Abstract
Drug-Coated Balloons (DCB) have been widely used in interventional treatment for coronary artery de novo lesions. However, DCB treatment still have a certain proportion of target vessel restenosis (TLR) and adverse follow-up events. Quantitative flow ratio (QFR) loss are important indicators for evaluating long-term vascular functional changes. However, in patients with de novo lesions treated by DCB, the potential risk and protective factors affecting QFR loss remain unclear. The aim of this study was to explore the factors affecting QFR loss in patients with de novo lesion after DCB-angioplasty. Patients who underwent DCB-only intervention de novo lesions and underwent coronary angiography within 12 ± 3 months were enrolled. The QFR loss was defined as difference between the immediate post-procedure QFR and follow-up QFR. The subjects were divided into high QFR loss and low QFR loss groups according to the binary method. The predictors of QFR loss were then analyzed. A total of 115 patients with 1-year follow-up were included in this study, and the median follow-up time was 357 days. Multivariate Logistic analysis showed that patients with diabetes mellitus (OR = 4.937, 95%CI 1.497–16.278, P = 0.009) and LDL-C > 1.8 mmol/L (OR = 2.575, 95%CI 1.021–6.493, P = 0.045) was significantly associated with higher QFR loss 1 year after surgery. In patients undergoing DCB treatment for coronary de novo lesions, diabetes is an independent risk factor for late QFR loss at 1 year. Conversely, achieving LDL-C targets during follow-up is an independent protective factor against late QFR loss at 1 year.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI) is currently the primary treatment for revascularization in coronary artery disease patients. Although drug-eluting stents (DES) remains the primary choice in PCI, concerns persist regarding the endothelial damage and in-stent restenosis (ISR) it may cause1. Therefore, the concept of avoiding permanent implants is gaining increased attention.
Drug-coated balloons (DCB) have emerged as a favorable alternative to DES, delivering high concentrations of antiproliferative drugs to the endothelium without leaving a permanent scaffold2,3. Clinical practice is increasingly favoring the use of DCBs for treating coronary artery disease, particularly for ISR, with the 2018 European Guidelines on Myocardial Revascularization recommending DCBs as the first-line therapy4. Additionally, the feasibility of DCBs in treating de novo coronary lesions is also being validated5,6,7. However, there is limited research on changes in coronary physiological function during follow-up after DCB treatment, largely due to the invasive and complex procedure of fractional flow reserve (FFR).
Recently, the quantitative flow ratio (QFR), a novel index for coronary physiological function assessment, has been increasingly adopted in daily practice as well as clinical trials. QFR is a simple, non-invasive method for simulating FFR, based on 3D quantitative coronary angiography and hemodynamic analysis8, and has been validated to have good correlation and consistency with FFR9,10,11.
Therefore, in this study, we retrospectively analyzed and compared lumen diameter and QFR changes during follow-up in patients treated with DCB. Our aim was to identify risk factors affecting late lumen loss and QFR loss, and to explore their predictive value for optimizing outcomes with DCB intervention.
Methods
Study design and population
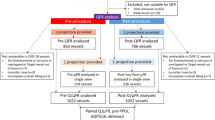
This single-center retrospective cohort study included 120 cases with de novo lesions treated with DCB from January 2017 to December 2021 at Sir Run Run Shaw Hospital, Hangzhou, China. Follow-up coronary angiography was performed 12 ± 3 months post-procedure. Exclusion criteria encompassed: (1) TIMI flow grade < 3 at baseline; (2) major procedural complications requiring DES implantation; (3) inadequate angiographic image quality limiting the QFR analysis.
The flowchart outlining the study process is depicted in Fig. 1. Following the screening process, a total of 115 patients with available QFR data and follow-up were included. The research was carried out in accordance with the guidelines established by the Declaration of Helsinki and was approved by the Institutional Ethics Committee at Sir Run Run Shaw Hospital. Informed consent was obtained from all participants prior to their involvement in the study.
Procedure protocol
All patients received a pre-procedural loading dose of dual antiplatelet therapy, comprising 300 mg of aspirin and either 300 mg of clopidogrel or 180 mg of ticagrelor, administered one day in advance. CAG was performed with conventional technique and transradial approach. The target lesion was clearly visualized from multiple angles. Patients were considered suitable for DCB-only intervention based on clinical and angiographic assessments, following current guideline recommendations and expert consensus. Specifically, DCB treatment was selected for de novo coronary artery disease in lesions with favorable morphological characteristics, such as non-severely calcified, non-ostial, and non-bifurcation lesions without significant dissection after pre-dilatation. Coronary de novo lesions were treated using paclitaxel-coated balloons, including Sequent Please (B Braun Melungeon, Germany), Vesselin (Lepu Medical, Beijing, China), and Swide (Shenqi Medical, Shanghai, China). The specific strategy for PCI and the utilization of intravascular imaging were left to the discretion of the operator. The success of angioplasty was determined by visually assessing the lumen after PCI, with a maximum diameter stenosis of less than 30%.
QFR assessment
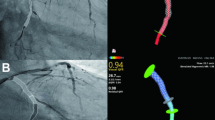
QFR measurements were conducted using an offline QFR system software (AngioPlus, Pulse Medical Imaging Technology, Shanghai Co. Ltd., Shanghai, China). At least two angiographic sequences were transferred to the QFR system. These sequences had to meet specific criteria, including a spatial angle of ≥ 25°, clear visualization of target lesion features, and proper filling of contrast agent. The system automatically identified the vessel type, traced the trajectory of the target lesion, and accurately outlined the vessel contour to exclude artifacts and branch vessel effects. The quantitative coronary angiography (QCA) data were provided. The following parameters were assessed: reference vessel diameter (RVD), minimal lumen diameter (MLD), percent diameter stenosis (%DS) and the QFR in selected vessels. The QFR was measured by two experienced researchers with a QFR reading license.
Follow-up and endpoints
In this study, patients underwent follow-up coronary angiography and QFR analysis 1 year after DCB intervention. The primary endpoint of this study was QFR loss, expressed as the difference between the immediate post-procedure QFR and follow-up QFR.
Statistical analysis
Categorical variables were reported as frequency (percentage), and group comparisons of categorical variables were assessed using the Pearson chi-square test. Continuous variables were presented as mean ± SD or median [interquartile range]. For the analysis of continuous variables, comparisons between two groups were conducted using the t-test or Kruskal-Walli’s test. Univariate and multivariate regression analysis were used to determine the predictive factors of QFR loss. Data analysis and graph production were performed using SPSS 26 (IBM SPSS Statistics), Prism 8.4.3 (GraphPad Software), and R 4.2.2 (R Foundation for Statistical Computing). The significance level was set at P < 0.05.
Results
Baseline clinical characteristics
A total of 115 patients, including 93 males and 22 females, with average age of 60.88 ± 11.15 years were enrolled. Patients were dichotomized into two groups: a low QFR loss group (QFR loss ≤ 0.05) with 58 patients and a high QFR loss group (QFR loss > 0.05) with 57 patients. The baseline characteristics of the QFR loss group are presented in Table 1. There were no significant differences in age, gender and laboratory examinations between patients with low or high QFR loss. The incidence rates of diabetes were significantly higher in the high QFR loss group compared to the low QFR loss group (34.7% vs. 13.3%, p = 0.030).
Procedure and QFR data
The characteristics of the lesions, procedures and QFR data are presented in Table 2. Out of the 115 lesions, 64(55.7%) were located in the left anterior descending arteries (LAD), 34(29.6%) in the left circumflex arteries (LCX), and 17 (14.8%) in the right coronary arteries (RCA). The high QFR loss group had a higher proportion of LAD lesions (68.4% vs. 43.1%, p = 0.020). The baseline average MLD was 1.07 ± 0.38 mm, and median QFR was 0.68[0.50, 0.76] prior DCB treatment. 54(47.0%) lesions used DCBs with a diameter greater than 3 mm, and 52(45.2%) lesions used DCBs with a length greater than 20 mm. The immediate post-procedure QFR value was 0.93 [0.89, 0.96]. The There were no significant differences between the two groups in terms of DCB specifications used and post-procedure QFR.
Regression of QFR loss predictors
To investigate the factors affecting QFR loss 1-year after DCB treatment, we analyzed the impact of different factors on QFR loss using a univariate logistic regression model, as shown in Table 3. The results indicate that diabetes mellitus (OR = 3.453, 95%CI 1.219–9.785, p = 0.020), lesions in LAD (OR = 2.143, 95%CI 1.008–4.555, p = 0.048), and LDL-C > 1.8 mmol/L at the 1-year follow-up (OR = 2.550, 95%CI 1.178–5.519, P = 0.017) were significantly associated with QFR loss in the univariate regression. When these three variables were included in a multivariate logistic regression model, the results showed that diabetes mellitus (OR = 4.937, 95%CI 1.497–16.278, p = 0.009) and LDL-C > 1.8 mmol/L at the 1-year follow-up (OR = 2.575, 95%CI 1.021–6.493, p = 0.045) were significantly associated with QFR loss greater than 0.05 at 1-yaer after DCB-treatment. Other factors, including hypertension, smoking, preoperative MLD, preoperative QFR, lesion location, intraoperative use of IVUS and special balloons, DCB specifications, postoperative MLD, and postoperative QFR, were not significantly associated with QFR loss at one year.
Discussion
This retrospective study explored the factors influencing QFR loss in patients with coronary de novo lesions treated with DCBs. The study included 115 patients who underwent DCB therapy and had follow-up coronary angiography 12 ± 3 months post-procedure. The findings revealed that diabetes and 1-year LDL-C > 1.8 mmol/L were identified as independent factors associated with QFR loss.
Coronary restenosis after PCI involves multiple complex pathophysiological processes, primarily driven by excessive neointimal hyperplasia, including endothelial injury and activation, inflammatory response, and the migration and proliferation of vascular smooth muscle cells12,13. In contemporary coronary interventional therapy, where DES implantation is the predominant approach, late in-stent restenosis remains a troubling and common complication, and it is one of the major factors affecting patient prognosis14. The presence of a stent as a foreign body induces endothelial cell damage and inflammatory responses, which promote neointimal hyperplasia15.Previous research has extensively explored the mechanisms and influencing factors of ISR following DES implantation. Multiple factors are associated with the occurrence of ISR, including common cardiovascular risk factors such as sex, age, obesity, smoking, hypertension, and diabetes, as well as patient-specific coronary lesion characteristics, such as lesion location and nature. Additionally, procedural factors related to PCI, including operator technique and decision-making, are also closely linked to the incidence of ISR1,16,17.
With the increasing use of DCBs in coronary interventions, the incidence of restenosis and late lumen loss following DCB treatment appears to be lower compared to drug-eluting stents (DES)18. DCBs apply antiproliferative drugs uniformly on the balloon surface, which are rapidly released and have prolonged effects upon contact with the vessel endothelium, thereby helping to prevent restenosis3. Furthermore, the absence of metallic or polymeric implants in DCBs reduces the risk of further lumen narrowing, giving DCBs certain advantages over DES. However, some studies have reported that the restenosis rate after DCB implantation can be higher than that after DES implantation19. Unlike DES, which induces restenosis through direct endothelial injury and inflammatory responses due to its wall-adhering design, DCBs lack mechanical structural support. Consequently, vascular elastic recoil and coronary dissection post-DCB implantation may be significant factors contributing to restenosis after DCB treatment20.
Late lumen loss (LLL) is an angiographic measure of luminal diameter reduction over time and serves as a morphological marker for restenosis. While LLL provides valuable anatomical information, it does not directly reflect the functional impact about the hemodynamic significance of the lesion, which is critical for evaluating clinical outcomes. Moreover, LLL assessment relies on two-dimensional angiographic measurements, which may not fully capture the complexity of vessel remodeling and microvascular dysfunction. Therefore, it is essential to evaluate the loss of vascular function from a physiological perspective during follow-up, with FFR serving as the gold standard for such assessments. FFR is an invasive physiological assessment that requires the use of a pressure wire and pharmacologically induced hyperemia to evaluate the functional significance of coronary stenosis. Given that restenosis after DCB treatment can lead to impaired blood flow despite preserved angiographic lumen dimensions, a functional assessment approach is crucial for detecting hemodynamically significant lesions. However, FFR measurement requires additional procedural time, specialized equipment, and pharmacological induction of hyperemia, which significantly limits its application during post-procedural angiographic follow-up. QFR offers a unique advantage as a less invasive, and cost-effective physiological assessment alternative to FFR. It enables a functional evaluation of lesion severity by computationally estimating pressure gradients, making it a practical and patient-friendly alternative for follow-up after DCB treatment. Since vessel function loss over time is a key concern in these patients, QFR serves as an important follow-up tool for evaluating functional deterioration. Based on this, our study investigate the risk factors for QFR loss at one year post-DCB treatment and found significant associations with diabetes and LDL-C target attainment during follow-up.
Previous studies have confirmed that diabetes is an independent risk factor for ISR following DES implantation16,21, with a 2- to eightfold increased risk of ISR in diabetic patients across various coronary disease populations. Our study similarly found that diabetes was associated with a 4.9-fold increased risk of QFR loss greater than 0.05 one year after DCB treatment, indicating that diabetes poses a high risk for both anatomical lumen narrowing and loss of blood flow function. The reasons for diabetes-induced restenosis and loss of physiological function are complex and multifactorial. Hyperglycemia in diabetic patients increases erythrocyte viscosity and promotes protein glycation and oxidation, leading to endothelial cell damage, impaired vasomotor function, and reduced vascular active substances22. Furthermore, chronic hyperglycemia induces oxidative stress and systemic inflammation via activation of the NLRP3 inflammasome, leading to elevated interleukin-1β (IL-1β) and C-reactive protein (CRP) levels, which promote vascular smooth muscle cell (VSMC) migration and neointimal hyperplasia23. Advanced glycation end-products (AGEs) reduce nitric oxide (NO) bioavailability, impairing endothelium-dependent vasodilation and promoting microvascular rarefaction24. Additionally, diabetic patients with glucose metabolism imbalance are more likely to have activated endothelial cell inflammation, contributing to new plaque formation25. Hyperglycemia also disrupts lipid metabolism, further promoting target vessel lumen and functional loss. Furthermore, HbA1c, a product of glucose binding to hemoglobin that reflects average blood glucose levels over the past 3 months, is a stable indicator of long-term glucose control. Higher levels of HbA1c have been associated with in-stent restenosis and adverse outcomes26, suggesting that rigorous glucose control may protect against post-procedural restenosis. These mechanisms collectively contribute to progressive progressive functional loss despite an apparently stable angiographic appearance. Prior studies have demonstrated that diabetic patients often experience worse long-term vascular healing following PCI, which may help explain the greater decline in QFR observed in our cohort.
Elevated levels of lipids, particularly LDL-C, are well-recognized as strong risk factors for coronary atherosclerotic heart disease. LDL-C plays a critical role not only in the formation of atherosclerotic plaques27 but also as an independent risk factor for restenosis following PCI28. LDL-C is ingested by monocytes or macrophages and migrates into the endothelium, where lipid and lipoprotein deposits beneath the endothelium are a crucial step in the development of neointimal atherosclerosis. Additionally, LDL and VLDL, along with their oxidized forms, have pro-inflammatory effects that activate endothelial cells, contributing to the occurrence of restenosis after PCI29. LDL-C oxidation triggers inflammatory responses, leading to endothelial activation and increased vascular stiffness, which may explain why patients with higher LDL-C levels experience greater QFR loss. The latest European guidelines on the management of dyslipidemia recommend an LDL-C target of less than 1.4 mmol/L for high-risk patients with established atherosclerotic events, representing a more stringent goal than previous guidelines30. A large-scale clinical study showed that, despite aggressive lipid-lowering therapy, only about 60% of high-risk patients achieved the LDL-C target, falling short of guideline recommendations31. In our study, the LDL-C target rate (LDL-C ≤ 1.8 mmol/L) at one year of follow-up was only 49.6%, and this target rate was significantly negatively correlated with post-procedural QFR loss, indicating that achieving LDL-C targets during follow-up is an independent protective factor against long-term QFR loss. This finding is consistent with conclusions from studies on DES implantation, where achieving LDL-C targets significantly reduced the incidence of ISR32. Chen et al.29 also found that, at one-year follow-up after PCI, patients achieving LDL-C targets had lower maximum diameter stenosis, higher QFR, and lower rates of major adverse cardiovascular events. This suggests that intensifying lipid-lowering treatment and strictly controlling lipid levels post-PCI, whether DES or DCB is used, is beneficial in reducing lumen loss and QFR loss.
Interestingly, we found a higher proportion of LAD lesions in the high QFR loss group (68.4% vs. 43.1%, p = 0.02). Although LAD was not significantly associated with QFR loss in the multivariate regression analysis, this finding suggests that anatomical factors may play a role in QFR loss following DCB treatment. The LAD supplies a large myocardial territory, making flow impairment in this artery more clinically significant compared to other coronary vessels. Even minor changes in lumen diameter or endothelial function can have a greater hemodynamic impact in the LAD due to its high flow demand. The LAD typically has a longer course with more bifurcations, increasing susceptibility to flow turbulence and endothelial shear stress disturbances after DCB treatment. These biomechanical forces may promote neointimal hyperplasia and accelerate QFR loss. Additionally, the DCB-specific challenges in LAD lesions also deserve attention. LAD lesions are more likely to involve calcified or tortuous segments compared to non-LAD arteries, potentially compromising DCB’s uniform drug delivery and increasing the risk of suboptimal vascular healing. Given these considerations, LAD lesions may require more stringent surveillance to detect early functional deterioration, even in the absence of angiographic restenosis.
Furthermore, other factors related to ISR or loss of post-procedural physiological function have been identified in previous studies. Nozue et al. reported that immediate post-procedural maximum diameter stenosis is a significant determinant of FFR-CT. However, in this study, we did not find significant correlations between pre-procedural 3D-QCA and QFR data, or post-procedural 3D-QCA and QFR data with one-year lumen loss, %DS increase, and QFR loss. The segmentation for lumen loss, lumen narrowing, and QFR loss based on median values may have occurred earlier in the restenosis process, resulting in lower sensitivity of potential risk factors to endpoint events.
In summary, this study highlights the potential value of QFR loss as a surrogate marker for functional deterioration following DCB treatment. Early identification of patients at risk for functional decline could enable more personalized follow-up strategies. This could lead to closer monitoring and tailored interventions for patients at higher risk, potentially improving long-term outcomes. Clinicians should consider more aggressive management of these comorbidities to preserve vascular function and reduce the risk of late functional deterioration. This could include better glycemic control in diabetic patients and more effective lipid-lowering strategies.
Limitation
This study has some limitations. First, it is a single-center retrospective cohort study, including only patients who received DCB treatment for in-stent restenosis and had one-year follow-up angiographic data. The proportion of patients undergoing one-year follow-up angiography at our center is relatively low, introducing a degree of selection bias. The sample size is small, and the level of evidence is relatively low; larger-scale randomized controlled trials are needed for further validation. The cutoff value for QFR loss (ΔQFR = 0.05) was selected based on the median value of our cohort. We acknowledge the need for future studies to validate specific QFR loss thresholds against hard clinical endpoints to establish clinically meaningful cutoffs. Additionally, other biochemical indicators at follow-up were not included in the study; future research could explore the assessment and impact of laboratory test data on post-procedural lumen or QFR loss. Furthermore, while QFR loss serves as a surrogate marker for functional deterioration, its direct correlation with adverse cardiovascular outcomes remains to be fully validated. Further research is needed to strengthen the reliability of QFR loss as a clinically applicable parameter.
Conclusion
In patients undergoing DCB treatment for coronary de novo lesions, diabetes is an independent risk factor for late QFR loss at one year. Conversely, achieving LDL-C targets during follow-up is an independent protective factor against late QFR loss at one year.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Giustino, G. et al. Coronary in-stent restenosis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 80(4), 348–372 (2022).
Scheller, B. et al. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 110(7), 810–814 (2004).
Scheller, B. et al. Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J. Am. Coll. Cardiol. 42(8), 1415–1420 (2003).
Sousa-Uva, M. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardio-thoracic Surg. Off. J. Eur. Assoc. Cardio-thoracic Surg. 55(1), 4–90 (2019).
Latib, A. et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: The BELLO (balloon elution and late loss optimization) study. J. Am. Coll. Cardiol. 60(24), 2473–2480 (2012).
Yu, X. et al. Treatment of large de novo coronary lesions with paclitaxel-coated balloon only: results from a Chinese institute. Clin. Res. Cardiol. Off. J. German Cardiac Soc. 108(3), 234–243 (2019).
Schulz, A., Hauschild, T. & Kleber, F. X. Treatment of coronary de novo bifurcation lesions with DCB only strategy. Clin. Res. Cardiol. Off. J. German Cardiac Soc. 103(6), 451–456 (2014).
Tu, S. et al. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc. Interv. 7(7), 768–777 (2014).
Tu, S. et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc. Interv. 9(19), 2024–2035 (2016).
Xu, B. et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J. Am. Coll. Cardiol. 70(25), 3077–3087 (2017).
Zhang, R. et al. Post-PCI outcomes predicted by pre-intervention simulation of residual quantitative flow ratio using augmented reality. Int. J. Cardiol. 352, 33–39 (2022).
Gori, T. Restenosis after coronary stent implantation: Cellular mechanisms and potential of endothelial progenitor cells (a short guide for the interventional cardiologist). Cells 11(13), 2094 (2022).
Aoki, J. & Tanabe, K. Mechanisms of drug-eluting stent restenosis. Cardiovasc. Interv. Ther. 36(1), 23–29 (2021).
Cassese, S. et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart (Br. Cardiac Soc.) 100(2), 153–159 (2014).
Indolfi, C. et al. Molecular mechanisms of in-stent restenosis and approach to therapy with eluting stents. Trends Cardiovasc. Med. 13(4), 142–148 (2003).
Cho, J. Y. Identification of risk factors influencing in-stent restenosis with acute coronary syndrome presentation. Chonnam Med. J. 53(3), 203–210 (2017).
Jakubiak, G. K. et al. Pathogenesis and clinical significance of in-stent restenosis in patients with diabetes. Int. J. Environ. Res. Public Health 18(22), 11970 (2021).
Jeger, R. V. et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet (London, England) 392(10150), 849–856 (2018).
Chen, Y. et al. Comparison of 2 different drug-coated balloons in in-stent restenosis: The RESTORE ISR China randomized trial. JACC Cardiovasc. Interv. 11(23), 2368–2377 (2018).
Song, Z. & Li, G. Role of specific microRNAs in regulation of vascular smooth muscle cell differentiation and the response to injury. J. Cardiovasc. Transl. Res. 3(3), 246–250 (2010).
Flaherty, J. D. & Davidson, C. J. Diabetes and coronary revascularization. JAMA 293(12), 1501–1508 (2005).
Wilson, S. et al. Diabetes and restenosis. Cardiovasc. Diabetol. 21(1), 23 (2022).
Li, D. J. et al. Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress. Redox Biol. 15, 22–33 (2018).
Freidja, M. L. et al. AGEs breaking and antioxidant treatment improves endothelium-dependent dilation without effect on flow-mediated remodeling of resistance arteries in old Zucker diabetic rats. Cardiovasc. Diabetol. 3(13), 55 (2014).
Daemen, J. et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet (London, England) 369(9562), 667–678 (2007).
Ueda, H. et al. Glycosylated hemoglobin is a predictor of major adverse cardiac events after drug-eluting stent implantation in patients with diabetes mellitus. Cardiology 116(1), 51–57 (2010).
He, X. et al. Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol. Res. 165, 105447 (2021).
Mori, K. et al. Enhanced impact of cholesterol absorption marker on new atherosclerotic lesion progression after coronary intervention during statin therapy. J. Atheroscler. Thromb. 24(2), 123–132 (2017).
Kattoor, A. J., Kanuri, S. H. & Mehta, J. L. Role of Ox-LDL and LOX-1 in atherogenesis. Curr. Med. Chem. 26(9), 1693–1700 (2019).
Mach, F. et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 41(1), 111–188 (2020).
Duarte Lau, F. & Giugliano, R. P. Lipoprotein(a) and its significance in cardiovascular disease: A review. JAMA Cardiol. 7(7), 760–769 (2022).
Shimono, H. et al. Characteristics of recurrent in-stent restenosis after second- and third-generation drug-eluting stent implantation. Coron. Artery Dis. 32(1), 36–41 (2021).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82070408) and the Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (2023ZJ496).
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070408) and the Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (2023ZL496).
Author information
Authors and Affiliations
Contributions
XL H and WB Z conceived and designed the study. YH Z organized these data and drafted the manuscript. YH Z and TL H analyzed the data and drew the pictures. QQ B, FY C and TP Z conducted QCA analyses and QFR measurements. J L, and GS F detected any errors in the whole process. All authors have read and approved the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study was approved by the Ethics Committee of Sir Run Run Shaw Hospital of Zhejiang University. (20201217-36).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Y., Hu, T., Bian, Q. et al. Predictors for quantitative flow ratio loss in patients with de novo coronary artery disease treated with drug-coated balloons. Sci Rep 15, 21479 (2025). https://doi.org/10.1038/s41598-025-05578-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05578-w