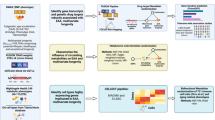
Abstract
Plasmapheresis is a medical procedure that separates plasma from blood cells, potentially removing pro-aging factors from circulation. Some studies suggest it may have rejuvenating effects by altering biomarkers of aging, but evidence on its impact on epigenetic aging in humans is limited. This study aimed to assess whether plasmapheresis without volume replacement with young plasma or albumin affects epigenetic age and other biomarkers in healthy adults. An automatic plasma collection system, the Haemonetics PCS2, was used for plasmapheresis. Healthy blood donors were divided into two groups using stratified randomization in a cross-over study with subjects undergoing either 8 plasmaphereses (8 pp) or 4 plasmaphereses (4 pp) for an 18-week period, with a minimum interval between plasmaphereses of 2 weeks (14 days). Samples were tested for biochemical, hematological analyses and epigenetic clocks. We documented the alteration in serum minerals, decreased serum lipids, mainly total cholesterol, non-HDL, triglycerides, apolipoprotein A levels, total proteins and albumin. Among hematologic parameters, we found an increase in Red Cell Distribution Width (RDW) and Mean Corpuscular Hemoglobin Concentration (MCHC). No significant epigenetic rejuvenation was observed based on epigenetic clock measurements. Instead, plasmapheresis was associated with increases in DNAmGrimAge, the Hannum clock, and the Dunedin Pace of Aging. Plasmapheresis can rapidly change the levels of pro-inflammatory and other pro-aging molecules in the circulation. However, the selected protocol has not provided conclusive data supporting benefits. Based on epigenetic clock parameters, it may accelerate epigenetic aging. More research into the long-term safety of this specific protocol is needed.
Similar content being viewed by others
Introduction
Aging is characterized by dynamic changes throughout life and a gradual decline in biological functions that lead to deterioration of the organism and ultimately death. Primary aging is the result of inevitable and innate maturation processes, while secondary aging is the loss of function accelerated by unhealthy lifestyle, environmental factors, and the presence of chronic diseases. Aging manifests itself at the level of organs (decline of function, e.g. pulmonary, cardiovascular, nervous, muscular, skeletal, endocrine systems), tissues, cells, organelles, and molecules1,2.
There are many theories of aging in humans that currently fall into three main categories: non-stochastic (programmed), stochastic (damage/error) theories, and their combinations1,2. Stochastic aging theories are based on the presence of episodic random events that cause cellular damage that accumulates in the organism over time. It is associated with elevated ROS production, organelle dysfunctions (mitochondria, lysosomes, endoplasmic reticulum, ribosomes), altered protein homeostasis (increased production of misfolded proteins and limited elimination and accumulation of pathological proteins), cellular senescence, DNA/RNA damage and genome instability, declination of reparatory processes, altered intercellular communication, dysbiosis, stem cell depletion, altered nutrient sensing, inflammation, accelerated telomere shortening, epigenetic changes, etc.3,4,5. Non-stochastic theory are based on genetically programmed events and individual biological clock that are associated with cellular damage: cell division and cell longevity are encoded in genes and lengths of telomeres. These theories also include the endocrine and immunological theory, in which biological clocks are controlled by hormones and the programmed decline of immune system functions1,6. Internal and external factors drive changes that are able to silence or activate genes; some of them are involved in the aging processes (gerontogenes)7.
DNA methylation-based biomarkers, particularly epigenetic clocks, are widely used as molecular indicators of biological aging. These biomarkers capture specific age-related changes in DNA methylation patterns, including locus-specific hypo- or hypermethylation, which can be used to estimate both chronological age, mortality risk, and the rate of aging. The epigenetic clock is influenced by various forms of cellular stress that contribute to the aging phenotype4,8,9.
Knowledge of the mechanisms of aging allows one to develop anti-aging strategies to target specific areas, pathways, or genes and to prevent or slow down pathological processes. The key is to support reparative processes and eliminate damaging processes and molecules. The hallmark goals of anti-aging strategies include reducing oxidative stress, remove senescent cells, inducing autophagy, rejuvenating stem cells, restoring telomere lengths, influencing the nutrition sensing pathways, and reducing inflammation.
According to some studies, plasmapheresis may have a positive effect on aging, as it can remove factors involved in the aging process10,11. Plasmapheresis is a medical procedure that separates whole blood into cellular components and plasma that is collected either as transfusable plasma or for further manufacturing into plasma-based derivates (such as intramuscular or intravenous immunoglobulins, coagulation factor concentrates, albumin)12. Plasmapheresis also serves as a therapeutic procedure that can alleviate a variety of diseases by removing dangerous molecules from the circulation. It is a second-line treatment for patients with autoimmune disorders such as vasculitis, myasthenia gravis, systemic lupus, or systemic sclerosis. It removes autoantibodies, circulating immune complexes, and inflammatory cytokines. Other diseases treatable by plasmapheresis include infectious diseases, acute hyperlipidemia, thyrotoxicosis, hypercoagulation disorders, and drug or chemical poisoning13,14,15,16,17,18,19.
Plasmapheresis has the potential to rejuvenate tissues by removing aging supporting blood factors such as proinflammatory cytokines, substances produced by senescent cells. Mehdipour et al. documented that replacing half of the plasma in mice with saline containing 5% albumin has a rejuvenating effect: it improves muscle regeneration and hippocampal neurogenesis, reduces liver adiposity and fibrosis in old mice. They also found that this procedure reduced neuroinflammation caused by activation of CD68 + microglia and reduced SA-βGal signal in the old brain, subsequently improving its function20,21. A positive effect of plasmapheresis on the central nervous system was also described by Boada et al., who treated patients with Alzheimer’s disease by replacing plasma with 5% albumin. This therapy led to a reduction in amyloid A1-42 level of amyloid Aβ1-42 in patients22.
Li et al. measured biomarkers of aging in volunteers before and after double filtration plasmapheresis. Based on their results, they concluded that the procedure altered the levels of biomarkers associated with aging (alkaline phosphatase, albumin, low-density lipoprotein, immunoglobulin M, urea, creatinine, blood glucose, β2-microglobulin, lactate dehydrogenase, homocysteine, cystatin C) resulting in a decrease of 4.47 years in men and 8.36 years in women according to their biological age assessment based on multiple linear regression analysis23.
Although evidence that plasmapheresis affects DNA methylation and the epigenetic clock in humans is still lacking, recent studies in animal models have shown that plasma exchange can improve tissue function and reduce age-related decline by removing circulating factors that contribute to aging. In humans, systemic factors such as pro-inflammatory cytokines, PAI-1, and leptin—known to influence epigenetic modifications—were identified as potential mediators of biological aging, forming the basis of our hypothesis that plasmapheresis might induce epigenetic rejuvenation20.
The aim of our study was to determine whether plasmapheresis without albumin or young plasma replacement affects epigenetic age and other selected plasma biomarkers. We analyzed biochemical, hematological, and epigenetic markers, determined by Horvath epigenetic clock analysis using multiple unique parameters of the biological age estimation such as DNAmAge, which measures the cumulative effect of an epigenetic maintenance system, DNAmPhenoAge (phenotypic age of DNA methylation) which is novel aging biomarker and predictor of chronic disease risk, lifespan, healthspan and all-cause mortality, DNAmSkinBloodAgeClock, which is an epigenetic clock for skin and the blood, and composite biomarker DNAmGrimAge, which strongly predicts lifespan and healthy lifespan based on a combination of chronological age, sex as well as several DNAm biomarkers of plasma proteins and smoking9,10,24,25,26,27.
Materials and methods
Observed groups of subjects
Subjects undergoing plasmaphereses were first time blood donors. They were divided into two groups using stratified randomization focusing on chronological age, sex, and BMI. Group 1 (G1) consisted of 28 subjects (13 females, 15 male). Group 2 (G2) consisted of 13 subjects (7 females, 6 males). Subjects in G1 underwent 8 plasmaphereses (plasma donations) during 18 weeks from April to August. The minimum interval between plasmaphereses was 2 weeks (14 days). After 4 plasmaphereses, specimens were collected (before the fifth plasmapheresis). Subjects in G2 did not undergo the first four plasmaphereses serving as a cross-control group. Last specimens were collected at least seven days after the last plasmapheresis, no more than 21 days. Given the challenges of recruiting first-time plasma donors and maintaining adherence to an intensive protocol, we opted for this design that involved sampling after 4 sessions (9 weeks) and 8 sessions (18 weeks). Although 18 weeks is relatively short in the context of aging research, the intensity of the plasmapheresis intervention was anticipated to rapidly alter circulating biomarkers, including epigenetic aging markers.
One male subject (G1) decided to end his participation in the study before the first specimen collection. One female (G1) subject ended her participation in the study during the first plasmapheresis due to low blood pressure. Another female subject (G1) stopped participating before the second withdrawal of the sample after being diagnosed with Lyme disease, which is one of the exclusion criteria for plasma donations. One male, one female from G1, and two females from G2 stopped participating in the study during its second half (before third last specimen withdrawals) due to a minor infectious disease or a COVID vaccination in the meantime that excluded them from a plasma donation program for a period longer than possible according to study design and its deadline. A total of 34 participants finished the study (see Table 1).
38 healthy blood donors (20 females, 19 males) successfully finished the first half of our study. 34 persons finished the study till the end. The median age of the 38 participants was 49.6 (Q1 = 45.4; Q3 = 51.9; min = 43.4; max = 59.3).
Only participants aged 40 to 60 were allowed in the study. Participants were eligible to participate in the study only if they met the criteria of becoming volunteer plasma donors.
Permanent exclusion of plasmapheresis donation included: diabetes mellitus with insulin medication, hepatitis B or C, extrapulmonary TBC, severe blood disease, malignant tumors, myocardial infarction, stroke, psychiatric disease, transplantation.
The two-year exclusion criteria included pulmonary TBC, rheumatic fever. One year exclusion criteria include: mononucleosis, hepatitis A, sepsis, polytrauma, risky sexual contact.
Six-month exclusion criteria included: borreliosis, toxoplasmosis, surgery, anesthesia, childbirth, interruption, severe accidents, arthroscopy, gastro/colono/cystoscopy, catheterization, thrombosis, medication affecting hemocoagulation, gastric ulcers, tattoos, piercing, acupuncture, sexually transmittable disease, blood transfusion.
Four-week exclusion criteria include: viral infection with fever, antibiotic treatment, vaccination for hepatitis B, flu, typhus, cholera, yellow fever, minor surgery without anesthesia, breastfeeding, Lyme.
Two-week exclusion criteria included: vaccination for covid-19, viral infection without fever, coughing or sneezing, tooth extraction, cold sore on the mouth.
Plasmapheresis procedure
At the beginning of each plasmapheresis, 10 mL of blood was always drawn from the donor for mandatory tests (in addition to the designated tubes for testing after the 4th and 8th collections). An automatic plasma collection system (Haemonetics PCS2, Braintree, MA, USA) was used for plasmapheresis. The procedure was conducted under the accredited and approved protocol for plasma donations at the Transfusion Department, University Hospital in Hradec Kralove. No change to the protocol was introduced (see Fig. 1 for more details). Physiological saline solution was given to donors when 750 ml or more of plasma was collected in a single donation. A 250 ml of saline was administered and only circulated to the donor on the last return (returning the contents of the centrifuge vessel).
Plasma collection scheme Legend: Withdrawal from 1 vein, alternating withdrawal (draw mode) and return phase (return mode). During the collection phase, whole blood (WB) is collected and a 4% citrate solution (CITRASOL 4%, Imuna Pharm a.s., Sarisske Michalany, Slovakia) is added to it. In the centrifuge separator (bowl), the WB is separated into its individual components and the plasma is transported to the collection bag. Once the centrifuge bowl is full (of blood components without plasma), the return phase is activated and erythrocytes, leukocytes and platelets are returned to the donor’s circulation. Once the vessel is empty, the next collection phase is triggered. Draw and return mode alternate until the specified amount of plasma has been collected (see Table 2).
DNA methylation and epigenetic clock analyses
One 6 ml BD Vacutainer K2EDTA tube was collected for molecular analysis. All the blood samples were processed within 4 h after collection. Firstly, 750 µl of whole blood was stored at -80 °C for further analyses. Secondly, double centrifugation of plasma was performed; the tube was centrifuged at 1,300 g at laboratory temperature, plasma (above buffy coat) was collected to microcentrifuge tube and centrifuged at 16,000 g at 4 °C to remove any remaining cell debris. Three plasma aliquots were stored at -80 °C for further analysis. Approximately 500 µl of buffy coat was recovered, 100 µl of buffy coat was added to 100 µl PBS for DNA isolation. DNA was isolated using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) and eluted in 60 µl of elution buffer. DNA was quantified by DeNovix DS-11 Spectrophotometer (DeNovix Inc., Wilmington, DE, USA) and Qubit 1.0 Fluorometer (Invitrogen, Thermo Fisher Scientific Waltham, MA, USA) with Qubit dsDNA BR Assay Kit (Invitrogen). Samples with minimum concentration of 50 µl of ds DNA were stored at -20 °C and ultimately shipped for DNAmAge analysis.
Performing the DNA methylation and epigenetic clock assays included first bisulfite conversion of extracted DNA using a Zymo EZ DNA methylation kit (Zymo Research, Irvine, CA) for downstream processing by MethylationEPICv1 BeadChip array kits (Illumina, San Diego, CA), which include all the reagents required for DNA amplification, labeling, and array. Scanning of the completed beadchip arrays for final DNA methylation readout. Normalization was performed using the Bioconductor minfi package28 with Noob background correction, followed by computation and analysis of multiple epigenetic aging clocks including the pan-tissue Horvath clock (predictor of chronological age based on DNA methylation at specific CpG sites)27, Skin&BloodAge (Horvath2)25, PhenoAge (estimation of phenotypic aging, reflecting chronic disease risk and overall health)24, and GrimAge (methylation surrogates for plasma proteins and smoking pack-years to predict mortality risk and healthspan)26.
DNAmGrimAge: This measure of biological age strongly predicts future healthspan and lifespan. A 7.5-year acceleration of GrimAge relative to chronological age corresponds to a doubling of mortality risk, while a 7.5-year reduction equates to about a 50% reduction in mortality risk26.
DNAmGrimAge2: An updated version of the original GrimAge clock. GrimAge2 improves the accuracy of biological age and lifespan predictions by incorporating additional biomarkers related to lifestyle and mortality risk. This clock enhances predictions of age-related outcomes, such as chronic disease risk and overall mortality29.
DNAmGrimAge2Calibrated: Since DNAmGrimAge2 was originally trained using blood samples, it may overestimate epigenetic age when applied to other tissue samples. This clock has been calibrated using The Clock Development Foundation’s model on a reference database of 1154 healthy samples (ages 18 to 91) from various sources (blood, saliva, buccal). The calibration corrects the overestimation trend by accounting for sample source, methylation array type, and age.
DNAmGrimAge_Tuned and DNAmGrimAge2_Tuned: These versions of DNAmGrimAge and DNAmGrimAge2 involve tuning the beta-value matrices before applying the epigenetic age predictors. The tuning process addresses missing data and estimates methylation beta-values for specific CpG sites using a regression model based on a coefficient matrix.
Using mixed effects models with plasmapheresis number variable we evaluated GrimAge clock components: Adrenomedullin (ADM), Beta-2-Microglobulin (B2M), Cystatin C, Growth Differentiation Factor 15 (GDF15), Leptin (Leptin), Pack-Years of Smoking (PACKYRS), Plasminogen Activator Inhibitor-1 (PAI1), Tissue Inhibitor of Metalloproteinases 1 (TIMP1), Cyclooxygenase (COX).
DunedinPACE: A novel blood biomarker for aging pace developed as part of the Dunedin Longitudinal Study. DunedinPACE demonstrates high test–retest reliability and associations with morbidity, disability, and mortality. It indicates faster aging in young adults with childhood adversity30.
Robust clocks: Albert Higgins-Chen and Morgan Levine retrained principal-component versions of six epigenetic clocks. Their work demonstrated that these PC-based clocks showed close agreement with the original clocks, with discrepancies generally within 1.5 years. They also observed improved detection of clock associations and intervention effects, and consistent longitudinal trajectories in both in vivo and in vitro settings. The PC clocks selected for retraining included proxies for Horvath1 (Pan tissue clock), Horvath2 (Skin&Blood clock), Hannum, DNAmTL, and GrimAge. Additionally, PCPhenoAge was trained directly on phenotypic age scores derived from clinical biomarkers. Elastic net regression was used to select principal components, resulting in the following selections: 121 out of 4280 for Horvath1, 140 out of 894 for Horvath2, 390 out of 655 for Hannum, 652 out of 4504 for PhenoAge, 599 out of 3934 for DNAmTL, and 1936 out of 3934 for GrimAge. These PC clocks demonstrated high correlation with their original counterparts in both training and test datasets. By reducing technical noise, these PC clocks offer enhanced sensitivity in detecting additional factors that influence epigenetic age31.
Immune cell count markers, including: CD8( +) T- lymphycytes (CD8T), CD4( +) T-lymphocytes (CD4T), NK cells, B cells, Mononuclears, granulocytes, plasmablasts, CD8( +) naive and CD4( +) naive cells were determined using methylation data and the Houseman algorithm32.
Biochemical and hematological data
For the biochemical and hematological analyses, 10 ml of blood were withdrawn using standard clinical practice and transported in EDTA treated or serum sep C/ANR tubes. Blood count was assessed using CELL-DYN Ruby, Abbott Laboratories, Abbott Park, IL, USA. Biochemical data were assessed using standardized accredited methods in the University Hospital in Hradec Kralove at the Department of Clinical Biochemistry.
Statistical analyses
For the statistical comparison of biochemical and hematological data, R version 4.4.1 was used with the “nortest” and "compute.es" packages. Based on the Anderson–Darling test for data distribution, either a parametric or non-parametric test was applied for comparisons. Changes in laboratory parameters over time were assessed using either Student’s paired t-test or the Wilcoxon signed-rank test. Associations between epigenetic markers and other laboratory values were evaluated using Pearson’s or Spearman’s rank correlation tests. A comparison was considered statistically significant if the probability level (p) was less than an alpha level of 0.05. As the study was exploratory in nature, results were not adjusted for multiple comparisons.
For the analysis of epigenetic clocks, a linear mixed-effects model with Satterthwaite’s degrees of freedom method was used to estimate p-values for each coefficient. Patient ID was included as a random effect. Epigenetic age or blood celltype estimates were used as response variables, while treatment week, chronological age, sex assigned at birth, and other relevant covariates were modeled as fixed effects.
Results
We documented the alteration in serum minerals. Calcium and phosphorus levels decreased after 8 plasmapheresis procedures (pp) (p = 0.0010; p = 0.0004, respectively), and calcium levels also decreased after 4 pp (p = 0.0175). Potassium levels increased after 8 pp (p = 0.0103, Fig. 2).
Significant changes in electrolyte levels after 8 plasmapheresis sessions. Legend: Box plot depicting serum calcium, phosphorus, and potassium levels before and after 8 plasmapheresis sessions (8 pp). The box represents the interquartile range (IQR) with a median line; whiskers extend to the minimum and maximum values within 1.5 IQR, and outliers are shown as dots. Significant differences are denoted by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001). This figure highlights the electrolyte alterations induced by plasmapheresis.
Pp decreased serum lipids, mainly total cholesterol (chol), non-HDL, triglycerides (TAG) and apolipoprotein A levels after 4 and 8 pp (chol: p < 0.05; p < 0.01; nonHDL: p < 0.05, p < 0.01; TAG: p < 0.05, p < 0.05; APO A: p < 0.001, p < 0.001). Levels of apolipoprotein B1 decreased only after 8 pp (p < 0.05), Fig. 2.
Plasmapheresis decreased serum lipids, mainly total cholesterol (Chol), non-HDL cholesterol, triglycerides (TAG), and apolipoprotein A (Apo A) levels after both 4 and 8 pp. For total cholesterol, p-values were p = 0.0207 after 4 pp and p = 0.0042 after 8 pp; for non-HDL cholesterol, p = 0.0101 and p = 0.0010; for TAG, p = 0.0381 and p = 0.0225; and for Apo A, p = 0.0009 and p = 0.0003, respectively. Levels of apolipoprotein B1 decreased only after 8 pp (p = 0.0323), see Fig. 3.
Significant lipid profile alterations following 8 plasmapheresis sessions. Legend: Box plot comparing levels of total cholesterol, non-HDL cholesterol, triglycerides (TAG), apolipoprotein A, and apolipoprotein B1 before and after 8 plasmapheresis sessions. The plot displays the median, quartiles, and range, with significant changes marked as * (p < 0.05), ** (p < 0.01), and *** (p < 0.001). ApoA-I is the main protein component of HDL cholesterol (the “good” cholesterol).
Higher levels of ApoA are generally good because. ApoB is the main protein component of LDL cholesterol (the “bad” cholesterol) as well as VLDL and IDL particles. Higher ApoB levels are bad because it correlates with the number of atherogenic particles.
Significant changes in the levels of proteins and amino acids were found. Total proteins decreased after 4 and 8 pp (p = 5.42 × 10⁻⁷; p = 0.0001), as well as albumin (p = 3.02 × 10⁻⁶; p = 0.0011). After 8 pp, we also detected elevated homocysteine levels (p = 0.0099), see Fig. 4. Vitamin D levels increased significantly after 4 and 8 pp (p = 0.0003; p = 7.58 × 10⁻⁶). These results indicate that plasmapheresis reduces the levels of certain plasma proteins, including total proteins and albumin, likely due to their removal during the plasma exchange process. A potential side effect observed was an increase in homocysteine levels, which may raise concerns regarding vascular health and warrants careful monitoring. Although an increase in vitamin D levels was also detected, this finding should be interpreted with caution, as the study was conducted in the spring—a period when natural vitamin D levels typically rise due to increased sunlight exposure.
Significant changes in protein and homocysteine levels after 8 plasmapheresis sessions. Legend: Box plot showing serum total protein, albumin, and homocysteine levels before and after 8 plasmapheresis sessions. The plot illustrates the median and quartile ranges with statistical significance indicated by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001). This figure demonstrates the reduction in protein levels alongside an increase in homocysteine following the procedure.
Among the hematologic parameters, significant increases in RDW (Red Cell Distribution Width) and MCHC (Mean Corpuscular Hemoglobin Concentration) were observed after 8 pp, with p = 0.0004 and p = 0.0432, respectively (Fig. 5). Additional details are provided in summary Table 3 or the supplementary files.
Significant hematological changes after 8 plasmapheresis sessions. Legend: Box plot comparing red cell distribution width (RDW) and mean corpuscular hemoglobin concentration (MCHC) before and after 8 plasmapheresis sessions. Significant changes are indicated by * (p < 0.05) and ** (p < 0.01). This figure underscores alterations in blood cell parameters associated with plasmapheresis.
To understand the effect the plasmapheresis sessions have on DNA methylation clocks we used linear mixed effects models with the following independent variables: the number of plasmapheresis sessions, sex assigned at birth, age, scaled monocyte and CD4( +) naive cell proportions, and patient ID as a random effect variable.
Seven epigenetic clock models showed that the number of plasmapheresis sessions was positively associated with the DNA methylation clock estimates (DNAmGrimAgeBasedOnRealAge increased by 0.26 ± 0.05 standard errors, p = 5 × 10–7; DNAmGrimAge2BasedOnRealAge increased by 0.22 ± 0.05, p = 0.0002; DNAmGrimAge2BasedOnRealAge_Tuned increased by 0.16 ± 0.03, p = 1.26 × 10–5; DNAmGrimAge2Calibrated increased by 0.22 ± 0.05, p = 0.0002), Hannum clock (DNAmAgeHannum increased by 0.17 ± 0.04, p = 0.0002; RobustHannum increased by 0.13 ± 0.03, p = 2.42 × 10–5), and DunedinPACE increased by 0.003 ± 0.001, p = 0.0058). The corresponding results are presented in Fig. 6. Other clock markers did not reach statistical significance. It is important to note that a period of more than 14 days elapsed between each plasmapheresis session to minimize the influence of acute, short-term physiological effects and to allow sufficient recovery of plasma components. This interval was chosen to reduce potential confounding factors related to transient changes in biomarkers, ensure participant safety, and align the study design with the evaluation of longer-term biological effects, including those measured by epigenetic clocks.
Relationship between plasmapheresis sessions and epigenetic age estimates. Legend: Bar plot presenting the estimates of the plasmapheresis number coefficient from a linear mixed-effects regression model for each epigenetic clock assay. The model accounts for age, sex, and scaled levels of monocytes and CD4 + naïve T cells, with individual ID as a random effect. Dark blue bars indicate statistically significant effects (p ≤ 0.05), while light blue bars denote non-significant effects (p > 0.05). This figure illustrates how increasing plasmapheresis sessions are associated with changes in epigenetic aging markers. For additional details on all results, including estimates of the plasmapheresis session coefficient without adjustment for blood count measures such as monocytes and CD4 + naïve T cells, please refer to the supplementary file.
There were statistically significant changes in multiple methylation-based surrogates that are components of DNAmGrimAge with accumulating plasmapheresis sessions. The methylation changes suggested increases in Adrenomedullin (ADM, p = 7.09 × 10–5), Beta-2-Microglobulin (B2M, p = 8.24 × 10–5), smoking packyears PACKYRS (p = 0.0077), plasminogen activator inhibitor 1, PAI1 (p = 1.96 × 10–5), cyclooxygenase (COX) (p = 0.0008) and decreased leptin (p = 2.9 × 10–6), see supplementary Fig. 1 for further details.
Evaluating methylation-based predictors of immune cell counts using mixed effects models with plasmapheresis number variable, sex assigned at birth, age and patient ID as random effect, monocytes and naive CD4 + T cells were significantly elevated (p = 6.1 × 10–6; p = 0.0241) while granulocytes were downregulated, supplementary Fig. 2.
Discussion
Aging is a physiological process that can be shaped by numerous internal and external factors that can accelerate or slow it down. One of those factors that may slow the rate of biological aging may be plasmapheresis27. The process involving the separation of cellular components of blood and plasma, which can then be used to produce drugs such as clotting factors, i.v. or i.m. immunoglobulins if taken from healthy donors. Cellular components are returned to the same donor with/without colloidal solutions or with albumin-containing solutions that replace the withdrawn plasma. Plasmapheresis can also be used as a treatment33,34. Together with plasma, many substrates are also removed from the body, including substances that damage the body and accelerate aging.
Various biochemical, hematological, and epigenetic markers were measured in volunteers enrolled in our study. We described the status of these markers before, after four and eight plasmapheresis sessions, compared them with each other. Mostly, the changes were more profound after 8 pp compared to 4 pp.
We documented the decrease in calcium levels in the female group and in the group of all participants after 4 and 8 pp. Interestingly, there was no statistical decrease in males. These gender specific differences may be partly attributed to hormonal influences, such as the role of estrogen in regulating calcium metabolism35. Although the current study’s sample size limited our ability to perform comprehensive subgroup analyses, these findings suggest that sex-specific hormonal factors could modulate the physiological effects of plasmapheresis. Pp also influenced potassium levels, which increased after 8 pp in males and all volunteers, and phosphorus level, which decreased in all groups after 8 pp. The loss of calcium can be caused by the calcium being withdrawn with plasma and the use of citrate (citrate toxicity). Mineral imbalance (calcium, phosphorus, potassium) was documented in multiple studies, mainly using therapeutic plasma exchange36,37. These changes can cause problems, e.g., cardiac dysfunction, changes in blood pressure, hypocalcemic tetany, paresthesia, and in long term was also concerned that decreased levels of calcium after frequent plasmapheresis can induce a decrease in bone density38,39. Bialkowski et al. conducted a longitudinal study and found that in men it did not affect the bone quality40. This is in agreement with our study because the levels of calcium in male did not drop. We found no study that addressed calcium loss after plasmapheresis particularly in women, who are generally more prone to develop osteoporosis. However, the potentially higher risk of developing osteoporosis after periods of plasmapheresis is of concern. In our study the levels of vitamin D significantly increased in all groups after 4 and 8 pp, but it should be noted that the study was conducted in spring in Europe, meaning a higher production of vitamin D in the skin of participants due to higher UV exposure. Hiemstra et al. measured the levels of plasma vitamin D after 5 plasma exchanges and documented the vitamin D deficiency41. We therefore believe that our results reflect the length and intensity of sunshine / UV light due to the season.
Not only electrolytes but also lipids are affected by plasmapheresis. In our study, we detected decrease in total cholesterol, non-HDL, triglycerides, and apolipoproteins. Rosa-Bray et al. analyzed blood samples from 663 plasma donors that were withdrawn before and after plasmapheresis. They confirmed that plasmapheresis lowered level of LDL and total cholesterol42. Plasmapheresis is also used therapeutically in patient with hyperlipidemia, thus our results are with agreement with other studies42,43. It is therefore safe to state that plasmapheresis affects the level of cholesterol in blood. Higher amount of cholesterol is considered to be not only a risk factor of cardiovascular disease, but also a cardiovascular mortality predictor. This way plasmapheresis might be able to lower the risk of cardiovascular mortality in plasma donors. The size of the effect must be further studied44.
Plasma contains many proteins, the concentration of which may decrease with plasma removal. 4 and 8 pp reduced levels of total proteins and albumin. Albumin plays a critical role in maintaining oncotic pressure, transporting various endogenous and exogenous substances, and serving as an antioxidant and anti-inflammatory agent. A significant reduction in albumin levels, as observed in our study, could contribute to hemodynamic instability, impaired immune responses, and increased susceptibility to infections. Additionally, low albumin has been linked to frailty, poor wound healing, and increased mortality risk in clinical settings, raising concerns about its depletion in frequent donors.
Beyond albumin, plasmapheresis appears to lead to the loss of immunoglobulins. Taborski et al. analyzed IgG levels that after initial drop after plasmapheresis subsequently regenerated and stabilized45. Bechtloff et al. observed plasma donors over a 3-year period. Markers were measured every 15th donation. Plasmapheresis decreased the levels of total proteins, albumin, IgG, hemoglobin, and ferritin. However, the decrease was not severe enough to require donors to be excluded from the donation46. Ciszewski et al. evaluated parameters in donors who underwent plasmapheresis weekly and after 14 days or longer. They confirmed that the rate of decline in total protein and IgG levels was higher in people who underwent plasmapheresis once a week compared to donors who underwent plasmapheresis at longer intervals. However, stabilization occurred over a 6-month period, and the authors suggest that weekly plasmapheresis (500 to 600 ml volume) has minimal impact on protein levels47. Plasmapheresis is also used as a therapy for protein removal (immunoglobulins, immunocomplexes, cytokines, protein aggregates)33. Speaking of immunoglobulins, we also found changes in the number of immune cells. Especially in the female group, a decrease in white blood count, lymphocytes, and basophils was found after 8 pp, while the number of total mononuclear cells increased in all groups after 4 and 8 pp, except for men after 4 pp. Hashemian et al. performed plasmapheresis in hospitalized patients with severe SARS-CoV-2 infection and described the decline in the number of T cells48. However, Yeh et al. conducted the study with healthy volunteers and documented an increase in the number of immune cells and suggested that a single session of double filtration plasmapheresis activate the cellular immune system. They also found that the levels of helper T cells and B cells increased while the levels of T regulatory cell decrease49. These results are consistent with an increase in mononuclear cell counts and potentially a decrease in absolute lymphocyte counts in our study. Activation of the immune system, especially in women, can be associated with an increase in CRP that we found. Plasmapheresis had a more profound impact on hematological parameters in females; not only on immunological parameters, but also on mean corpuscular haemoglobin concentration (decrease; MCHC) and red cell distribution width (increase without iron deficiency; RDW). The observed changes in MCHC and RDW may reflect compensatory changes in erythropoiesis or indicate an adaptive response to oxidative stress induced by the removal of plasma components. For instance, elevated RDW has been associated with inflammatory conditions and altered red blood cell turnover, while changes in MCHC might suggest shifts in red blood cell morphology or membrane integrity50,51,52.
Surprisingly, plasmaphereses, especially 8 pp, in the male group were associated with an increase in estrogen, progesterone and thyroxine levels. Plasmapheresis is known to remove the hormones from the circulation53. Therefore, we assumed that the increase of hormone levels depended on the increased production. Estrogen and progesterone are produced in men mainly in adrenal gland; therefore, enhanced production may be related to stress associated with plasma donation53,54. In women, elevated levels of progesterone stimulate the release of thyroxine55. Thus, there is an assumption that thyroxine and progesterone levels may be closely related, even in men56. It is known that thyroid tissue expresses the receptor for estrogen that stimulate these cells57. It is possible that while in women the thyroid tissue is used to higher concentrations and fluctuations in men, even a small deviation could lead to an effect on thyroid function and increase the release of thyroxine. We documented a decrease in DHEA levels, which is consistent with other studies showing that plasmapheresis removes hormones from the circulation.
We also analyzed the levels of amino acid homocysteine which is associated with a wide variety of pathological processes and diseases and aging58,59. Homocysteine production increased in the female group and all participants group after 8 pp. It can be suggested that changes associated with plasmapheresis may induce the synthesis of homocysteine60. Although our study did not incorporate nutritional supplementation, future investigations could evaluate whether targeted interventions—such as supplementation with folate, vitamin B6, and vitamin B12, which have been shown to lower homocysteine concentrations—might mitigate this effect. Such supplementation strategies could be particularly valuable in minimizing potential adverse metabolic impacts in the context of plasmapheresis.
Our study evaluated the effect of plasmapheresis on the epigenetic clocks which show the difference between chronological and biological age, risk of chronic disease and death. DNAmGrimAge, RobustHannum clocks and DundedinPACE showed significant acceleration of the biological age. The most profound changes were found in the DNAmGrimAge methylation clocks, as it showed elevation in the mixed effects model. The hypothesis of epigenetic rejuvenation due to plasmaphereses has not been proven right in our study.
There is no easy explanation for the elevation of DNAmGrimAge (and other clocks). Although therapeutical plasmaphereses showed promise in mouse models and even studies on human subjects showed positive changes in biochemical parameters, there seems to be a lot of unanswered questions in the impact of plasmaphereses on blood methylation profile for now20,21,22,23. Ward-Caviness et al. associated epigenetic markers of accelerated aging (Extrinsic Epigenetic Age Acceleration Difference, Epigenetic Age Acceleration Difference) with hemostatic factors (Fibrinogen, PAI-1, aPTT), where the advanced epigenetic age correlated with prothrombotic hemostatic profile61. Other studies point out to the diversity of cell populations tested when testing blood methylation clocks. This certainly could have impacted our study. Fahy et al. describe 2-year decrease in epigenetic opposite (assessed by DNAmGrimAge) to chronological age after treatment focused on regeneration of thymus. The epigenetic clock methylation-based methods seem valid as it shows rejuvenation after certain procedures or lifestyle changes and as it can successfully predict lifespan and health-span of subject24,62.
While our hypothesis was that plasmapheresis might promote epigenetic rejuvenation by clearing pro-aging factors, the observed acceleration in epigenetic clocks suggests an alternative biological response. The observed increase in epigenetic aging markers (e.g., DNAmGrimAge, Hannum Clock, DunedinPACE) following plasmapheresis may reflect a stress response triggered by the procedure, particularly given the short duration of the study. Recent studies have demonstrated that epigenetic age can increase in response to acute stressors such as surgery but may be reversible upon recovery. For example, Poganik et al. reported transient increases in epigenetic age following major surgery, with subsequent restoration over weeks to months, suggesting that such changes may not always indicate permanent aging acceleration. Future studies could plan to measure levels of cortisol, inflammatory cytokines (e.g., IL-6, TNF-α), and oxidative stress markers (e.g., 8-OHdG) to determine whether the observed epigenetic shifts correlate with systemic stress responses, providing a clearer interpretation of the changes in epigenetic aging markers63,64,65.
The increase in naïve CD4 + T cells (predicted by methylation profile) is considered an improvement as generally naïve T cells decline with aging, and they have a lower epigenetic age than other immune cells. This may indicate a short-term recovery response is countering this response, and that longer-term follow up could reveal more benefits of treatment66,67.
Other epigenetic clock markers did not reach statistical significance, none of them showing signs of rejuvenation when using the protocol suggesting more research is needed to specify what the protocol of therapeutical plasmapheresis should look like and whether it is beneficial in the long run for a healthy human cohort, or should it be used only in a subgroup of specific patients (such as those with hypercholesterolemia, obesity or others).
Our findings contrast with some of the previous human studies that investigated the effects of plasmapheresis and plasma exchange on aging biomarkers. Li et al. reported a reduction in biological age after double-filtration plasmapheresis, estimating a decrease of 4.47 years in men and 8.36 years in women based on a multi-biomarker approach. Their study indicated a potential rejuvenating effect of plasmapheresis, particularly in metabolic and inflammatory markers, which contradicts our findings where epigenetic aging markers, such as DNAmGrimAge and DunedinPACE, showed an increase. One possible explanation for this discrepancy could be the differences in protocol—while our study utilized a standard plasmapheresis protocol without albumin or young plasma replacement, Li et al. used a specific double-filtration approach, which might selectively remove different plasma components, leading to distinct biological responses. The methodological variation suggests that the effects of plasmapheresis on aging biomarkers are highly dependent on the procedure specifics and warrants further investigation into optimal parameters for potential rejuvenation, especially the albumin replacement23.
Although all participants in this study were screened to be healthy blood donors, we acknowledge that individual lifestyle factors—such as diet, exercise, and baseline inflammatory status—may also modulate epigenetic modifications and contribute to the variability in epigenetic clock measurements. Previous studies have demonstrated that dietary patterns and physical activity can influence systemic inflammation and DNA methylation profiles, potentially affecting markers such as DNAmGrimAge, the Hannum clock, and DunedinPACE68,69.
Similarly, the study by Mehdipour et al. demonstrated that repositioning therapeutic plasma exchange (TPE) to dilute systemic pro-aging factors can recalibrate critical signaling pathways to a more youthful state. However, our data do not support such claims in the absence of albumin replacement. Instead, we observed an increase in epigenetic age acceleration markers, which may be attributed to acute physiological stress or the loss of beneficial plasma components that were not replenished. More recent work by Gilmutdinova et al. suggests that hardware plasmapheresis can positively impact aging biomarkers when performed with albumin supplementation. The discrepancy between these results suggests that the presence of albumin, or possibly other plasma constituents, may play a critical role in mitigating the potentially adverse effects of plasmapheresis on epigenetic aging. Further clinical studies incorporating albumin replacement or alternative modifications to plasma exchange protocols are necessary to determine whether plasmapheresis can be optimized for longevity benefits without accelerating epigenetic aging10,11.
In the context of our findings, where the methylation-based predictors for ADM, B2M, pack-years, PAI-1, and COX increased with the number of plasmapheresis sessions, and leptin levels decreased, we certainly cannot state a major rejuvenation effect. PAI-1 and COX have been shown to be tightly linked to age-related diseases and processes. For instance, studies demonstrate that PAI-1 levels, a critical marker of cellular senescence and inflammation, predict lifespan and healthspan, suggesting that the increase in PAI-1 methylation with plasmapheresis might reflect changes related to aging rather than rejuvenation26. Similarly, elevated COX methylation has been implicated in cancer progression and age-related pathologies, and methylation of COX-2, for example, is considered an early event in carcinogenesis70. On the other hand, the observed decrease in leptin, a hormone closely tied to metabolic aging and inflammation, might suggest a modulatory effect of plasmapheresis on metabolic pathways, though it remains unclear whether this indicates a rejuvenating effect or simply metabolic adaptation to repeated interventions. These results align with broader patterns seen in epigenetic studies, indicating that while certain markers of senescence and inflammation increase, the systemic effects of plasmapheresis on aging remain complex and warrant further investigation to clarify their potential rejuvenating properties.
Conclusions
No participant had to stop the study due to severe negative long-term effects or sickness caused by the plasmapheresis. The observed reductions in total cholesterol, non-HDL cholesterol, and triglycerides are clinically relevant, as dyslipidemia is a major risk factor for cardiovascular disease. These lipid-lowering effects of plasmapheresis suggest its potential role in cardiovascular risk management. However significantly lower levels of albumin and proteins in general were measured. No epigenetic rejuvenation was shown.
Plasmapheresis has the potential to change the levels of proinflammatory and other pro-aging molecules in the circulation of donors, however the selected protocol of 8 pp in 18 weeks (4 pp in 9 weeks respectively) has not shown conclusive data supporting benefits of plasma extraction in general population. Our data do not provide evidence for a rejuvenating effect of plasmapheresis under the current protocol. In fact, the observed increase in certain epigenetic aging markers implies that repeated sessions may contribute to biological alterations consistent with accelerated aging. This finding underscores the necessity for further research to determine whether protocol modifications, such as the reintroduction of albumin or adjustment of donation frequency, might mitigate these effects. The protocol of donating plasma every two weeks, although deemed safe by many countries around the world, is not yet well researched and cannot therefore be marked as benefiting to the donor right now.
The procedure of plasma donation is, mostly due to economic incentives, increasingly adopted worldwide, often following a biweekly protocol without the reintroduction of albumin. While this approach has demonstrated reductions in plasma triglycerides and LDL cholesterol levels, our study has not established a definitive therapeutic benefit. Moreover, there are emerging concerns regarding potential risks associated with this practice. Specifically, epigenetic clock analyses failed to show any rejuvenation effects. In fact, some components of the epigenetic clocks or epigenetic clock analyses themselves indicated an accelerated aging process, thereby potentially increasing long-term health risks associated with regular plasma donation under these conditions.
Plasmapheresis, although according to world literature a promising technique for body rejuvenation, is not yet ready for a rejuvenation technologies spotlight and should be further studied. The main proposition for future studies to be learned from our publication is to consider prolonging the intervals between each plasmapheresis and to substitute for vital parts of plasma that are being diminished by the procedure, such as albumin. On the other hand, the depletion of cholesterol, could point to a decreased risk of cardiovascular mortality after plasmaphereses cycles.
The future promise of this technology lies in the possibility of reducing cholesterol and possibly other damaging pro-inflammatory or pro-aging molecules, thus lowering the amount of medication needed to lower risks of chronic diseases. However, further refinement to balance the clearance of pro-aging factors with the maintenance of systemic homeostasis is needed. Future research should therefore focus on integrating supportive measures and comparing plasmapheresis with alternative interventions to ensure a safe and effective rejuvenating regimen best for the individual.
Study limitations
While our study provides novel insights into the biochemical, hematological, and epigenetic impacts of plasmapheresis, several limitations should be acknowledged. First, the relatively small sample size of 34 finishing participants comprising of first-time plasma donors limits the statistical power and generalizability of our findings. Additionally, our cohort was restricted to individuals aged 40 to 60 years in accordance with Czech regulatory guidelines, which, although intentional to focus on an older population where rejuvenating effects might be most apparent, constrains the evaluation of age-related differences across a broader demographic. Furthermore, the 18-week duration of the study, while sufficient to detect rapid alterations in key biomarkers under an intensive plasmapheresis protocol, may not fully capture the long-term implications of these changes. Due to our trial taking place during spring and summer months, we cannot fully separate the effects of increased sunlight exposure, outdoor physical activity, and dietary changes from the observed rises in Vitamin D and concurrent shifts in DNAm-based aging metrics. We did not collect objective measures of activity or diet, so these factors remain potential confounders. Future studies with larger, more diverse cohorts, subgroups with albumin or other replacements and extended follow-up periods will be essential to further elucidate the underlying mechanisms and clinical relevance of plasmapheresis-induced modifications in epigenetic aging.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153(6), 1194–1217 (2013).
Bwiza CP, Son JM, Lee C. Integrated Theories of Biological Aging. (2019).
Schmeer, C., Kretz, A., Wengerodt, D., Stojiljkovic, M. & Witte, O. W. Dissecting aging and senescence-current concepts and open lessons. Cells (2019).
Oh, J., Lee, Y. D. & Wagers, A. J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 20(8), 870–880 (2014).
Haran, J. P. & McCormick, B. A. Aging, frailty, and the microbiome-how dysbiosis influences human aging and disease. Gastroenterology 160(2), 507–523 (2021).
da Costa, J. P. et al. A synopsis on aging-theories, mechanisms and future prospects. Ageing Res. Rev. 29, 90–112 (2016).
Rattan, S. I. Ageing genes: Gerontogenes. eLS https://doi.org/10.1002/9780470015902.a0003059.pub3 (2018).
Simpson, D. J., Olova, N. N. & Chandra, T. Cellular reprogramming and epigenetic rejuvenation. Clin Epigenet. 13(1), 170 (2021).
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19(6), 371–384 (2018).
Mehdipour, M., Etienne, J. & Liu, C. Attenuation of age-elevated blood factors by repositioning plasmapheresis: A novel perspective and approach. Transfus. Apheres. Sci. 60(3), 103162 (2021).
Gilmutdinova, I., Kudryashova, I. & Kostromina, E. The use of therapeutic plasmapheresis in preventive and sports medicine. BIO Web. Conf. 48, 01009 (2022).
Faria, R., Bucur, A. & Gordinho, A. Therapeutic Plasmapheresis: Seven Year Experience of an Intensive Care Unit in Portugal. Acta. Med. Port. (2021).
Scherer, N. V. & Bista, D. Management of asymptomatic severe hypertriglyceridemia. Proc. (Bayl. Univ. Med. Cent). 35(1), 58–59 (2022).
Jha, P. & Gebhard, D. Plasmapheresis for rescue in severe encephalopathy and multiorgan failure from fulminant influenza (H3N2) infection. Pediatr. Infect. Dis. J. 39(12), e464–e466 (2020).
Walters, G. Role of therapeutic plasmapheresis in ANCA-associated vasculitis. Pediatr. Nephrol. 31(2), 217–225 (2016).
Builes-Montano, C. E., Rodriguez-Arrieta, L. A. & Román-González, A. Therapeutic plasmapheresis for the treatment of thyrotoxicosis: A retrospective multi-center study. J. Clin. Apher. 36(5), 759–765 (2021).
Stahl, K., Schmidt, J. J. & Seeliger, B. Effect of therapeutic plasma exchange on endothelial activation and coagulation-related parameters in septic shock. Crit. Care. 24(1), 71 (2020).
Shariat, S. S., Zoofaghari, S. & Gheshlaghi, F. Effectiveness of plasmapheresis in aluminum phosphate poisoning. J. Res. Pharm. Pract. 10(1), 57 (2021).
Nye, R. & Singh, T. Use of CRRT and plasmapheresis to treat simultaneous iron and acetaminophen overdose. Blood Purif. (2021).
Mehdipour, M., Skinner, C. & Wong, N. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. Aging (Albany NY). 12(10), 8790–8819 (2020).
Mehdipour, M., Mehdipour, T. & Skinner, C. M. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. Geroscience. 43(1), 1–18 (2021).
Boada, M., Anaya, F. & Ortiz, P. Efficacy and safety of plasma exchange with 5% albumin to modify cerebrospinal fluid and plasma amyloid-? concentrations and cognition outcomes in Alzheimer’s disease patients: A multicenter, randomized. Controll. Clin. Trial. J. Alzheimers Dis. 56(1), 129–143 (2017).
Li, X., Zhang, J. & Sun, C. Application of biological age assessment of Chinese population in potential anti-ageing technology. Immun. Ageing. (2018).
Levine, M. E., Lu, A. T. & Quach, A. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10(4), 573–591 (2018).
Horvath, S., Oshima, J. & Martin, G. M. Epigenetic clock for skin and blood cells applied to hutchinson gilford progeria syndrome and ex vivo studies. Aging (Albany NY). 10(7), 1758 (2018).
Lu, A. T., Quach, A. & Wilson, J. G. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 11(2), 303 (2019).
Horvath, S. DNA methylation age of human tissues and cell types. Genome. Biol. (2013).
Aryee, M. J., Jaffe, A. E. & Corrada-Bravo, H. Minfi: A flexible and comprehensive bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30(10), 1363–1369 (2014).
Lu, A. T., Binder, A. M. & Zhang, J. DNA methylation GrimAge version 2. Aging (2022).
Belsky, D. W., Caspi, A., Corcoran, D. L. & DunedinPACE, A. DNA methylation biomarker of the Pace of Aging. Elife (2022).
Higgins-Chen, A. T., Thrush, K. L. & Wang, Y. A computational solution for bolstering reliability of epigenetic clocks: Implications for clinical trials and longitudinal tracking. Nat. Aging. 2(7), 644–661 (2022).
Houseman, E. A. et al. Reference-free deconvolution of DNA methylation data and mediation by cell composition effects. BMC Bioinform. (2016).
Reeves, H. M. & Winters, J. L. The mechanisms of action of plasma exchange. Br. J. Haematol. 164(3), 342–351 (2014).
Kaplan, A. A. Therapeutic plasma exchange: A technical and operational review. J. Clin. Apher. 28(1), 3–10 (2013).
Bansal, N. et al. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin. Endocrinol. Metab. 98(12), 4890–4898 (2013).
Nayak, S., Bajpai, M., Maiwall, R. & Mohapatra, A. Changes in pH and electrolytes during therapeutic plasma exchange in patients with liver diseases and factors predictive of these changes. Ther. Apher. Dial. 24(6), 725–730 (2020).
Ypma, P. F., Muradin, A. & Kerkhoffs, J. L. Large volume apheresis: Electrolyte imbalance and loss of platelets; watch for clinically relevant disturbances. Transfus. Apheres. Sci. 48(2), 149 (2013).
Krishnan, R. G. & Coulthard, M. G. Minimising changes in plasma calcium and magnesium concentrations during plasmapheresis. Pediatr. Nephrol. 22(10), 1763–1766 (2007).
Weinstein, R. Hypocalcemic toxicity and atypical reactions in therapeutic plasma exchange. J. Clin. Apher. 16(4), 210–211 (2001).
Bialkowski, W., Blank, R. D., Zheng, C., Gottschall, J. L. & Papanek, P. E. Impact of frequent apheresis blood donation on bone density: A prospective, longitudinal, randomized, controlled trial. Bone Rep. (2018).
Hiemstra, T. F., Casian, A., Boraks, P., Jayne, D. R. & Schoenmakers, I. Plasma exchange induces vitamin D deficiency. QJM 107(2), 123–130 (2014).
Rosa-Bray, M. et al. Prospective multicentre study of the effect of voluntary plasmapheresis on plasma cholesterol levels in donors. Vox Sang. 105(2), 108 (2013).
Kardas, F., Çetin, A. & Solmaz, M. Successful treatment of homozygous familial hypercholesterolemia using cascade filtration plasmapheresis. Turkish J. Hematol. 29(4), 334–341 (2012).
Jung, E., Kong, S. Y., Ro, Y. S., Ryu, H. H. & Shin, S. D. Serum cholesterol levels and risk of cardiovascular death: A systematic review and a dose-response meta-analysis of prospective cohort studies. Int. J. Environ. Res. Pub. Health (2022).
Taborski, U. & Laitinen, T. Donor safety in an individualized plasmapheresis program – Results of an interim analysis. Transfus. Apheresis Sci. https://doi.org/10.1016/j.transci.2022 (2022).
Bechtloff, S. et al. A prospective trial on the safety of long-term intensive plasmapheresis in donors. Vox Sang. 88(3), 189–195 (2005).
Ciszewski, T. S., Ralston, S., Acteson, D., Wasi, S. & Strong, S. J. Protein levels and plasmapheresis intensity. Transfus. Med. 3(1), 59–65 (1993).
Hashemian, S. M. R., Shafigh, N. & Afzal, G. Plasmapheresis reduces cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Pulmonology 27(6), 486–492 (2021).
Yeh, J. H., Chien, P. J., Hsueh, Y. M., Shih, C. M. & Chiu, H. C. Changes in the lymphocyte subset after double-filtration plasmapheresis. Am. J. Clin. Pathol. 128(6), 940–944 (2007).
Inuzuka, R. & Abe, J. Red blood cell distribution width as a link between ineffective erythropoiesis and chronic inflammation in heart failure. Circ. J. 79(5), 974–975 (2015).
Arkew, M., Gemechu, K., Haile, K. & Asmerom, H. Red blood cell distribution width as novel biomarker in cardiovascular diseases: A literature review. J. Blood Med. 13, 413–424 (2022).
Fornal, M. et al. Association of red blood cell distribution width, inflammation markers and morphological as well as rheological erythrocyte parameters with target organ damage in hypertension. Clin. Hemorheol. Microcirc. 56(4), 325–335 (2014).
Romeo, R. D., Bellani, R. & McEwen, B. S. Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress. 8(4), 265–271 (2005).
Wirth, M. M., Meier, E. A., Fredrickson, B. L. & Schultheiss, O. C. Relationship between salivary cortisol and progesterone levels in humans. Biol. Psychol. 74(1), 104–107 (2007).
Sathi, P., Kalyan, S., Hitchcock, C. L., Pudek, M. & Prior, J. C. Progesterone therapy increases free thyroxine levels - Data from a randomized placebo-controlled 12-week hot flush trial. Clin. Endocrinol. (Oxf). 79(2), 282–287 (2013).
Krajewska-Kulak, E. & Sengupta, P. Thyroid Function in Male Infertility. Front. Endocrinol. (Lausanne) (2013).
Santin, A. P. & Furlanetto, T. W. Role of estrogen in thyroid function and growth regulation. J. Thyroid Res. https://doi.org/10.4061/2011/875125 (2011).
Moretti, R. & Caruso, P. The controversial role of homocysteine in neurology: From labs to clinical practice. Int. J. Mol. Sci. 20(1), 231 (2019).
Ganguly, P. & Alam, S. F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 14(1), 1–10 (2015).
Tselmin, S., Rodionov, R. N., Müller, G., Bornstein, S. & Julius, U. Homocysteine in lipoprotein apheresis patients - Retrospective data analysis in apheresis center of a university hospital. Atheroscler Suppl. 14(1), 123–128 (2013).
Ward-Caviness, C. K., Russell, A. G. & Weaver, A. M. Accelerated epigenetic age as a biomarker of cardiovascular sensitivity to traffic-related air pollution. Aging 12(23), 24141–24155 (2020).
Bell, C. G., Lowe, R. & Adams, P. D. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. (2019).
Levine, M. E. & Hagg, S. DNA methylation: Cause or consequence of aging?. Innov. Aging. 3(Suppl 1), S32–S33 (2019).
Venney, C. J., Anastasiadi, D., Wellenreuther, M. & Bernatchez, L. The evolutionary complexities of DNA methylation in animals: From plasticity to genetic evolution. Genome Biol. Evol. (2023).
Poganik, J. R. et al. Biological age is increased by stress and restored upon recovery. Cell Metab. 35(5), 807–820 (2023).
Kilpatrick, R. D., Rickabaugh, T. & Hultin, L. E. Homeostasis of the naive CD4+ T cell compartment during aging. J. Immunol. 180(3), 1499 (2008).
González-Bermúdez, B., Kobayashi, H. & Abarca-Ortega, A. Aging is accompanied by T-cell stiffening and reduced interstitial migration through dysfunctional nuclear organization. Immunology 167(4), 622–639 (2022).
Fiorito, G. et al. DNA methylation-based biomarkers of aging were slowed down in a two-year diet and physical activity intervention trial: The DAMA study. Aging Cell 20(10), e13439 (2021).
Quach, A. et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY). 9(2), 419–446 (2017).
Kim, H. G., Ryu, S. Y., Lee, K. H., Lee, J. H. & Kim, D. Y. Quantitative analysis of COX-2 promoter methylation in gastric carcinoma. Ann. Surg. Treat Res. 95(2), 111–120 (2018).
Acknowledgements
The authors would like to express their gratitude to the patients whose willingness to cooperate enabled the study to come into being.
Funding
The study was supported by Cooperatio UK – Health Sciences and Charles University, Faculty of Medicine in Hradec Kralove, the Czech Republic, project SVV-2025–260776.
Author information
Authors and Affiliations
Contributions
PB and PS conceptualized the study; HP, RB, MM, JG, CA in control of methodology; PB, VR investigated; PB, RB, MM, JG curated the data; PB and DH wrote the original draft of the manuscript; HP, RB, MM, JG, VR, IB, NM reviewed and edited the manuscript; RB, MM, JG, JK conducted the statistical analyses and visualizations; SH, ZF, LB supervised; PB administered the project; PS, ZF, LB acquired the funding.
Corresponding author
Ethics declarations
Competing interests
Steve Horvath and his UCLA team developed the first epigenetic clocks for human saliva, for all tissues (pan-tissue clock), for human mortality risk prediction (PhenoAge, GrimAge), and pan-mammalian clocks. The Regents of the University of California are the sole owner of patents and patent applications directed at epigenetic biomarkers for which SH is a named inventor; SH and Robert Brooke are founders and paid consultant of the non-profit Epigenetic Clock Development Foundation that licenses these patents. SH is a Principal Investigator at the Altos Labs, Cambridge Institute of Science. The remaining authors declare no interest.
Ethics
The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the University Hospital in Hradec Kralove, the Czech Republic (project identification code: PROGRES Q40-09, Q40-10 and Q40-11; reference number: 201705 183P; date of approval: May 2, 2017) with an expanded Ethics committee approval for the project “Studie vlivu darování krevní plazmy – Plasmapheresis”. Informed written consent was obtained from all subjects. The clinical study was recorded in clincaltrials.gov under the number NCT05004220, first submitted on 30/07/2021.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Borsky, P., Holmannova, D., Parova, H. et al. Human clinical trial of plasmapheresis effects on biomarkers of aging (efficacy and safety trial). Sci Rep 15, 21059 (2025). https://doi.org/10.1038/s41598-025-05396-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05396-0