Abstract
Small-molecule inhibitors targeting prostate-specific membrane antigen (PSMA) have demonstrated promising results in the theranostics of prostate cancer (PCa). We designed and synthesized a novel small-molecule PSMA inhibitor, SC691. This study aimed to conduct a preliminary clinical prospective investigation of 68Ga-SC691. Following predefined inclusion/exclusion criteria, this study enrolled 7 healthy volunteers and 12 patients with PCa. Patients underwent 68Ga-SC691 and 68Ga-PSMA-11 PET/CT imaging within one week. PET/CT data were analyzed using a uWS-MI workstation.68Ga-SC691 demonstrated favorable safety profiles in all participants. 68Ga-SC691 was primarily excreted via the urinary system, exhibiting a biodistribution similar to that of 68Ga-PSMA-11. Compared to 68Ga-PSMA-11, 68Ga-SC691 showed lower non-specific organs uptake, particularly in the liver: 2.5 ± 0.7 vs. 4.1 ± 1.6 (P = 0.002). Furthermore, 68Ga-SC691 and 68Ga-PSMA-11 detected the same number and location of PSMA-positive lesions in PCa primary tumors, bone metastases, and lymph nodes metastases. Notably, 68Ga-SC691 demonstrated higher tumor-to-non-tumor ratios (T/NT) (P < 0.05). In conclusions, as a novel small-molecule PSMA inhibitor, SC691 exhibits a favorable safety profile, biodistribution, and diagnostic performance, suggesting its potential as a valuable agent for PCa imaging and therapy. The lower non-specific organ uptake further supports its promise for theranostic applications.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the most common malignancy of the male genitourinary system, with an increasing incidence worldwide1. Prostate-specific membrane antigen (PSMA) PET/CT has demonstrated high sensitivity and specificity for detecting primary, metastatic, and recurrent PCa lesions2,3,4.
Among the various PSMA-targeting probes, small-molecule PSMA inhibitors have been extensively investigated due to their well-defined structure-activity relationships and small molecular weight5. 68Ga-PSMA-11 is one of the most widely studied small-molecule targeting tracers3,6,7developed based on the Lys-urea-Glu motif, which exhibits favorable affinity and specificity for PCa cells. Although small-molecule PSMA tracers offer significant diagnostic advantages, their relatively lower detection rates for PCa lesions with low prostate-specific antigen (PSA) levels and higher non-specific uptake in normal organs indicate certain limitations8,9. Inspired by the efficacy of lysine residue side-chain modification strategies and the iodo-DCFPyL structure10,11 we developed a novel Lys-urea-Glu-based PSMA tracer, SC691. Preclinical studies have demonstrated ideal uptake of 68Ga-SC691 in PSMA-positive tumors in animal models, along with a favorable safety profile in toxicology studies12.
To further investigate the clinical feasibility of 68Ga-SC691, we evaluated its safety and biodistribution in humans and conducted a prospective head-to-head comparison with 68Ga-PSMA-11 to assess their diagnostic performance in PCa patients. This study aimed to determine the clinical value of 68Ga-SC691 and facilitate its further translation into clinical practice.
Results
Safety
No significant changes were observed in vital signs or clinical laboratory parameters in any healthy volunteer or patient before and after 68Ga-SC691 administration. The tracer was well tolerated by all participants, and no adverse events or side effects were observed during the study, with no difference between male and female participants.
Biodistribution
Visual analysis of images from all healthy volunteers revealed low whole-body background activity of 68Ga-SC691, facilitating the detection of both primary and metastatic lesions. Increased accumulation in the kidneys and bladder was observed with increasing time post-injection, suggesting that 68Ga-SC691 is primarily cleared through the urinary system. Moderate uptake was observed in the lacrimal glands, salivary glands, liver, spleen, small intestine, and cardiac blood pool. No significant uptake was observed in the brain, lungs, muscles, bones, thyroid gland, gallbladder, pancreas, stomach, and large intestine. Maximum-intensity-projection (MIP) images at multiple time points after intravenous administration of 68Ga-SC691 in a representative healthy volunteer are shown in Fig. 1.
Maximum intensity projection (MIP) images were acquired at 5, 30, 60, and 90 min following intravenous administration of 68Ga-SC691 in healthy volunteers. The MIP images revealed low whole-body background activity, with prominent tracer accumulation in the kidneys and bladder. Moderate tracer distribution was observed in the lacrimal and salivary glands, liver, spleen, small intestine, and cardiac blood pool. No significant tracer uptake was detected in other organs, including the brain, lungs, skeletal system, thyroid gland, pancreas, stomach, and large intestine.
Comparison of semi-quantitative analysis of normal organ uptake
Overall, both tracers exhibited similar biodistribution patterns in participants; however, some differences in uptake values were observed. Semi-quantitative analysis was performed using images acquired 60 min post-injection. Compared to 68Ga-PSMA-11, 68Ga-SC691 demonstrated significantly lower SUVmean values in normal organs, including the kidneys, salivary glands, spleen, liver, and small intestine. Conversely, 68Ga-SC691 showed higher activity in bladder urine than 68Ga-PSMA-11, indicating its relatively rapid urinary clearance. Regarding blood pool activity, 68Ga-SC691 exhibited higher accumulation than 68Ga-PSMA-11, suggesting potentially slower and differential plasma clearance (P = 0.002). No significant tracer uptake was observed in tissues and organs such as the brain, lungs, thyroid glands, muscles, and bones. The SUVmean values for gluteal muscle background were 0.58 ± 0.09 for 68Ga-SC691 and 0.44 ± 0.1 for 68Ga-PSMA-11 (P = 0.002). Detailed data are presented in Table S1, and a bar graph comparison is shown in Fig. 2A.
(A) Quantitative analysis of 68Ga-SC691 and 68Ga-PSMA-11 uptake was performed by measuring the SUVmean in selected normal organs (salivary glands, liver, spleen, kidneys, and small intestine), urinary bladder, and blood pool. Data are presented as bar graphs with error bars representing standard deviations. Compared to 68Ga-PSMA-11, 68Ga-SC691 showed significantly lower uptake in all evaluated normal organs (P = 0.002). In contrast, higher activity of 68Ga-SC691 was measured in the urinary bladder, indicating faster renal excretion, the elevated activity of 68Ga-SC691 in the blood pool suggested a potentially slower plasma clearance rate (P = 0.002). (B) Quantitative analysis of 68Ga-SC691 and 68Ga-PSMA-11 uptake was performed by measuring the SUVmax in primary PCa lesions, bone metastases, and lymph nodes metastases. Data are presented as bar graphs with error bars representing standard deviations of the mean. 68Ga-SC691 showed slightly lower SUVmax values compared to 68Ga-PSMA-11 in all three lesion types (primary lesions, bone metastases, and lymph node metastases); however, both radiotracers demonstrated high accumulation within these lesions, with mean SUVmax values exceeding 10.
Comparison of semi-quantitative analysis of lesions
Overall, 68Ga-SC691 and 68Ga-PSMA-11 showed no significant difference in the detection of primary PCa lesions, bone metastases, and lymph nodes metastases, both demonstrating excellent performance. A total of 176 PSMA-positive lesions were identified in 12 patients, including 10 primary PCa lesions, more than 121 bone metastases, and 53 lymph node metastases (some patients had extensive multiple metastases); two liver metastases were detected in one patient. Primary lesions were confirmed by biopsy. Both tracers detected the same lesions, with no lesions detected exclusively by either 68Ga-SC691 or 68Ga-PSMA-11 (Table S2, Fig. 3).
Semi-quantitative analysis was performed on 63 lesions (10 primary PCa lesions, 26 bone metastases, and 27 lymph nodes metastases), with a maximum of 5–6 lesions selected per patient (maximum of 2 lesions per organ). The results showed that the SUVmax values of 68Ga-SC691 were 18.3 ± 16.2 for primary PCa lesions, 12.0 ± 10.9 for bone metastases, and 17.1 ± 15.2 for lymph nodes metastases. The corresponding SUVmax values of 68Ga-PSMA-11 were 23.1 ± 17.7 for primary PCa lesions, 16.1 ± 13.2 for bone metastases, and 22.5 ± 19.3 for lymph node metastases. Although most lesions showed slightly lower uptake with 68Ga-SC691 compared to 68Ga-PSMA-11, there was no significant difference in lesion detection efficacy, and the mean SUVmax values were all > 10 (Table S3, Fig. 2B).
Correlation and agreement analysis
Linear regression analysis was used to assess the correlation of SUVmax values between 68Ga-SC691 and 68Ga-PSMA-11 in different lesions. Overall, the uptake values of the two tracers in primary PCa lesions, bone metastases, and lymph nodes metastases were highly correlated, with coefficients of determination R2 of 0.975 (P < 0.001), 0.963 (P < 0.001), and 0.968 (P < 0.001), respectively. This indicates that the detection of lesions in different locations of PCa by the two tracers is mutually corroborative and reliable.
Bland-Altman analysis was performed to assess the agreement of SUVmax values between 68Ga-SC691 and 68Ga-PSMA-11 in primary PCa lesions, bone metastases, and lymph nodes metastases. The mean bias values were − 4.7 ± 3.0 for primary PCa lesions, -4.1 ± 3.3 for bone metastases, and − 5.4 ± 5.1 for lymph node metastases. In conjunction with the Bland-Altman plots, the bias distribution of all analyzed data was within the 95% limits of agreement, indicating high agreement between the results of the two tracers (Fig. 4).
(A) The correlation of SUVmax values obtained with 68Ga-SC691 and 68Ga-PSMA-11 in various lesions was assessed using scatter plot analysis. Regression lines (solid lines), 95% confidence intervals (thin dashed lines), and 95% prediction intervals (thick dashed lines) are displayed on the scatter plots. All data points were contained within the 95% prediction intervals. Strong correlations were observed for primary PCa lesions, bone metastases, and lymph nodes metastases, suggesting comparable diagnostic accuracy of the two radiotracers. (B) Agreement between 68Ga-PSMA-11 and 68Ga-SC691 SUVmax measurements in different lesions was evaluated using Bland-Altman analysis. The mean bias (difference between 68Ga-PSMA-11 and 68Ga-SC691 SUVmax values) is indicated by a red dashed line, and the 95% limits of agreement (LOA) are represented by thin dashed lines. The mean bias ± standard deviation was − 4.7 ± 3.0 for primary PCa lesions, -4.1 ± 3.3 for bone metastases, and − 5.4 ± 5.1 for lymph nodes metastases. All data points were within the 95% LOA, confirming good agreement between the two radiotracers across all lesion types.
Comparison of T/NT and tumor-to-background ratio (TBR)
The T/NT ratios of primary PCa lesions to representative normal tissues (salivary glands, liver, and kidneys) were analyzed. The T/NT ratios on 68Ga-SC691 and 68Ga-PSMA-11 PET/CT were 2.79 ± 2.74 vs. 2.20 ± 1.96 (P = 0.09), 8.36 ± 7.84 vs. 6.62 ± 6.05 (P = 0.02), and 1.11 ± 0.95 vs. 0.96 ± 0.77 (P = 0.06), respectively. The T/NT ratios of primary PCa lesions to all three normal organs were relatively higher with 68Ga-SC691, with the difference in the primary lesion-to-liver T/NT ratio being statistically significant. Further analysis of the T/NT ratios of bone and lymph nodes metastases to the liver showed values of 12.67 ± 12.05 vs. 11.3 ± 10.68 (P = 0.037) and 10.6 ± 8.18 vs. 8.68 ± 6.92 (P = 0.013) for 68Ga-SC691 and 68Ga-PSMA-11, respectively, indicating relatively superior accumulation of 68Ga-SC691 in primary PCa lesions, bone metastases, and lymph node metastases, along with lower liver uptake (Fig. 5A).
(A) Quantitative analysis of tumor-to-normal tissue ratios (T/NT ratios) was performed to compare the uptake of 68Ga-SC691 and 68Ga-PSMA-11 in primary PCa lesions relative to selected normal organs (salivary glands, liver, and kidneys). Mean T/NT ratios with standard deviations are presented as bar graphs. The results indicated that 68Ga-SC691 showed relatively better accumulation in primary PCa lesions, as well as in bone and lymph node metastases, coupled with significantly lower liver uptake compared to 68Ga-PSMA-11 (P < 0.05). (B) Quantitative analysis of tumor-to-background ratios (TBRs) was conducted to compare 68Ga-SC691 and 68Ga-PSMA-11 uptake in primary PCa lesions, bone metastases, and lymph node metastases. Data are presented as bar graphs showing mean TBRs with standard deviations as error bars. While 68Ga-SC691 demonstrated slightly lower TBR values than 68Ga-PSMA-11 in all lesion types (primary lesions, bone metastases, and lymph node metastases), the TBRs for both radiotracers remained above 25, indicating excellent tumor-to-background contrast.
The TBR is a valuable parameter for evaluating the diagnostic performance of tracers. TBR values of 68Ga-SC691 and 68Ga-PSMA-11 were calculated by comparing the SUVmax of different lesions with the SUVmean of the gluteal muscle. The specific TBR data were 30.5 vs. 47.5 for primary PCa lesions, 26.0 vs. 45.8 for bone metastases, and 34.4 vs. 56.2 for lymph node metastases (Fig. 5B).
Discussion
The high specificity and sensitivity of PSMA for PCa have made it one of the most important targets for PCa diagnosis, staging, and follow-up5. The Lys-urea-Glu scaffold, due to its excellent affinity and specificity for PSMA, is the most common and important targeting motif in PSMA inhibitor structures. Modification of this motif has led to the synthesis of various high-affinity and high-specificity PSMA inhibitors13,14. By introducing a p-iodo benzoyl moiety to modify the lysine residue and selecting the macrocyclic chelator DOTAGA (with four acetate donor arms), which exhibits higher tumor uptake and retention, we synthesized a novel small-molecule PSMA inhibitor, SC691. 68Ga (III) was chosen to label SC691 due to its easier availability and simpler labeling procedure. Our previous animal studies demonstrated favorable uptake of 68Ga-SC691 in PCa lesions, indicating that SC691 is a highly specific and effective targeting agent12.
This preliminary clinical study demonstrated good safety and tolerability of 68Ga-SC691 in all participants, with no significant difference observed between male and female subjects. The biodistribution of 68Ga-SC691 was similar to that of 68Ga-PSMA-11 and other small-molecule PSMA inhibitors, exhibiting moderate to high physiological uptake in organs known to express PSMA, such as the lacrimal glands, salivary glands, liver, spleen, kidneys, and small intestine2,15. The urinary system, as the primary route of excretion, resulted in the highest dose distribution in the kidneys and bladder wall among normal organs.
Regarding the semi-quantitative analysis, the SUVmean uptake values of 68Ga-SC691 in the salivary glands, liver, spleen, small intestine, and kidneys were all lower than those of 68Ga-PSMA-11, indicating lower uptake in non-target organs. However, the higher accumulation in the bladder suggests more rapid urinary clearance of 68Ga-SC691. Conversely, the higher uptake of 68Ga-SC691 in the blood pool compared to 68Ga-PSMA-11 suggests slower blood clearance. This is consistent with our previous observations, and we hypothesize that the macrocyclic chelator and the p-iodo benzoyl modification simultaneously increased the hydrophilicity and plasma protein binding affinity of SC691, as SC691 has a higher hydrophilicity than PSMA-11 (log P − 3.530 ± 0.086 vs. −2.91 ± 0.06)12. Therefore, the early clearance of 68Ga-SC691 from the kidneys to the bladder may interfere with the detection of lesions near the urethra. To overcome this limitation, appropriate hydration and post-void delayed imaging should be considered in future 68Ga-SC691 imaging protocols. The slower blood clearance may be one of the factors influencing the TBR, prolonged blood circulation and higher accumulation in target organs may also be beneficial in practice. As diagnostic radiotracer doses generally do not cause significant adverse reactions but have the potential to improve prognostics. Furthermore, compared to PSMA-11, the introduction of DOTAGA makes it possible to label SC691 with therapeutic radionuclides (e.g., 177Lu, 90Y, or 225Ac). Unlike the high non-specific uptake of 68Ga-PSMA-11 and 177Lu-PSMA-617 in the salivary glands, kidneys, and bone marrow, the lower uptake of 68Ga-SC691 in non-target organs and its longer blood circulation time suggest its greater potential for application in PCa radionuclide ligand therapy (RLT)3,9,16.
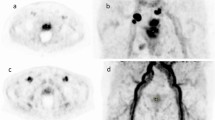
Of note, the SUVmean uptake of 68Ga-SC691 in the liver was lower than that of 68Ga-PSMA-11: 2.5 ± 0.7 vs. 4.1 ± 1.6 (P = 0.002). This results in improved contrast for visualizing liver lesions in cases of PCa liver metastasis with 68Ga-SC691 (Fig. 6). Although liver metastasis of PCa is a relatively rare late-stage event, the potential for liver involvement is higher when considering the application of PSMA targeting in other tumors, such as breast cancer and renal cell carcinoma. Furthermore, lower radiation absorbed dose to the liver during 177Lu/225Ac-SC691 therapy for PCa is also a noteworthy advantage.
A 63-year-old male patient with a recent diagnosis of prostate cancer (PCa) two weeks prior to imaging and one cycle of goserelin treatment presented with a PSA level of approximately 68.1 ng/mL. (A) 68Ga-SC691 PET/CT MIP image demonstrated high tracer uptake in the primary PCa lesion(red circle), multiple right iliac para-vascular lymph node metastases, and a focal hypermetabolic lesion in the right lobe of the liver consistent with metastasis. (B) Transaxial fused PET/CT images, the liver lesion showed an SUVmax of approximately 6.9, compared to a normal liver parenchyma SUVmean of approximately 3.1. (C) The prostate showed intense and homogenous tracer uptake with an SUVmax of approximately 29.7. (D) 68Ga-PSMA-11 PET/CT MIP image showed similar distribution patterns of tracer uptake(PCa lesion, red circle); however, the liver metastasis was less clearly visualized due to higher background activity in the normal liver parenchyma. (E) The corresponding transaxial fused PET/CT image of the liver showed the lesion with a higher SUVmax of approximately 9.0 (normal liver parenchyma SUVmean ≈ 4.5). (F) The prostate also showed intense uptake with an SUVmax of approximately 35.3. The TBRs for 68Ga-SC691 compared to 68Ga-PSMA-11 were higher for both the liver metastasis-to-normal liver (2.2 vs. 2) and the PCa lesion-to-normal liver (9.58 vs. 7.84), suggesting improved contrast on visual analysis.
In this prospective head-to-head comparison study, the SUVmax and TBR of 68Ga-SC691 in lesions were slightly lower than those of 68Ga-PSMA-11, but both still demonstrated high accumulation in tumor lesions and excellent background contrast (average SUVmax > 10, TBR > 25), indicating that both agents have excellent targeting ability and affinity for PSMA-expressing lesions, with no difference in detection efficacy. Furthermore, both agents exhibited excellent correlation and concordance in PCa diagnosis. Moreover, the T/NT analysis showed that 68Ga-SC691 performed better than 68Ga-PSMA-11, particularly with significantly higher ratios in various lesions (primary PCa lesions, bone metastases, and lymph nodes metastases) to the liver, indicating relatively superior accumulation of 68Ga-SC691 in these lesions, along with lower liver uptake. This offers improved value for image analysis.
In recent years, PSMA-targeted PET/CT has significantly impacted the diagnosis and management of PCa at various stages3,5. In particular, the small molecule inhibitors 68Ga-PSMA-11 and 68Ga-PSMA-617 have garnered widespread research and attention; the former was approved by the FDA for clinical use in December 202017, while 177Lu-PSMA-617 (Pluvicto®) received FDA approval for Pica therapy in March 202418. PSMA PET/CT imaging has become an integral component of standard clinical practice for PCa diagnosis and management worldwide19. Furthermore, with advancements in PET and hybrid imaging technologies, PET/MRI, combining the molecular imaging capabilities of PET with the multiparametric imaging advantages of MRI, holds considerable promise for urogenital malignancies20. Our study demonstrates the imaging characteristics of 68Ga-SC691 and its potential therapeutic applications, suggesting its viability as a valuable diagnostic/therapeutic alternative in PCa management.
This study has several limitations. First, the limited number of participants is a primary limitation, precluding broad generalizations of our findings. However, this is a common constraint in prospective pilot studies, and the head-to-head comparison demonstrated consistent differences in biodistribution between the two compounds. Nonetheless, larger cohort studies are warranted to validate our observations. Second, the identification of lymph nodes and bone metastases on imaging was based solely on tracer uptake, without pathological confirmation. Third, dynamic imaging to assess the multi-time-point retention of 68Ga-SC691 in various lesions was not performed. Furthermore, blood and urine samples were not collected in our study, thus precluding an assessment of in vivo radiopharmaceutical stability. Despite these limitations, the promising preliminary results support further investigation through larger-scale and head-to-head comparative studies.
Patients and methods
Patients
This clinical study was approved by the Institutional Review Board of the Affiliated Hospital of Southwest Medical University (KY2021051), and all participants provided written informed consent. Healthy volunteers were screened based on their medical history and pre-enrollment physical examinations. PCa patients were enrolled according to predefined inclusion and exclusion criteria. This study included seven healthy volunteers (five males and two females) and twelve patients with PCa. Among the PCa patients, four were newly diagnosed via sextant biopsy and had not received any prior treatment. The remaining eight patients had histologically confirmed PCa and had undergone prostatectomy, with or without androgen deprivation therapy (ADT), and were in various stages of treatment, follow-up, or recurrence. Detailed demographic and clinical characteristics of the participants are summarized in Table S4.
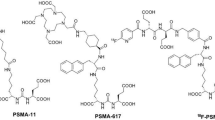
Radiopharmaceutical preparation
The synthetic route and chemical structure of SC691, as previously described in preclinical studies, involve a straightforward synthesis12. The structure of SC691 is shown in Fig. 7, with a molar mass of 1092.92 g/mol (compared to 946.99 g/mol for PSMA-11). 68Ga radiolabeling was performed in a sterile hot cell. Briefly, 25 µL of SC691 (1 mg/mL in pure water) was added to 100 µL of sodium acetate/acetic acid (NaAc/HAc) buffer (0.5 M/0.5 M, pH 4.45), followed by the addition of 400 µL of 68Ga(III) eluate (20–30 mCi; ITG, Munich, Germany). The reaction mixture was incubated at 80 °C for 10 min. The final product was diluted with sterile saline and sterilized by filtration through a 0.22 μm sterile filter to ensure sterility and apyrogenicity. Quality control of the product was performed using reversed-phase high-performance liquid chromatography (RP-HPLC; Shimadzu, Kyoto, Japan), confirming a radiolabeling yield > 95% and a radiochemical purity > 98%. 68Ga-PSMA-11 was synthesized according to previously reported procedures21with the PSMA-11 precursor obtained from ABX (Radeberg, Germany).
Imaging protocol
The injected activity was calculated based on the participant’s body weight (0.05–0.1 mCi/kg). Participants were instructed to drink ample water (approximately 500 mL) after injection and to void their bladder before PET/CT imaging. Data acquisition was performed using a PET/CT scanner (uMI780, United Imaging Healthcare, Shanghai, China) for both visual and quantitative analysis. Healthy volunteers underwent four whole-body scans at 5, 30, 60, and 90 min post-injection for biodistribution analysis. Patients underwent a single scan 60 min post-injection, in accordance with European Association of Nuclear Medicine (EANM) guidelines19. The field of view covered the skull base to the mid-femur. Low-dose CT scans were acquired using the following parameters: tube voltage 120 kV, tube current 50 mAs, slice thickness 3.00 mm, and a 128 × 128 matrix. PET scans were acquired in 3D acquisition mode for 3.0 min per bed position, with 5–6 bed positions per participant (depending on height), matching the bed positions of the CT scan. PET data were reconstructed using an ordered subset expectation maximization (OSEM) iterative algorithm (2 iterations, 20 subsets). All patients underwent comparative imaging with both 68Ga-SC691 and 68Ga-PSMA-11 PET/CT within a one-week period with no intervening treatment. The injected activity, imaging protocol, and equipment remained consistent for both scans.
The safety of all participants was assessed and graded. Urine analysis and complete blood counts, along with liver and renal function tests, were performed before and 24 h after scanning to evaluate safety. Participants were also followed up within one week after the 68Ga-SC691 PET/CT scan to collect and analyze any potential side effects for adverse event monitoring.
Image analysis
All acquired data were transferred to the uWS-MI workstation (Version R002, United Imaging Healthcare, Shanghai, China). To minimize bias, 60-minute images from both 68Ga-SC691 and 68Ga-PSMA-11 PET/CT were independently reviewed in a blinded manner by two experienced readers (board-certified nuclear medicine physicians and radiologists with senior professional titles) without access to other clinical or imaging data. Discrepancies were resolved by consensus. PET/CT images were analyzed frame by frame, and volumes of interest (VOIs) were manually drawn over lesions using spherical volume activity. For semi-quantitative analysis of normal organs uptake, the salivary glands, liver, spleen, kidneys, and small intestine were selected as representative organs. The bladder was delineated to obtain data on tracer urinary excretion. Blood pool activity was assessed by drawing VOIs on the inferior vena cava on 5-mm thick transaxial images. Normal organ distribution, bladder activity, and blood pool activity for both tracers were quantified as mean standardized uptake values (SUVmean). Additionally, VOIs were applied to the gluteal muscles to obtain SUVmean as a background reference value.
Visual analysis was performed to assess the biodistribution and number of lesions detected by 68Ga-SC691 and 68Ga-PSMA-11 PET/CT. Any focal uptake in the prostate exceeding the surrounding prostatic tissue was considered a positive finding for prostate lesions. Outside the prostate, areas of increased uptake exceeding adjacent tissues and not attributable to physiological variation were defined as pathological lesions. Results are expressed as maximal standardized uptake values (SUVmax). For semi-quantitative comparative analysis, up to 5–6 lesions per patient were randomly selected for SUVmax analysis (maximum of 2 lesions per organ). T/NT were calculated using the salivary glands, liver, and kidneys as non-target organs. The TBR was defined as the ratio of lesion SUVmax to g luteal muscle SUVmean to characterize background activity.
Statistical analysis
Statistical analyses were performed using Prism version 8.0 (GraphPad Software, La Jolla, California, USA) and SPSS version 26.0 (IBM Corp., Armonk, New York, USA). Continuous variables, such as age, SUVmean, and SUVmax, are presented as mean ± standard deviation, median, and interquartile range (IQR). Categorical variables are presented as frequencies and percentages. The Shapiro-Wilk test was used to assess the normality of data. The Wilcoxon signed-rank test was used to compare SUVmean values in normal organs between the two tracers. Linear regression and Bland-Altman analysis were performed to assess the correlation and agreement, respectively, of lesion SUVmax values between the two tracers. A two-tailed P value < 0.05 was considered statistically significant.
Conclusions
In conclusion, as a novel PSMA-targeting small molecule inhibitor, SC691’s favorable safety profile, biodistribution characteristics, and diagnostic efficacy suggest its potential as a valuable option for PCa imaging and therapy, warranting further clinical investigation. The low uptake of SC691 in non-target organs and its prolonged blood circulation time highlight its potential for theranostic applications when labeled with therapeutic radionuclides such as 177Lu, 90Y, or 225Ac.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to protect study participant privacy, but are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Afshar-Oromieh, A. et al. PET imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging. 40, 486–495 (2013).
Afshar-Oromieh, A. et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 42, 197–209 (2015).
Jochumsen, M. R. & Bouchelouche, K. PSMA PET/CT for primary staging of prostate cancer-an updated overview. Semin. Nucl. Med., Elsevier, pp. 39–45 (2024).
Capasso, G., Stefanucci, A. & Tolomeo, A. A systematic review on the current status of PSMA-targeted imaging and radioligand therapy. Eur. J. Med. Chem. 263, 115966 (2024).
Morigi, J. J. et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J. Nucl. Med. 56, 1185–1190 (2015).
Hofman, M. S. et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 395, 1208–1216 (2020).
Fendler, W. P. et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. 5, 856–863 (2019).
Paganelli, G. et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate Parotid gland protector: Preliminary results in metastatic castration-resistant prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging. 47, 3008–3017 (2020).
Banerjee, S. R. et al. 177Lu-labeled low-molecular-weight agents for PSMA-targeted radiopharmaceutical therapy. Eur. J. Nucl. Med. Mol. Imaging. 46, 2545–2557 (2019).
Chen, Y. et al. 2-(3-{1-Carboxy-5-[(6-[18F] fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F] dcfpyl, a PSMA-based PET imaging agent for prostate cancer. Clin. Cancer Res. 17, 7645–7653 (2011).
Chen, H. et al. In vitro and in vivo comparative study of a novel 68Ga-labeled PSMA-targeted inhibitor and 68Ga-PSMA-11. Sci. Rep. 11, 19122 (2021).
Kuo, H-T. et al. What a difference a methylene makes: Replacing Glu with asp or Aad in the Lys-urea-Glu pharmacophore of PSMA-targeting radioligands to reduce kidney and salivary gland uptake. Theranostics 12, 6179 (2022).
Wester, H-J. & Schottelius, M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin. Nucl. Med., Elsevier, pp. 302–312 (2019).
Gühne, F. et al. Differences in distribution and detection rate of the [68Ga] Ga-PSMA ligands PSMA-617, -I&T and-11—inter-individual comparison in patients with biochemical relapse of prostate cancer. Pharmaceuticals 15, 9 (2021).
Delker, A. et al. Dosimetry for 177Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 43, 42–51 (2016).
Carlucci, G. et al. 68Ga-PSMA-11 NDA approval: A novel and successful academic partnership. J. Nucl. Med. 62, 149–155 (2021).
Hennrich, U. & Eder, M. [177Lu] Lu-PSMA-617 (PluvictoTM): The first FDA-approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals 15, 1292 (2022).
Fendler, W. P. et al. PSMA PET/CT: Joint EANM procedure guideline/snmmi procedure standard for prostate cancer imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging. 50, 1466–1486 (2023).
Evangelista, L. et al. PET/MRI in prostate cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging. 48, 859–873 (2021).
Sasikumar, A. et al. Diagnostic value of 68Ga PSMA-11 PET/CT imaging of brain tumors—preliminary analysis. Clin. Nucl. Med. 42, e41–e48 (2017).
Acknowledgements
This work was supported by Science and Technology Fund of Chengdu Medical College (CYZYB22-19), Isotope and Drug Innovation Fund of the National Engineering Research Center for Isotopes and Drugs(TWSCX-2023-CXJJ-07-1), National Natural Science Foundation of China (U20A20384), Sichuan Provincial Radiotherapy and Therapy Clinical Medicine Research Center Open Project(2024ZX07).
Author information
Authors and Affiliations
Contributions
Guarantors of integrity of entire study, J. C., Z. Z., Y. C.; study concepts/study design, J. C., Z. Z., Y. C.; data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; Drafting or revising of the manuscript critically for important intellectual content, Z. Z., Y. C.; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, J. C., Z. S., Y. H.; clinical studies, J. C., Z. S., Y. H., D. P., X. J.; statistical analysis, J. C., Z. S., Y. H.; and manuscript editing, J. C., Z. S.; manuscript revising, Z. Z., Y. C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, J., Sun, Z., Zhou, Z. et al. Preliminary clinical study of p-iodo benzoyl moiety-modified PSMA inhibitors and prospective comparison with 68Ga-PSMA-11. Sci Rep 15, 20000 (2025). https://doi.org/10.1038/s41598-025-05344-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05344-y