Abstract
The study aimed to synthesize and evaluate the effects of an experimental adhesive system containing different concentrations of riboflavin in combination with Lipoid/phosphatidylcholine solutions on the dentin-bonding interface. Lipoid solutions were prepared in riboflavin (RF) experimental self-etching adhesive, (Ct0, RF0.5%, RFLi0.5%/0.25%, RFLi0.5%/0.5%). Resin-dentin slabs were prepared for hybrid layer evaluation and microtensile strength was tested. Interfacial leakage was also determined using silver tracer nanoleakage. The mechanical properties of collagen fibrils were evaluated. The adhesive was assessed for contact angle and degree of conversion. A biofilm based on Streptococcus mutans, Actinomyces naeslundii, and Streptococcus sanguis was used to evaluate fluorescence in-situ hybridization for antimicrobial analysis. Collagen was examined using a transmission electron microscope with in-situ hybridization. Human macrophages were stained for immunofluorescence. Resin tags were detectable in all adhesive specimens. RFLi preserved adhesive bond strength after long-term aging. Sizes and dispersion of fibrils in RF0.5%, RFLi0.5%/0.25%, RFLi0.5%/0.5% were substantially larger (p < 0.05). Contact angle values exhibited significant differences (p < 0.05). Both Ei and Hi were impacted by different adhesives. RFLi 0.5%/0.5% group exhibited an increase in the degree of conversion. RFLi0.5%/0.5% displayed well-preserved collagen fibrils. Confocal images showed the presence of dead bacteria amongst RFLi0.5%/0.25%/RFLi0.5%/0.5% groups. CD80 + markers on macrophages were detected in the RFLi0.5%/0.25%/RFLi0.5%/0.5% groups. RFLi0.5%/0.5% modified adhesives show enhanced bonding to dentin and can be expected to prolong the long-term integrity of the resin-dentin interface.
Similar content being viewed by others
Introduction
The foremost objective in adhesive dentistry is to produce restorations with a long-lasting bonding to enamel and dentin1. Dentin is a vital tissue consisting of organic and inorganic components. The collagen crosslinks with calcium and phosphates in a complex structure to provide strength and elasticity to the tooth2. It is due to this intrinsic structure, that dentin bonding remains far more challenging when compared to enamel bonding. Currently, the hybridization in dentin leads to the removal of inorganic hydroxyapatite by acid etching, acidic monomers, or calcium chelating agents, making way for adhesive resin infiltration and achieving micromechanical retention3,4. This hybrid layer is a critical zone in dentin bonding where the quality of the interface directly impacts the resin-dentin interface. More uniform hybrid layers are associated with increased bond strength, especially in etch-and-rinse systems. This is crucial for the long-term success of adhesive restorations5.
It is crucial to acknowledge that the overstretching of dentin collagen following acid etching, combined with the high moisture content of dentin, renders it impossible for certain hydrophobic resin monomers in the adhesive to permeate the full thickness of the demineralised collagen due to an unfavourable diffusion gradient among the various components at the bonding interface5. Along with the exposure of the collagen fibrils, the endogenous matrix metalloproteinases and cysteine-cathepsins are activated and cause the degradation of the unprotected collagen fibrils within the resin-dentin hybrid layer6. In addition, the hydrophilic resin monomers absorb water and hydrolyze over time reducing the longevity of the resin-dentin bonds7. Therefore, enhancing the dentin durability of hybrid layers constitutes a pressing practical issue that requires resolution. The biomodification of the dentin collagen via cross-linking agents have shown results in mechanical stability of the resin dentin interface and better physicomechanical characteristics of the collagen structure8. Several reports indicate that the use of crosslinking agents can increase the rigidity of collagen by strengthening the hybrid layer through the addition of hydrogen bonding and the creation of covalent inter or intramolecular crosslinks9. Collagen cross-linking agents have a two-fold effect; they maintain the collagen structure in an expanded state preventing the collagen structure from collapsing. This enhances the diffusion of resin monomers to penetrate deeper into the acid-etched dentin collagen structure, thus creating a stronger and more durable hybrid layer. Furthermore, the cross-linking agents have been demonstrated to inhibit dentin proteases, therefore reducing the biodegradation of the collagen fibrils within the hybrid layer10.
Riboflavin (RF), or vitamin B2, is a biocompatible substance that may connect molecules and can fight against viruses and bacteria. It has been effectively employed in the field of ophthalmology to enhance the strength of the cornea and address various corneal ailments11. Activated by ultraviolet-A radiation, RF generates radicals (1O2) that form covalent intermolecular collagen crosslinks by photo-oxidation12. Moreover, it has been suggested that including riboflavin/UVA in dentin adhesives might boost the initial bond strength, stabilize the adhesive interface, and inhibit the action of dentin matrix metalloproteinases. This, in turn, can increase the durability of resin-dentin interfaces. Several chemical carriers, including benzalkonium chloride, gentamicin, d-alpha-tocopheryl polyethylene-glycol 1000 succinate (D-Alpha), and ethylenediamine tetraacetic acid, have been used to enhance the permeation of riboflavin collagen crosslinkers. Moreover, several chemical agents, also known as permeation enhancers, can enhance the penetration of cross-linking agents into the collagen fibrillar network10,13. The precise method by which permeation enhancers work is not entirely understood. However, it has been observed that these chemical agents can increase the absorption of riboflavin-5-phosphate (RF) into the cornea and accelerate collagen crosslinking14.
One possible approach to enhance the capacity of RF to pass through and be absorbed by collagen is by utilizing liposomal formulations. Liposomes are spherical structures made up of phospholipids and can vary in size from 20 nm to 15 µm. Their unique configuration allows for the containment of many active chemicals without the need for chemical bonding or any previous chemical alterations. Hydrophilic medicines are either confined within the inner water-based phase or attached to the outer layer through electrostatic interactions. Lipophilic medications bind to the lipid area, whereas amphipathic molecules are positioned between these two regions. Liposomes have demonstrated many benefits compared to other drug delivery methods due to their biodegradability, biocompatibility, non-toxicity, and non-immunogenicity15,16. Moreover, riboflavin, when used, exhibits antimicrobial properties against pathogenic microbes17. The antibacterial effects of ROS-mediated RF and lipoid mixture have not been previously investigated. The use of riboflavin in combination with liposomes in combating bacteria also seems to be a motivating approach. Liposomes may be a promising approach to bypass the dentinal matrix and enhance the penetration of riboflavin into the dentinal matrix, which might be beneficial for the longevity of the resin-dentin interface. In this study, we used liposomes (lecithin) together with riboflavin-5’-monophosphate investigating the effects in a laboratory setting.
Dental adhesives are generally composed of cross-linking monomers, acidic functional monomers, and solvents, all enclosed inside a single bottle system. Thus, these systems, classified as 7 th-generation one-step self-etching systems, may present comparable issues regarding bonding efficacy, degradation, and longevity18. Utilizing a universal adhesive system in self-etch mode minimizes the likelihood of dentin recontamination by water when rinsing. This characteristic reduces the susceptibility of these systems to variations in method, in contrast to systems that need application in etch-and-rinse mode19. However, there are significant constraints in these bonding systems, despite the simplification. These limitations encompass reduced adhesive strength between resin and dentin, as well as significant leakage at the nanoscale level over a prolonged period20. The objective of the study was to create novel dentin adhesive systems by combining riboflavin at various concentrations with lipoid (phosphatidylcholine) as a chemical enhancer carrier. This aim was accomplished by assessing the dentin microtensile bond strength, resin-dentin interface morphology, and nanoleakage evaluation expression after prolonged water storage. Furthermore, the quality of dentin collagen fibrils in the dentin and hybrid layer after adhesive, exposure along with substrate mechanical and polymerization conversion properties were also evaluated. Antibacterial and biocompatibility tests were also performed to assess the viability properties of the modified adhesive used. The null hypotheses tested were that the use of a newly modified universal adhesive would not (i) improve the microtensile bond strength; (ii) have any antibacterial properties; (iii) completely prevent deterioration of the bonded interface over time.
Results
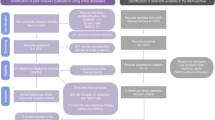
The schematic representation of the experiments performed in this study is presented in Fig. 1.
Scanning electron microscopy (SEM)
Resin tags were detectable in the dentinal tubules in all the adhesive specimen groups. The control adhesive on dentin was fully penetrated showing an intact resin-dentin hybrid layer (Fig. 2A; white arrow). Similarly, the application of RF0.5% (Fig. 2B), RFLi0.5%/0.25% (Fig. 2C), and RFLi0.5%/0.5% (Fig. 2E) to dentin resulted in the presence of a visibly intact resin-infiltrated hybrid layer (white arrows). In some of the specimens using control adhesives (Fig. 2F; white arrow), only brief resin tags were created while bonding to dentin. The hybrid layer remained mostly intact, and the specimens generated with the RFLi0.5%/0.5% adhesive showed lengthy resin tags (Fig. 2D; white arrow) that penetrated deeply. Nevertheless, the RFLi0.5%/0.25% specimens exhibited varying thicknesses, albeit the hybrid layer was more visible. The control specimens (Fig. 2A) exhibited a hybrid layer development that was not homogeneous in some areas (Fig. 2F), which may not accurately represent the adhesive model.
Representative SEM images of resin tags and hybrid layer detectable in all the adhesive specimen groups. (A) The control adhesive on dentin was penetrated with the adhesive showing a resin-dentin hybrid layer (white arrow). Similarly, the application of (B) RF0.5%, (C) RFLi0.5%/0.25% and (E) RFLi0.5%/0.5% to dentin resulted in the presence of a visibly intact resin-infiltrated hybrid layer (white arrow). (D) The specimens generated with the RFLi0.5%/0.5% adhesive showed lengthy resin tags (white arrow) that penetrated deeply. In some of the specimens using (F) control adhesives, only brief resin tags (white arrow) were created while bonding towards dentin (D: intertubular sound dentin).
Confocal microscopy (CLSM)
The confocal laser microscopy images taken at the resin dentin interface for all experimental groups are displayed in Fig. 3. All specimens showed resin permeation and the presence of a hybrid layer. The resin-dentin interface formed by the control adhesive system (Fig. 3A-B) revealed gaps commonly seen between resin tags; these were probably due to mineral precipitation inside the resin-dentin interface. The resin permeated the dentin, forming satisfactory hybrid layers in both RFLi0.5%/0.25% (Fig. 3D-E) and RFLi0.5%/0.5% groups (Fig. 3F) modified adhesives. None of the RF0.5% (Fig. 3C) or RFLi0.5%/0.25% (Fig. 3D-E) and RFLi 0.5%/0.5% groups (Fig. 3F) depicted interfacial gaps. The creation of a hybrid layer containing resin tags was detected in all specimens, with the length of the tags being more prominent in RFLi0.5%/0.5% (Fig. 3J) and RFLi0.5%/0.25% (Fig. 3I) groups adhesives as compared to (Fig. 3G) control, and RF0.5% groups (Fig. 3H). Figure 3K-L represented lateral angles of RFLi0.5%/0.5% groups indicating a comprehensive seal of adhesive with prominent resin tags.
Representative confocal laser microscopy images taken at the resin dentin interface for all experimental groups. The resin-dentin interface formed by the (A-B) control adhesive system revealed the occurrence of commonly seen resin tags. The resin permeated the dentin, forming thick hybrid layers in both (3D-E) RFLi0.5%/0.25% and (3 F) RFLi0.5%/0.5% groups modified adhesives. None of the (C) RF0.5% or (D-E) RFLi0.5%/0.25% (gaps seen between resin tags due to mineral precipitation inside the interface have been identified with white arrows) and (F) RFLi 0.5%/0.5% groups depicted resin-dentin interface gaps. The creation of a hybrid layer containing resin tags was detected in all specimens (HL identified with white arrows), with the length of the tags being more prominent in (J) RFLi0.5%/0.5% and (3I) RFLi0.5%/0.25% groups adhesives as compared to (Fig. 3G; arrow represents the direction of resin tags) control, and RF0.5% groups (H; arrow pointing towards hybrid layer); (K-L) lateral angles of RFLi0.5%/0.5% groups indicating a comprehensive penetration of adhesive. (red rhodamine dye within adhesive and resin tags; dentin is represented as green fluorescence; RT = resin tags; HL = hybrid layer).
Nanoleakage evaluation
The backscattered SEM images of the control group (Fig. 4A) showed a significant amount of silver deposited along the resin-dentin interface and within the dentinal tubules. The RF0.5% adhesive groups (Fig. 4B) displayed a slightly lower amount of silver deposition as observed in the resin-dentin interface as compared to the control groups (Fig. 4A). This deposition is considered a thin and discontinuous silver deposit that can be observed along the resin-dentin interface. In some parts, some silver deposits appeared between some resin tags and tubule walls. As for RFLi0.5%/0.25% (Fig. 4C) and RFLi0.5%/0.5% groups (Fig. 4D) are concerned, no silver deposition is observed in the resin-dentin interfaces. Increasing the concentration of riboflavin and lipoid also increases the crosslinking effect of the collagen and decreases nanoleakage in the dentin hybrid layer. The nanoleakage score for all groups is depicted in Table 1.
In the backscattered SEM images of (A) control group, which is the adhesive without riboflavin and lipoid, a significant amount of silver was deposited along the resin-dentin interface and within the dentinal tubules. The (B) RF0.5% adhesive groups displayed a slightly lower amount of silver deposition as observed in the resin-dentin interface. As for (C) RFLi0.5%/0.25% and (D) RFLi0.5%/0.5% groups were concerned, no silver deposition is observed in the resin-dentin interfaces; (E) commercial control adhesive.
Microtensile bond strength (μTBS)
The µBS values for each group at 24 h, 6 months, and 12 months are presented in Table 1. A three-way ANOVA analysis revealed that the factors“crosslinking agent”(p < 0.003, F = 37.4) and“time”(p < 0.003, F = 11.54) had a significant impact on the bond strength to dentin. The correlations between“time”and“crosslinking agents”(p < 0.003, F = 12.44) and between“adhesive”and“crosslinking agent”(p < 0.01, F = 2.33) were found to be statistically significant. The variation in bond strength during storage was not influenced by either the crosslinking agent or the adhesive. A statistically significant variation in bond strengths was seen between all groups at 24 h (p > 0.05). There was no statistically significant variation in bond strength detected between the RFLi0.5%/0.25% and RF0.5%, groups within 6 months of evaluation (p > 0.05). The application of RFLi had no negative impact on the initial adhesive bond strength. In contrast, the bonding strength of all groups showed a significant decrease after 12 months of exposure to artificial saliva. Similarly, there was no notable alteration in the bonding strength of both adhesives in the RFLi0.5%/0.25%and RFLi0.5%/0.5% groups after 12 months, as compared to 24 h. Table 3 displays the proportionate breakdown of failure mechanisms for the debonded dentin specimens in the adhesive groups after 12 months. Typically, lower microtensile bond strength values were linked to a greater likelihood of failure occurring inside the adhesive itself, known as an adhesive failure. The failure mechanisms seen in all test specimens exhibited predominantly failed in mixed mode.
Small angle Xray scattering (SAXS)
Scatter patterns and intensity vs. q-range plots for the dentin specimens modified with different adhesive treatments are depicted in Fig. 5A. The 5 th-order peak was utilized to measure the D-spacing, whereas the entire q-range (0.01–0.1 Å − 1) was employed to determine the fibril diameter (Table 2). Compared to the controls, the sizes and dispersion of fibrils in the RF0.5%, RFLi0.5%/0.25%, RFLi0.5%/0.5% were substantially larger (p < 0.05). Similarly, the RFLi0.5%/0.25%, RFLi0.5%/0.5% groups exhibited considerably greater fibril sizes and distributions compared to the control group and the RF0.5%, RF0.5%, as well as significantly smaller D-spacing (p < 0.05). SAXS analyses conducted on dentin sections during expansion across a cone reveal an augmentation in orientation index in the RFLi0.5%/0.25%, RFLi0.5%/0.5% groups, indicating a greater alignment of fibrils in parallel to each other, an indication of better crosslinking also (Fig. 5A). However, no noticeable alteration in direction (Fig. 5A) and no substantial modification within control groups were observed. The next highest orientation was found amongst RF0.5% groups, indicative of riboflavin crosslinking alone; this was an increase seen as statistically significant when compared to control groups (p < 0.05). Among all the preservatives, the samples in RF0.5% groups exhibited values that were most like the controls.
(A) Display of scatter patterns and intensity vs. q-range plots for the dentin specimens modified with different adhesive treatments using SAXS. The colored graphical lines depict SAXS/WAXD patterns for collagen matrices (sizes and dispersion of fibrils) seen in controls, RF0.5%, RFLi0.5%/0.25%, RFLi0.5%/0.5%. The RF experimental groups displayed substantially larger changes within the patterns. The inset within the SAXS graphical streams show the deconvolved (blue profile) collected at a point representing deionised pattern of acquisition. The patterns represented (n = 2 per group) groups from control from the lowest intensity to higher intensities within no modification, RF0.5%, > RFLi0.5%/0.25%, > RFLi0.5%/0.5% (labeled). The shifts in X-ray diffraction peaks (black arrow) indicate changes in collagen fibril dimensions due to chemical changes and the crosslinking effect (p < 0.05) (D space of collagen fibril within RFLi0.5%/0.5%; yellow arrow). (B) The figure displays the most suitable positions assumed by the riboflavin molecule and lipoid as it is docked into collagen and enzymes (yellow arrow). The inset image pointed by yellow is the free radical oxidation by the presence of RF molecules. Schematic representation of the collagen network and the positioning of the simulation for an individual chain showing a simplified depiction of the collagen fibril network and the intermolecular cross-links connecting adjacent collagen molecules. The hydroxyproline residues of the molecule are denoted by fixed grey spheres. The blue and red denote the MD-modelled curves, corresponding to the colour scheme of amino acids and intramolecular hydrogen, respectively. The riboflavin binding mechanism has been confirmed to successfully bind to the catalytic sites of MMP-2 and −9 enzymes (white arrow). The blue, yellow and green ribbons resented when the enzyme’s active site was blocked by the crosslinking agent.
Molecular modelling simulation
The introduction of the riboflavin molecule into the binding pocket of 3 AYU was achieved utilizing the Glide XP procedure (Fig. 5B). In this protocol, the ligand was maintained in a flexible state while the receptor was kept rigid. Both compounds exhibited favorable negative binding energies. The XP GScores of riboflavin was −2.593. Figure 5B displays the binding posture (in both 3D) as well as the important amino acid that is involved in the binding of riboflavin. The essential amino acids of MMP-2 interact with riboflavin and lecithin lipoid (data not shown). Both riboflavin and lecithin exhibited advantageous binding energies with the active site of MMP-9, as indicated by negative ‘XP G-Scores’ of −7.212 and −0.668, respectively.
Contact angle
During the comparison of the findings of these latest data (Table 2), it could be inferred that the contact angle values estimated using wetting angle analysis had statistical differences (p < 0.05). The RFLi-modified groups exhibited reduced (p < 0.05) contact angles relative to the control and RF-modified groups. Furthermore, a statistical difference was observed between the RFLi and other groups, indicating an effect of the crosslinking agent. However, it could be determined that the trend observed in the data is consistent. This means that the same numerical model may be utilized for optimization purposes (Fig. 6).
(A) Macromolecular adhesion angle; contact angle images estimated using wetting angle analysis exhibit statistical differences for (B) control (C) RF0.5% (D) RFLi0.5%/0/25% (E) RFLi0.5%/0/5%; (F) numerical model for the RFLi formulation (G) RF model and (H) Nanoindentations for AFM of resin dentin hybrid layer.
Mechanical properties using atomic force microscopy (AFM)
Both elastic moduli (Ei) and nano hardness (Hi) were influenced by the different adhesive treatments (p < 0.05), or the adhesive system choice and the position along the interface from the 1 st to 6th point of indent (Table 3). The statistical analysis results of the DC for the control and modified groups after 30 min are displayed in Table 1. Although the DC (degree of conversion) of the RFLi0.5%/0.25% modified specimens was somewhat lower, there was no statistically significant difference seen between the control experimental, commercial, and the RFLi0.5%/0.25% modified adhesive groups. The specimens subjected to a RFLi0.5%/0.5% alteration exhibited an increase in DC after photo-activation, in comparison to the control group (p < 0.05).
Transmission electron microscopy (TEM)
The TEM results of the dentin collagen network after different adhesive application groups are presented in Figs. 7 and 8. Overall, all specimens exhibited different levels of structural alterations after 3 months of aging. Nevertheless, the impact of the chemical enhancer RF/Li on maintaining the integrity of dentin-collagen structure cross-linked with RF is easily noticeable when compared to the non-cross-linked (Fig. 7A-C) and RF0.5% cross-linked (Fig. 7D-F) specimens that do not contain lipoids after being aged for 3 months. Clear evidence of thin collagen spread within the control dentin specimens was observed (Fig. 7A-C) as indicated by the identifiable typical collagen fibril structure. The collagen mesh looks slightly deteriorated, resulting in some loss of structure and periodicity, making it unidentifiably amorphous. The specimens treated with a modified RF0.5% adhesive (Fig. 7D-F) exhibited well-preserved collagen fibril structure. Nevertheless, the collagen network had a less compact arrangement characterized by greater intervals between collagen fibrils and shorter collagen fibrils. On the other hand, dentin specimens treated with a RFLi0.5%/0.25% (Fig. 7G-I) exhibited a thicker collagen network modified with the permeation enhancer exhibiting a well-preserved and compact collagen network. The collagen fibrils had a distinct cross-banding pattern, which is a hallmark feature of collagen. Furthermore, the periodicity of collagen fibrils and areas where either gaps or overlap could be easily observed by TEM after aging (3 months) (Fig. 8). The specimens that were treated with the permeation enhancer RFLi0.5%/0.5% (Fig. 7J-L) displayed a well-preserved thick structure of collagen fibrils with distinct collagen crossbanding (Fig. 8). High magnification transmission TEM imaging showed that the collagen fibrils structure in dentin specimens crosslinked with RFLi0.5%/0.5% remained intact and exhibited a consistent periodicity pattern even after 3 months of storage. The periodicity of the collagen fibrils indicated the precise alignment of the 1 and 2 chains of collagen, which was kept and maintained by extensive crosslinking (Fig. 8).
Representative TEM images exhibited different levels of structural alterations after 3 months of storage. (A-C) non-cross-linked and (D-F) RF0.5% cross-linked specimens. The specimens treated with a modified (D-F) RF0.5% adhesive exhibited well-preserved collagen fibril structure as pointed by white arrows. Specimens treated with a (G-I) RFLi0.5%/0.25% exhibited a thicker collagen network modified with the permeation enhancer exhibiting a well-preserved and compact collagen network as indicated by a dotted circle. Specimens that were treated with the permeation enhancer (J-L) RFLi0.5%/0.5% displayed a similar well-preserved thick structure of collagen fibrils with distinct collagen crossbanding (white arrows).
High magnification transmission electron microscopy (TEM) imaging, showing that the collagen fibrils structure in dentin specimens crosslinked with RFLi0.5%/0.5% remained intact and exhibited a consistent periodicity pattern even after 3 months of storage. (A-C) Control; (D-F) RFLi0.5%/0.25%; (G-I) RFLi 0.5%/0.5%. In a higher magnification of the TEM images, it can be seen even clearer where the crosslinking is being formed, shown by the red circles and yellow arrows.
Fluorescence in situ hybridization (FISH) for antimicrobial analysis
FISH accurately identified almost all bacteria that were stained as streptococci displayed in Fig. 9. The application of different concentrations of adhesives on dentin had a substantial influence on the quantity of bacteria that were attached (ANOVA, p < 0.05). There was a considerable rise in the number of bacteria on control specimens in the third week. The combined pictures in the"orthogonal section display mode"clearly showed the presence of dead bacteria amongst the RFLi0.5%/0.25% and RFLi0.5%/0.5% groups. Bacterial penetration was shown by the detection of a fluorescent signal from the oligonucleotide probes EUB 338 and STR 405, which could be seen in the tubules up to a depth of 10 μm below the surface (Fig. 9).
FISH accurately identified almost all bacteria that were stained as streptococci. The combined pictures in the"orthogonal section display mode"clearly showed the presence of dead bacteria amongst the (A) RFLi0.5%/0.25% and (B-C) RFLi0.5%/0.5% groups. There was a considerable rise in the number of bacteria on (D-E) control specimens. (green live bacteria; red dead bacteria).
Immunohistochemical staining
Confocal laser scanning microscopy images of invading cells that express CD163 and CD80 are shown in Fig. 10. The mononuclear macrophages CD68 + (without stimulation) were observed in all examined cells during the whole duration of the investigation. The phenotype of the macrophages was analyzed qualitatively after applying the adhesives. In both the control group (Fig. 10A) and the RF0.5% groups (Fig. 10B), the macrophages showed a strong CD163 + phenotype, indicating the presence of M2 markers. In contrast, the CD80 + markers were also detected in the RFLi0.5%/0.25% (Fig. 10C-D) and RFLi0.5%/0.5% groups (Fig. 10E-H). The results of the one-way analysis of variance (ANOVA) indicated that the M1/M2 ratio was considerably lower in the RFLi0.5%/0.25% and RFLi0.5%/0.5% groups compared to the control, and RF0.5% groups (p < 0.05). Values greater than 1.0 indicate a pro-inflammatory M1-type reaction, whereas values lower than 1.0 indicate an anti-inflammatory M2-type response (Fig. 10I).
Confocal laser scanning microscopy images of invading cells that express CD163 (red) and CD80 (blue). (A) Control group and the (B) RF0.5% groups, the macrophages showed a strong CD163 + phenotype, indicating the presence of M2 markers. In contrast, the CD80 + markers were also detected in the (C-D) RFLi0.5%/0.25% and (E–H) RFLi0.5%/0.5% groups. (I) M1/M2 ratio was considerably lower in the RFLi0.5%/0.25% and RFLi0.5%/0.5% groups compared to the control, and RF0.5% groups (p < 0.05). Values greater than 1.0 indicate a pro-inflammatory M1-type reaction, whereas values lower than 1.0 indicate an anti-inflammatory M2-type response.
Discussion
The degradation of dentin collagen fibrils within the hybrid layer is a primary factor contributing to the diminished bonding strength of adhesive systems applied to dentin21. Research has shown that phosphoric acid and acidic functional monomers can remove minerals from collagen and promote the activation of different proteolytic enzymes (such as MMPs)22, which in turn digest the resin-unprotected collagen fibrils at the base of the hybrid layer causing gaps and microleakage at the resin-dentin interface. The process of hydrolytic aging of adhesive surfaces starts at the early stage of acid etching in the bonding process23,24. The high organic content and presence of fluids in the dentinal tubules make dentin a less ideal substrate for resin bonding compared to enamel25. The integrity of the bond at the interface between dentin and adhesive resin is crucial for the overall success of composite restorations in clinical practice. Bonding methods encompass complete etch (also known as etch and rinse), self-etch, or selective etch techniques when employing universal adhesive systems. The objective, irrespective of the approach employed, is to create a hybrid layer by adhesive impregnation and establish a long-lasting connection between the composite resin and dentin, which remains a dreadful task26,27.
The main concept of this laboratory investigation lay in demonstrating how various concentrations of riboflavin could improve dentin collagen cross-linking. However, the current formulation has been established and adjusted based on earlier protocols28. These formulations involve the use of liposomes as a crosslinking enhancer, together with UVA-activated riboflavin. Riboflavin acts as a protective barrier against potential UVA absorption by generating proteoglycan-core proteins and collagen crosslinking bonds29. Nevertheless, molecules with a high viscosity molecular weight might restrict the deeper penetration of RF in the extracellular matrix30. In this study, we also evaluated the possible impact of permeation enhancers such as lipoid lecithin on improving the penetration and accumulation of RF in acid-etched dentin via the application of a universal adhesive system, as well as its effect on crosslinking efficiency. Liposomal formulations can bring about photo stabilization of riboflavin31,32. Currently, there is no existing research that investigates the impact of incorporating RF into these liposomal carriers within a Universal adhesive system. The primary incentive for studying the enhancement of RF permeability through the dentin with liposomes, rather than studying RF, was its solubility in water, which allowed us to create more concentrated solutions and obtain higher overall permeability through the dentin, as demonstrated in sections of this study, enhancing collagen crosslinking. Upon the addition of riboflavin-5-phosphate to the produced liposomes, there is an electrostatic interaction between the positively charged choline moieties of the liposome headgroup and the monophosphate groups of riboflavin-5-phosphate. The lipoid headgroups become inclined and the negatively charged surface is exposed, resulting in a further reduction in surface charge33. The reason for using this technique was that liposome lecithin may act as a cross-linking enhancer, aiding riboflavin in its binding to collagen fibrils. This is particularly important in countering the effects of high-viscosity molecular weight compounds that are generated during UVA absorption. Based on these extensive pieces of evidence, we investigated the potential importance of this new collagen enhancer in improving the permeation and accumulation of RF in dentin collagen-based protein matrices, as well as its effectiveness in crosslinking when it was combined with an experimental self-etching adhesive. It is well known that the presence of functional monomer groups leads to the formation of a genuine chemical link in dentin. However, this bond has a low affinity for etched dentin34, thus, it was crucial to examine the impact of using such an experimental adhesive when used in self-etch mode.
According to the findings of this study, the first two null hypotheses that were examined must be rejected. The reason is that the results obtained during the µTBS tests demonstrated a bond strength of the experimental adhesives comparable to those of several commercial adhesive systems currently on the market35. Upon careful examination of the scanning electron microscope images, it was seen that the RFLi0.5%/0.5% groups had a distinct interfacial integrity. This finding provides evident support to the high values of µTBS, particularly in the RFLi0.5%/0.5% groups, even after a storage period of 12 months. There was no negative impact on the bond strength values obtained after 12 months of storage in artificial saliva, despite the rise in concentration of liposomes within the RFLi0.5%/0.5% groups. Furthermore, the presence of RFLi0.5%/0.5% content in the experimental adhesive did not impact on the polymerization reaction, although there could have been a conflict between RF and the photo-activators/initiators of the adhesive system in absorbing polymerization light. The use of blue light in the study potentially had a double impact by stimulating both the riboflavin and the polymerization process of the resin monomers in the experimental adhesives. There was no discernible rise in RF content, indicating that it did not produce any of its protective effects against blue light. The correlation between the µTBS findings may be substantiated by the SEM finding. RFLi0.5%/0.5%groups may have conserved collagen fibrils by preserving the axially constricted telopeptide domains at the N- and C-termini36. The liposome component, in conjunction with the hydrophobic alteration37, forms a bond with collagen fragments38 that are extremely mobile, hence preserving the helical shape. The failure mode distribution analysis revealed that most fractures seen in both immediate and 12-month storage specimens were characterized as mixed failures. The rise in adhesive failures seen in the control and RF groups suggests that the modified experimental adhesives have inferior mechanical characteristics, which aligns with the lower µTBS. Water penetration during aging causes the polymeric resin within the hybrid layer to swell, leading to the deterioration of covalent bonds at the interface and the detachment of filler particles from the resin39. These findings are consistent with the results obtained in the confocal analysis, which demonstrated the creation of an optimal hybrid layer amongst the RFLi groups. The versatile monomers successfully created resin tags and hybrid layers in the RFLi groups. Nanoleakage increased considerably over time, in control specimens. There was no obvious variation in the nanoleakage score between the RFLi of both adhesives from the beginning to the end of the 12 months. Nevertheless, nanoleakage exhibited a notable increase in control, and RF groups after 12 months. Therefore, the third hypothesis must be partially rebutted. Furthermore, when the modification was raised, the degree of conversion was not impaired, which might partially account for and clarify the optimal µTBS values seen.
Water storage may adversely impact bond strength values. The creation of resin tags and the durability of tags are mostly influenced by the application technique and the dentin adhesive used40. Adhesive specimens of RFLi0.5%/0.5% exhibited a satisfactory bond interaction. The presence of such an effect may elucidate the reason behind the presence of a well-defined hybrid layer along the whole interface in all the RFLi0.5%/0.5% specimens treated in this work. Untreated control specimens exhibited a sound and well-preserved hybrid layer (Fig. 1A). Based on our observations, it is reasonable to hypothesize that RFLi0.5%/0.5% may also deactivate proteases. Additionally, the crosslinking effect likely contributes to the formation of a complex collagen fibrillar network, as described in our earlier study28, which may enhance adhesion. Integrating RFLi0.5%/0.5% into the dentin adhesive model and incorporating it into the production of modern adhesive systems can be seen as a crucial and recommendable method to achieve specific desired effects of riboflavin on collagen crosslinking. This is particularly important when the dentin is being etched and endogenous MMPs (matrix metalloproteinases) are activated41.
The distinctive scattering arises from the well-organized quarter-stagger arrangement of collagen molecules underneath the hybrid layer (Fig. 5), which has recurring areas of gap and overlaps in a specific axial periodicity (D-period)42. The change in intensity across distinct rings or peaks indicates the distribution of electron density in collagen43,44. Following the cross-linking process with various concentrations of adhesive modification and without, there is a noticeable alteration in the strength of the diffraction peaks (as shown in Fig. 5A). This interaction has an impact on the organization of collagen molecules, which subsequently leads to variations in the intensity of certain peaks. The enhanced crosslinking has enhanced the ability of the collagen intermolecular structure to withstand contraction during the dehydration of specimens and after etching. Additionally, we noticed a more significant rise in the peak of RFLi0.5%/0.5% adhesive specimen compared to the other order peaks. Nevertheless, before this investigation, adhesive specimens without modifications showed a significant dried result under the same conditions, with alterations in the relative strength of the peaks in comparison to the crosslinked specimens suggestive of changes within the collagen fibrils (data not shown). The increase in overall peak intensity of RFLi0.5%/0.5% adhesive specimens may be attributed to the heightened contrast in electron density resulting from lipoid-induced RF crosslinking, as well as the structural modifications caused by the covalent and non-covalent interactions of the modifier with collagen.
TEM investigation revealed a similar expected result in the RFLi0.5%/0.5% groups, and to some extent within the RFLi0.5%/0.25% and RF groups which showed undamaged collagen. This work presents the initial findings on the ultra-morphology of the dentin collagen formed by utilizing adhesives that have been modified using a unique crosslinking formulation technique. These adhesives were applied in self-etch mode on dentin. Water is more challenging to extract from dentin using ethanol compared to acetone because ethanol has a higher ability to create hydrogen bonds with water45. Therefore, it is possible to hypothesize that the crosslinking formulation examined in this study may have had a beneficial impact on the removal of water from dentin. Additionally, the correlation between particle size and density is a distinctive characteristic in adhesive systems, and it requires additional research on size distribution to confirm its validity.
The most suitable positions assumed by the riboflavin molecule and lipoid as it is docked into collagen and enzymes are shown in Fig. 5B. The riboflavin binding mechanism has been confirmed to successfully bind to the catalytic sites of MMP-2 and −9 enzymes. Upon analysis, it was shown that RF exhibited electrostatic attractions that hindered the functionality of the MMPs’ active sites. The findings play a crucial role in evaluating the interaction between the oxygen of the carbonyl group in riboflavin and the nitrogen atoms of the Gly120 amino acid residue (2.52 Å). Therefore, it is quite likely that the cysteine repeats are hindered in their movement due to the presence of negatively charged aspartate, histidine, and cysteine residues. This aligns with the observation that the docked location and structure of riboflavin likely hindered the normal catalytic activity. The binding energy also demonstrated a substantial ligand-receptor complex with the MMP-2 and −9 enzymes. Additionally, we hypothesize that a concentration of RFLi0.5%/0.25% yields the most favorable decreased values, allowing for the long-term crosslinking of the dentin matrix. This enables operators to compare the findings with other adhesives. Moreover, including RF inside the adhesive rather than using it as a separate pretreatment guarantees the crosslinking effect, since the effectiveness of the agent may decrease following acid etching when water rinses might eliminate the crosslinking agent.
The FISH technique was employed in this study to detect spatiotemporal organization in early forming biofilm against the adhesive specimens. The use of these methods to visualize bacterial adherence is deemed preferable. The prevalence of streptococci identified in this study is consistent with previous research that examined the bacterial colonization of the oral cavity throughout 4 to 48 h46. Thus, the use of FISH to identify bacterial colonies and to assess their proliferation and differentiation capabilities allows for a precise evaluation of the accuracy of antibacterial analysis. Negatively charged surfaces might impact the struggle among bacteria for cation nutrients47, or hinder respiration by blocking the release of waste products48. Hence, it is plausible that the RFLi0.5%/0.25% adhesive groups have more efficacy in suppressing pathogenic strains. In addition to its overall negative charge, lecithin also includes quaternary ammonium cations with a determined positive charge. This feature allows lecithin to attract and bind bacterial colonies through electrostatic interactions, providing another plausible mechanism for this process49.
This study investigated the impact of modified riboflavin/lecithin adhesive on macrophage phenotype, identifiable and distinguishable by cell surface markers50. M1 and M2 macrophages participate in the early and late phases of inflammation and tissue remodelling, respectively51. CD 80 + denotes M1-activated cells, while CD163 + indicates elevated expression in M2-activated macrophages. Imbalances in M1/M2 ratios might result in pathological alterations52, whereas modulating the M1/M2 ratio via systemic infusion of polarised M1 and M2 macrophages can exacerbate or ameliorate chronic inflammation53. This study’s results demonstrated that the application of the adhesive can alter the macrophage polarization profile towards a primarily M2 anti-inflammatory phenotype and, hence can become a factor for promoting tissue repair and regeneration. This makes a profound statement on its biological compatibility on the substrate applied too. The results indicate that modulating the M1 to M2 ratio may offer a new treatment approach.
A key limitation of this study was that the various characterization methods employed to evaluate the modified adhesive had differing aging durations. Although the aging methods varied, the tests were structured to replicate real scenarios in which aging may transpire under diverse situations. Highlighting this limitation demonstrates our team awareness of the study design identifying areas of future research with standardized aging protocols. Another possible limitation of this study is represented by the lack of a commercial adhesive used as a control to evaluate and compare the results except for testing the bond strength values. Moreover, assessing the antimicrobial ability of riboflavin-contained adhesives at a single time interval maybe not be enough to determine its possible antibacterial effectiveness features in a possible clinical scenario. In future studies, it would be interesting to test such experimental materials in a monomicrobial biofilm. Further research will be necessary to examine the impact of the blue diode laser-riboflavin combination on various microorganisms and clinical strains. A further limitation of this study is represented by the specimens not exposed to oral environmental conditions or masticatory forces. Furthermore, the study did not consider the salivary pellicle, which serves many functions such as protecting and lubricating the adhesive, as well as promoting hydration with buffering capacity. It is also important to consider that our experimental materials can release or discharge RFLi. Thus, it is advisable to implement a strong experimental strategy to evaluate the therapeutic use of this modified universal adhesive under relevant and changing oral environmental conditions. In addition, conducting a water sorption/solubility test using adhesive discs would provide a more accurate representation of the clinical situation. Tests are now underway as part of an ongoing study.
Conclusion
Considering the limitations of this in vitro study, it can be inferred that RFLi0.5%/0.5% modified adhesives enhance the bonding ability of dentin via the formation of optimal resin-dentin bonded interfaces with long-term integrity and stable bonding strength to dentin over time. This is related to the fact that increasing the concentrations of riboflavin and lipoid within the adhesive’s composition enhances the crosslinking effect of collagen with greater fibril sizes and distributions reducing nanoleakage within the hybrid layer. Moreover, RFLi0.5%/0.5% exhibits greater biocompatibility, antibacterial properties, and DC after photo-activation compared to the control group with desired biocompatibility.
Methodology
All methods, in addition to the use of extracted teeth, were performed and used in accordance with the relevant guidelines and approvals of the ethical committee. No animal studies were conducted.
The lipoid solution was prepared using 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol, and polycarbonic membranes (Alabaster, AL). The liposomes were synthesized using a 90/10 ratio and a concentration of 10 mM. The DPPC and Cholesterol were mixed in predetermined amounts. The mixture was subjected to stream drying followed by vacuum drying for 20 min. The desiccated lipid layer was forced through 100 nm polycarbonate membranes to produce single-layered liposomes. The dehydrated lipid film was rehydrated using solutions containing 0.5 mM and 10 mM riboflavin-5-phosphate. In addition to this, a 10 mM concentration of liposomes was maintained in different concentrations of Riboflavin as per weight percent (control with no modification; 0.5% RF, 0.5% RF/0.25% Li, 0.5% RF/0.5%Li). The groups were designated as Ct0, RF0.5%, RFLi0.5%/0.25%, RFLi0.5%/0.5%. A 3M Universal commercial adhesive (3M ESPE in St. Paul, MN, USA) was used as an additional control material to compare the bond strength results.
Preparation of modified experimental universal resin adhesives
The RFLi Universal adhesive system (Fig. 1) was formulated by adjusting the mass ratios of Bisphenol glycidyl methacrylate, 2-hydroxyethyl methacrylate, and decandiol methacrylate/D3 MA [Sigma-Aldrich, Malaysia] to 60:30:10. The solvent used was a solution of ethanol (Spektrum-3D) with a concentration of 30% by mass. The experiment utilized diphenyliodonium hexafluorophosphate, and camphorquinone at a concentration of 0.5% by weight. These were mixed with DPIHP at a concentration of 1.0 wt% and ethyl (4-dimethylamino) benzoate at a concentration of 0.5 wt%. The diphenyliodonium hexafluorophosphate was introduced to enhance electron absorption, contingent upon the total amount of monomers. All the chemical reagents used in this part of the study were purchased from Sigma-Aldrich, Malaysia.
The selection of the intricate photoinitiator system containing camphorquinone was based on its ability to enhance the level of conversion for all the evaluated elements in the formulation based on weight %. Additional monomers, including dipentaerythritol pentacrylate phosphoric acid ester (PENTA) (Sigma Aldrich, Malaysia) and Biphenyl dimethacrylate (5%) (BPDM), were incrementally mixed with 14% Methacryloyloxydecyl Dihydrogen phosphate (MDP) at a ratio below the minimum threshold of 5% while maintaining a water concentration of 10%. To prevent spontaneous polymerization, all chemicals were exposed to dark/red light and maintained at a pH of 3.2. The supramolecular polymer, Tetrahydroxycalix4 arene-5,11,17,23-tetrasulfonic acid sodium salt (Sigma Aldrich, Malaysia) was added at 0.1wt% concentration at room temperature. The mixing and dissolution procedures were conducted using a flask equipped with a magnetic stirrer (SA300; Sansyo, Tokyo, Japan), and a vent condenser. The groups were designated as Ct0, RF0.5%, RFLi0.5%/0.25%, RFLi0.5%/0.5%. The modification combination was introduced directly into the solvent, resulting in a pH of 2.5 (± 0.05). The adhesives were stored at a temperature of 4◦C and utilized within a month of being prepared. To achieve particles of uniform size, the adhesive dispersion was subjected to ultrasonication at a temperature of 4◦C for 60 min. Subsequently, it was centrifuged with a force of 1000 g for 30 min. The Zetasizer (Nano-ZS, Malvern Panalytical Instruments, Worcestershire, WR14 1XZ, United Kingdom) was used to measure the particle size distribution in the supernatant. The supernatant (100 μL) was diluted in 1 mL of a 0.1 mol/L HCl aqueous solution and agitated at a steady rate for 1 h at 37 ◦C until a transparent solution was obtained.
Sample size
To calculate the sample size needed for analysing specimens, we use the following equation while ensuring a study power of 90% and a significance level of 5%. (Z1 is the normal deviation, \({\sigma }_{1}\) is the difference means of 2, \({Z}_{1-\beta }\) is the is the normal deviate at 1-β% power.
n = mean difference = 5.2
Preparation of resin-dentin bonded specimens
The occlusal surface of each tooth was precisely sectioned perpendicular to its longitudinal axis using a low-speed saw manufactured by Buehler (Lake Bluff, IL, USA). This procedure allowed the exposure of the middle dentin surface, while with a second cut at 1 mm below the dentin-enamel junction, it was possible to remove the roots. To provide a consistent bonding area, a double-sided adhesive tape with a 5-mm diameter aperture was applied onto the dentin surface. The experimental adhesives were applied to the exposed dentin surface using a rubbing action for 20 s. The surface was air-dried for 5 s and then light-cured for 10 s using a light-curing system (Curing Light 2500,3M ESPE in St. Paul, MN, USA), positioned 10 mm away from the surface. The resin composite was applied in four increments of 2 mm each, with each layer being light-cured for 20 s. Subsequently, specimens were stored in artificial saliva for 24 h before undergoing cutting procedures to prepare resin-dentin beams measuring 1 mm2 for bond strength testing. The specimens prepared in this session were also used for scanning electron microscopy, confocal imaging, nanoleakage, and antimicrobial and nanoindentation analysis.
Scanning electron microscopy of resin-dentin interface
Four teeth (n = 5 slabs per tooth) were immersed in artificial saliva for 24 h before being subjected to cutting techniques to create resin-dentin interface slabs with 2 mm thickness. Two parallel grooves were created on the outside surfaces of each tooth, running in the mesiodistal direction, to enable split fracture. A chisel and hammer were used for the ultimate separation. The specimens were attached to aluminum stubs using adhesive tape that conducts electricity on both sides. They were then coated with a thin layer of gold and palladium using a process called sputter-coating. The fragmented pieces were affixed to aluminum stubs, coated with a layer of gold/palladium, and analyzed using a scanning electron microscope (SEM) at an acceleration voltage of 10 kilovolts (kV).
Confocal laser scanning microscopy evaluation (CLSM)
A total of 3 resin-dentin specimen teeth (n = 5 slabs per tooth) were allocated to each group. The dentin specimens were bonded using the adhesives previously mentioned, which were doped with 0.1 wt% Rhodamine B (Sigma Chemicals, St. Louis, MO, USA). The specimens underwent a final rinsing process in an ultrasonic bath with distilled water for 2 min. Afterward, they were divided into slabs measuring 1 mm in the mesiodistal direction using a slow-speed diamond saw that was cooled with water (Lab cut, Agar Scientific, Stansted, UK). The resin-dentin slabs were ultimately polished using 1200-grit SiC papers for 30 s, followed by an ultrasonic bath in water at 1 m. A thorough examination was conducted on each resin-dentin contact, and five photos were randomly taken and recorded to illustrate the ultra-morphology of the resin-dentin interface. The imaging procedures were conducted using a confocal laser scanning microscope (Leica SP2 CLSM, Heidelberg, Germany) with a 63 ×/1.4 NA oil immersion lens. The microscope was outfitted with a 633 nm krypton ion laser. Fluorescence pictures were obtained performing 20 optical sections with a z-step of 1 m below the surface. These were processed using Leica SP2 CLSM image-processing software (Leica, Heidelberg, Germany) and finally pseudo-colored to improve their visualization. The system setup was uniformly standardized and consistently used throughout the whole research.
Nanoleakage evaluation using ammoniacal silver nitrate solution
Four teeth were first bonded with adhesives and divided into slabs (n = 5 slabs per group) measuring 5 mm in the mesiodistal direction. The specimens were prepared as mentioned previously. subsequently, the teeth were immersed in a solution containing 50% ammoniacal silver nitrate (pH 9.5), following a method reported by Tay et al.54. After 24 h, they were washed and soaked in a photo-developing solution under fluorescent light for 8 h. This induced the silver ions to turn into silver grains. The specimens were polished with diamond pastes and cleaned before being examined. The latter was performed on the central part of each specimen (determining if a clear image of the hybrid layer is present on at least 75% of the interface’s length) using a scanning electron microscope (SEM) operating at 15 kV.
Microtensile bond strength
Sections of 0.9 mm × 0.9 mm were obtained from four bonded teeth (n = 5 beams per group) in the occluso-gingival region. The beams were randomly assigned for the measurement of microtensile bond strength (µTBS) after being stored in artificial saliva at a temperature of 37 °C for periods of 24 h, and 12 months. The composition of the artificial saliva included CaCl2 at a concentration of 0.7 mM/L, MgCl2•6H2O at a concentration of 0.2 mM/L, KH2PO4 at a concentration of 4.0 mM/L, KCl at a concentration of 30 mM, NaN3 at a concentration of 0.3 mM, and HEPES buffer. A weekly replacement of the storage medium was conducted. Before performing the microtensile test, each beam was glued to a Bencor Multi-T device, made by Danville Engineering in San Ramon, CA, USA, using cyanoacrylate adhesive (Zapit, Dental Ventures of North America in Corona, CA, USA). The tensile force was evaluated at a crosshead speed of 1 mm/min (Universal testing machine model 4440, Instron, Inc., Canton, MA, USA).
Small angle xray scattering (SAXS)
SAXS was performed to determine the ultrastructure and microstructural characteristics of collagen fibrils. Sections of resin dentin interface specimens from four teeth in each group (n = 5 slabs per tooth) were divided into two thinner segments, running parallel to the resin-dentin interface. These segments roughly corresponded to the transitional zone of resin and dentin. These parts will be denoted as the upper half and the lower half, respectively. Data was gathered using a NanoSTAR II Small-Angle X-ray Scattering (SAXS) equipment (Bruker AXS in Karlsruhe, Germany). The experiment utilized a copper rotating anode X-ray source with a 0.3 mm filament. The source operated at 45 kV and 110 mA, generating Cu Kα radiation with a wavelength of 0.15418 nm. The beam had a 1 mm diameter and a full width at half maximum at the sample location. A VÅNTEC 2D detector, manufactured by Bruker AXS in Karlsruhe, Germany, was placed in the center of the beam axis. It has a resolution of 2048 × 2048 pixels and each pixel measures 68 × 68 µm. Additionally, a beam-stop with a diameter of 2.85 mm was placed directly in front of the detector. The distance between the specimen and the detector was calibrated by utilizing a standard sample of a dentin disc. The measured location was consistently accurate to within 0.01 mm. The data was obtained at two different orientations relative to the X-ray beam direction. 2D SAXS data were acquired for each specimen, varying the scattering vector q within the range of 0.15 to 3.92 nm−1.
The superscripts"m“and”e"indicate the meridional and equatorial dispersion, respectively. The orientation distribution of collagen fibrils in each specimen was estimated as g (∅), which was computed using equations.
Molecular docking simulations (MDS)
The Schrödinger small-molecule drug discovery suite was utilized to conduct molecular docking experiments. These experiments aimed to gain molecular insights into the binding mechanism of lecithin/liposome, both alone and in conjunction with riboflavin, on the active sites of matrix metalloproteinase (MMP)−2/MMP-9 proteins and collagen. The numerals 29 and 30 are bracketed by square brackets. The crystal structures of MMP-2 (PDB ID: 3 AYU), MMP-9 (PDB ID: 6ESM), and collagen (1Q7D) were obtained from the Protein Data Bank (PDB), which is managed by the Research Collaboratory for Structural Bioinformatics (http://www.pdb.org). The proteins designated for docking were subjected to processing using the ‘protein preparation’ module of Schrödinger, with the default configurations. Water molecules with less than three hydrogen bonds were eliminated during the protein synthesis process. Subsequently, hydrogen bonds were established under a pH of 7.0, and any missing side chain atoms and loops were incorporated into the protein structure. Subsequently, the OPLS 2005 force field was employed to induce energy reduction. MMP-9 shows the presence of an incoming ligand, whereas MMP-2 and collagen do not display any inbound ligand. Therefore, the ‘Sitemap’ module of Schrödinger was utilized with the default settings to identify the active sites in MMP-2 and collagen. Sitemap provides a fast and effective approach to identify possible binding sites and provide accurate predictions about their suitability for drug targeting29. The active site in MMP-9 was determined depending on the specific ligand being targeted. A grid box measuring 10 ¼ units was generated using the ‘receptor grid generation’ module of Schrödinger, using the default parameters. The entering ligand served as the focal point. The ‘receptor grid construction’ module of Schrödinger was utilized to generate a grid surrounding the binding location exhibiting the highest score. The grids produced using this approach were utilized for empirical investigations including molecular docking. Vitamin E and riboflavin’s chemical structures were drawn using Maestro 11.8, and the Ligprep module was utilized to generate three-dimensional structures. The OPLS 2005 force field was used to optimize and generate low-energy conformers. The binding site was subjected to docking of low-energy conformations of the compounds using the extra precision (XP) approach. This method incorporates vocabulary on the energy required to remove water molecules and the structural patterns of protein–ligand interactions into the scoring function used to calculate binding free energy.
Atomic force microscopy and nanoindentation of resin-dentin interface and contact angle measurement
The specimens bonded (four teeth per group) with resin were cut perpendicular to the bonding zone using a diamond saw as previously described. Therefore, resin-dentin slabs were obtained, with each slab measuring 2 mm in thickness. The resin-dentin slabs from four teeth (n = 5 slabs per tooth) were utilized in an initial study to evaluate the biomechanical nano-properties at the interface between the resin and dentin. Six indentations were done in a straight line, with a force of 4000 nN and a time function of 10 s. The confocal laser was used to identify the precise position and breadth of the hybrid layer. To standardize the number of indentations in hybrid layers with varying thicknesses, a single indentation was made in the middle of each hybrid layer for every line. The indentation lines started from the hybrid layer and went down to the intertubular dentin. These discs were then analyzed to determine the reference nano-elasticity and nano-hardness values. The student–Newman–Keuls test was used to do multiple comparisons. The statistical analysis was conducted with a significant threshold of p < 0.05.
The contact angle at the macroscopic scale is determined by placing a droplet of the adhesive on the surface of the dentin and measuring the angle formed. The technique employed was derived from a static sessile droplet approach. The droplet is deposited onto the surface using a capillary. Over time, a state of balance is reached between these components, which was explained by the formula commonly referred to as Young’s equation:
Degree of conversion
The degree of conversion (DC) of experimental adhesives (n = 5 adhesive specimens per group) was assessed using a transmission FTIR (FTIR-8800; Shimadzu, Kyoto, Japan). To conduct FTIR analysis on both the unmodified and modified bonding agents, a 15 µL volume of adhesive was deposited into a well before the testing procedure. The quantity of carbon–carbon double bonds in the experimental adhesives were ascertained, and the absorbance peaks were quantified in transmission mode. A modest quantity of adhesive was administered onto potassium bromide discs (2.032.0) (Shimadzu, Kyoto, Japan) with a resolution of 4 cm-1. The adhesive was left undisturbed until it hardened, and then it was dried using a controlled stream of oil-free air. During the spectrum research, the sensor maintained continuous contact with the uncured resin. The FTIR spectrometer was employed to quantify the absorbance peaks of the uncured specimens in a range from 400 to 4000 cm−1. After measuring the absorbance peaks in the uncured resin, the adhesives underwent photo-curing as previously described. Following the start of irradiation, the FTIR measurements were performed at different time intervals, ranging from 10 s to 30 min. The absorbance peak of C = C at 1638 cm−1, which represents the stretching vibration of unpolymerized methacrylate, and the reference peak of C = C at 1607 cm−1, which represents the stretching vibration of the aromatic ring, were determined using the baseline technique. The absorbance intensities of (C = C)/(C–C) were measured before and after polymerization to ascertain the proportion of unreacted double bonds, as denoted by Eq. (2):
Where.
-
Caliphatic is the absorption peak at 1638 cm-1 of the polymerized resin,
Caromatic is the absorption peak at 1 607 cm-1 of the polymerized resin,
Ualiphatic is the absorption peak at 1 638 cm-1 of the unpolymerized resin,
Uaromatic is the absorption peak at 1607 cm-1 of unpolymerized resin.
The spectra were obtained three times in each specimen to identify significant changes in our approach that may result in a minimal standard deviation.
TEM of dentin collagen
Two resin-bonded teeth from each group were dissected according to the protocol described in the "Microtensile bond strength" section after 3-months of storage in artificial saliva. The specimens (n = 5 dentin slabs per group) were coated with nail varnish in two layers, except for a narrow zone measuring 1 mm diameter at the resin-dentin contact. The teeth were divided into bucco-lingual sections, each measuring 0.9 mm in thickness. The sections were immersed in Karnovsky’s fixative, a solution containing 2.5% glutaraldehyde and 2% paraformaldehyde in a 0.1 M cacodylate buffer with a pH of 7.3. The fixative was applied for a maximum duration of 4 h. After that, the sections were thoroughly washed with a 0.1 M sodium cacodylate solution. The specimens underwent a 2-week decalcification process using an EDTA solution, with the solution being changed every 24 h. Following fixation, a 1% solution of osmium tetraoxide was employed in a 0.1 M sodium cacodylate buffer with a pH of 7. The method had a duration of 1 h at room temperature. Subsequently, all specimens were treated with a 2% uranyl acetate solution for a duration of 1 h. Subsequently, a series of drying methods were employed, including ethanol solutions with increasing concentrations: 50%, 75%, 80%, 95%, and 1.0%. Subsequently, the specimens were immersed in epoxy resin, and thin slabs measuring 70nm were produced using an ultramicrotome. The slabs were further examined using a transmission electron microscope (TEM) (EM208S, Philips, Eindhoven, Netherlands) operating at 300 kV.
Fluorescence in situ hybridization (FISH) for antimicrobial analysis
Streptococcus mutans ATCC 35,668, Actinomyces naeslundii ATCC 12,104, and Streptococcus sanguis ATCC 10,556 were used to create a multi-species biofilm. The bacteria were grown in a controlled anaerobic environment at a temperature of 37◦C for 48 h. The growth medium used was BHI broth, which was supplemented with 8% sucrose (pH 7.4) and a tiny amount of xylitol (0–2%). The scarcity of xylitol increased the probability of bacterial colonies reaching the end of their logarithmic growth phase. After being subjected to centrifugation at a speed of 4000 revolutions per minute for 10 min, the solid masses of cells were cleansed three times using sterile PBS (0.01 M, pH 7.2) and then suspended again in 100 mL of growth medium. The suspension was subsequently diluted to a concentration of McFarland 0.5 (107 cells/mL) before further use. A bacterial solution was made and homogenized to attain a targeted bacterial population (McFarland 107 CFU/mL) for the development of multi-species biofilms. The purpose of this was to regulate the cloudiness of the liquid/bacterial mixture to keep a consistent quantity of bacteria within a predefined range, hence standardizing microbiological testing. The aseptic dentin discs coated with experimental adhesives were positioned onto a 12-well tissue culture plate (Thermo Fisher Scientific Inc.) containing 2 mL of a multispecies biofilm. Subsequently, they were cultured for 120 min at a temperature of 37 ◦C in an orbital shaker incubator operating at a speed of 50 rpm. The multi-species biofilms were cultivated in an anaerobic environment containing 85% nitrogen, 10% hydrogen, and 5% carbon dioxide over three days. The concentration of the biofilms was 107 CFU/mL. The media was refreshed daily with BHI broth to maintain a uniform atmosphere and pH of 6.1.
FISH and CLSM-analysis were conducted on biofilm specimens (n = 5 biofilm specimens per group) that were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) containing 1.7 mMKH2PO4, 5 mM Na2HPO4, and 0.15 M sodium chloride at pH 7.2 for 8 h at 4 °C. Afterward, the slabs were rinsed twice with sterile PBS and then placed in a solution consisting of equal parts (1:1, v/v) of 50% ethanol and 50% PBS. They were held at a temperature of 4 °C for 3 h. Subsequently, the specimens underwent two rounds of washing with PBS. The slabs were immersed in a solution containing 7 mg/ml of lysozyme (specifically, hen egg white lysozyme from Fluka, Buchs, Switzerland, with an activity of 105,000 U/mg in a solution of 0.1 M Tris–HCL, 5 mM EDTA, pH 7.2) for 9 min at a temperature of 37 °C within a chamber with controlled humidity. Subsequently, the specimens were washed with double distilled water and subjected to dehydration using a series of increasing ethanol concentrations. The slabs were hybridized in a hybridization buffer of 600 mL which consisted of 5 M NaCl, 1 M Tris–HCL (pH 8.0), 25% formamide (v/v), and 10% SDS (sodium dodecylsulfate) (w/v). The buffer included 5 L??? of oligonucleotide probe for eubacteria and 2 L?? of oligonucleotide probe for streptococci, with each probe having a concentration of 50 ng/l. The oligonucleotide probe was marked with the fluorochrome cy3 at the 5’ end, whereas the EUB 338 probe was labeled with -GCT GCC TCC CGT AGG AGT- at the 5’ end. The specimens were analyzed using confocal laser scanning microscopy (CLSM) with a Leica TCS SP2 AOBS system from Mannheim, Germany, after conducting FISH (fluorescence in situ hybridization). The adhesive slabs were examined using CLSM (Leica TCS SP2 AOBS) with a 63-water immersion objective (Leica) in a chambered cover glass (Slide 8 Well, ibidi GmbH, Munich, Germany). Images were taken at different levels of magnification and with an electrical zoom of 6.3x. Each biofilm was examined at certain points. A Z-series of optical sections was obtained by vertically sectioning the biofilm at 0.97-m intervals.
Isolation and culturing of macrophages
Human peripheral blood mononuclear cells (PBMC) (SC cell line, CRL-9855 #LOT: 61,834,527) were obtained from ATCC. The cell concentration per flask remained at 2 × 105 cells. At a concentration of 1 × 10^6 cells/mL, the cells were cultured in Iscove’s Modified Dulbecco’s Media (IMDM; ATCC, Lot:63,331,110-Manassas/VA). To enhance the culture, 10% FBS, 0.05 mM 2-mercaptoethanol, 0.1 mM hypoxanthine, and 0.016 mM thymidine were added. Day 0 saw the introduction of coverslips from Fisher Scientific ™ Richard-Allan Scientific into 6-well plates from Sigma-Aldrich to produce resting macrophages. A solution containing 60ng/ml of phorbol myristate acetate (Sigma P1585-Lot # SLBQ7454 V) was used to culture the SC CRL cells for at least 6 h. Following 6 h, the macrophage cells were exposed to a lipopolysaccharide solution (LPS-PG Ultrapure/Porphyromonas gingivalis-TLR4 ligand) at a concentration of 0.1 µg/ml. To promote M1 polarization, interferon-gamma (IFN γ-) was also administered at a concentration of 20ng/ml. In addition, the cytokine interleukin 4 was used to promote M2 polarization. The following numbers are from the Pep Rotech Human IL-4 catalog: 200–04/Lot# 011,614–1 and the concentration was 20ng/ml. After that, each well was put into an incubator for 66 h. First, the cells were treated with 1 µL of adhesive on the third day. After rinsing the cells with PBS, the IMDM medium was completely removed. To further stabilize the cells, they were next exposed to 4% paraformaldehyde (PFA) for 20 min at room temperature. X100 Sigma Aldrich in PBS, which contains 0.1% Triton X-100, was added to the cells after rinsing them with PBS. The cells were left to sit at room temperature for 20 min. When that was done, the Triton X was discarded, and the wells were sealed for about twenty mins at room temperature using 2% Bovine Serum Albumin -BSA (P1585-1 mg in PBS).
Immunohistochemical staining
The same PBMC cells were also used in this part of the study. The flask was maintained at a cell concentration of 2 × 105 cells per flask. The cells were counted and cultured in Iscove’s Modified Dulbecco’s Media (IMDM; ATCC, Lot:63,331,110-Manassas/VA) at a density of 1 × 106 cells per milliliter. The culture was enriched with 10% fetal bovine serum (FBS), 0.05 mM (mM) 2-mercaptoethanol, 0.1 mM hypoxanthine, and 0.016 mM thymidine. On Day 0, coverslips from Fisher Scientific ™ Richard-Allan Scientific were placed into 6-well plates from Sigma-Aldrich to generate resting macrophages (MӨ). The SC CRL cells were cultured in a solution with a concentration of 60ng/ml of phorbol myristate acetate (PMA; Sigma P1585-Lot # SLBQ7454 V) for at least 6 h. After 6 h, a solution containing lipopolysaccharide (LPS-PG Ultrapure/Porphyromonas gingivalis-TLR4 ligand) at a concentration of 0.1 µg/ml was added to the macrophage cells without removing the existing contents of the well. In addition, a concentration of 20ng/ml of interferon-gamma (IFN γ-) was administered to promote M1 polarization. In addition, the cytokine interleukin 4 (IL-4 Pep Rotech Human IL-4-Catalog # 200–04/Lot# 011,614–1; 20ng/ml) was added to promote M2 polarization. Afterward, all the wells were put in an incubator for 66 h. On the third day, the cells were pre-treated with 1 µL of adhesive. The IMDM medium was completely removed, and the cells were washed with PBS. The cells were then exposed to a 4% solution of paraformaldehyde (PFA) for 20 min at room temperature to stabilize them. The cells were washed with PBS and subsequently exposed to a 0.1% solution of Triton X-100 (X100 Sigma Aldrich in PBS) for 20 min at room temperature. Subsequently, the Triton X was eliminated, and the wells were obstructed with a 2% solution of Bovine Serum Albumin (BSA) in PBS (P1585-1 mg) for about 20 min at ambient temperature.
Statistical analysis
The bond strength data was represented as the mean values together with their corresponding standard deviations and examined using the statistical software program Sigma Stat Version 22, (SPSS, Chicago, IL, USA). The bond strength values were found to have a normal distribution according to the Shapiro–Wilk test, and they were also found to have equal variances according to the modified Levene test. Hence, a three-way analysis of variance (ANOVA) was performed to examine the impact of“agents”,“adhesives”, and“time”, as well as the interaction between these factors on microtensile bond strength. The nanoindentation values were evaluated with a one-way analysis of variance (ANOVA). Tukey’s test was employed for doing post hoc multiple comparisons. The evaluation of nanoleakage entailed analyzing both the consistency across different observers and the consistency within the same observer. These consistencies were quantified using the weighted Kappa (kw) statistics. The nanoleakage scores were categorized as ordinal data. The Cochran-Mantel–Haenszel (CMH) method was used to evaluate the statistical significance of the differences among the groups at the specified time intervals. A statistically significant criterion of p < 0.05 was used.
Data availability
All data, code, and resources utilized in the study are accessible to any researcher for the aim of replicating or expanding upon the analysis. The datasets produced and/or examined during the present investigation are not accessible to the public owing to confidentiality concerns. However, interested parties may get them from the corresponding author upon a reasonable request.
References
Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion - not there yet. Jpn. Dent. Sci. Rev. 56, 190–207 (2020).
Mohammad, A. S., Julia, V., Anna, V. & Steven, M. M. Functional role of inorganic trace elements in dentin apatite tissue—part III: Se, F, Ag, and B. J. Trace Elem. Med. Biol. https://doi.org/10.1016/j.jtemb.2022.126990 (2021).
Pashley, D. H. et al. State of the art etch-and-rinse adhesives. Dent. Mater. 27, 1–16 (2011).
Tjäderhane, L. Dentin bonding: Can we make it last?. Op. Dent. 40, 4–18 (2015).
Betancourt, D. E., Baldion, P. A. & Castellanos, J. E. Resin-dentin bonding interface: Mechanisms of degradation and strategies for stabilization of the hybrid layer. Intern. J. Biomater. https://doi.org/10.1155/2019/5268342 (2019).
Pashley, D. H. et al. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 83, 216–221 (2004).
Li, K. et al. Enhancing resin-dentin bond durability using a novel mussel-inspired monomer. Mater. Today Biol. 12, 100174 (2021).
Daood, U. et al. Antimicrobial and self-crosslinking potential of experimentally developed dioctadecyldimethyl ammonium bromide and riboflavin dentin adhesive. J. Biomed. Mater. Res. Part A. 109, 2392–2406 (2021).
Frassetto, A. et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability-A literature review. Dent. Mater. 32, 41–53 (2016).
Daood, U. et al. Novel riboflavin/VE-TPGS modified universal dentine adhesive with superior dentine bond strength and self-crosslinking potential. Dent. Mater. 36, 145–156 (2020).
Frederik, R. et al. Corneal crosslinking with riboflavin and UVA light in progressive keratoconus: Fifteen-year results. Am. J. Ophthalmol. 250, 95–102 (2023).
Nsairat, H. et al. Liposomes: Structure, composition, types, and clinical applications. Heliyon. 8, e09394 (2022).
Hardan, L. et al. Effect of collagen crosslinkers on dentin bond strength of adhesive systems: A systematic review and meta-analysis. Cells 11, 21417 (2022).
Osracolo, C. et al. Enhancement of corneal permeation of riboflavin-5-phosphate through vitamin E TPGS: A promising approach in corneal trans-epithelial cross-linking treatment. Intern. J. Pharmacol. 440(148), 153 (2013).
Lamy, R. et al. Ultrasound enhanced permeation of topical riboflavin into the corneal stroma. Investig. Ophthalmol. Vis. Sci. 54, 5908–5912 (2013).
O’Brart, D. P. S. Corneal collagen cross-linking: A review. J. Ophthalmol. 7, 113–124 (2014).
Farah, N. et al. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. https://doi.org/10.1016/j.crmicr.2022.100111 (2022).
Chen, C. et al. Bonding of universal adhesives to dentine—old wine in new bottles?. J. Dent. 43, 525–536 (2015).
Bernardo, M. et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J. Am. Dent. Assoc. 138, 775–783 (2007).
Maciel, P. P. et al. Bonding performance and ultramorphology of the resin-dentine interface of contemporary universal adhesives. Clin. Oral Invest. 26, 4391–4405 (2022).
Tezvergil-Mutluay, A., Pashley, D. H. & Mutluay, M. M. Long-term durability of dental adhesives. Curr. Oral Health Rep. 2, 174–181 (2015).
Sebold, M. et al. Bonding interface and dentin enzymatic activity of two universal adhesives applied following different etching approaches. Dent. Mater. 38, 907–923 (2022).
Sauro, S. et al. Micro permeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: A comparison study using a double-staining/confocal microscopy technique. Eur. J. Oral Sci. 116, 184–193 (2008).
Mazzoni, A. et al. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 91, 467–472 (2012).
Perdigao, J., Araujo, E., Ramos, R. Q., Gomes, G. & Pizzolatto, L. Adhesive dentistry: Current concepts and clinical considerations. J. Esthet. Restor. Dent. 33, 51–58 (2021).
Yoshihara, K. et al. Etching efficacy of self-etching functional monomers. J. Dent. Res. 97, 10101–10116 (2018).
Cuevas-Suarez, C. E., da Rosa, W. L. O., Lund, R. G., da Silva, A. F. & Piva, E. Bonding performance of universal adhesives: An updated systematic review and meta-analysis. Journal of Adhesive Dentistry. 21, 726 (2019).
Daood, U., Matinlinna, J. P. & Fawzy, A. S. Synergistic effects of VE-TPGS and riboflavin in crosslinking of dentin. Dent. Mater. 35, 356–367 (2019).
Hayes, S., Kamma-Lorger, C. S. & Boote, C. The effect of riboflavin/UVA collagen cross-linking therapy on the structure and hydrodynamic behaviour of the ungulate and rabbit corneal stroma. PLoS ONE 8, e52860 (2013).
Hass, V. et al. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent. Mater. 32, 732–741 (2016).
Ahmad, I. et al. Formulation and stabilization of riboflavin in liposomal preparations. J. Photochem. Photobiol. B. Biol. 153, 358–366 (2015).
Beztsinna, N. et al. Amphiphilic phospholipid-based riboflavin derivatives for tumor targeting nanomedicines. Bioconj. Chem. 27, 2048–20161 (2016).
Morini, M. A. et al. Influence of temperature, anions and size distribution on the zeta potential of DMPC, DPPC and DMPE lipid vesicles. Colloid. Surf. B. Biointerfac. 131, 54–58 (2015).
Taschner, M. et al. Influence of preliminary etching on the stability of bonds created by one-step self-etch bonding systems. Eur. J. Oral Sci. 120, 239–248 (2012).
Salvio, L. A., Hipólito, V. D. & Martins, A. L. Hybridization quality and bond strength of adhesive systems according to interaction with dentin. Eur. J. Dent. 7, 315–326 (2013).
Joseph, P. O., Tim, J. W. & Andrew, M. The in-situ conformation and axial location of the intermolecular cross-linked non-helical telopeptides of type I collagen. Structure. 8, 137–142 (2000).
Meier, R., Tomizaki, T., Schulze-Briese, C., Baumann, U. & Stocker, A. The molecular basis of vitamin E retention: Structure of human alpha-tocopherol transfer protein. J. Mol. Biol. 331, 725–734 (2003).
Cole, D. J., Paynea, M. C. & Ciacchibc, L. C. Water structuring and collagen adsorption at hydrophilic and hydrophobic silicon surfaces. Phys. Chem. Chem. Phys. 11, 11395–11409 (2009).
Yiu, C. K. et al. Effect of resin hydrophilicity and water storage on resin strength. Biomaterial. 25, 5789–5796 (2004).
Daood, U., Iqbal, K., Nitisusanta, L. I. & Fawzy, A. S. Effect of chitosan/riboflavin modification on resin/dentin interface: Spectroscopic and microscopic investigations. J Biomed. Mater. Res. A. 101A, 1846–1856 (2013).
Daood, U. et al. In vitro assessment of ribose modified two-step etch-and-rinse dentine adhesive. Dent. Mater. 34, 1175–1187 (2018).
Petruska, J. A. & Hodge, A. J. A subunit model for the tropocollagen macromolecule. Proc. Natl. Acad. Sci. U. S. A. 51, 871–876 (1964).
Claffey, W. Interpretation of the small-angle X-ray diffraction of collagen in view of the primary structure of the alpha1 chain. Biophys. J. 19, 63–70 (1977).
Hulmes, D. J., Miller, A., White, S. W. & Doyle, B. B. Interpretation of the meridional X-ray diffraction pattern from collagen fibres in terms of the known amino acid sequence. J. Mol. Biol. 110, 643–666 (1977).
Hansen, C. M. & Skaarup, K. The three-dimensional solubility parameter key to paint component affinities. III. Independent calculation of the parameter components. J. Paint Technol. 39, 11–20 (1967).
Dige, I., Nilsson, H. & Kilian, M. In situ identification of streptococci and other bacteria in initial dental biofilm by confocal laser scanning microscopy and fluorescence in situ hybridization. Eur. J. Oral Sci. 115, 459–467 (2007).
Williams, L. B. Natural antibacterial clays: Historical uses and modern advances. Clay. Clay. Miner. 67, 7–24 (2019).
Sezonov, G., Joseleau-Petit, D. & D’Ari, R. Escherichia coli physiology in luria-bertani broth. J. Bacteriol. 189, 8746–8749 (2007).
Ge, M. Q., Li, J., Song, S. J., Meng, N. & Zhou, N. L. Development and antibacterial performance of silver nanoparticles-lecithin modified montmorillonite nanoparticle hybrid. Appl. Clay Sci. 183, 105334 (2019).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trend. Immunol. 25, 677 (2004).
Andrade, I. Jr., Silva, T. A., Silva, G. A., Teixeira, A. L. & Teixeira, M. M. The role of tumor necrosis factor receptor type 1 in orthodontic tooth movement. J. Dent. Res. 86, 1089 (2007).
Zhang, Q. et al. IL-17-mediated M1:M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin. Cancer Res. 19, 3176 (2013).
Wang, Y. et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int https://doi.org/10.1038/sj.ki.5002275 (2007).
Tay, F. R., Pashley, D. H., Garcìa-Godoy, F. & Yiu, C. K. Single-step, self-etch adhesives behave as permeable membranes after polymerization. Part II. Silver tracer penetration evidence. Am. J. Dent. 2004, 315–322 (2004).
Acknowledgements
The authors express gratitude to the laboratories at IMU and Mimos Research Centre for their assistance in conducting research experiments and analysis. The authors would like to express our special thanks to our mentor Professor Frederick Smales for his advice and suggestions and help reading the draft for its grammatical check.
Funding
The study protocol was funded by IMU University project number 537/2021.
Author information
Authors and Affiliations
Contributions
The authors assert that they have no conflict of interest. The funders did not contribute to the study’s design, data collection, analysis, interpretation, manuscript writing, or decision to publish the results. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The project obtained permission from the Institutional Review Board of International Medical University Kuala Lumpur. Obtained written informed consent from all persons who contributed extracted teeth for the laboratory in-vitro experiments approved by the Institutional Research Ethical Committee (537/2021).
Consent for Publication
Consent for publishing has been obtained from all authors. All authors read and approved the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Er-Vin, W., Marn, Y.C., Shuai, W.S. et al. Synergistic strategy of riboflavin and lipoids to bioengineer resin dentin hybrid layer. Sci Rep 15, 20877 (2025). https://doi.org/10.1038/s41598-025-04923-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04923-3