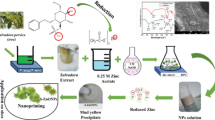
Abstract
Growing nanotechnology use in agriculture can transform traditional practices. This study investigated the application of zinc oxide nanoparticles (ZnO NPs) in Hibiscus syriacus L. cultivation, examining: particle size (30, 50, and 90 nm), concentration (10, 50, 100, 500, and 1000 mg/L), and application method (seed priming and foliar application). In seed priming experiments, low concentrations (10–100 mg/L) of all tested NP sizes enhanced germination parameters, while higher concentrations showed inhibitory effects. The optimal treatment was 30 nm ZnO NPs at 50 mg/L, achieving a 68.89% germination rate. Seed priming also significantly improved seedling growth and physiological biochemistry. In pot experiments, foliar application studies showed that while both ZnSO4 and ZnO NPS increased leaves Zn content, nanoparticle treatments (especially smaller particles at 30 and 50 nm) produced more sustained growth benefits, with ZnO NPS outperforming traditional zinc fertilizers, particularly at 50 mg/L with 50 nm particles. These findings highlight dual advantages of ZnO NPs as an effective seed priming and foliar application, demonstrating potential as an efficient fertilizer for ornamental woody plants. In addition, significant concentration thresholds were found in both application methods (with varying thresholds), above which phytotoxic effects were observed.
Similar content being viewed by others
Introduction
The rapid development of nanotechnology has led to its application in many fields, such as disease treatment, biomedical instrumentation, nanosensors, biomarker development, display technologies, and environmental protection1. Nanoparticles have also been widely used in plant science and agricultural production in recent years, due to their size, surface properties and low ecotoxicity, with demonstrated positive effects on improving seed germination and increasing crop productivity2,3.
Seed nano-priming is an efficient process that affects not only germination but also the entire plant lifecycle4. Several reports have shown that nanomaterials of different particle sizes and concentrations have positive or negative effects on seed germination of different plants5,6,7. Also, the effect of ZnO NPs on plants can be biphasic. Prasad et al.8 reported that a 1000 ppm treatment of ZnO NPs promoted the seed germination and seedling vigor of Cucumis sativus, but an inhibitory effect was found at 2000 ppm. Kim et al.9 showed that NPs may cause phytotoxicity by forming aggregates with themselves or other cellular materials inside the cell, and when a certain threshold is reached the NPs may dissolve to metal ions. Nano-fertilizers are an efficient way to promote plant nutrients by improving nutrient absorption rates and reducing environmental hazards10. Zinc (Zn) is an essential plant nutrient but it is deficient in many agricultural soils worldwide11 which adversely impacts plant growth and productivity. Moreover, zinc is an essential trace element for human health, and oral zinc supplementation has been demonstrated to enhance immune function12. Due to the inefficient use of chemical fertilizers in current agricultural practices the loss of fertilizer to the surrounding environment can have a serious impact on natural ecosystems13. Nutrient uptake in the soil is not always entirely effective because of leaching, soil fixations, blockages, and other losses. These problems can be minimized by arranging a foliar application14. In agriculture, many NPs are used in nano-fertilizers or nano-pesticides to enhance plant nutrition or strengthen resistance due to their unique physicochemical properties15. Several studies have investigated the effect of foliar applications for plant growth. The foliar application of ZnO NPs positively affects the fresh and dry weights of coffee (coffea Arabica L.) roots and leaves16. The foliar application of low NP concentrations to Helianthus annuus significantly improved the plant physiological traits and nutrition17. Foliar application of Zn and boron (B) nano-fertilizers have been shown to significantly improve Punica granatum fruit yield and quality18.
Although there have been many studies of the effects of NPs on seed germination and seedling growth, they have mainly focused on crops, such as Cucumis sativus19,20 Triticum aestivum21,22 Sorghum bicolor23 and Solanum lycopersicum24,25,26 and the effects on ornamental plants, especially Hibiscus syriacus are not fully understood. H. syriacus has beautiful flowers and a long flowering stage, and is therefore often grown in gardens or as a natural fence27. It has strong adaptability and resistance28,29 and can be used for soil reclamation and ecological remediation. H. syriacus not only plays an important role in ornamental horticulture, but also in medicine due to its anti-oxidant30 anti-inflammatory31 and anti-cancer effects32 enabling it to be used to treat various diseases. Furthermore, the fresh flowers can be made into flower juice or wine, or used directly in dishes33. The leaves are edible and can be made into a tea27. As a result of these properties, H. syriacus is used as an ornamental plant with great potential for other applications. However, H. syriacus suffers from seed abortion and a low germination rate, and thus improving the seed germination rate is of great significance for germplasm resource management and novel applications. Enhancing the growth and nutritional quality of H. syriacus seedlings can significantly increase its economic value.
This study on the ornamental shrub Hibiscus syriacus L., we aimed to investigate the feasibility of using ZnO NPs as a fertilizer for ornamental woody plants and to compare the effects of ZnO NPs with different particle sizes and concentrations on the seed germination, growth and development of H. syriacus, thereby determining the safest and most beneficial application protocol.
Materials and methods
Experimental materials
The seeds were collected from the Hibiscus syriacus L. planted by the Central South University of Forestry and Technology, and seedlings were germinated and cultivated from the collected seeds. The culture substrate was purchased from the local flower market and consisted of a 1:1 mix of peat soil and perlite.
Characterization of ZnO NPs
Three different NP sizes (30 ± 10, 50 ± 10, and 90 ± 10 nm) of ZnO NPs (Fig. 1A-C) were purchased (Shanghai Macklin Biochemical Technology Co.) with purity > 99.8% and a density of 5.61 g/cm3. Zinc sulfate heptahydrate (ZnSO4.7H2O) was purchased from Sinopharm Chemical Reagent Co. Transmission electron microscopy (TEM) characterization revealed that the ZnO NPs used in this study exhibited irregular polygonal shapes.
Experimental design
The experiment was completed at the College of Landscape Architecture, Central South Forestry University of Science and Technology, Changsha, Hunan, China, under natural light irradiation at a temperature of 20–25 °C, a photoperiod of 16/8 h (light/dark), and a relative humidity of 60%. ZnO NPs were dispersed by ultrasonic dispersion (360 W, 40 kHz) to form suspensions, and ZnSO4.7H2O was dissolved in deionized water to make a solution. The solutions used in the experiment were all made before use, and have ensured that the suspension is free from visible agglomerations during use.
Seed priming with ZnO NPs
Intact and robust full-grown H. syriacus seeds were selected, these seeds were soaked in 0.4% potassium permanganate solution for 1 h for disinfection, then rinsed several times with deionized water. The seeds were placed in 100 mL conical flasks and were treated with: (1) deionized water (CK), (2) 30 nm ZnO NPs, (3) 50 nm ZnO NPs, and (4) 90 nm ZnO NPs, at concentrations of 10, 50, 100, 500, and 1000 mg/L, with 100 seeds in each conical flask. All conical flasks were sealed with sealing film and then placed in a constant temperature shaker at 24 °C and 100 r/min for 6 h. After 6 h, the seeds were rinsed several times with deionized water to remove the suspension.
Seeds were randomly placed in disinfected Petri dishes (10 centimeters in diameter) lined with two layers of filter paper. Each dish contained 30 seeds, and three replicates were performed per treatment. The petri dishes were incubated in a constant temperature incubator at 26 °C. The number of seeds germinated was observed and recorded daily. After 17 days, the following formulas were used to calculate the germination parameters:
Gt: number of seeds germinated on day t; Dt: number of days to germinate.
All germinated seeds were sown in plastic potsand, with five seeds in each pot under the same treatment. Three months after sowing, three seedlings were randomly selected for each treatment group to determine different traits.
Foliar application
The intact and robust full-grown H. syriacus seeds were soaked in 0.4% potassium permanganate solution for 1 h for disinfection, then rinsed several times with deionized water and then shaking for 6 h. After 6 h, the seeds were randomly spotted in disinfected petri dishes with two layers of filter paper and the diameter is 10 centimeters, with 30 seeds in each petri dish. Seeds were sown in hole trays after germination and the cultivation substrate was a mixture of peat soil: perlite (1:1).
After two months, healthy and uniformly growing seedlings were selected and transplanted to plastic pots (each pot had five seedlings). The experiment was divided into 11 treatment groups, 5 seedlings per treatment, and repeated three times: (1) control (no foliar application), (2) 50 mg/L zinc sulfate heptahydrate (ZnSO4.7H2O), and (3) 30, 50, and 90 nm ZnO NP suspensions (10, 50, 100 mg/L). A 100 mL sprayer was used for foliar application, 20 ml of of the different treatments were sprayed uniformly on both sides of the leaves of H. syriacus seedlings. With three applications during the experiment: the start of experiment, and 15 and 30 days after the initial application. The differences in traits were determined two days after each foliar application (i.e. days 2, 17, and 32).
Growth traits
Three seedlings were randomly selected for each treatment, and leaf length and width as well as plant height were determined using vernier calipers and measuring tape. The plants were rinsed with deionized water, blotted with filter paper and then divided into roots, stems and leaves. The plants were dried at 80 °C to constant weight to determine the biomass of each plant part.
Chlorophyll content
The chlorophyll content was determined according to Lichtenthaler and Wellburn34. Briefly, 0.05 g of each fresh leaf tissue sample was homogenized in 4 mL of 80% acetone. The resulting homogenate was centrifuged at 12,000 r/min for 5 min at 4 °C. The absorbance of the supernatant was taken at 663 and 646 nm against a blank of pure 80% acetone using a UV spectrophotometer (T6 series, PG Scientific, Auburn, CA, USA).
Physiological parameters
Three leaves from each pot were randomly selected as samples to determine a range of biochemical parameters. The soluble sugar content was determined according to the anthrone sulfuric acid method, as described by Irigoyen35. For the extraction, 0.05 g of each sample was weighed out, homogenized in 4 mL of distilled water. The homogenate was heated in a water bath at 100 °C for 20 min, centrifuged at 12000 r/min for 15 min. Take the supernatant and fix the volume with distilled water to 20 mL. 1 mL of the supernatant was added to 5 mL of anthrone sulfuric acid. The mixture was boiled in a water bath for 10 min and quickly cooled in an ice bath. The absorbance was taken at 620 nm. The soluble protein content was measured following the method described by Bradford36. The samples were homogenized in 5 mL of 0.05 mol/L phosphate buffer saline (PBS) solution (pH = 7.8). The homogenates were centrifuged at 12000 r/min for 15 min. 1mL of the supernatant was mixed with 5 mL G-250 Coomassie brilliant blue solution. Waited for 5 min to note the absorbance at 595 nm. The superoxide dismutase (SOD) activity assay procedure was conducted according to the photochemical reduction of nitro blue tetrazolium (NBT) described by Constantine37. Samples were extracted in the same way as the soluble protein extraction.
Zn content
Determination of foliar zinc content was performed by flame atomic absorption spectrometry (FAAS)38.
Statistical analysis
Data were analyzed by an analysis of variance (ANOVA) using SPSS version 29 for Windows, and a Duncan test was used to determine significance at P < 0.05, the effect size is reported as partial η² (Partial Eta-Squared). The results were presented as the mean ± standard deviation (SD).
Results
Effect of ZnO NPs on germination
The germination rate, germination energy, germination speed, and germination index of H. syriacus seeds significantly increased compared to the control (CK) at the 10, 50, and 100 mg/L treatments for all three NP sizes, whereas at higher ZnO NP concentrations (500 and 1000 mg/L), these indicators were significantly lower than at low ZnO NP concentrations (10, 50, and 100 mg/L) (Fig. 2A-E). The 30 nm ZnO NPs at 50 mg/L (N30C50) treatment significantly increased the seed germination rate by 51.22% compared with the control. At a ZnO NPS concentration of 50 mg/L, 30 nm ZnO NPs enhanced germination more than the other NP sizes, but for the 500 and 1000 mg/L treatments the toxicity trend reversed. At high concentrations, the smaller the particle size, the more significant the inhibition of germination. The inhibitory effects were slightly higher for the 30 nm ZnO NPs than the 50 and 90 nm ZnO NPs. For the 30, 50, and 90 nm ZnO NPs at 1000 mg/L, the germination rate was significantly reduced by 31.71%, 26.83%, and 21.95% compared with the control, respectively.
We have observed that seed priming with ZnO NPs resulted in early germination, with control (CK) seeds initiating germination on day 6, whereas most NP treatments seeds commenced on days 3–4. In addition, the 50 and 100 mg/L treatments for all three NP sizes, the mean germination time (MGT) of the seeds was significantly lower, the MGT of the 30 nm NPs at 50 mg/L (N30C50) and 50 nm NPs at 100 mg/L (N50C100) treatments were about 7 days, whereas the MGT of CK was about 11 days. The MGT of the three particle sizes in the 500 and 1000 mg/L treatments was closer to that of CK at 10–11 days.
The seed germination rate (A), germination energy (B), germination speed (C), mean germination time (D), and germination index (E) in H. syriacus treated with 30, 50, and 90 nm ZnO NPs at 0, 10, 50, 100, 500, and 1000 mg/L. Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
Effect of seed priming with ZnO NPs on H. syriacus seedlings
Growth traits
Effects of different NP sizes and ZnO NP concentrations on the leaf length, leaf width, seedling height, and leaf, stem, and root biomass of seedlings are shown in Table 1. Effects of different treatments on phenotypes in H. syriacus seedlings (Fig. 3A-C in order 30, 50, and 90 nm ZnO NPs). The N30C50 treatment stimulated leaf growth in seedlings, with a clear increase in length and width, along with longer and denser root systems. The maximum seedling height reached 19.77 cm, which was 64.27% higher than CK. And the 50 and 90 nm treatments at lower ZnO NP concentrations (10, 50, and 100 mg/L) resulted in a significant increase in leaf growth and seedling height, while higher concentrations (500 and 1000 mg/L) caused inhibitory effects. The same trend was found for the leaf, stem, and root biomass. All treatments at 500 and 1000 mg/L showed a significant decreased in leaf, stem, and root biomass compared with the other treatments, the 30 nm NPs at 1000 mg/L (N30C1000) treatment showing the strongest inhibition.
Chlorophyll content
Compared with CK, the 30 nm ZnO NPs at 10 and 50 mg/L significantly improved the chlorophyll content (Fig. 4), and there was no significant difference when the concentration at 100 mg/L, while higher concentrations (500 and 1000 mg/L) caused inhibitory effects. The N30C50 significantly increased the chlorophyll a content by 37.87% and chlorophyll b content by 35.02%. The 50 and 90 nm ZnO NPs at low concentrations consistently increased the chlorophyll content, with a peak at 100 mg/L, and then decreased at higher concentrations. The chlorophyll content for all three NP sizes at high concentrations was significantly reduced. As the treatment concentration increased, the decrease in the chlorophyll content became larger. The smaller the particle size, the more obvious the inhibition of the chlorophyll content.
Effect of seed priming (30, 50, and 90 nm ZnO NPs at 0, 10, 50, 100, 500, and 1000 mg/L) on chlorophyll content of H. syriacus. Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
Physiological parameters
Physiological parameters, the soluble sugar and protein contents and SOD activity, were observed to study the effects of the different NP sizes and concentrations in the treatments (Fig. 5A-C). We observed that the content of soluble sugars and proteins tended to increase and then decrease with increasing concentrations of the different NP sizes, but with different peaks at different concentrations for different particle sizes. The soluble sugar and protein contents of all three NP sizes were lower than those of CK at high concentrations, as the treatment concentration increased, the decrease content became larger. And at high concentration (1000 mg/L), all indicators reached their lowest values.
Compared with CK, some of treatments at lower ZnO NP concentrations, the soluble sugar contents significantly increased (Fig. 5A). Among them, the soluble sugar content of N30C50 treatment was significantly increased by 79.81% and reached the highest value of 21.17 mg/g, while the soluble sugar content of 50 nm NPs at 100 mg/L (N50C100) and 90 nm NPs at 100 mg/L (N90C100) treatments significantly increased by 58.27% and 45.15%, respectively. In contrast, the 30, 50, and 90 nm ZnO NPs at 1000 mg/L significantly decreased the soluble sugar content by 55.10%, 37.01%, and 36.18%, respectively.
Similar tended occurred for soluble protein content (Fig. 5B). Compared with CK, the soluble sugar content of N30C50 treatment significantly increased by 137.50%, the N50C100 and N90C100 treatments, the protein content were found to be significantly increased by 61.44% and 30.81%, respectively. The 30, 50, and 90 nm ZnO NPs at 1000 mg/L significantly decreased the soluble protein content by 51.21%, 30.75, and 34.33%, respectively.
SOD is a component of the antioxidant system and changes in its activity reflect the plant’s ability to combat ROS. The highest increase in SOD activity (43.40%) was observed in N30C50 treatment, followed by a significant increase of 35.43% and 26.88% in N50C100 and N90C100 treatments, respectively, compared with CK (Fig. 5C). At higher ZnO NP concentrations (500 and 1000 mg/L), all three NP sizes there were significant decreases in the SOD activity. The 30, 50, and 90 nm ZnO NPs at 1000 mg/L significantly decreased SOD activity by 61.84%, 54.72%, and 44.43%, respectively.
Effect of seed priming (30, 50, and 90 nm ZnO NPs at 0, 10, 50, 100, 500, and 1000 mg/L) on (A) soluble sugar content, (B) soluble protein content, and (C) SOD activity of H. syriacus. Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
Determining the interactions between various ZnO NP concentrations, sizes, and variables through GLM and ANOVA
The interactions between the various ZnO NP concentrations, sizes and variables are shown in Table 2. In terms of seedling growth, the leaf length was significantly related to the particle size and concentration of ZnO NPs. However, the leaf width, seedling height, and biomass were not significantly related to particle size but were significantly related to concentration and their interaction. The biochemical parameters were affected by the particle size, concentration, and their interaction. Overall the effect of concentration on the indicators is stronger.
The Partial Eta - squared (ηp2) is a measure of effect size that represents the proportion of variance in the dependent variable accounted for by a particular factor while controlling for the effects of other factors.
Effects of the foliar application of ZnO NPs to seedlings
Growth trait responses to the foliar application of ZnO NPs
Foliar application of different ZnO NP sizes and concentrations affected seedling growth (Fig. 6A-C). Effects of foliar application of different treatments on phenotypes in H. syriacus seedling (Fig. 7A-D). Seedling height of the foliar application of ZnSO4 was closer to that of CK, but there were more leaves and longer roots. The 30 nm NPs at 10 mg/L (N30C10) and 50 nm NPs at 50 mg/L (N50C50) treatments increased seedling leaf and seedling height growth, and roots were longer and denser. At higher ZnO NP concentrations (100 mg/L), the 50 and 90 nm treatments significantly inhibited hibiscus seedling growth.
Compared with CK, ZnSO4 (50 mg/L) treatment slightly increased leaf length and width on day 2, but seedling height was significantly lower; it significantly increased leaf length and seedling height on day 17; however, there was no significant difference in leaf length, leaf width, and seedling height, which were only increased by 0.77%, 1.17%, and 2.32%, respectively, on day 32.
On day 2, the leaf length increased by 30.96% and 25.23% under the 50 mg/L treatment for 30 and 50 nm ZnO NPs, respectively, compared with CK. The leaf width increased in the treatments with 30 nm ZnO NPs at all three concentrations. The seedling height increased at lower concentrations (10 and 50 mg/L) treatments for 30 and 50 nm ZnO NPs, while higher concentrations (100 mg/L) caused inhibitory effects. On day 17, there was an increase in leaf length and width at lower concentrations (10 and 50 mg/L) of 30 and 50 nm ZnO NPs, compared with CK. The seedling height significantly increased by 58.72% and 30.64% under the N30C10 and N30C50 treatments, and the highest increase was observed at N50C50 treatment, with the seedling height reaching 15.43 cm, an increase of 97.02% compared with CK. On day 32, the seedling height was highest under the N50C50 treatment (25.13 cm), leaf length and width were largest under the N30C50 treatment. Overall, N50C50 and N30C50 treatments had a better promotion effect on H. syriacus seedling growth, and all three sizes of ZnO NPs at high concentration (100 mg/L) showed negative effect. In addition, 90 nm NPs at 50 mg/L (N90C50) treatment promoted leaf growth better but did not significantly improve plant height.
Growth of H. syriacus seedlings at different time periods after foliar application with ZnSO4 (50 mg/L) and ZnO NPs (30, 50 and 90 nm at 10, 50 and 100 mg/L), (A) leaf length, (B) leaf width, (C) seedling height. Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
Compared with CK, the ZnSO4 (50 mg/L) treatment showed significantly increased in leaf, stem and root biomass, by 29.35%, 39.15%, and 20.43%, respectively (Fig. 8). The leaf, stem, and root biomass significantly increased under the N30C10 treatment, while the greatest increase was observed under the N50C50 treatment, by 97.90%, 281.92%, and 248.83%, respectively. The N90C50 treatment slightly increased the biomass. The highest decrease was observed under the N50C100 treatment, with 70.87% and 72.83% decreases in stem and root biomass, respectively.
Biomass of H. syriacus seedlings under foliar application with ZnSO4 (50 mg/L) and ZnO NPs (30, 50 and 90 nm at 10, 50 and 100 mg/L), (A) leaf biomass, (B) stem biomass, (C) root biomass. Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
The response of chlorophyll content to the foliar application of ZnO NPs
The foliar application of different ZnO NP sizes and concentrations affected the chlorophyll content (Fig. 9A, B). We observed that both chlorophyll a and chlorophyll b tended to increase with time in all treatments. Under the same period of time, the chlorophyll content of higher concentration of ZnO NP in 30 nm was lower, while the chlorophyll content of 50 nm and 90 nm ZnO NPs was the highest at 50 mg/L. Compared with the CK, the treatment with ZnSO4 (50 mg/L) treatment showed a smaller increase in chlorophyll content at all periods. on day 2, the most significant increase in chlorophyll a and chlorophyll b content was observed at N50C50(36.82% and 43.64%, respectively). On day 17 and 32, the most significant increase in chlorophyll content was observed under N30C10 and N50C50 treatments. The chlorophyll a content was significantly lower than that of CK at high ZnO NP concentrations (100 mg/L with 30, 50 and 90 nm ZnO NPs), whereas the chlorophyll a content of N30C100, N50C100, N90C100 treatments were significantly reduced by 10.08%, 20.54%, and 23.08%, respectively, on day 32. Overall, N50C50 treatment showed the most outstanding performance in enhancing chlorophyll content, by contrast the improvement in the ZnSO4 (50 mg/L) treatment was relatively weaker.
The (A) chlorophyll a content, and (B) chlorophyll b contentunder under foliar application with ZnSO4 (50 mg/L) and ZnO NPs (30, 50 and 90 nm at 10, 50 and 100 mg/L) on day 2, day 17, and day 32. Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
Physiological parameters in response to the foliar application of ZnO NPs
The soluble sugar and soluble protein contents showed a gradual increase over time in the different treatments (Fig. 10A, B), whereas the SOD activity showed a first increase and then a decrease in most of the treatments (Fig. 10C).
The soluble sugar content of the ZnSO4 (50 mg/L) treatment increased by 12.81% and 14.23% on day 2 and day 17, respectively, while the difference was not significant on day 32, compared with CK (Fig. 10A). The soluble sugar content of all three NP sizes, at concentrations of 10 mg/L and 50 mg/L increased significantly at different periods compared to CK, the N50C50 treatment showing the greatest increase, 65.70%, 43.54%, and 43.62% on days 2, 17, and 32, respectively. Also the N30C10 treatment, increased by 49.00%, 37.34% and 32.69% at 2, 17 and 32 days, respectively. During all periods, the 100 mg/L treatments with all three NP sizes significantly decreased the soluble sugar content. The highest decrease was found with the N50C100 treatment.
Compared to CK, the ZnSO4 (50 mg/L) treatment showed non-significant difference in soluble protein content on day 2, significant increase of 43.30% on day 17 and significant decrease of 7% on day32 (Fig. 10B). The N50C50 treatment showed the most significant increase, 73.30%, 75.30% and 29.90% on days 2, 17 and 32, respectively. In addition the N30C10 treatment, increased by 48.60%, 59.10%, 11.90% on days 2, 17, and 32, respectively.When the ZnO NPs concentration was 100 mg/L, soluble protein exhibited similar changes as soluble sugar, and the soluble protein content of all three NP sizes was significantly reduced during all periods.
Compared to CK, the SOD activity of the ZnSO4 (50 mg/L) treatment showed insignificant difference on day 2, increased by 6.7% on day 17 and was 2% lower on day 32 (Fig. 10C). The 30 and 50 nm treatments of ZnO NPs at lower concentrations (10 mg/L and 50 mg/L) had similar effects on SOD activity, and these treatments were significantly improved SOD activity in each period. The highest increase in SOD activity was observed on day 32 for N50C50 treatment (19.51%), followed by N30C10 treatment (6.62%). However, at the higher ZnO NP concentration (100 mg/L), the SOD activity of all three particle sizes increased in the early period and decreased significantly on day 32. The SOD activity was significantly increased under the N50C100 treatment on day 2 (60.81%), but on days 17 (42.36%) and 32 (60.23%) a large decrease was observed.
The (A) soluble sugar, (B) protein contents, and (C) SOD activity under foliar application with ZnSO4 (50 mg/L) and ZnO NPs (30, 50 and 90 nm at 10, 50 and 100 mg/L) on days 2, 17, and 32. Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
The response of Zn accumulation in H. syriacus leaves to the foliar application of ZnO NPs
Foliar application of both ZnO NPs and ZnSO4 increased Zn accumulation in leaves of H. syriacus seedlings (Fig. 11). Zn content of leaves of ZnSO4 (50 mg/L) treatment was significantly increased by 25.09%, compared with CK. As the concentration of ZnO NPs increased, the Zn content of leaves increased continuously, and the smaller the particle size, the more significant the increase was at the same concentration. The highest Zn content of 38.03 µg/g was found in the N30C100 treatment, which was 60.36% higher than that of CK. The N50C100 and N90C100 treatments had 36.99 and 27.69 µg/g Zn, respectively, with significant increases of 55.95% and 16.75%, respectively, compared with CK. Among the three sizes, ZnO NPs with 30 and 50 nm particle sizes improved leaves Zn content better than 90 nm, while the N90C10 treatment showed no significant difference.
The Zn content in leaves of H. syriacus under foliar application with ZnSO4 (50 mg/L) and ZnO NPs (30, 50 and 90 nm at 10, 50 and 100 mg/L). Data are shown as mean ± standard deviation of three replications. Significant differences (p < 0.05) are indicated by column lines labelled with different letters according to Duncan’s test.
Comparison of growth parameters for seedlings developed from seeds and seedlings receiving a foliar application
In seed priming, the concentrations of 50 and 100 mg/L treatments promoted seedling growth, but in foliar fertilization the concentration of 100 m g/L treatment inhibited plant growth (Fig. 12A-F). The greatest seedling height was reached with seed priming under the N30C50 treatment, while the greatest seedling height for foliar application was observed under the N50C50 treatment. The leaf, stem, and root biomass increased more with the foliar application than with seed priming under the N30C10 treatment. Seed priming increased seedling biomass at 100 mg/L of ZnO NPs for all three sizes, but the same treatment resulted in a decrease with the foliar application. For both seed priming and foliar application, the biomass under low concentration treatments with 90 nm ZnO NPs was slightly increased.
Effects of seed priming and foliar application with ZnO NPs A (seed priming of 30 nm), B (seed priming of 50 nm), C (seed priming of 90 nm), D (foliar application of 30 nm), E (foliar application of 50 nm), and F (foliar application of 90 nm) on phenotypes in H. syriacus seedling, Bars = 5 cm in A, B.
Under the N30C10 treatment, the chlorophyll a content with seed priming was similar to that with the foliar application on day 17, but the rate of increase in the chlorophyll a content with the foliar application was higher than that with seed priming. The chlorophyll b content was increased much more with the foliar application than with seed priming. Similar results were also found under the 50 mg/L treatment with 50 and 90 nm ZnO NPs.
The soluble sugar and protein contents increased the most with seed priming under the N30C50 treatment, but with the foliar application the most significant increase was observed under the N30C10 treatment. For the 50 and 90 nm treatments with ZnO NPs, seed priming under the 100 mg/L treatments significantly increased the soluble sugar and protein contents, but with the foliar application their contents were significantly decreased under the 100 mg/L treatment. The SOD activity was significantly improved with seed priming under the N30C50 treatment, but it slightly increased with foliar application in all three periods. Under the 100 mg/L treatments with 50 and 90 nm ZnO NPs there was a significant increase in SOD activity with seed priming, but with the foliar application SOD activity only increased with the N50C100 treatment ZnO NPs on day 2, and significantly decreased on days 17 and 32.
Discussion
The effect of ZnO NPs on seed germination
When lower concentrations of ZnO NPs were used for seed priming in H. syriacus, they had positive effects on seed germination, germination speed, and germination index, with N30C50 treatment having the highest germination rate, and the shortest MGT. While higher concentrations acted as an inhibitor, which is similar to the results of the studies on Capsicum annuum L39. Pisum sativum L40. and Abelmoschus esculentus41. The increased germination rate due to NP treatments is related to the particle penetration into seeds42. The frequent bombardment of NPs enables them to enter the seed coat, shorten seed germination time and promote seed germination through enzyme stimulation, osmoregulation and breaking dormancy43,44. As the particle size increases fewer particles can enter the cells45. This explains why the smaller ZnO NPs promoted seed germination and seedling growth more than larger NPs under the low concentration treatments. In contrast, smaller-sized nanoparticles are more likely to enter into the cell, excessive NPs may also negatively influence seed germination by promoting lipid peroxidation, promoting hormone imbalance, and inducing seed dormancy and stress events46. Thus, at high concentrations, the smaller the size of nanoparticles, the stronger the inhibitory effect on seed germination.
The effect of seed priming and foliar application of ZnO NPs on seedling growth
In this study, appropriate ZnO NP concentrations in the seed priming and foliar application treatments significantly increased the seedling biomass, especially for the stem and root, provides the supply of Zn for plant growth and development, whereas Zn promotes cell elongation and division, thus increasing fresh and dry weight47. In addition, ZnO NPs up-regulate the expression of genes involved in nutrient transport and carbon/nitrogen metabolism in plants48. Youssef et al.49 showed that the effect of ZnO NPs on Vicia faba could probably be attributed to an increase in the cell division index due to the interaction between dissolved Zn ions and auxin.
The reason why the phytotoxic effect occurred at lower concentrations for foliar sprayed ZnO NPs compared with seed priming may be that ZnO NPs are better able to penetrate the stomata than the seed coat, and seed priming seedlings take up less ZnO NPs than seedlings fertilized with foliar fertilizers. The inhibition of seedling growth caused by ZnO NPs may be due to their effect on the root system. Nanoparticles may adhere to epithelial root cells causing mechanical damage to cells and blocking pores, and large NPs may induce the formation of larger pores on the cell surface, thus reducing root hydraulic conductivity and water availability50,51. Also, NPs may damage the root membrane and impact on physical adsorption at the root surface, thus blocking nutrient uptake by plant roots52.
The effect of seed priming and the foliar application of ZnO NPs on seedling physiology
In this study, seed priming and foliar application of of lower ( 10 and 50 mg/L) concentrations of ZnO NPs showed improvement in photosynthetic performance, osmoregulation and antioxidant capacity of H. syriacus seedlings. This promotion of chlorophyll by ZnO NPs was obtained by Trujillo-Reyes et al.53 in Lactuca sativa and Mukherjee et al.54 in peas. Zn in photosynthesis keeps photosystem II active, which would incidentally increase chlorophyll synthesis55. However, the foliar application of ZnO NPs increased the chlorophyll content more significantly than seed priming at a lower concentrations for the same particle size. We speculated that this was because the foliar application was applied directly to the leaves, while seed priming affected the radicle. Low concentrations of Zn have a positive effect on the metabolic processes of plants48,56 Carbon metabolism is closely related to the soluble sugar content57. Zn is involved in the synthesis of several proteins58. Which may explain the significant effect of ZnO NPs on soluble sugar and protein. The three sizes of ZnO NPs at low concentrations improved the SOD activity, but this was reversed at high concentrations. This may be attributed to the Zinc plays a critical role in stabilizing biomembranes and proteins by balancing the production of scavenging reactive oxygen species (ROS)59 but when the degree of oxidative stress was increased the cell membrane system was damaged. Wang et al.60 found that ZnO NPs enhanced the transcription of genes related to antioxidant capacity. When Zn concentration reach phytotoxic levels, plants activate defense mechanisms to reduce metal uptake. These responses include cellular regulation and root exudate secretion, which restrict heavy metal entry into cells61,62 and mitigate reactive oxygen species (ROS) imbalance63.
The effect of foliar application of ZnSO4 and ZnO NPs on seedling
Foliar application of ZnSO4 and low concentrations of ZnO NPs promotes seedling growth and physiology. ZnO NPs at 30 and 50 nm particle sizes had more significant and sustained effect on the growth and physiology of seedlings than ZnSO4. The observed phenomenon may be due to the fact that ZnO NPs provide more soluble and available Zn, which have a better slow-release effect, as well as the small size of the nanoparticles allows them to penetrate biological membranes more easily64,65,66.
Conclusion
The application of ZnO NPs to seedlings caused a range of phenotypic and physiological changes with both positive and negative effects depending on their size and concentration, and also on the way that they were applied. ZnO NPs at low concentrations (10 and 50 mg/L), 30 and 50 nm nano-sizes performed better than 90 nm. Seed priming under the N30C50 treatment best improved seed germination (51.22% increase), seedling growth (64.27% increase in seedling height). Furthermore, the N50C50 treatment was the most effective in foliar application of ZnO NPs to promote the growth of H. syriacus seedlings (248.83%, 281.92%, and 97.90% increase in root, stem, and leaf biomass, respectively). These results provide a scientific basis for the use of ZnO NPs as a nanofertilizer for ornamental plants, providing theoretical foundations for the nano-enabled horticultural management of woody ornamentals.
In this study, although the pot test conditions could precisely control the variables, it was not possible to fully simulate the effects of field environment (e.g., soil microbial community, climate change, etc.) on the effects of ZnO NPs. On the other hand, the perennial nature of H. syriacus, as both a woody ornamental and edible plant, makes it difficult to assess the long-term effects of ZnO NPs in short-term experiments, especially on ornamental traits (bloom period, flower volume) and the nutritive value of edible part of the plant. In future studies, it is essential to systematically investigate the effects of ZnO NPs on ornamental traits (e.g., flower size, flowering duration) and nutritional composition in H. syriacus. Further analysis of Zn transport and distribution patterns among organs, as well as key regulatory genes, will provide theoretical support for the application of nano-fertilizers in woody ornamentals.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Verma, S. K. et al. Engineered nanomaterials for plant growth and development: a perspective analysis. Sci. Total Environ. 630, 1413–1435 (2018).
Kumari, A., Rana, V., Yadav, S. K. & Kumar V. Nanotechnology as a powerful tool in plant sciences: recent developments, challenges and perspectives. Plant. Nano Biology. 5, 100046 (2023).
Shelar, A. et al. Nanoprimers in sustainable seed treatment: molecular insights into abiotic-biotic stress tolerance mechanisms for enhancing germination and improved crop productivity. Sci. Total Environ. 951, 175118 (2024).
Pereira, A. D. E. S., Oliveira, H. C., Fraceto, L. F. & Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials (basel Switzerland). 11, 267–295 (2021).
Yeasmin, S. et al. Nanopriming and AI for sustainable agriculture: boosting seed germination and seedling growth with engineered nanomaterials, and smart monitoring through deep learning. Nano Mater. 7, 8703–8715 (2024).
Lv, Z. Y. et al. Interaction of different-sized ZnO nanoparticles with maize (Zea mays): accumulation, biotransformation and phytotoxicity. Sci. Total Environ. 796, 148927 (2021).
Lizzi, D. et al. Germination and early development of three spontaneous plant species exposed to nanoceria (nCeO2) with different concentrations and particle sizes. Nanomaterials 10, 2534 (2020).
Prasad, T. N. V. K. V. et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant. Nutr. 35, 905–927 (2012).
Kim, S., Lee, S. & Lee, I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut. 223, 2799–2806 (2012).
Kopittke, P. M. et al. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. Nano. 6, 3513–3524 (2019).
Wang, X. X., Sun, W. J., Zhang, S., Sharifan, H. & Ma, X. M. Elucidating the effects of cerium oxide nanoparticles and zinc oxide nanoparticles on arsenic uptake and speciation in rice (Oryza sativa) in a hydroponic system. Environ. Sci. Technol. 52, 10040–10047 (2018).
Amling, L., Rink, L. & Bennstein, S. B. Short-term oral zinc supplementation enhances natural killer cell functionality and decreases circulating innate lymphoid cell counts and frequencies in healthy young adults. J. Translational Med. 23, 333 (2025).
Kah, M., Kookana, R. S., Gogos, A. & Bucheli, T. D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 13, 677–684 (2018).
Ahmed, R., Samad, M. Y. A., Uddin, M. K., Md. Quddus, A. & Hossain, M. A. M. Recent trends in the foliar spraying of zinc nutrient and zinc oxide nanoparticles in tomato production. Agronomy 11, 2074 (2021).
Rastogi, A. et al. Impact of metal and metal oxide nanoparticles on plant: a critical review. Front. Chem. 5, 1–16 (2017).
Rossi, L., Fedenia, L. N., Sharifan, H., Ma, X. M. & Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant. Physiol. Biochem. 135, 160–166 (2019).
Kolenčík, M. et al. Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nanomaterials 10, 1619 (2020).
Davarpanah, S., Tehranifar, A., Davarynejad, G., Abadía, J. & Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. ardestani) fruit yield and quality. Sci. Hort. 210, 57–64 (2016).
Zhao, L. J. et al. Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and zn: a life cycle study. J. Agric. Food Chem. 61, 11945–11951 (2013).
Moghaddasi, S. et al. Effects of coated and non-coated ZnO nanoparticles on cucumber seedlings grown in gel chamber. Archives Agron. Soil. Sci. 63, 1108–1120 (2017).
Yanık, F. & Vardar, F. Toxic effects of aluminum oxide (Al2O3) nanoparticles on root growth and development in Triticum aestivum. Water Air Soil. Pollut. 226, 296 (2015).
Rizwan, M. et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214, 269–277 (2019).
Maswada, H. F., Djanaguiraman, M. & Prasad, P. V. V. Seed treatment with nano-iron (iii) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J Agron. Crop Sci. 204, 577–587 (2018).
Song, U. et al. Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2 and ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 93, 60–67 (2013).
Wang, Q., Ma, X. M., Zhang, W., Pei, H. C. & Chen, Y. S. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 4, 1105–1112 (2012).
Ramesh, R., Nair, R., Chavalmane, S., Wang, W. N. & Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics: Integr. Biometal Sci. 7, 1584–1594 (2015).
Park, Y. et al. Nutritional composition and phytochemical screening in different parts of Hibiscus syriacus L. Food Sci. Nutr. 10, 3034–3042 (2022).
Lu, W. J. et al. A comparative analysis of photosynthetic function and reactive oxygen species metabolism responses in two hibiscus cultivars under saline conditions. Plant. Physiol. Biochem. 184, 87–97 (2022).
Wei, J. et al. Identification and characterization of Hibiscus mutabilis varieties resistant to Bemisia tabaci and their resistance mechanisms. Insects 15, 545 (2024).
Jeffery, T. D. & Richardson, M. L. A review of the effectiveness of hibiscus for treatment of metabolic syndrome. J. Ethnopharmacol. 270, 113762 (2021).
Eo, H. J. et al. GC/MS analysis and anti-inflammatory effect of leaf extracts from Hibiscus syriacus through Inhibition of Nf-κb and Mapks signaling in Lps-stimulated Raw264.7 macrophages. Plant. Biotech. Rep. 14, 547–547 (2020).
Yeon, S. W. et al. Three new naphthalenes from the roots of Hibiscus syriacus. Phytochem. Lett. 33, 110–113 (2019).
Zhang, P. et al. Identification and quantitative analysis of anthocyanins composition and their stability from different strains of Hibiscus syriacus L. flowers. Ind. Crops Prod. 177, 114457 (2022).
Lichtenthaler, H. K. & Wellburn, A. R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11, 591–592 (1983).
Irigoyen, J. J., Einerich, D. W. & Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 84, 55–60 (1992).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: I. occurrence in higher plants. Plant. Physiol. 59, 309–314 (1977).
Zhang, J. et al. Determination of trace elements in hibiscus by flame atomic absorption spectrometry. J. Guangdong Trace Elem. Sci. 20, 23–26 (2013).
García-López, J. et al. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 8, 215 (2018).
Nagdalian, A. et al. Nano-priming of pea (Pisum sativum L.) seeds with CuO nanoparticles: synthesis, stabilization, modeling, characterization, and comprehensive effect on germination and seedling parameters. Food Chem. 478, 143569 (2025).
Ramzan, M. et al. Enhancing physio-biochemical characteristics in okra genotypes through seed priming with biogenic zinc oxide nanoparticles synthesized from halophytic plant extracts. Sci. Rep. 14, 23753 (2024).
Feizi, H., Kamali, M., Jafari, L. & Moghaddam, P. R. Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 91, 506–511 (2013).
Samad, A., Khan, M. J., Shah, Z. & Tariq Jan, M. Determination of optimal duration and concentration of zinc and phosphorus for priming wheat seed. Sarhad J. Agric. 30, 89 (2014).
Wu, S. G. et al. Electrospray facilitates the germination of plant seeds. Aerosol Air Qual. Res. 14, 632–641 (2014).
Slomberg, D. L. & Schoenfisch, M. H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Techno. 46, 10247–10254 (2012).
Ahmad, A., Hashmi, S. S., Palma, J. M. & Corpas, F. J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 290, 133329 (2022).
Elsilk, S. E., El-Shenody, R. A., Afifi, S. S. & Abo-Shanab, W. A. Green-synthesized zinc oxide nanoparticles by Enterobacter sp.: unveiling characterization, antimicrobial potency, and alleviation of copper stress in Vicia faba (L.) plants. BMC Plant. Biol. 24, 474 (2024).
Sun, L. et al. Physiological, transcriptomic, and metabolomic analyses reveal zinc oxide nanoparticles modulate plant growth in tomato. Environ. Sci. :Nano. 7, 3587–3604 (2020).
Youssef, M. S. & Elamawi, R. M. Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in Vicia faba. Environ. Sci. Pollut Res. 27, 18972–18984 (2018).
Miralles, P., Church, T. L. & Harris, A. T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 46, 9224–9239 (2012).
Martínez-Fernández, D., Barroso, D. & Komárek, M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut Res. 23, 1732–1741 (2015).
Zhang, W. L. et al. Uptake and accumulation of bulk and nanosized cerium oxide particles and ionic cerium by radish (Raphanus sativus L). J. Hazard. Mater. 63, 382–390 (2015).
Trujillo-Reyes, J., Majumdar, S., Botez, C. E., Peralta-Videa, J. R. & Gardea-Torresdey, J. L. Exposure studies of core-shell Fe/Fe3O4 and cu/cuo Nps to lettuce (Lactuca sativa) plants: are they a potential physiological and nutritional hazard. J. Hazard. Mater. 267, 255–263 (2014).
Mukherjee, A. et al. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6, 132–138 (2014).
Simonin, M. et al. Negative effects of copper oxide nanoparticles on carbon and nitrogen cycle microbial activities in contrasting agricultural soils and in presence of plants. Front. Microbiol. 9, 03102 (2018).
Akhtar, S., Adnan, M., Sharif, S. & Khan, S. A. Modulation response of biologically synthesized ZnO nanoparticles using Mentha Piperita L. on the physio-chemical parameters of Pisum sativum L. J. Plant Nutr. Soil Sci. 188, 151–164 (2025).
Zhang, P. et al. Growing rice (Oryza sativa) aerobically reduces phytotoxicity, uptake, and transformation of CeO2 nanoparticles. Environ. Sci. Technol. 55, 8654–8664 (2021).
Stanton, C. et al. Zinc in plants: integrating homeostasis and biofortification. Mol. Plant. Elsevier. 15, 65–85 (2022).
Faizan, M., Faraz, A., Yusuf, M., Khan, S. T. & Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56, 678–686 (2018).
Wang, X. P., Li, Q. Q., Pei, Z. M. & Wang, S. C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 62, 801–808 (2018).
Lin, Y. F. & Aarts, M. G. M. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 69, 3187–3206 (2012).
Ghori, N. H. et al. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 16, 1807–1828 (2019).
Wang, Y. et al. Physiological and metabolomic insights into molecular mechanisms of root sensitivity to zinc toxicity in rice (Oryza sativa L). J. Hazard. Mater. 492, 138204 (2025).
Zhang, H. et al. Optimizing the basal application dosage of ZnO nanoparticles for enhancing rice yield, rice quality, and zinc content. Environ. Sci. :Nano. https://doi.org/10.1039/D5EN00229J (2025).
Mahmood, F. et al. Bioinspired cobalt oxide nanoball synthesis, characterization, and their potential as metal stress absorbants. ACS Omega. 8, 5836 (2023).
Duhan, J. S. et al. Nanotechnology: the new perspective in precision agriculture. Biotech. Rep. 15, 11–23 (2017).
Acknowledgements
The authors wish to acknowledge the research facilities provided by the College of Landscape Architecture, Central South University of Forestry and Technology. At the same time, the authors wish to acknowledge the financial support provided by the “Double First-Class” Cultivation Discipline of Hunan Province (XiangJiaoTong (2018) No. 469) and Hunan Provincial Department of Education Support Project(No. 24C0113). Appreciation to the editors and reviewers of Scientific Reports.
Funding
This research was funded by the “Double First-Class” Cultivation Discipline of Hunan Province (XiangJiaoTong (2018) No. 469), Hunan Provincial Department of Education Support Project( No. 24C0113).
Author information
Authors and Affiliations
Contributions
XW: Conceptualization; Funding acquisition; Writing review and editing; YZ: Methodology; Investigation; Resources; Writing original draft preparation; Software; Formal analysis; YT : Investigation; Writing review and editing; Software; Formal analysis; LH: Methodology; Investigation; Resources; Writing original draft preparation; JX&XZ: Software; Formal analysis; Investigation; XD: Software; Formal analysis; SZ: Provision of research materials; LH: Funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Y., Tang, Y., Hu, L. et al. Growth and physiology effects of seed priming and foliar application of ZnO nanoparticles on Hibiscus syriacus L.. Sci Rep 15, 22606 (2025). https://doi.org/10.1038/s41598-025-05059-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05059-0