant carbohydrate, which may affect the balance of the secondary metabolites. The soil application of biochar with high cation exchange capacity and rich in micronutrients and macronutrients (potassium, zinc, iron, calcium, phosphorus, etc.) provided the necessary nutrients as cofactors for the optimal functioning of specific enzymes involved in the synthesis pathways of essential oil compounds, resulting in increased hydrocarbon and oxygenated monoterpenes and sesquiterpenes in yarrow under both stress and non-stress conditions41,47. Additionally, in the present study, the chitosan elicitor likely increased these essential oil compounds in yarrow under both stress and non-stress conditions by activating and expressing the genes in the biosynthetic pathways of monoterpenes and sesquiterpenes and also antioxidant enzyme activity, and compatible solutes, sending a series of chemical signals to the plant48.
Conclusions
Based on the results of this study, water deficit meaningfully reduced the biological yield, dry flower weight, and essential oil yield of yarrow (A. millefolium), however, enzymatic activity of catalase, peroxidase, and ascorbate peroxidase, and antioxidant activity and as well as the contents of proline, essential oil, and some major constituents of the essential oil under deficit irrigation significantly improved. The soil application of biochar and foliar spraying of chitosan could be effective on the stability of the growth parameters and dry herbal yield under deficit irrigation condition, improving its tolerance to water deficit stress caused by decreased irrigation water at 60 and 40% F.C. Interestingly, the quality of yarrow essential oil was improved by an enhancement in the biosynthesis and accumulation of monoterpenes e.g. α/β –pinene, β-myrcene, camphor, borneol, 1,8-cineole etc., which the soil amendment of biochar along the foliar-spraying of chitosan under severe water deficit stress had the highest amounts in the yarrow essential oil. However, the highest production of sesquiterpenes in A. millefolium e.g. nerolidol and caryophyllene oxide were obtained from the yarrow plants under the non-applications of biochar and chitosan under optimum irrigation. Generally, the interactive of the biochar amendment and the foliar-spraying of chitosan can mitigate the adverse impacts of water deficit stress and improved the active biologically compounds like essential oil in A. millefolium under dehydration conditions especially in arid and semiarid climate. According to these findings, it is evident that incorporating biochar and chitosan sustainable agricultural practices could be a viable strategy for farmers to ensure better the quality of yarrow essential oil in the future. The action mechanism of the biochar and chitosan effects on the biosynthesis pathways of secondary metabolites particularly monoterpenes in essential oil of the yarrow plant is not addressed currently. In general, the analysis at molecular levels, identify of secondary metabolites biosynthesis paths need to be studied in A. millefoliumunder soil-based biochar, foliar-spraying of chitosan and water deficit stress conditions.
Materials and methods
Experimental treatments and design, and agronomic practices
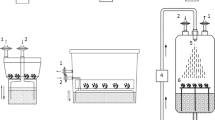
To investigate the impact of the chitosan and biochar applications on the biochemical and phytochemical properties of yarrow (Achillea millefolium L.) under different irrigation regimes, an experiment was conducted during consecutive two years (2020 and 2021) at the Islamic Azad University Experimental Farm located in the Malard region (semiarid climate) of Tehran Province, Iran. The experiment was designed as a split-plot factorial in a completely randomized block design with three replications. the foliar spraying of chitosan (control and 2.5 g/L chitosan) along the use of biochar (control and soil application at 2.0 kg/m2) under different irrigation levels (irrigation at 80–85% field capacity or F.C., 60–65% F.C., and 40–45% F.C., respectively) that these treatments were assigned to the main plots. The application of chitosan and biochar treatments were set at two levels including non-application of chitosan and the spraying of chitosan at 2.5 g/L chitosan and the soil application of biochar including non-application of biochar and soil application biochar at a rate of 2 kg per square meter of soil which were factorial placed in subplots (Fig. 8). Biochar was produced by pyrolysis of woody pruning residues of the branches of forest trees (as the major residue in wood factory in the northern Iran).
Before planting, a one-kilogram soil sample was taken from a depth of 0 to 30 cm at the planting site of yarrow to determine the soil’s physicochemical properties. This sample was sent to the water and soil laboratory, and nitrogen, phosphorus, and potassium base fertilizers were applied to the experimental field based on the results of the soil test (Table 3) and according to fertilizer recommendations for this plant.
Soil preparation operations included one plowing and two perpendiculars disking before planting. Each experimental plot measured 1.5 m in length and 2 m in width, consisting of three planting rows. The distance between the main plots was 1.5 m, and the distance between blocks was also 1.5 m.
For planting of yarrow, the seeds were purchased from Pakaneh Seed Company, Esfahan, Iran. The collection site access has been approved by A. Modaresi, Ph.D., the local owner. Plant identities were confirmed by H.A. Shirmardi, Ph.D. (Research Center for Agriculture and Natural Resources, Iran). A voucher specimen of the plant was preserved in the TARI herbarium of the Research Institute of Forests and Rangelands (RIFR), Iran (Herbarium specification No. 104120 TARI). We confirm that field studies on cultivated plants in this investigation comply with international, national and/or institutional guidelines. To produce seedlings, the seeds were sown in the greenhouse of Islamic Azad University, Shahriyar branch, in planting trays filled with perlite and peat moss, spaced 5 cm apart and sown to a depth of 0.5 cm, then covered with coco coir. The seedlings were transferred to the main field at the 5-leaf stage. At the time of transplanting, the seedlings in the biochar application treatments, 2 kg of biochar per square meter was mixed with the soil to a depth of 15 cm. The transplanting operations were conducted with planting distances of 20 cm within rows and 50 cm between rows in December 2021 and 2022, according to the planting map. Immediately after planting, the first irrigation was performed. Irrigation of each plot was performed using the soil moisture curve. F.C. and permanent wilting point (P.W.P.) were − 33 and − 1500 kPa, respectively. Soil moisture was measured by a TDR device three times a week following the manufacturer’s protocol49.
After the plants were established, the irrigation treatments were applied. For the chitosan application treatment, the yarrow plants were foliar sprayed at three stages: establishment, stem elongation, and budding at dew point i.e. early morning (~ 150 mL/plant). Maintenance operations included mechanical control of weeds, pests, and diseases throughout the plant’s growth. The flowering tops of yarrow were harvested on July 15th of each growing year. No systemic pesticides and herbicides were used during the experiment, and weeds were controlled manually.
Measurement of biological and flower yields
To determine the biological and flower yields of yarrow, during the flowering stage (~ 50%), the plants from the middle row of each plot were selected and harvested. After harvesting, the flowering tops were separated and dried in the shade with exposure to free air. The dried samples were weighed and converted to kg/ha. The aerial parts of the plants were placed in an oven at 70 °C for 48 h, then weighed, and combined with the flower yield to record the total biological yield50. Subsequently, the essential oil from yarrow flowers was extracted.
Measurements of biochemical traits
The enzyme extraction process was carried out at 4 °C using freshly homogenised samples that had been treated with EDTA-Na2 and ascorbate in potassium phosphate buffer. Centrifugation was used to produce the raw enzyme extract. According to the method of Change and Maehly51, 10 mM guaiacol, 70 mM hydrogen peroxide, 250 µL of potassium phosphate buffer, and 467 µL of sterile distilled water were added to a quartz cell and read in a Perkin Elmer UV–Vis spectrophotometer (Perkin Elmer, USA) as a control. Then, the extracted yarrow plant extract was added to the above mixture, and the antioxidant peroxidase enzyme activity was recorded at a wavelength of 470 nm over 180 s. The activity of this enzyme was reported based on the number of micromoles of hydrogen peroxide decomposed by the peroxidase enzyme per milligram of protein in the extracted plant sample.
According to the method by Bergmeyer52, 500 µL of sterile distilled water was added to 250 µL of 100 mM potassium phosphate buffer in a quartz cell. Then, 250 µl of 70 mM hydrogen peroxide were added to the mixture and read in a spectrophotometer at a wavelength of 240 nm as a control. After that, 20 µL of the extract from each treatment was added to the above mixture, and the highest absorbance of hydrogen peroxide by the catalase enzyme was reported at a wavelength of 240 nm.
Using the method of Nakano and Asada53 the antioxidant enzyme activity of ascorbate peroxidase in yarrow was measured. Briefly, 850 µL of 0.5 mM ascorbate (dissolved in 100 mM potassium phosphate buffer) were placed in a quartz cell along with 150 µL of 2 mM hydrogen peroxide (dissolved in double-distilled water) and considered as a control in the spectrophotometer at a wavelength of 290 nm. Then, 20 µL of the extracted enzyme was added to the reaction mixture, and the activity of ascorbate peroxidase was recorded over 180 s. The activity of ascorbate peroxidase was expressed as the number of micromoles of hydrogen peroxide decomposed per minute per milligram of protein.
To measure the amount of proline in the leaves of the yarrow plant, 0.5 g of plant tissue was ground with 10 mL of 3% sulfosalicylic acid in a porcelain mortar54. The resulting solution was transferred to a test tube with a lid, and these tubes were centrifuged for 15 min at 3000 RPM. One milliliter of the centrifuged extract was transferred to another test tube along with 1 mL of ninhydrin and 1 mL of glacial acetic acid, and placed in a 100-degree water bath for one hour. After the extract changed color to a pink hue, to stop the reactions, the extract was placed in ice water. After cooling, 4 mL of toluene solution was added to each of the tubes containing the extract. In each test tube containing the extract, two distinct phases were observable, with the color of the upper phase varying from red to pink based on the concentration of proline in the extract. Therefore, to measure the proline content in the extract, the upper phase was used, and its absorbance was calculated at a wavelength of 541 nm using a spectrophotometer. Standard solutions were prepared to draw the standard curve, and the curve was plotted based on their light absorbance. The concentration of proline in each extract sample was reported in micromoles per gram of fresh weight.
Essential oil content and yield
To measure the essential oil present in the flowers of yarrow, the water distillation method was used. For this purpose, 40 g of dried flowers from each treatment were weighed and then ground. The ground flower samples were transferred into a flask containing 500 mL of water in a Clevenger apparatus (made by Glass Fabricating of Ashk-e-Shishe Co., Tehran, Iran). The flower and water mixture was boiled for four hours, after which the volume of the extracted essential oil was recorded. By opening the apparatus valve, the essential oil was transferred into dark-colored bottles, and anhydrous sodium sulfate was added to the extracted essential oil. Based on the essential oil obtained per 100 g of dried flowers for each treatment, the essential oil yield of yarrow was calculated in liters per hectare.
Gas chromatography (GC) and gas chromatography–mass spectrometry (GC/MS)
To separate and identify the components of the essential oil, the essential oil obtained from each treatment was injected into GC-FID and GC/MS devices with the following specifications. GC was a Younglin Acme6000 with a column length of 30 m, an internal diameter of 0.25 mm, and a film thickness of 0.25 µm of type BP5. To identify the components of the essential oil, the sample was diluted with n-hexane and 1 µL was injected into GC. The temperature program for the column was set as follows:—initial oven temperature of 50 °C, held for 5 min;—temperature gradient of 3 °C/min;—increased to 240 °C, and then increased to 300 °C at a rate of 15 °C/min, with a hold at this temperature for 3 min, and a total response time of 75 min.
GC/MS analyses of the samples were performed on an Agilent Technologies 6890 gas chromatograph coupled to an Agilent 5973 C mass selective detector (MSD) and quadrupole EI mass analyzer (Agilent Technologies, Palo Alto, CA, USA) with a column length of 30 m, an internal diameter of 0.25 mm, and a film thickness of 0.25 µm of type BPX5. Mass spectra were recorded at 70 eV. Mass range was from m/z 40–500. For identifying the components of the essential oil, a sample diluted with n-hexane was injected at a rate of 1 µL into the GC/MS device. The temperature program for the column was set same GC method.
The injection chamber temperature was set at 290 °C with a split ratio of 1 to 35. The FID detector temperature was set at 300 °C, and helium gas was used as the carrier gas with a flow rate of 1 mL/min. Helium gas was used as the carrier gas with a flow rate of 0.5 mL/min. The software used was Chemstation. The identification of the spectra was performed using their retention indices and compared with indices available in reference books and articles, using mass spectra of standard compounds and information available in the computer library55,56,57.
Statistical analysis
All data were tested for normality and homogeneity of variance. The observations were statistically analyzed based on the generalized linear model (GLM) procedure of the SAS statistical package (v.9.2.). The Duncan multiple range test at 5% level was employed for comparing means, and Excel software was used for drawing tables and graphs.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- F.C.:
-
Field capacity
- RCBD:
-
Randomized complete block design
- P.W.P.:
-
Permanent wilting point
- CAT:
-
Enzymatic activity of catalase
- POD:
-
Peroxidase
- APX:
-
Ascorbate peroxidase
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazy
- GC–FID:
-
Gas chromatography (GC)–Flame ionization detector (FID)
- GC/MS:
-
Gas chromatography/mass Spectrometry
- GLM:
-
Generalized linear model
References
Farhadi, N., Babaei, K., Farsaraei, S., Moghaddam, M. & Ghasemi Pirbalouti, A. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crops Prod. 152, 112570 (2020).
Alvarenga, I. C. A., Pacheco, F. V., Alvarenga, A. A., Bertolucci, S. K. V. & Pinto, J. E. B. P. Growth and production of volatile compounds of yarrow (Achillea millefolium L.) under different irrigation depths. An. Acad. Bras. Ciênc. 90, 3901–3910 (2018).
Ghasemi Pirbalouti, A. Chemical composition of the essential oils from the leaves and flowers of two Achillea species from Iran. J. Essent. Oil Bearing Plants 20, 205–214 (2017).
Konarska, A. et al. Herb and flowers of Achillea millefolium subsp. Millefolium L.: Structure and histochemistry of secretory tissues and phytochemistry of essential oils. Molecules 28, 7791 (2023).
Mohammed, H. A. et al. Variability in the volatile constituents and biological activities of Achillea millefolium L. essential oils obtained from different plant parts and by different solvents. Arab. J. Chem. 16, 105103 (2023).
Babaei, K., Moghaddam, M., Farhadi, N. & Ghasemi Pirbalouti, A. Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes minuta L.) to drought stress. Sci. Hort 284, 110116 (2021).
Bakhtiar, A., Khaghani, S., Ghasemi Pirbalouti, A., Gomarian, M. & Chavoshi, S. Essential oil variation among different populations of Ziziphora tenuior L. cultivated at semiarid climate. J. Essent. Oil Res. 33(4), 385–393 (2021).
Danesh-Shahraki, H., Ghasemi Pirbalouti, A., Rajabzadeh, F. & Kachouei, M. A. Foliar application of salicylic acid and proline to mitigate water deficit impact on purple coneflower (Echinacea purpurea (L.) Moench). Acta Sci. Pol. Hortorum Cultus 22, 89–97 (2023).
Humbal, A. & Pathak, B. Influence of exogenous elicitors on the production of secondary metabolite in plants: A review (“VSI: secondary metabolites”). Plant Stress 8, 100166 (2023).
Demehin, O., Attjioui, M., Goñi, O. & O’Connell, S. Chitosan from mushroom improves drought stress tolerance in tomatoes. Plants 13, 1038 (2024).
Ghasemi Pirbalouti, A., Malekpoor, F., Salimi, A. & Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 15(217), 114–122 (2017).
Vosoughi, N., Gomarian, M., Ghasemi Pirbalouti, A., Khaghani, S. & Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Indus. Crops Prod. 1(117), 366–374 (2018).
Malerba, M. & Cerana, R. Chitin-and chitosan-based derivatives in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water. Polysaccharides 1, 21–30 (2020).
Alavi Samany, S. M., Ghasemi Pirbalouti, A. & Malekpoor, F. Phytochemical and morpho-physiological changes of hyssop in response to chitosan-spraying under different levels of irrigation. Ind. Crops Prod. 176, 114330 (2022).
Chandrasekaran, M. & Paramasivan, M. Chitosan derivatives act as a bio-stimulants in plants: A review. Int. J. Biol. Macromol., 271, 132720 (2024).
Hafez, Y. et al. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 10, 630 (2020).
Lee, Y.-E., Jeong, Y., Kim, I.-T., Ahn, K.-H. & Jung, J.-H. Enhancing the potential application of food-waste biochar as a sustainable bio-solid fuel: Analysis of post-treatment and combustion behavior. Chemosphere 364, 143216 (2024).
Pradhan, S., Parthasarathy, P., Mackey, H. R., Al-Ansari, T. & McKay, G. Food waste biochar: A sustainable solution for agriculture application and soil–water remediation. Carbon Res. 3, 1–29 (2024).
Hazrati, S., Rostami, N., Mohammadi, H. & Ebadi, M.-T. Improving the growth parameters, yield, and oil quality of camelina in rainfed farming due to the combined use of biochar and supplementary irrigation. J. Agric. Food Res. 16, 101160 (2024).
Dong, X. et al. Enhancing the growth performance of Sesbania cannabina using Ensifer alkalisoli and biochar under salt stress. Rhizosphere 30, 100888 (2024).
Yang, A. et al. Biochar mitigates combined effects of drought and salinity stress in quinoa. Agronomy 10, 912 (2020).
Tavali, I. E. Short-term effect of biochar on the improvement of calcareous soil biological properties and marjoram (Origanum majorana L.) growth under greenhouse conditions in a Mediterranean climate. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 50, 12688–12688 (2022).
Seham, M., El-Din, W. M. S., Farouk, S. & Mokhtar, N. Integrated effects of biochar and potassium silicate on borage plant under different irrigation regimes in sandy soil. J. Hortic. Sci. Ornamental Plants 13, 60–76 (2021).
Alharbi, K. & Alaklabi, A. Alleviation of salinity induced growth and photosynthetic decline in wheat due to biochar and jasmonic acid application involves up-regulation of ascorbate-glutathione pathway, glyoxylase system and secondary metabolite accumulation. Rhizosphere 24, 100603 (2022).
Bistgani, Z. E., Siadat, S. A., Bakhshandeh, A., Ghasemi Pirbalouti, A. & Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 5(5), 407–415 (2017).
Mosaedi, H., Mozafari, H., Sani, B., Ghasemi Pirbalouti, A. & Rajabzadeh, F. Foliar-applied silicon and zinc nanoparticles improve plant growth, biochemical attributes, and essential oil profile of fennel (Foeniculum vulgare) under different irrigation regimes. Funct. Plant Biol 51, FP24149 (2024).
Novak, J. M. et al. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 174, 105–112 (2009).
Hidangmayum, A., Dwivedi, P., Katiyar, D. & Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol Mol Biol Plants 25, 313–326 (2019).
Hidangmayum, A., Dwivedi, P., Katiyar, D. & Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 25, 313–326 (2019).
Bistgani, Z. E., Barker, A. V. & Hashemi, M. Physiology of medicinal and aromatic plants under drought stress. Crop J. 12(2), 330–339 (2024).
Samani, A. S., Ghasemi Pirbalouti, A., Yadegari, M. & Rajabzadeh, F. Effects of foliar application of salicylic acid and proline on the growth traits and the essential oil content of Dracocephalum kotschyi Boiss. under water deficit conditions. J. Plant Environ. Physiol. 69, 160–171 (2023).
Maghsoudi, E., Abbaspour, H., Ghasemi Pirbalouti, A. & Saeidi-Sar, S. Influence of the foliar applications of paclobutrazol and 24-epibrassinolide on the quantitative and qualitative traits of sage (Salvia officinalis L.) volatile oil under different soil moisture conditions. J. Plant Growth Regul. 42(9), 5495–5506 (2023).
Liang, X., Zhang, L., Natarajan, S. K. & Becker, D. F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 19, 998–1011 (2013).
Shams, H., Omidi, H. & Seif, S. M. The impact of phytochemical, morpho-physiological, and biochemical changes of Lallemantia royleana (Benth.) on drought tolerance. Plant Prod. Sci. 25(4), 440–457 (2022).
Zhang, X. et al. Biochar regulates 2-acetyl-1-pyrroline, grain yield and quality in fragrant rice cropping systems in Southern China. Agronomy 13, 2860 (2023).
Zemanova, V., Břendová, K., Pavlikova, D., Kubátová, P. & Tlustoš, P. Effect of biochar application on the content of nutrients (Ca, Fe, K, Mg, Na, P) and amino acids in subsequently growing spinach and mustard. Plant Soil. Environ. 63, 322–327 (2017).
Mehmood, S. et al. Chitosan modified biochar increases soybean (Glycine max L.) resistance to salt-stress by augmenting root morphology, antioxidant defense mechanisms and the expression of stress-responsive genes. Plants 9, 1173 (2020).
Parcheta, M. et al. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 14, 1984 (2021).
Arabsalehi, F. et al. Metabolic and physiological effects of water stress on Moshgak (Ducrosia anethifolia Boiss) populations using GC–MS and multivariate analyses. Sci. Rep. 12, 22148 (2022).
Zulfiqar, F. et al. Biochar, compost, and biochar–compost blend applications modulate growth, photosynthesis, osmolytes, and antioxidant system of medicinal plant Alpinia zerumbet. Front. Plant Sci. 12, 707061 (2021).
Alharbi, K. et al. Synergistic effect of β-sitosterol and biochar application for improving plant growth of Thymus vulgaris under heat stress. Chemosphere 340, 139832 (2023).
Arif, Y., Siddiqui, H. & Hayat, S. Sustainable Agriculture Reviews 53: Nanoparticles: A New Tool to Enhance Stress Tolerance (eds. Faizan, M., Hayat, S. & Yu, F.) 53, 399–413 (Springer, 2022).
Abd-Rabbu, H. S., Wahba, H. E. & Khalid, K. A. The effects of foliar application of chitosan on the morphological and chemical characters of French lavender against water deficiency. Vegetos 37, 997–1008 (2024).
Eghlima, G., Mohammadi, M., Mirjalili, M. H. & Ghorbanpour, M. Exploring the potential impact of biochar amendments in promoting redox reactions, agro-morphological, and phytochemical characteristics in Satureja khuzistanica Jamzad under salt stress. J. Soil Sci. Plant Nutr. 24, 190–202 (2024).
Caser, M. et al. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 129, 85–96 (2019).
Ghassemi, S. & Raei, Y. Evaluation of ion content, productivity and essential oil quality of garlic under saline conditions and biochar and polyamine treatments. J. Food Compos. Anal. 96, 103720 (2021).
Kiani, H., Khalesro, S., Mokhatssi-Bidgoli, A. & Sharifi, Z. Biochar and conservation tillage affect the agronomic performance and fatty acid composition of Nigella sativa L. Under both irrigated and dryland conditions. Sci. Rep. 14, 2648 (2024).
Kahromi, S. & Khara, J. Chitosan stimulates secondary metabolite production and nutrient uptake in medicinal plant Dracocephalum kotschyi. J. Sci. Food Agric. 101, 3898–3907 (2021).
Jafari, S., Garmdareh, S. E. H. & Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 253, 128–133 (2019).
Lee, J. W. & Son, J. E. Nondestructive and continuous fresh weight measurements of bell peppers grown in soilless culture systems. Agronomy 9, 652 (2019).
Change, B. & Maehly, A. Assay of catalases and peroxidase. Methods Enzymol 2, 764–775 (1955).
Bergmeyer, H.-U. Methods of Enzymatic Analysis (Elsevier, Amsterdam, 2012).
Nakano, Y. & Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 28, 131–140 (1987).
Bates, L. S., Waldren, R. & Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Adams, R. P. & Sparkman, O. D. Review of identification of essential oil components by gas chromatography/mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 803–806 (2007).
McLafferty, F.W., Stauffer, D.B., Stenhagen, E. & Heller, S.R. The Wiley/NBS registry of mass spectral data (Wiley, New York, 1989).
McLafferty, F. W., Stauffer, D. A., Loh, S. Y. & Wesdemiotis, C. Unknown identification using reference mass spectra. Quality evaluation of databases. J. Am. Soc. Mass Spectromet. 10, 1229–1240 (1999).
Acknowledgements
The authors are thankful to the research and technology deputy of Islamic Azad University of Shahr-e-Qods Branch and also Research Center for Medicinal Plants of Islamic Azad University of Shahr-e-Qods Branch for providing facilities.
Funding
There was no funding.
Author information
Authors and Affiliations
Contributions
F. Rezaei-Adl carried out the field experiment, collected the plant sample, and essential oil extraction; A. Ghasemi Pirbalouti shared the work idea, revising and writing the draft of the manuscript, supervising, and administering of project; T. Rahimi edited of the manuscript and supervising, analyzing of data; F. Rajabzadeh edited of the manuscript and consulting of project; H. Mozafari edited of the manuscript and consulting of project manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study is complied with relevant institutional, national, and international guidelines and legislation. This study does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rezaei-Adl, F., Ghasemi Pirbalouti, A., Rahimi, T. et al. Soil-based biochar and foliar-spraying of chitosan enhances the phytochemical traits of yarrow (Achillea millefolium L.) under varying moisture levels. Sci Rep 15, 30881 (2025). https://doi.org/10.1038/s41598-025-04308-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04308-6