Abstract
The oral administration of insulin offers significant clinical benefits due to its simplicity. This study evaluated piperine’s efficacy in improving insulin’s intestinal absorption in Wistar rats. Confocal microscopy demonstrated that prolonged retention of insulin-loaded chitosan-coated solid lipid nanoparticles (Ch-In-SLNs) on intestinal mucosa was due to chitosan’s mucoadhesive properties. These studies also revealed Ch-In-SLNs permeation in rat intestinal segments. Piperine enhanced the permeation of insulin-loaded nanoparticles, with more particles transported into deeper intestinal layers. The study assessed Ch-In-SLNs with piperine administered orally to diabetic rats. The formulated SLN’s effectiveness was evaluated in vivo in overnight-fasted Wistar rats. Male Wistar rats (n = 5) were divided into 8 groups, with the first as diabetic control, and others receiving various treatments, including blank SLN, oral insulin solution (25 IU/kg), subcutaneous (SC) insulin (5 IU/kg), and insulin nanoformulations (25 IU/kg and 50 IU/kg with and without piperine, respectively). Ch-In-SLN with piperine showed a statistically significant reduction (p < 0.05) in blood glucose levels in streptozotocin-induced (STZ) diabetic rats compared to SLNs without piperine and SC insulin. The Ch-In-SLN with piperine formulation demonstrated a noteworthy correlation between pharmacokinetics (PK) and pharmacodynamics (PD). This approach could replace traditional insulin injections and mitigate associated side effects, simplifying insulin therapy.
Similar content being viewed by others
Introduction
Diabetes Mellitus (DM) is a chronic illness that affects an estimated 380 million individuals by 2025. This metabolic disorder results from the ineffective functioning of the pancreas, insulin resistance, and insufficient insulin secretion. DM can generally be categorized into two primary types: type 1 diabetes mellitus (T1DM), commonly known as insulin-dependent diabetes, and type 2 diabetes mellitus (T2DM), commonly referred to as non-insulin-dependent diabetes. In T1DM, the immune system specifically targets and destroys the insulin-producing beta cells in the pancreas, necessitating lifelong insulin therapy. In contrast, T2DM is characterized by insulin resistance and impaired insulin secretion, commonly associated with lifestyle factors, such as obesity, and genetic predisposition. The classification of diabetes into these two types facilitates the application of appropriate treatment approaches for managing the disease and mitigating its impact on individuals’ health1,2,3,4,5,6. Diabetes management typically entails the use of antidiabetic medications, either individually or in conjunction with one another. In cases where these treatments are unsuccessful, insulin administration is the only option for lower ring elevated blood glucose levels. Although SC injection is the most commonly used method for insulin administration, it has several drawbacks, including hyperinsulinemia, physiological stress, pain from daily injections, infections, and localized insulin deposition. Since the discovery of insulin nearly a century ago, there has been continuous and significant effort to develop strategies for oral peptide administration. Although orally administered insulin is considered the most preferable route, gastrointestinal enzymes rapidly degrade insulin, resulting in poor oral bioavailability. The primary objective of oral peptide administration is to enhance patient convenience and improve the overall management of conditions such as diabetes that require peptide-based therapies4,7,8,9,10,11. The oral administration of insulin is limited by its instability in the GIT and low permeability across intestinal membranes, resulting in poor oral bioavailability12,13. Oral administration of insulin may replicate the effects of endogenously secreted insulin in the portal circulation14.
An effective oral insulin delivery system must possess biocompatibility, safeguard insulin from the adverse conditions of the GI tract while maintaining high drug loading efficiency, and be capable of releasing insulin in a pH-dependent manner15. Despite numerous approaches that have been attempted globally, none have been able to achieve satisfactory results in addressing these concerns. The significant challenge posed by the limited permeability of peptide and protein drugs across the intestinal mucosal membranes remains a major obstacle to the development of oral formulations16. Researchers have long addressed the challenges of delivering insulin orally, and in recent years, various strategies have been explored to address these issues. Among these, the development of innovative drug delivery systems has garnered significant attention. Numerous drug delivery systems have been investigated to overcome the hurdles of oral insulin delivery and safeguard the protein from the harsh conditions of the GIT17,18. Nanoparticles are commonly used because of their ability to improve the permeation and oral bioavailability of hydrophilic macromolecular peptides. The primary advantage of nanoparticles is their ability to traverse the GI tract and be absorbed by M cells of Peyer’s patches, a type of lymphatic tissue in the intestine that serves as the principal site of nanoparticle absorption in comparison to microparticles19,20,21.
The capacity of SLN to traverse the intestinal epithelial barrier is unique, allowing for the efficient release of entrapped drugs upon physiological degradation. The hydrophobic nature of SLNs facilitates favorable interaction with the hydrophilic surfaces of the intestinal epithelium, promoting particle uptake. In the case of insulin-loaded SLNs, it is expected that the release of insulin within the intestinal lumen will lead to direct internalization, potentially causing hypoglycemia22,23,24.
The bioadhesive characteristics inherent in lipid-based materials contribute to a gradient of insulin diffusion, with insulin moving from a higher concentration SLN matrix to a lower concentration within intestinal cells. This dual mechanism, which combines the hydrophobicity and bioadhesive properties of lipids, is expected to result in a prolonged physiological effect of insulin following oral administration. These characteristics enhance the potential of SLNs as effective carriers for oral delivery of insulin, with promising sustained and controlled release to manage blood glucose levels over an extended period25,26,27,28.
The pursuit of an effective and convenient oral peptide delivery system, particularly for insulin, is driven by the potential benefits for patient compliance and quality of life. Additionally, the incorporation of permeation enhancers and mucoadhesive agents, like chitosan has been investigated to improve the transport of peptides across the intestinal epithelium. It is widely recognized that chitosan, a natural cationic polysaccharide, can interact with anionic glycoproteins present on the epithelial cell surface. This interaction leads to the opening of tight junctions, thereby enhancing the permeation of administered proteins29,30,31. Piperine, a primary constituent in Piper longum, is a widely recognized bioenhancer, known for its ability to significantly increase the oral bioavailability of various drugs, ranging from 30 to 200%. It accomplishes this by inhibiting drug-metabolizing enzymes, such as P-glycoprotein (Pgp) and cytochrome P450 (CYP 450), altering membrane dynamics, and promoting vasodilation32,33,34,35,36.
Our earlier research has shown that Ch-In-SLNs, formulated with glyceryl behenate, soya lecithin, and poloxamer 407, coated with chitosan and piperine, exhibited sustained release and significant protection in simulated gastric and intestinal fluids for 12 h. The formulation was monodisperse, with a positive zeta potential and an optimal particle size for oral delivery. In vitro studies conducted on excised goat intestinal mucosa and Caco-2 cell lines showed favorable results, indicating enhanced permeation in the presence of piperine. These findings suggest that the combination of insulin-loaded nanoparticles with natural permeation enhancers such as chitosan and piperine has the potential to improve insulin absorption by opening tight junctions and modulating membrane dynamics. Ch-In-SLNs were observed to maintain stability at 4 °C, wherein the composition preserved its particle size, PDI, and association efficiency within acceptable parameters. This study aimed to investigate the effect of piperine on enhancing the permeation and absorption of Ch-In-SLNs in diabetic rats and to assess its potential in lowering blood glucose levels37.
Materials and methods
Materials used. Male Wistar rats, Streptozotocin, Glucometer (Accu-Check) and strips, Insulin Elisa kit (Cloud Clone, Juniper Life Sciences Pvt Ltd), Isoflurane. Piperine (97%) was purchased from Sigma-Aldrich (Merck, Germany).
Methodology. All animal experiments were performed in accordance with the Institutional Animal Ethics Committee of the NGSM Institute of Pharmaceutical Sciences, Mangalore (protocol approval number: NGSMIPS/IAEC/JAN-2023/333) following Committee for Control and Supervision of Experiments on Animals (CCSEA) guidelines. The research conducted and reported accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Male Wistar rats with average weights ranging from 250 g to 300 g were housed in standard collective cages at a room temperature of 30 °C. The rats were subjected to a 12-h light and 12-h dark cycle, and were provided with a standard laboratory diet in pellet form along with access to fresh water38. The wistar rats used in this study were obtained from Animal house Facility of the NUCARE (NU Centre for Animal Research and Experimentation), NGSM Institute of Pharmaceutical Sciences, Paneer, Deralakatte, Mangalore, Karnataka, India which is licensed to breed Wistar rats (Licence number: 1781/PO/ReBi/S/14/CCSEA).
Mucoadhesion studies using rat intestine
Male Wistar rats with an initial weight of 280 g were used to investigate the mucoadhesive behavior of chitosan. Four animals were selected for this study and were subjected to a 12-h fasting period. The rats were administered oral gavage with chitosan coated SLNs (Ch-SLNs) and uncoated SLNs both labeled with rhodamine (0.2 mg/ml). Rats were sacrificed at the end of 3rd hour by isoflurane overdosage. The study was conducted at the third hour post-administration, and intestinal segments from the duodenum, jejunum, and ileum were isolated and dissected. The segments were thoroughly washed with saline, and their surfaces were examined under a confocal microscope to assess the mucoadhesive properties of SLN resulting from the presence of chitosan39,40.
In vivo permeation studies in rat intestine
Four male wistar rats with initial weight 280 g were selected and subjected to a 12-h fasting period. To evaluate the effects of piperine as a permeation enhancer in vivo, rats were administered oral doses of Ch-SLNs, both with and without piperine (administered uncoated SLNs without piperine, chitosan coated SLNs without piperine and chitosan coated SLNs with 2, 4 & 6 mg piperine in Wistar rats without insulin. Piperine was incorporated in the chitosan coating and SLN was coated with the same and was administered orally in Wistar rats). The administered nanoparticles were labeled with rhodamine for tracking. Three hours following administration, the rats were sacrificed by isoflurane overdosage, and the duodenum, jejunum, and ileum were isolated and thoroughly rinsed with saline. The isolated intestinal segments were then horizontally sectioned to a thickness of 5 μm using a microtome, processed in wax and stained with eosin-hematoxylin. The tissue sections were examined under a confocal microscope in order to compare the permeation of SLNs with and without piperine39,41.
Induction of diabetes
Animals were categorized into eight groups, with each group consisting of five animals (n = 5). Each rat received a single intraperitoneal (IP) injection of STZ at a body weight of 40 mg/kg42. STZ was freshly prepared by dissolving the STZ powder in citrate buffer at pH 4.5. Before STZ administration, the blood glucose levels (BGL) of all rats were assessed using glucometer strips, and readings were observed within the range of 75–105 mg/dL. Following the IP injection of STZ, BGL was measured again43,44. Animals with blood glucose levels exceeding 250 mg/dL were identified as diabetic and were subsequently included in the study45,46,47. The animals were weighed both before and after STZ injection.
Group 1: Diabetic control without any treatment.
Group 2: Empty SLN solution (Blank).
Group 3: Oral insulin solution (25 IU/kg, in Phosphate Buffered Saline).
Group 4: Subcutaneous insulin (5 IU/kg).
Group 5: Insulin nanoformulation 25 IU/kg with bioenhancer (F1).
Group 6: Insulin nanoformulation 25 IU/kg without bioenhancer (F2).
Group 7: Insulin nanoformulation 50 IU/kg with bioenhancer (F3).
Group 8: Insulin nanoformulation 50 IU/kg without bioenhancer (F4).
Pharmacodynamic studies (blood glucose estimation)
Diabetes was successfully induced in all study groups using the established method. Prior to and during the experiment, the animals underwent a 12-h fast, with unlimited access to water. Various treatments were administered to groups 2 through 8, including no treatment for the first group serving as the diabetic control. The second group received oral administration of blank SLN, while the third group received 25 IU/kg of insulin solution in phosphate buffer (PBS) via oral administration. The fourth group received SC insulin 5 IU/kg. The fifth and sixth groups were administered Ch-In-SLNs at a dose of 25 IU/kg with and without piperine, respectively (F1 and F2). The seventh and eighth groups received Ch-In-SLNs at a dose of 50 IU/kg, with and without piperine, respectively (F3 and F4)48,49.
Blood samples were drawn from the tail vein via needle puncture. To assess blood glucose levels, glucometer measurements were taken at various intervals (0, 1, 2, 4, 6, and 12 h) over a period of 12 h. One-way ANOVA was used to statistically evaluate the results, and significant differences (p < 0.05) between the groups were identified using a post-hoc test (Scheffe). Graph Pad was utilized for all statistical analyses. Plasma glucose levels were graphically represented as a percentage of baseline glucose levels over time to observe trends. Additionally, the cumulative hypoglycemic effect of treatment was calculated by comparing the area under the curve (AUC) of oral insulin with that of the treatment group47,48.
The pharmacological availability (PA) of the administered formulations was examined to evaluate the effectiveness of the nanoformulations50,51.
Pharmacokinetic studies (plasma insulin level estimation)
Insulin plasma levels following oral administration of insulin-loaded SLNs at doses of 25 and 50 IU/kg, either with or without piperine (F1, F2, F3, F4), as well as SC insulin, blank SLN, and oral insulin solution in diabetic Wistar rats, were determined using an ELISA kit (Juniper Life Sciences Pvt Ltd, Bangalore) according to the manufacturer’s instructions. Blood samples were obtained from animals in groups 2–8 via the tail vein. The relative bioavailability percentage was calculated for the aforementioned groups47.
To evaluate the bioavailability of insulin nanoformulations with and without piperine in comparison to SC insulin, the plasma insulin concentrations over time were plotted, and the relative bioavailability was calculated using the following formula:
Results
Mucoadhesion studies using rat intestine
The results of the confocal microscopy analysis demonstrated that rhodamine-labeled Ch- SLNs adhered to the surface of the intestinal epithelium, as illustrated in Fig. 1. In contrast, minimal particle adherence was observed for the uncoated SLNs (Fig. 2).
In vivo permeation studies in rat intestine
The results of in vivo permeation studies involving Ch-SLNs with and without the incorporation of piperine and uncoated SLNs are illustrated in Fig. 3. The addition of piperine significantly enhanced SLN permeation into the deeper layers of the small intestine. Furthermore, comparison of uncoated SLNs and Ch-SLNs indicated that Ch-SLNs exhibited greater permeation than their uncoated counterparts (Fig. 3).
Confocal microscopic images of (A) rhodamine-labeled Ch-SLNs with piperine, (B) rhodamine-labelled Ch-SLNs without piperine and (C) rhodamine-labelled uncoated SLNs (red spots in the figures represent rhodamine labelled SLNs) permeated into different layers of rat duodenum, jejunum, and ileum of 5 μm thickness after 3 h of oral administration. Scale bar represents 500 μm.
Pharmacodynamic studies (blood glucose estimation)
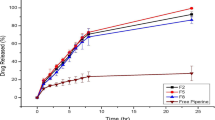
The effect of various SLN formulations on post-administration blood glucose levels is illustrated in Fig. 4. The plasma glucose level over time profiles demonstrated that SC injection of insulin resulted in a decrease in blood glucose levels that persisted for up to four h, followed by a gradual increase. In contrast, oral insulin and blank SLN formulations did not exhibit a substantial reduction in blood glucose levels compared to the control group (Supplementary file: Blood glucose level estimation).
The values reported are mean ± SEM (n = 5 per group). When compared with oral insulin solution all Ch-In-SLNs were found to be significant (P < 0.05). * Indicates statistical significance (p < 0.05) with oral insulin solution. Mean values were significant (P < 0.05) for disease control, blank SLN, oral insulin and F2 when compared with SC insulin. P > 0.05 for F1, F3 & F4 and was comparable to that of SC insulin at the end of 24 h.
Pharmacokinetic studies (plasma insulin level estimation)
The pharmacokinetic profile of the formulated SLN treatments in animal subjects is depicted in Fig. 5. The results indicate that SC insulin administration resulted in a rapid decrease in blood glucose levels within the first hour, followed by a continued decrease until the fourth hour (p > 0.05), and a subsequent gradual increase at the sixth hour. Conversely, all insulin-loaded SLN formulations demonstrated an elevation in insulin levels for up to six h (p < 0.05).
The values reported are mean ± SEM (n = 5). When compared with disease control, SC insulin and insulin nanoformulations (F1, F2, F3 & F4) were significant (P < 0.05). * Indicates statistical significance (p < 0.05) with disease control, Blank SLN & oral insulin was not significant (P > 0.05).
Discussion
The present investigation aimed to evaluate the effect of piperine incorporated in Ch-In-SLNs on enhancing the intestinal absorption of insulin and it’s in vivo efficacy in diabetic rats. The study also demonstrated the mucoadhesive property of chitosan with the sustained retention of Ch-In-SLNs on the rat intestinal surface.
The sustained retention of Ch-SLNs on the intestinal mucosa was attributed to the mucoadhesive properties of chitosan. The positive charge of chitosan allows for effective interaction with the negatively charged intestinal membranes, facilitating retention in the GI mucosa52. Uncoated SLNs exhibited rapid diffusion through the intestinal membrane, while Ch-SLNs accumulated in the upper layers of the mucous membrane with controlled deeper permeation. The strong ionic interaction between chitosan and mucin increases the residence time and delays clearance from the mucosal surface. The mucoadhesive and absorption-enhancing properties of chitosan are crucial in enhancing insulin concentration on the intestinal surface. Chitosan also disrupts tight cellular junctions, and facilitates absorption26,53,54,55,56.
In accordance with in vivo permeation studies, uncoated SLNs exhibited limited penetration into the mucosal layers, whereas Ch-SLNs demonstrated significant permeation extending through the submucosal to muscularis layers. The exceptional properties of chitosan as a permeation enhancer, capable of opening tight cellular junctions, contribute to this heightened permeation. Additionally, the effectiveness of chitosan as a mucoadhesive was evident, prolonging the residence time on mucous membranes and facilitating sustained drug release57. Figure 3 A further show that Ch-SLNs, when combined with piperine, demonstrated augmented permeation compared with those without piperine (Fig. 3B and C). Notably, a greater number of particles permeated the duodenum, jejunum, and ileal regions of the rat intestine in the presence of piperine. It is noteworthy that the incorporation of piperine facilitated a higher concentration of nanoparticles within the muscularis layer in Ch-SLNs, highlighting a significant difference when compared to Ch-SLNs without piperine. Piperine, a well-established bioenhancer, exerts its influence by inhibiting drug efflux pumps and metabolizing enzymes (such as P-gp and CYP3A4), enhancing drug absorption, promoting increased blood circulation, and modulating membrane dynamics to facilitate drug transport across biological barriers58,59,60,61,62,63,64,65,66.
The uniform distribution of particles across the three regions of the small intestine (duodenum, jejunum, and ileal regions) in rats challenges the precise prediction of nanoparticle permeation through a specific segment of the small intestine. This uniformity in distribution suggests that the nanoparticles have permeated effectively throughout the entire small intestine rather than being concentrated in a particular section. This even distribution may be indicative of a comprehensive and widespread permeation profile, implying that the nanoparticles are capable of traversing the diverse physiological environments present in each of the aforementioned regions. Consequently, these findings underscore the potential of the studied nanoparticles to exhibit a broad and consistent permeation pattern across the entire small intestine.
Pharmacodynamic data confirmed that oral administration of insulin has been found to be less effective, with enzymatic degradation and poor permeability leading to low bioavailability67,68. In contrast, Ch-In-SLN formulations showed sustained reductions in blood glucose levels for up to 6 h compared with SC insulin injections (p < 0.05). It is noteworthy that groups 5 and 7, which received F1 and F3, respectively, demonstrated a significant decrease in blood glucose levels when compared to groups 6 and 8, which received the same doses of SLNs without piperine (F2 and F4) (Fig. 4). Furthermore, the results indicated that groups 5 and 7, which were treated with F1 and F3, demonstrated cumulative hypoglycemia of 51.22 ± 0.1% and 65.45 ± 0.3%, respectively (Table 1). When compared to SC insulin, group 7 displayed a 1.1-fold higher cumulative hypoglycemia, providing convincing evidence of piperine’s role as a permeation enhancer in facilitating insulin absorption.
These findings corroborated the efficacy of the formulated SLN in improving the stability of insulin in both gastric and intestinal environments. Furthermore, the incorporation of piperine in the SLN formulation was found to significantly enhance insulin permeation, leading to a substantial decline in blood glucose levels in diabetic rats. The % cumulative hypoglycemia in rats treated with SLN without piperine was 34.57 ± 0.2% and 58.23 ± 0.1% (groups 6 & 8), which is significantly lower compared to groups 5 & 7 (51.22 ± 0.1 & 65.45 ± 0.3 respectively) that received SLNs with piperine. In contrast, SC-administered insulin resulted in sudden and severe hypoglycemia, leading to hyperinsulinemia within the first few h of injection without achieving further glycemic control. This may result in patient non-compliance due to the need for a multiple-dose regimen.
From the pharmacokinetic studies, F1 exhibited an AUC value of 242.53 ± 12.87, resulting in a relative bioavailability (%BAR) of 17.62 ± 0.94%. Similarly, F3 attained a higher AUC value of 355.21 ± 7.15 compared to SC insulin, accompanied by a %BAR of 12.9 ± 0.25% (Table 2). Conversely, Ch-In-SLNs without piperine, F2 & F4, exhibited lower AUC values (191.09 ± 12.54 & 306.86 ± 5.60 respectively) and diminished bioavailability (13.88 ± 0.91 & 11.14 ± 0.203 respetively) when compared to their piperine-enhanced counterparts. The utilization of piperine in the delivery of insulin through SLNs resulted in increased permeation and absorption, as indicated by the higher AUC and %BAR values compared to the absence of piperine. Even with the administration of a more significant insulin dose (50 IU), the absence of piperine resulted in lower AUC and %BAR values. These findings strongly validate the role of piperine in enhancing the permeation of insulin across biological barriers and its subsequent absorption.
Ideally, there should be a predictable relationship between the amount of drug the body is exposed to (PK AUC) and the magnitude of the drug’s effect (PD). Both AUC & Cmax give a reasonable summary of the bioavailability of the drug in the body. In pharmacokinetic studies AUC & Cmax values of F3 > F1, so an increased cumulative hypoglycemia can be expected for F3 than F1. While F1 has a higher overall exposure (AUC, reflected in % BAR), F3 might have a faster rate of absorption, leading to a higher peak concentration (Cmax). For drugs like insulin or hypoglycemic agents, a rapid rise in concentration can be more effective in lowering blood glucose, even if the total exposure is lower. Thus, F3 might induce a more rapid and intense hypoglycemic effect, leading to a greater cumulative effect over time. Even with a lower systemic exposure, F3 might have a better distribution to the target tissues (e.g., liver, muscle) responsible for glucose regulation. This could result in higher concentrations at the site of action, leading to a greater pharmacodynamic effect69,70.
The role of bioenhancer is to improve the drug permeation and hence the absorption. F1(25 IU insulin with piperine) with 51% cumulative hypoglycemia was achieved in the presence of piperine. Because F2 with the same dose of insulin but without piperine showed just 34.5% cumulative hypoglycemia. Similarly, F3 (50 IU insulin with piperine) & F4 (50 IU insulin) showed 65% & 58% cumulative hypoglycemia respectively. From the results it is very clear that if F4 is incorporated with piperine, % cumulative hypoglycemia should have been increased to a greater extend. A mere increase in dose of insulin will only result in undesirable side effects like severe hypoglycemia. Absorption rate is clearly increased in presence of piperine compared to the formulations without it71,72.
According to the MRT values, the residence time of SC insulin is only 6.62 h, indicating the necessity for frequent injections to maintain normal blood sugar levels. In contrast, the Ch-In-loaded SLNs demonstrate a more extended residence time of 16 h, potentially allowing for a significant reduction in the dosing schedule, except for the 6th group administered with F2 (MRT-12 h). This finding emphasizes the role of piperine as a permeation enhancer in enhancing insulin absorption.
Role of bioenhancer is to improve the absorption rate of the drugs by improving their permeation. Only a shorter MRT can result in poor absorption as the drug will get less time for getting dissolved and absorbed before being eliminated from the GI tract. MRT of F1 is 16.15% and that of F4 is 16.03% even if it’s a small difference F1 showed higher MRT. From the MRT of F1 & F2 its clear that with piperine MRT of F1 increased with a better cumulative hypoglycemia than F2. In F3 & F4 insulin doses are double when compared to F1 & F2, obviously time taken for excretion will also be higher than it will take for a smaller dose.
Also, in all the formulations (F1-F4) insulin is encapsulated in a nanocarrier (SLN) which is further coated with a well-known mucoadhesive, chitosan. Chitosan can also open the tight cellular junctions and improve the drug absorption. It is proved that SLN as carriers and chitosan coating can improve the drug permeation as well as absorption to a certain extend37,73.
The findings reveal a strong association between PK and PD for all Ch-In-SLNs. This relationship is supported by the close correspondence observed between the percentage of antidiabetic activity (% PA) and the percentage of bioavailability (% BAR) in pharmacokinetics. This association signifies a significant connection between how the body processes the formulation and the resulting therapeutic effects. These results are advantageous in the development of drug delivery systems, demonstrating the potential of this particular formulation to achieve both optimal drug absorption and the desired pharmacological responses.
The in vivo scenario suggests that the combination of lipids and chitosan coating functions as a protective mechanism for entrapped insulin, safeguarding it from the adverse gastric environment and promoting its release in the intestinal pH. Moreover, the simultaneous presence of chitosan and piperine is linked to an improved permeation of Ch-In-SLNs through biological barriers, resulting in a remarkable hypoglycemic effect.
The results from this study suggest a promising direction for the improvement of insulin delivery systems. The favorable correlations observed between PK and PD, the protective effects demonstrated in the GI environment, the enhanced permeation across biological barriers, and the significant hypoglycemic effects all contribute to this positive outlook.
Conclusion
The incorporation of piperine enhanced the permeation of Ch-SLNs across various segments of the rat intestines, potentially mitigating the occurrence of adverse effects. The presence of chitosan in the formulation confers mucoadhesive properties, contributing to prolonged residence time in the GIT and facilitating sustained insulin release. In vivo studies conducted on male Wistar rats have demonstrated that the optimized formulation of Ch-In-SLNs with piperine provides a sustained reduction in blood glucose levels compared to SC insulin administration. The significant hypoglycemic effects observed, coupled with an excellent PK/PD correlation, indicate the potential of this formulation as a promising and industrially relevant oral insulin delivery system. This system could provide a viable alternative to conventional SC insulin administration, offering sustained and effective blood glucose control. The successful development of such systems could represent a significant advancement in the field of therapeutics and have broad implications for the oral delivery of a range of peptide-based drugs.
Data availability
The datasets/information used for this study is available on reasonable request to the corresponding authors.
Abbreviations
- Ch-In-SLNs:
-
Insulin-loaded chitosan-coated solid lipid nanoparticles
- SC:
-
Subcutaneous
- STZ:
-
Streptozotocin
- PK:
-
Pharmacokinetics
- PD:
-
Pharmacodynamics
- DM:
-
Diabetes Mellitus
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- Pgp:
-
P-glycoprotein
- CYP 450:
-
Cytochrome P450
- Ch-SLNs:
-
Chitosan coated SLNs
- IP:
-
Intraperitoneal
- PBS:
-
Phosphate buffer
- AUC:
-
Area under the curve
- PA:
-
Pharmacological availability
References
Shah, R., Patel, M., Maahs, D. & Shah, V. Insulin delivery methods: past, present and future. Int. J. Pharm. Investig. 6, 1 (2016).
Rahman, S. K. & Kawatra, P. Insulin delivery: what is new in the queue? Int. J. Basic. Clin. Pharmacol. 6, 229 (2017).
Cernea, S. & Raz, I. Insulin therapy: future perspectives. Am. J. Ther. 27, E121–E132 (2020).
Marín-Peñalver, J. J., Martín-Timón, I., Sevillano-Collantes, C. & Cañizo-Gómez, F. J. del. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes. 7, 354 (2016).
Wick, J. Y. & Insulin Almost a century of lifesaving. Consult Pharm. 32, 190–200 (2017).
George, S. & Roy, A. Novel approaches in insulin drug Delivery - A review. Int. J. Drug Dev. Res. 5, 25–29 (2013).
Articles, O. Hypoglycemic effect of insulin-loaded hydrogel-nanogel composite on streptozotocin-induced diabetic rats. 76, 364–371 (2021).
Ndisang, J. F., Vannacci, A. & Rastogi, S. Insulin Resistance, Type 1 and Type 2 Diabetes, and Related Complications 2017. J. Diabetes Res. 10–13 (2017). (2017).
Meneses, M. et al. Antidiabetic drugs: mechanisms of action and potential outcomes on cellular metabolism. Curr. Pharm. Des. 21, 3606–3620 (2015).
Heidarisasan, S., Ziamajidi, N., Karimi, J. & Abbasalipourkabir, R. Effects of insulin-loaded chitosan-alginate nanoparticles on RAGE expression and oxidative stress status in the kidney tissue of rats with type 1 diabetes. (2018). https://doi.org/10.22038/IJBMS.2018.28463.6899
Kalantarian, G. et al. Effect of insulin-coated trimethyl Chitosan nanoparticles on IGF-1, IGF-2, and apoptosis in the hippocampus of diabetic male rats. Restor. Neurol. Neurosci. 36, 571–581 (2018).
Bernkop-Schnürch, A., Kast, C. E. & Guggi, D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/gsh systems. J. Control Release. 93, 95–103 (2003).
Wagner, A. M., Gran, M. P. & Peppas, N. A. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm. Sin B. 8, 147–164 (2018).
Gedawy, A., Martinez, J., Al-Salami, H. & Dass, C. R. Oral insulin delivery: existing barriers and current counter-strategies. J. Pharm. Pharmacol. 70, 197–213 (2018).
Lopes, M. A. et al. Intestinal absorption of insulin nanoparticles: contribution of M cells. Nanomed. Nanatechnol. Biol. Med. 10, 1139–1151 (2014).
Kamei, N. et al. Effects of intestinal luminal contents and the importance of microfold cells on the ability of cell-penetrating peptides to enhance epithelial permeation of insulin. Eur. J. Pharm. Biopharm. 155, 77–87 (2020).
Hirlekar, R. S., Patil, E. J. & Bhairy, S. R. Oral insulin delivery: novel strategies. Asian J. Pharm. 11, S434–S443 (2017).
Renukuntla, J., Vadlapudi, A. D., Patel, A., Boddu, S. H. S. & Mitra, A. K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 447, 75–93 (2013).
Sonia, T. A. & Sharma, C. P. Oral delivery of insulin. Oral Deliv Insul. 1–345. https://doi.org/10.1016/C2013-0-18177-9 (2015).
Yu, F. et al. Enteric-coated capsules filled with mono-disperse micro-particles containing PLGA-lipid-PEG nanoparticles for oral delivery of insulin. Int. J. Pharm. 484, 181–191 (2015).
Xu, Y., Shrestha, N., Préat, V. & Beloqui, A. Overcoming the intestinal barrier: A look into targeting approaches for improved oral drug delivery systems. J. Control Release. 322, 486–508 (2020).
He, H., Wang, P., Cai, C., Yang, R. & Tang, X. VB12-coated Gel-Core-SLN containing insulin: another way to improve oral absorption. Int. J. Pharm. 493, 451–459 (2015).
Boushra, M. et al. Methocel-Lipid hybrid nanocarrier for efficient oral insulin delivery. J. Pharm. Sci. 105, 1733–1740 (2016).
Satyavani, K. & Gurudeeban, S. Insight on solid lipid nanoparticles: characterization and application in diabetes mellitus. J Crit. Rev 3, (2016).
Ansari, M. J. et al. Enhanced oral bioavailability of insulin-loaded solid lipid nanoparticles: Pharmacokinetic bioavailability of insulin-loaded solid lipid nanoparticles in diabetic rats. Drug Deliv. 23, 1972–1979 (2016).
Fonte, P. et al. Chitosan-coated solid lipid nanoparticles for insulin delivery. Methods Enzymol. 508, 295–314 (2012).
Boushra, M., Tous, S., Fetih, G., Xue, H. Y. & Wong, H. L. Development of bi-polymer lipid hybrid nanocarrier (BLN) to improve the entrapment and stability of insulin for efficient oral delivery. J. Drug Deliv Sci. Technol. 49, 632–641 (2019).
Koland, M., Anchan, R. B., Mukund, S. G. & Mulleria, S. S. Design and investigation of alginate coated solid lipid nanoparticles for oral insulin delivery. 55, 383–394 (2021).
Eilleia, S. Y., Soliman, M. E., Mansour, S. & Geneidi, S. A. Novel technique of insulin loading into porous carriers for oral delivery. Asian J. Pharm. Sci. 13, 297–309 (2018).
Ji, N. et al. Chitosan coating of zein-carboxymethylated short-chain amylose nanocomposites improves oral bioavailability of insulin in vitro and in vivo. J. Control Release. 313, 1–13 (2019).
Mumuni, M. A., Kenechukwu, F. C., Ofokansi, K. C., Attama, A. A. & Díaz, D. D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr Polym 229, (2020).
Kesarwani, K. & Gupta, R. Bioavailability enhancers of herbal origin: an overview. Asian Pac. J. Trop. Biomed. 3, 253–266 (2013).
Ajazuddin et al. Role of herbal bioactives as a potential bioavailability enhancer for active pharmaceutical ingredients. Fitoterapia 97, 1–14 (2014).
Han, H. K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 7, 721–729 (2011).
Han, Y., Tan, C., Lim, L. Y. & T. M. & In vitro and in vivo evaluation of the effects of Piperine on P-gp function and expression. Toxicol. Appl. Pharmacol. 230, 283–289 (2008).
Raghunath, I., Koland, M., Vadakkepushpakath, A. N., Kumar, L. & Shenoy, S. C. S. Herbal bioenhancers with nanocarriers: A promising approach for oral peptide delivery. Int. J. Pharm. Investig. 13, 07–13 (2022).
Raghunath, I., Koland, M., Sarathchandran, C., Saoji, S. & Rarokar, N. Design and optimization of chitosan-coated solid lipid nanoparticles containing insulin for improved intestinal permeability using Piperine. Int. J. Biol. Macromol. 280, 135849. https://doi.org/10.1016/j.ijbiomac.2024.135849 (2024).
Jafary Omid, N. et al. In-vitro and in-vivo cytotoxicity and efficacy evaluation of novel glycyl-glycine and alanyl-alanine conjugates of Chitosan and trimethyl Chitosan nano-particles as carriers for oral insulin delivery. Int. J. Pharm. 535, 293–307 (2018).
Sonaje, K. et al. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials 30, 2329–2339 (2009).
Rekha, M. R. & Sharma, C. P. Oral delivery of therapeutic protein/peptide for diabetes-Future perspectives. Int. J. Pharm. 440, 48–62 (2013).
Banerjee, A. et al. Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. U S A. 115, 7296–7301 (2018).
Jamshidi, M., Ziamajidi, N., Khodadadi, I., Dehghan, A. & Kalantarian, G. Biomedicine & pharmacotherapy the e Ff Ect of insulin-loaded Trimethylchitosan nanoparticles on rats with diabetes type I. Biomed. Pharmacother. 97, 729–735 (2018).
Akbarzadeh, A. et al. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 22, 60–64 (2007).
Abdollahi, M. & Hosseini, A. Streptozotocin. Encycl Toxicol. Third Ed. 4, 402–404 (2014).
Kumari, Y. et al. Modified Apple polysaccharide capped gold nanoparticles for oral delivery of insulin. Int. J. Biol. Macromol. 149, 976–988 (2020).
Momoh, M. A. et al. Microemulsion-based approach for oral delivery of insulin: formulation design and characterization. Heliyon 6, e03650 (2020).
Agrawal, A. K., Urimi, D., Harde, H., Kushwah, V. & Jain, S. Folate appended Chitosan nanoparticles augment the stability, bioavailability and efficacy of insulin in diabetic rats following oral administration. RSC Adv. 5, 105179–105193 (2015).
Kaur, I. et al. Exploring protein stabilized multiple emulsion with permeation enhancer for oral delivery of insulin. Int. J. Biol. Macromol. 167, 491–501 (2021).
Agrawal, A. K., Harde, H., Thanki, K. & Jain, S. Improved stability and antidiabetic potential of insulin containing folic acid functionalized polymer stabilized multilayered liposomes following oral administration. Biomacromolecules 15, 350–360 (2014).
Agrawal, A. K., Kumar, K., Swarnakar, N. K., Kushwah, V. & Jain, S. Liquid crystalline nanoparticles’: rationally designed vehicle to improve stability and therapeutic efficacy of insulin following oral administration. Mol. Pharm. 14, 1874–1882 (2017).
Liu, C. et al. Design of Virus-Mimicking polyelectrolyte complexes for enhanced oral insulin delivery. J. Pharm. Sci. 108, 3408–3415 (2019).
Baspinar, Y., Üstündas, M., Bayraktar, O. & Sezgin, C. Curcumin and Piperine loaded zein-chitosan nanoparticles: development and in-vitro characterisation. Saudi Pharm. J. 26, 323–334 (2018).
Fonte, P., Nogueira, T., Gehm, C., Ferreira, D. & Sarmento, B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv Transl Res. 1, 299–308 (2011).
Shalaby, T. I. & El-Refaie, W. M. Bioadhesive chitosan-coated cationic nanoliposomes with improved insulin encapsulation and prolonged oral hypoglycemic effect in diabetic mice. J. Pharm. Sci. 107 (2018).
Anchan, R. B. & Koland, M. Oral insulin delivery by Chitosan coated solid lipid nanoparticles: ex vivo and in vivo studies. J. Young Pharm. 13, 43–48 (2021).
Chitosan, E. O. F., Solid, C., Nanoparticles, L., Oral, F. O. R. & Delivery, I. Development and Evaluation of Chitosan Coated Solid Lipid Nanoparticles for Oral. (1956).
Weyers, M., Peterson, B., Hamman, J. H. & Steenekamp, J. H. Formulation of chitosan microparticles for enhanced intranasal macromolecular compound delivery: factors that influence particle size during ionic gelation. Gels 8 (2022).
Atal, N., Bedi, K. L. & Bioenhancers Revolutionary concept to market. J. Ayurveda Integr. Med. 1, 96–99 (2010).
K, K. Enhanced intestinal permeability of Silymarin by natural products as Bioenhancers - Assessment by Ex-Vivo noneverted rat gut sac study. Bioequivalence Bioavailab. Int. J. 2, 1–10 (2018).
Barve, K. & Ruparel, K. Effect of bioenhancers on amoxicillin bioavailability. ADMET DMPK. 3, 45–50 (2015).
Majumdar, S. H., Kulkarni, A. S., Kumbhar, S. M. & Bioenhancer, Y. Yogavahi - An ayurvedic concept used in modern medicines. Int. Res. J. Pharm. Med. Sci. 1, 20–25 (2018).
Islam, N. et al. Piperine phytosomes for bioavailability enhancement of Domperidone. J. Liposome Res. 32, 172–180 (2022).
Alsaad, A. A. A. Formulation & evaluation of β-glycyrrhetinic acid patches with natural bioenhancer. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2021.04.136 (2022).
Rathee, P., Kamboj, A. & Sidhu, S. Enhanced oral bioavailability of nisoldipine-piperine-loaded poly-lactic-co-glycolic acid nanoparticles. Nanotechnol Rev. 6, 517–526 (2017).
Singh, S. & Piperine An effective bioenhancer for drug absorption. Pharm. Drug Regul. Aff J. 4, 1–3 (2021).
Raghunath, I., Koland, M., Narayanan, A. V. & Piperine A possible permeation enhancer for oral protein delivery. J. Appl. Pharm. Sci. 14, 35–45 (2024).
Fonte, P., Araújo, F., Reis, S. & Sarmento, B. Oral insulin delivery: how Far are we? J. Diabetes Sci. Technol. 7, 520–531 (2013).
Rehmani, S. & Dixon, J. E. Oral delivery of anti-diabetes therapeutics using cell penetrating and transcytosing peptide strategies. Peptides 100, 24–35 (2018).
Arnolds, S., Kuglin, S., Kapitza, B., Heise, T. & C. & How Pharmacokinetic and pharmacodynamic principles pave the way for optimal basal insulin therapy in type 2 diabetes. Int. J. Clin. Pract. 64, 1415–1424 (2010).
Bolli, G. B. et al. Comparative pharmacodynamic and Pharmacokinetic characteristics of subcutaneous insulin Glulisine and insulin Aspart prior to a standard meal in obese subjects with type 2 diabetes. Diabetes Obes. Metab. 13, 251–257 (2011).
Khajuria, A., Thusu, A., Zutshi, U. & N. & Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 9, 224–231 (2002).
Veeresham, C., Sajatha, S. & Rani, T. S. Effect of Piperine on the pharmacokinetics and pharmacodynamics of glimepiride in normal and streptozotocin-induced diabetic rats. Nat. Prod. Commun. 7, 1283–1286 (2012).
Halarnekar, D. et al. Eco synthesized chitosan/zinc oxide nanocomposites as the next generation of nano-delivery for antibacterial, antioxidant, antidiabetic potential, and chronic wound repair. Int. J. Biol. Macromol. 242, 124764. https://doi.org/10.1016/j.ijbiomac.2023.124764 (2023).
Acknowledgements
The authors are thankful to Kampala International University, Nitte (Deemed to be University) and NGSM Institute of Pharmaceutical Sciences for providing the necessary facilities to conduct this research work.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
IR and MK initiated the conception. IR, MK, SPNB and SDS developed the design. IR, MK, SDS, SPNB, SDN, VA and NR conducted the experiments. MK, SPNB and CT analyzed the results. IR, MK, SDS, SPNB and CT prepared the first draft of the manuscript. IR, MK, SDS, SPNB, SDN, NR, VA and CT reviewed and edited the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by IAEC (NGSMIPS/IAEC/JAN-2023/333) and conducted according to the CPCSEA guidelines. All of the experiments conducted on animals in this study received prior approval from the Institutional Animal Ethics Committee (IAEC), Institutional Animal Ethics Committee of the NGSM Institute of Pharmaceutical Sciences, Mangalore, India, and they followed the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) protocols.
Informed consent
There are no human subjects in this article and informed consent is not applicable.
Consent for publication
All the authors have read and agreed to the final copy of the finding as contained in the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Raghunath, I., Koland, M., Saoji, S.D. et al. Effects of piperine on intestinal permeation, pharmacodynamics and pharmacokinetics of insulin loaded chitosan coated solid lipid nanoparticles in rats. Sci Rep 15, 22771 (2025). https://doi.org/10.1038/s41598-025-05137-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05137-3
Keywords
This article is cited by
-
Possibilities and challenges of next-generation therapeutics: solid lipid nanoparticles revolutionizing metabolic disorder treatment
Journal of Diabetes & Metabolic Disorders (2026)
-
The future of vesicular drug delivery: transferosomes in therapeutic advancement—applications, innovations and challenges
BioMedical Engineering OnLine (2025)
-
Lovastatin Loaded Piperine Enabled Nanostructured Lipid Carriers for Improvement of Ex vivo Permeation, Oral Bioavailability, and Antihyperlipidemic Activity
BioNanoScience (2025)