Abstract
Microbial-induced calcite precipitation (MICP) has emerged as a sustainable soil improvement method over the past few decades. Despite its promise, challenges persist in controlling the injection rate of the cementation solution (CS) and mitigating the emission of harmful gases during the bio-cementation process. This study introduces Persian Gum (PG) as an additive to the MICP process to address these issues, enhancing the practical applications of bio-cementation. Direct shear tests were conducted to evaluate the effects of PG on the cementation process and the shear strength characteristics of MICP-treated sands. Samples were treated with varying volumes of bacterial and cementation solutions, PG concentrations, and spraying intervals of the CS and PG solutions. Results indicate a substantial increase in soil shear strength, ranging from 3.1 to 9.8 times, depending on the normal stress level. Additionally, this method effectively controls the application rate of CS and significantly reduces the emissions of carbon dioxide and ammonia by up to 20 times compared to the conventional MICP process. The test results along with Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD) analyses, reveal that the incorporation of PG not only enhances soil strength by up to 20% compared to traditional MICP but also naturally regulates the cementation process rate. This innovation eliminates the need for external control of CS application, making the treatment procedure more time-efficient and eco-friendly.
Similar content being viewed by others
Introduction
Soil improvement methods using bio-mediated or bio-based approaches encompass biomaterials and microbial activities, primarily including biopolymer treatment and bio-cementation1,2,3. Biopolymer treatment utilizes various biopolymers, such as different types of gums, as cementing agents. The process involves mixing biopolymers with water and soils to enhance the soil strength, reduce permeability, and improve erosion resistance4,5,6. Despite the benefits of biopolymers, challenges related to water exposure weakness and biodegradation hinder their widespread use in soil treatment7,8,9,10. Hence, further research is essential to address this problem and to increase the practical applications of biopolymers in soil stabilization projects8.
On the other hand, over the past few decades, bio-cementation has emerged as a green and effective method for stabilizing soil, mainly due to its environmental sustainability and stability against humidity and water presence1,8,11,12. This method has versatile applications13,14,15, such as enhancing soil strength16,17,18,19,20, mitigating liquefaction potential21, enhancing shear strength parameters of soil-steel interfaces22,23, repairing cracks24,25,26, reducing dust generation27,28,29,30, reducing efflorescence rate31, improving erosion resistance in diverse environmental conditions32,33,34,35, and removing heavy metals from polluted soil36,37,38. The method enhances the strength and stiffness of the treated soil by precipitating calcite crystals between soil particles, a process known as microbially induced calcite precipitation (MICP)39.
Previous studies on MICP have shown that during the process, urea decomposes into NH3 and CO2, elevating pH and fostering bacterial growth. Calcium ions from the cementation solution (CS) react with urea decomposition byproducts in water to produce calcium carbonate, stabilizing soil. However, this procedure leads to the release of ammonia and carbon dioxide gases into the air, a drawback of the MICP method. Additionally, previous studies showed that optimal sediment quality is achieved at a lower injection rate of CS1,40,41,42,43.
The soil MICP treatments include injection, immersion, mixing, and surface percolation of biochemical solutions into soil44,45. Injection is costly and may lead to uneven distribution and clogging of added materials within soil pores. Immersion is limited to lab scale, while mixing is challenging and costly. Surface percolation is most suitable for field use but demands precise CS application control, hindering large-scale deployment44,46,47,48,49,50,51. This limitation makes the MICP method more time-consuming compared to conventional soil stabilization methods52,53.
This research endeavors to tackle the challenges associated with the application of CS in soil during MICP method. It employs Persian Gum (PG), a biopolymer, as a moisture absorbent to naturally control CS release and considers its impact on the emission of harmful gases during the MICP process. The method is referred to as Persian Gum Microbially Induced Calcite Precipitation (PG-MICP) in the following.
To date, several studies have investigated the use of gum biopolymer-biocement composites for soil stabilization, like xanthan and guar gums, aiming to reduce the costs, time, and resources required for the bio-cementation procedure23,32,54,55. Persian gum (PG), due to its anionic structure, shows stronger interaction with Ca²⁺, forms stable gels, and creates a robust biofilm network, making it more effective in soil stabilization compared to xanthan, arabic and guar gums56,57,58,59,60,61,62,63. Thus, PG superior performance justifies its selection in this study.
A previous study on PG properties has also shown that adding PG to a CaCl2 solution at concentrations above 0.015 mol/L can create a biofilm network due to the bridging effect of Ca2+ ions60. As CaCl2 is a main reactant in the MICP process, PG can allow for CS storage within the soil matrix and release it at an appropriately slow rate. This slow release not only improves the homogeneity of the soil stabilization process but also potentially reduces the emission of ammonia and carbon dioxide gases, a known drawback of the traditional MICP method.
By integrating PG into the bio-cementation process, this study aims to mitigate gas emissions and enhance the environmental sustainability of soil stabilization projects.
Therefore, this study aims to investigate the effectiveness of Persian Gum (PG) as a natural additive in the MICP process to enhance soil stabilization performance. The main objectives are to evaluate the impact of PG on the control of cementation solution application, the reduction of harmful gas emissions, and the improvement of mechanical properties, particularly shear strength, in treated sandy soils. It is hypothesized that the incorporation of PG not only facilitates a more controlled and eco-friendly bio-cementation process but also significantly enhances the mechanical strength of the soil.
Nevertheless, no studies have considered the combination of PG with the MICP method.
Materials and methods
Bacterial solution (BS)
The bacterium used as the biological catalyst in this study was the S. pasteurii strain (ATCC 11859). The bacteria were cultured in a completely sterilized medium consisting of nutrient broth (NB) and urea, and then harvested by centrifugation at 4000 rpm for 20 min at 25 ± 2℃. The resulting bacterial mass was washed with sodium phosphate buffer before being added to a solution of nutrient broth, urea, ammonium chloride, and sodium bicarbonate as BS. The concentration of bacteria in the BS was maintained at 109 cfu/ml (colony-forming units per milliliter) for all tests conducted, which falls within the optimal range of 108−1010 cfu/ml suggested in previous studies64,65. The pH of the culture medium was initially set to 7 at the start of the incubation phase.
Cementation solution (CS).
During a bio-cementation process, the main substances required are urea fertilizer and calcium chloride. In this study, CS was prepared by dissolving one mol of urea and one mol of calcium chloride in one liter of distilled water47.
Persian gum solution (PS).
To prepare PS, the PG, harvested from Prunus scoparia Spach in Fars province in Iran, was first ground into a fine powder and then sterilized by exposure to low-pressure UV lamps emitting light at a wavelength of 254 nm within a Laminar Flow Hood. The sterilized powder was kept in a sealed plastic bag during the sterilization process at a constant temperature of 24 °C. The dry PG powder passed through sieve No. 100 (77.1% passed through sieve No. 200) was then used to prepare the PS.
The powder was mixed with distilled water at 5, 10, and 15 g/L concentrations using a magnetic mixer for two hours at 150 rpm and 60 °C. Initial experiments determined that a PG concentration of 10 g/L provided the optimal balance between gel formation and ease of application. Concentrations lower than 10 g/L resulted in insufficient gel formation, while higher concentrations led to excessive viscosity, hindering the uniform distribution of the solution. Furthermore, the 10 g/L concentration allowed for a controlled CS application rate of 0.278 mol/L per hour, which is consistent with previous studies indicating superior quality and uniformity in bio-cemented soil samples49.
To ensure that the addition of PG does not negatively impact the urease activity of the S. pasteurii bacterium in the NB and Urea culture medium, the amount of ammonia released from hydrolyzed urea was measured using the Nessler method, to quantify the urease activity of the bacteria66 in the presence of various concentrations of PG. Table 1 presents the results of these experiments, where one unit of urease activity (U) is defined as the amount of urease enzyme in 1 ml of the medium that can hydrolyze one micromole of urea per minute67,68. The results show that the presence of PG has even enhanced the urease activity of the S. pasteurii bacterium, which could be attributed to the presence of diverse sources of nutrients and mineral elements in the PG60. It is worth noting that some parallel tests (not reported here) showed that, unlike the PG, adding Arabic and Xanthan gum could have a negative impact on the urease activity of the bacteria.
Soil characteristics
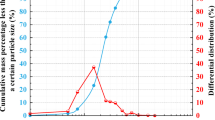
In the experiment, poorly graded quartz sand with sub-rounded particles (classified as SP according to USCS) and the friction angle of 31° for the untreated soil was obtained based on the results of a direct shear test. Figure 1 illustrates the gradation curve of the sand, while Table 2 outlines some basic geotechnical properties of the soil. Before preparing the samples, the soil was washed over a sieve #200 and dried for 24 h at 100 °C. The maximum dry density of the soil was found to be 16.1 kN/m3.
Gas measurement device
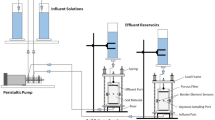
An attempt was made here to evaluate the effect of the combined method PG-MICP on the release rate of harmful gases like ammonia and carbon dioxide, during MICP-based soil stabilization. The experiment used a 250 mL flat-bottom glass balloon container with two openings, a 5 ml pipette, a 2 mm silicone hose, a rubber pipette, and a 10 mm gas flowmeter. A soil sample of 100 g was poured into the balloon and the bio-chemical solutions were added instantly in the case with Persian gum biopolymer and gradually at an injection rate of 0.278 mol/L per hour without Persian gum biopolymer. The quantification of gas emissions was ascertained through the motion of the ball float within the flowmeter. Figure 2 shows the components of the gas measurement device.
Experimental program
Sample Preparation
The soil samples were prepared in a dry state, with a relative density of 35%, using the dry pluviation method. The samples were directly placed in molds for the direct shear test. In both the conventional MICP and PG-MICP methods, the bacterial solution (BS) was first poured over the soil sample surface, followed by a resting period of 1 h. In the conventional MICP method, the cementation solution (CS) was sprayed onto the soil specimen at a controlled rate of 0.278 mol/L per hour49. In the PG-MICP method, a mixture of CS and Persian gum solution (PS) in equal portions was carefully and rapidly sprayed over the soil surface in two phases with time intervals of 12, 18, and 24 h, as shown in Fig. 322.
The treated soil samples were then placed in a temperature-controlled room (at 24 ± 1℃) for a curing period of 14 days. This allowed sufficient time for the bio-cementation process in the treated specimens to complete. Subsequently, the samples were oven-dried for 24 h to eliminate the potential impact of any suction on the test results before their shear strength was determined17,22.
Testing program
Given that the concentration of CaCl2 in the CS used in this study, exceeds 0.015 mol/L, the inclusion of PG is expected to result in the formation of a biofilm network that stores and gradually releases the CS60. Furthermore, the presence of PG enhances the urease activity of the S. pasteurii bacterium, as illustrated in Table 1. This can lead to improved performance of the MICP method, resulting in bio-cemented samples with higher shear strength parameters, achieved through higher urease activity and a lower release rate of CS.
In light of the above, the standard direct shear tests were performed on PG-MICP treated samples to determine their shear strength in accordance with ASTM D3080, using a shearing rate of 0.25 mm/min70. To eliminate the potential impact of temperature fluctuations on the results, all experiments were carried out in a controlled environment with a temperature of 24 ± 1℃71,72,73,74. The experiments were conducted at normal stresses of 50, 100, and 150 kPa, which are commonly encountered in geotechnical engineering projects. Additionally, SEM and XRD analyses were performed to investigate the calcium carbonate crystals and PG biofilms formed among and on the surfaces of the soil particles.
Previous studies75 have indicated that optimal microbial-induced calcite precipitation in soils can be achieved by utilizing equal volumes of BS and CS, referred to as VBS and VCS, respectively. Accordingly, in this study, VBS = VCS was adopted in all tests. In addition to the volume of BS and CS, and the injection interval of the CS/PS, some preliminary tests indicated that the concentration of PG in PS is also a key factor influencing the strength of the PG-MICP samples. Based on some previous studies [71,76], three levels of VBS = VCS = VV, 1.5 VV, and 2 VV were considered where VV is the volume of the voids in the sample prior to the treatment, as the first parameter. Additionally, this study investigated the effects of different mass concentrations of PG in PS at three levels: 5, 10, and 15 g/L, as the second parameter. The third parameter adopted was the spraying intervals of CS/PS, which were taken as 12, 18, and 24 h, as mentioned earlier.
To investigate the effect of the influencing parameters on the shear strength of the bio-treated soils, an L9 array was used to design the test layout based on the Taguchi Design Of Experiments (TDOE)77, as presented in Table 3. This test design approach has been frequently applied to various geotechnical topics, including slope stability78, determination of soil and rock strength parameters79, bearing capacity64,76, behavior of contaminated soils80, and improving shear strength parameters through the MICP method22,81. To ensure the accuracy of the test results, each test was conducted at least twice. Additionally, five control tests (in addition to the primary test program outlined in Table 3) were conducted to validate the TDOE predictions for five randomly selected combinations of the influencing factors.
Finally, the amounts of gas emissions from the conventional MICP method and the optimized PG-MICP procedure were compared to evaluate the effect of PG on the absorption of harmful gases.
Analysis of the experimental data
The shear strength characteristics of the PG-MICP sand samples were interpreted using the Mohr-Coulomb failure criterion. The shear strength parameters, the friction angle \(\:\text{(}\phi\:\text{)}\) and cohesion (\(\:\text{c}\)), were determined using the results of the standard direct shear tests. To further scrutinize the experimental data, standard statistical analyses were employed, and the signal-to-noise ratios (S/N) at various levels of the influencing parameters were calculated. The analysis of means (ANOM) and analysis of variance (ANOVA) were employed to determine the optimum level and the degree of participation (DOP) of each parameter. Further details on the formulation and application of ANOVA and ANOM can be found in Tan78, Bak, et al.64,, Mortazavi Bak, et al76,82., and Kariminia,, et al.81 (to name a few), and are not repeated here. Finally, SEM images and XRD analyses of soil samples were utilized to assess the effectiveness of microbial calcite precipitation and other possible binding materials resulting from the use of PG in soil pores and on the surface of soil particles after the bio-treatment.
Results and discussion
The Impact of PG-MICP on soil shear strength parameters
Table 4 displays the friction angles and cohesion values of PG-MICP soil samples obtained from direct shear tests and TDOE method predictions. Each test was conducted at three different stress levels and repeated at least twice, resulting in two reported friction angles (\(\:\phi\:\)) and cohesion values (c) for each case. Additionally, the table includes the results of the five randomly selected control tests (T10, T14, T21, T25, and T27) and their comparisons with the predictions of the TDOE method. Accordingly, a total of 84 separate direct shear tests were performed on PG-MICP samples in this study.
Figure 4 presents the results of the ANOM for the friction angle and cohesion of the PG-MICP samples. S/N values in Fig. 4 are derived from the data in Table 4, assuming that higher values of φ and c indicate better performance22.
From Fig. 4, it is evident that the optimal levels for VBS/Vv = VCS/Vv, the concentration of PG in PS, and the spraying interval are L2, L2, and L3, respectively, in terms of both friction angle and cohesion of the treated samples, corresponding to T5 sample. The results indicate that inducing a PG-MICP leads to a significant increase in both friction angle and cohesion of the soil (from 31.0 to up to 41.2 and from 0 to up to 251.5, respectively).
The optimal volume of BS and CS is found to be 1.5 times the soil volume void. This is in agreement with the volume used in previous MICP studies66,83. Moreover, The results agree with previous studies, indicating that soils with high permeability require higher amounts of BS for the surface percolation method compared to the injection method84.
The ideal concentration of PG in PS for optimal results was found to be 10 g/L, whereas a concentration of 15 g/L resulted in less uniform PG-MICP samples with reduced strength parameters. This behavior can primarily be attributed to the formation of a less permeable layer near the soil surface when a higher concentration of PG is used, resulting in a significant decrease in the rate of CS passing through the sample. This finding is consistent with several previous studies43, indicating that higher doses of PG do not necessarily result in better performance of the MICP treatment. It underscores the importance of achieving the right balance of BS, CS, and PS to induce the most efficient bio-cementation in soils.
The differences among S/N values of different levels are insignificant for the injection interval, indicating that changing the spraying interval has a minimal effect on the strength parameters of PG-MICP samples. This differs from normal MICP treatment of soils, as indicated by several previous findings49,50. This difference can be attributed to the use of PG in this study, which regulates the rate of CS/PS release, making the injection rate of CS/PS less influential.
Figure 5 presents the results of the ANOVA to determine the degree of importance of each influencing parameter in quantitative terms, expressed as DOPs.
The analysis indicates that among the affecting parameters, the concentration of PG in PS has the most significant impact on the strength parameters of the PG-MICP soil samples, with a DOP of 66.7% for friction angle and 73.4% for cohesion. On the other hand, the spraying rate is found to be the least influential parameter, with a DOP of 2.9% for friction angle and 10.8% for cohesion.
Model prediction accuracy was evaluated using the root mean square error (RMSE) and coefficient of determination (R²) According to Table 4. For friction, the RMSE was 0.2 for the control tests and 0.5 for the main test predictions, with a strong correlation (R² = 0.98) between predicted and observed values. For cohesion, the RMSE values were 7.7 kPa and 9.3 kPa for the control and main test predictions, respectively, with an R² of 0.81. As per the values of root mean square error and coefficient of determination, the TDOE predictions acceptably match the experimental data, and are consistent with previous findings on bio-cemented materials22, supporting the reliability of this method for predicting the other test outcomes in this study.
Shear strength enhancement
In this section, the shear strength of the PG-MICP samples is evaluated by utilizing the test data shown in Table 4. The evaluation the influence of the PG-MICP method on treated samples involves establishing a dimensionless parameter, termed the Shear Strength Efficiency Factor (SSEF). This factor is determined by the ratio of peak shear stress in MICP-treated soil to that in untreated soil and is defined as follows:
.
where superscripts PG-MICP and UTR stand for PG-MICP treated and un-treated, respectively, and \(\:{\sigma\:}_{n}\) and \(\:{\tau\:}_{p}\) are the effective normal stress and the peak shear stress, respectively, in the tests. Considering Eq. (1), higher values of SSEF indicate a more efficient PG-MICP process within the soil medium in terms of shear strength.
Figure 6 presents the SSEFs for all direct shear tests conducted during this study. The data clearly demonstrate that the SSEFs are significantly greater than 1 in all cases, typically ranging from 3.1 to 9.8. This indicates that the shear strength of the PG-MICP soil samples is several times greater than that of untreated samples under comparable conditions. The increase in shear strength is particularly pronounced at lower normal stresses, as the cohesion of the samples plays a more significant role in their shear strength under this condition17,85. To explain more, The improvement in shear strength due to PG-MICP treatment was more pronounced at lower normal stresses, which is in agreement with trends reported in the literature17,85,86. This behavior is primarily due to the dominant contribution of calcium carbonate bonding to the cohesion (c) of the soil matrix under low confining pressure. As the normal stress increases, particle interlocking and friction take over as the governing mechanisms, thereby reducing the relative impact of bio-cementation17,75. Additionally, at higher stresses, bond breakage or microcracks may form, limiting the strength gains17,75. Previous studies have also shown that MICP has a greater influence on cohesion than on internal friction angle (φ), further supporting the observed trends17,85,86.
T5 exhibits the highest SSEF values at all normal stress levels, consistent with the ANOM results discussed in earlier sections.
To separate the impact of adding PG to the MICP process, an Improvement Ratio (denoted as IR) is defined (Eq. 2) in which the reference shear strength is assumed as that of the corresponding MICP-treated sample without PS, but otherwise similar:
.
Table 5 summarizes the strength parameters and IRs for T5 sample. For the corresponding MICP sample, VBS, VCS and the spraying interval were adopted the same as those of T5; however, at each spraying interval, the target volume of CS was applied to the soil at the rate of 0.278 mol/L per hour49, as opposed to the instantaneous application used in the PG-MICP procedure.
As can be seen, the strength parameters of the PG-MICP sample are greater than those of the corresponding MICP sample, resulting in IR\(\:>\)1, which shows the added benefit of introducing PG to the MICP treatment.
To better demonstrate the enhancement in shear strength achieved by the optimized PG-MICP method (T5) compared to the conventional MICP process, Fig. 7 is presented as a plot. This plot illustrates the shear strength values at three confining stresses (50, 100, and 150 kPa) for both treatment approaches. The results clearly show that the PG-MICP method consistently delivers approximately 22–23% higher shear strength than the conventional MICP technique. This improvement is primarily attributed to the synergistic role of Persian gum in facilitating more uniform calcium carbonate precipitation and stronger interparticle bonding. In the corresponding MICP sample, the same VBS, VCS, and spraying intervals as in T5 were used; however, the cementation solution (CS) was applied at a controlled rate of 0.278 mol/L per hour49 at each interval, in contrast to the rapid spraying used in the PG-MICP process.
Previous studies have also shown that even when used on its own as a stabilizer, the introduction of PG to soil medium can boost the stiffness and strength characteristics of the treated soil56,87,88.
SEM and XRD analyses
SEM and XRD analyses were conducted to observe the calcite precipitation on and among particles in the untreated and MICP-treated and PG-MICP-treated soils (Fig. 8). In the MICP-treated soil samples, calcium carbonate crystals were identified on and among the soil particles (Fig. 8b). However, in the PG-MICP-treated soil sample with the highest shear strength (T5), sand particles were observed to be entirely coated with calcium carbonate crystals. Additionally, bio-cement bridges formed by PG biofilms were visible, connecting the particles (Fig. 8c). The biofilms formed amidst the soil particles are relatively small in amount owing to the minimal amount of PG biopolymer used. However, they generate a smooth surface, similar to the gel-type biofilms produced on the surface of soil particles in some previous studies89,90. These bridges enhance inter-particle bonding and reduce the void ratio, resulting in a higher shear strength compared to the untreated soil.
A variety of calcium carbonate sediments, such as amorphous carbonate, vaterite, and calcite, can be generated through a MICP process91. Among these, calcite is the most stable form of calcium carbonate and is, therefore, the preferred sediment for MICP treatments27,92,93. The XRD technique was utilized to determine the type of calcium carbonate crystals formed in the samples analyzed in this study. Figure 9 displays the XRD analysis results of the T5 sample, indicating that the calcium carbonate sediments in the sample were predominantly composed of calcite crystals. The findings show that the highest intensity of the calcite crystals in the MICP-treated sample was observed at 2θ of 29.6°, consistent with numerous prior studies [27,72,74,94]. Figure 6 illustrates a distinctive diffraction peak of quartz, which was expected as the soil employed in this investigation was composed of quartz sand. Although some vaterite traces were detected in the PG-MICP sample, their intensity was insignificant.
Gas emission and the PG-MICP efficiency
In this study, gas output during soil treatment procedure was measured using a gas flowmeter. It was found that the amount of gas produced from soil improved by the MICP method was 2 milliliters per 100 g of dried soil, whereas for soil improved by the PG-MICP method it was reduced to 0.1 milliliters per 100 g of dried soil. This significant reduction demonstrates that Persian gum not only aids in eliminating the time-consuming and expensive injection stage but also effectively absorbs harmful gases produced by the MICP method (i.e., ammonia and carbon dioxide), making it a completely eco-friendly approach.
As mentioned before, previous studies on the MICP methodology have highlighted that injecting CS at rates around 0.278 mol/L per hour yields bio-cemented samples of greater uniformity and superior quality49. Additionally, most studies suggest that the average volume of CS required for effective bio-cementation in sandy soils is about 1 to 1.5 times the soil’s void volume at one molarity22,66,83. Consequently, it takes between 3.5 and 5.5 h to inject the necessary CS into sandy soil, which poses challenges in terms of equipment, labor costs, and implementation time for field scale MICP soil stabilization projects.
The PG-MICP approach in large-scale soil stabilization projects, such as those aimed at combating desertification and suppressing dust storms, offers improved soil strength parameters compared to conventional MICP and opportunities to reduce both labor and fuel costs. The more efficient injection process facilitated by the new method can shorten project duration, reducing the need for frequent transportation of cementation materials and thereby lowering fuel expenses. By addressing injection challenges and enhancing overall project efficiency, this improved MICP method holds promise for significant cost reductions in desert soil stabilization projects.
Moreover, a 20-fold reduction in the release of harmful gases, such as carbon dioxide and ammonia, provides a much safer environment for executive personals during conducting the MICP operations.
Conclusion
The required low rate of applying the cementation solution (CS) in MICP presents a major challenge for field-scale projects. To this end, this study proposed a new bio-cementation method that eliminates the need for slow CS application. The proposed method enhances the conventional MICP by adding Persian gum (PG) to the CS. This naturally slows down the CS application rate in the MICP process due to PG’s storage capacity for soluble substances. Direct shear tests were conducted to determine and compare the strength characteristics of PG-MICP treated samples with those of the conventional MICP procedure. The test results confirmed the efficiency and superiority of the proposed PG-MICP approach. The main conclusions are outlined as follows:
-
(1)
The PG-MICP method shows significant promise for reducing costs and enhancing efficiency in field-scale soil stabilization projects. Unlike the time-consuming conventional inclusion of CS, the PG-MICP approach streamlines the inclusion process and results in significant cost reduction.
-
(2)
The optimal PG-MICP mixture for achieving the highest shear strength of the treated soil was determined through the ANOM approach. The ANOVA results confirmed that the PG concentration in PS has the greatest impact on the soil shear strength in consistent with the ANOM findings.
-
(3)
The shear strength of PG-MICP-treated soil samples significantly improved, ranging from 3.1 to 9.8 times that of untreated soil samples, depending on the applied normal stress level.
-
(4)
Comparing the improvement rate (IR) of bio-treated samples indicates that the PG-MICP method produced shear strength values up to 20% greater than those achieved through the conventional MICP method.
-
(5)
The SEM image and XRD analyses of the optimum PG-MICP sample confirmed the efficiency of the proposed method through presenting more concentrated calcite crystals bridging the soil particles.
-
(6)
Comparing gas emissions from the conventional MICP method with those from the PG-MICP procedure showed a 20-fold reduction in harmful gases, including carbon dioxide and ammonia, demonstrating the new PG-MICP approach as a more sustainable bio-cementation method.
Overall, this study demonstrates the high potential of the proposed PG-MICP method as an environmentally friendly soil stabilization technique. While this research emphasizes the potential benefits of using PG to enhance the efficiency and eco-friendliness of the MICP method, further investigation is required to evaluate the impact of other factors, such as temperature, pH, soil particle distribution, bacterial strain, and the effects of extended curing durations (beyond 14 days), on the strength of PG-MICP treated soils. Moreover, employing multiple mechanical characterization techniques will help achieve a more comprehensive understanding of the strength behavior of PG-MICP-treated soils. Future studies should consider spectroscopic or chromatographic methods to directly measure CO₂ and NH₃ produced during PG-MICP, quantify CS release using time-based and spectroscopic methods to clarify PG’s release behavior, and also include comparative assessments of various biopolymer-MICP combinations under consistent conditions to distinguish biopolymer effects from microbial-induced calcite precipitation. Future studies may incorporate direct quantitative methods such as SEM image analysis, TGA, or acid digestion techniques to more accurately measure calcite content and minimize variability between PG-MICP treated samples.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Choi, S. G. et al. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 246, 118415 (2020).
Saffari, R., Nikooee, E., Habibagahi, G. & Van Genuchten, M. T. Effects of biological stabilization on the water retention properties of unsaturated soils. J. Geotech. GeoEnviron. Eng. 145, 04019028 (2019).
Hobbenaghi, M., Baghbanan, A., Hashemolhoseini, H., Hosseini, M. & Ghazijahani, T. G. Presentation at Environment, Energy Security, and Sustainability Symposium and Exhibition E2S2. in Structures. 106915 (Elsevier).
Larson, S. L., Newman, J. K., Griggs, C. S., Beverly, M. & Nestler, C. C. Modified biopolymers as an alternative to petroleum-based polymers for soil modification; ESTCP ER-0920: treatability studies. (2012).
Khatami, H. R. & O’Kelly, B. C. Improving mechanical properties of sand using biopolymers. J. Geotech. GeoEnviron. Eng. 139, 1402–1406 (2013).
Nakamatsu, J. et al. Eco-friendly modification of earthen construction with carrageenan: water durability and mechanical assessment. Constr. Build. Mater. 139, 193–202 (2017).
Aaliya, B., Sunooj, K. V. & Lackner, M. Biopolymer composites: A review. Int. J. Biobased Plast. 3, 40–84 (2021).
Dagliya, M., Satyam, N. & Garg, A. Desert sand stabilization using biopolymers. Smart Constr. Sustainable Cities. 1, 5 (2023).
Terzis, D. & Laloui, L. A decade of progress and turning points in the Understanding of bio-improved soils: A review. Geomech. Energy Environ. 19, 100116 (2019).
Mahamaya, M., Das, S. K., Reddy, K. R. & Jain, S. Interaction of biopolymer with dispersive geomaterial and its characterization: an eco-friendly approach for erosion control. J. Clean. Prod. 312, 127778 (2021).
Zi, J. et al. Quantitatively characterizing sandy soil structure altered by MICP using multi-level thresholding segmentation algorithm. Journal Rock. Mech. Geotech. Engineering 16 (2024).
Hemayati, M., Nematollahi, A., Nikooee, E., Habibagahi, G. & Niazi, A. Non-ureolytic microbially induced carbonate precipitation: Investigating a cleaner biogeotechnical engineering pathway for soil mechanical improvement. The Journal of Engineering e12350 (2024). (2024).
Zhang, K. et al. Microbial–induced carbonate precipitation (MICP) technology: a review on the fundamentals and engineering applications. Environ. Earth Sci. 82, 229 (2023).
Fouladi, A. S., Arulrajah, A., Chu, J. & Horpibulsuk, S. Application of microbially induced calcite precipitation (MICP) technology in construction materials: A comprehensive review of waste stream contributions. Construction Building Materials, 131546 (2023).
Ahenkorah, I., Rahman, M. M., Karim, M. R. & Beecham, S. Unconfined compressive strength of MICP and EICP treated sands subjected to cycles of wetting-drying, freezing-thawing and elevated temperature: experimental and EPR modelling. J. Rock Mech. Geotech. Eng. 15, 1226–1247 (2023).
Su, F., Yang, Y., Qi, Y. & Zhang, H. Combining microbially induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 10, 107770 (2022).
Khaleghi, M. & Rowshanzamir, M. Biologic improvement of a sandy soil using single and mixed cultures: A comparison study. Soil Tillage. Res. 186, 112–119 (2019).
Mahawish, A., Bouazza, A. & Gates, W. P. Effect of particle size distribution on the bio-cementation of coarse aggregates. Acta Geotech. 13, 1019–1025 (2018).
Lee, L. M., Ng, W. S., Tan, C. K. & Hii, S. L. Bio-mediated soil improvement under various concentrations of cementation reagent. in Applied Mech. Materials 326–329 (Trans Tech Publ) (2012).
Saffari, R., Habibagahi, G., Nikooee, E. & Niazi, A. Biological stabilization of a swelling fine-grained soil: the role of microstructural changes in the shear behavior. Iran. J. Sci. Technol. Trans. Civil Eng. 41, 405–414 (2017).
Montoya, B., DeJong, J. & Boulanger, R. in Bio-and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013. 125–135 (ICE Publishing).
Bak, H. M., Kariminia, T., Shahbodagh, B., Rowshanzamir, M. A. & Khoshghalb, A. Application of bio-cementation to enhance shear strength parameters of soil-steel interface. Constr. Build. Mater. 294, 123470 (2021).
Mortazavi Bak, H., Khoshghalb, A., Shahbodagh, B. & Kariminiya, T. in International Conference on Transportation Geotechnics. 179–187 (Springer).
Choi, S. G., Wang, K., Wen, Z. & Chu, J. Mortar crack repair using microbial induced calcite precipitation method. Cem. Concr. Compos. 83, 209–221 (2017).
Son, H. M., Kim, H. Y., Park, S. M. & Lee, H. K. Ureolytic/non-ureolytic bacteria co-cultured self-healing agent for cementitious materials crack repair. Materials 11, 782 (2018).
Zhang, D., Shahin, M. A., Yang, Y., Liu, H. & Cheng, L. Effect of microbially induced calcite precipitation treatment on the bonding properties of steel fiber in ultra-high performance concrete. J. Building Eng. 50, 104132 (2022).
Duo, L. et al. Experimental investigation of solidifying desert aeolian sand using microbially induced calcite precipitation. Constr. Build. Mater. 172, 251–262 (2018).
Meng, H., Gao, Y., He, J., Qi, Y. & Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 383, 114723 (2021).
Naeimi, M., Chu, J. & Khosroshahi, M. Zenouzi, L. K. Soil stabilization for dunes fixation using microbially induced calcium carbonate precipitation. Geoderma 429, 116183 (2023).
Hemayati, M., Nikooee, E., Habibagahi, G., Niazi, A. & Afzali, S. F. New non-ureolytic heterotrophic microbial induced carbonate precipitation for suppression of sand Dune wind erosion. Sci. Rep. 13, 5845 (2023).
Qian, C., Chen, Y. & Xue, B. Microbial mineralization at the surface layer of cement-based materials and its effect on efflorescence performance. J. Building Eng. 52, 104480 (2022).
Sun, X., Miao, L., Chen, R., Wang, H. & Xia, J. Surface rainfall erosion resistance and freeze-thaw durability of bio-cemented and polymer-modified loess slopes. J. Environ. Manage. 301, 113883 (2022).
Gowthaman, S., Nakashima, K. & Kawasaki, S. Durability analysis of bio-cemented slope soil under the exposure of acid rain. J. Soils Sediments. 21, 2831–2844 (2021).
Chung, H., Kim, S. H. & Nam, K. Application of microbially induced calcite precipitation to prevent soil loss by rainfall: effect of particle size and organic matter content. J. Soils Sediments. 21, 2744–2754 (2021).
Sun, X., Miao, L., Wang, H., Chen, R. & Wu, L. Bio-cementation for the mitigation of surface erosion in loess slopes based on simulation experiment. J. Soils Sediments. 22, 1804–1818 (2022).
Rajasekar, A., Wilkinson, S. & Moy, C. K. MICP as a potential sustainable technique to treat or entrap contaminants in the natural environment: A review. Environ. Sci. Ecotechnology. 6, 100096 (2021).
Dong, Y. et al. Experimental study on solidification and remediation of lead–zinc tailings based on microbially induced calcium carbonate precipitation (MICP). Constr. Build. Mater. 369, 130611 (2023).
Zeng, Y. et al. Microbiologically induced calcite precipitation technology for mineralizing lead and cadmium in landfill leachate. J. Environ. Manage. 296, 113199 (2021).
Lin, H., Suleiman, M. T. & Brown, D. G. Investigation of pore-scale CaCO3 distributions and their effects on stiffness and permeability of sands treated by microbially induced carbonate precipitation (MICP). Soils Found. 60, 944–961 (2020).
Whiffin, V. S. Microbial CaCO3 Precipitation for the Production of Biocement (Murdoch University, 2004).
Mukherjee, S., Sahu, R., Mukherjee, J. & Sadhu, S. in Ground Improvement Techniques and Geosynthetics: IGC 2016 Volume 2. 85–94 (Springer).
Chen, Y., Wang, S., Tong, X. Y. & Kang, X. Crystal transformation and self-assembly theory of microbially induced calcium carbonate precipitation. Appl. Microbiol. Biotechnol. 106, 3555–3569 (2022).
Yu, T., Souli, H., Péchaud, Y. & Fleureau, J. M. Optimizing protocols for microbial induced calcite precipitation (MICP) for soil improvement–a review. Eur. J. Environ. Civil Eng. 26, 2218–2233 (2022).
Xu, X., Guo, H., Li, M. & Deng, X. Bio-cementation improvement via CaCO3 cementation pattern and crystal polymorph: A review. Constr. Build. Mater. 297, 123478 (2021).
Jiang, N. J. & Soga, K. Erosional behavior of gravel-sand mixtures stabilized by microbially induced calcite precipitation (MICP). Soils Found. 59, 699–709 (2019).
Zhao, Q., Li, L., Li, C., Zhang, H. & Amini, F. A full contact flexible mold for Preparing samples based on microbial-induced calcite precipitation technology. Geotech. Test. J. 37, 917–921 (2014).
Jiang, N. J., Tang, C. S., Yin, L. Y., Xie, Y. H. & Shi, B. Applicability of microbial calcification method for sandy-slope surface erosion control. J. Mater. Civ. Eng. 31, 04019250 (2019).
Cheng, L. & Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol J. 31, 396–406 (2014).
Tian, K. et al. Effect of reactant injection rate on solidifying aeolian sand via microbially induced calcite precipitation. J. Mater. Civ. Eng. 32, 04020291 (2020).
Mortensen, B., Haber, M., DeJong, J., Caslake, L. & Nelson, D. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 111, 338–349 (2011).
Tang, Q., Tian, A., Ling, C., Huang, Y. & Gu, F. Physical and mechanical properties of recycled aggregates modified by microbially induced calcium carbonate precipitation. Journal Clean. Production, 135409 (2022).
Gomez, M. G., DeJong, J. T., Anderson, C. M., Nelson, D. C. & Graddy, C. M. Large-scale bio-cementation improvement of sands. in Geotechnical Struct. Eng. Congress. 941–949. (2016).
Fang, C. & Achal, V. Enhancing carbon neutrality: A perspective on the role of microbially induced carbonate precipitation (MICP). Biogeotechnics 2, 100083 (2024).
Dubey, A. A., Hooper-Lewis, J., Ravi, K., Dhami, N. K. & Mukherjee, A. Biopolymer-biocement composite treatment for stabilisation of soil against both current and wave erosion. Acta Geotech. 17, 5391–5410 (2022).
Dubey, A. A., Ravi, K., Shahin, M. A., Dhami, N. K. & Mukherjee, A. Bio-composites treatment for mitigation of current-induced riverbank soil erosion. Sci. Total Environ. 800, 149513 (2021).
Ghasemzadeh, H. & Modiri, F. Application of novel Persian gum hydrocolloid in soil stabilization. Carbohydr. Polym. 246, 116639 (2020).
Garcıa-Ochoa, F., Santos, V., Casas, J. & Gómez, E. Xanthan gum: production, recovery, and properties. Biotechnol. Adv. 18, 549–579 (2000).
Mudgil, D., Barak, S. & Khatkar, B. S. Guar gum: processing, properties and food applications—a review. J. Food Sci. Technol. 51, 409–418 (2014).
Phillips, G. O. & Williams, P. A. Handbook of Hydrocolloids (Elsevier, 2009).
Dabestani, M., Kadkhodaee, R., Phillips, G. O. & Abbasi, S. Persian gum: A comprehensive review on its physicochemical and functional properties. Food Hydrocoll. 78, 92–99 (2018).
Suresh Kumar, A., Mody, K. & Jha, B. Bacterial exopolysaccharides–a perception. J. Basic Microbiol. 47, 103–117 (2007).
Lapasin, R. Rheology of Industrial Polysaccharides: Theory and Applications (Springer Science & Business Media, 2012).
Bak, J. & Yoo, B. Rheological characteristics of concentrated ternary gum mixtures with Xanthan gum, Guar gum, and carboxymethyl cellulose: effect of nacl, sucrose, pH, and temperature. Int. J. Biol. Macromol. 253, 126559 (2023).
Bak, H. M., Halabian, A. M., Hashemolhosseini, H. & Rowshanzamir, M. Axial response and material efficiency of tapered helical piles. J. Rock Mech. Geotech. Eng. 13, 176–187 (2021).
Mirmohammad sadeghi, M., Modarresnia, A. R. & Shafiei, F. Parameters effects evaluation of microbial strengthening of sandy soils in mixing experiments using Taguchi methodology. Geomicrobiol J. 32, 453–465 (2015).
Whiffin, V. S., Van Paassen, L. A. & Harkes, M. P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J. 24, 417–423 (2007).
Natarajan, K. Kinetic study of the enzyme urease from Dolichos biflorus. J. Chem. Educ. 72, 556 (1995).
Achal, V., Mukherjee, A., Basu, P. & Reddy, M. S. Lactose mother liquor as an alternative nutrient source for microbial concrete production by Sporosarcina pasteurii. J. Ind. Microbiol. Biotechnol. 36, 433–438 (2009).
Azadi, M., Ghayoomi, M., Shamskia, N. & Kalantari, H. Physical and mechanical properties of reconstructed bio-cemented sand. Soils Found. 57, 698–706 (2017).
Al Qabany, A., Soga, K. & Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. GeoEnviron. Eng. 138, 992–1001 (2012).
Cheng, L., Shahin, M. & Cord-Ruwisch, R. Bio-cementation of sandy soil using microbially induced carbonate precipitation for marine environments. Géotechnique 64, 1010–1013 (2014).
Kim, G., Kim, J. & Youn, H. Effect of temperature, pH, and reaction duration on microbially induced calcite precipitation. Appl. Sci. 8, 1277 (2018).
Sun, X., Miao, L., Tong, T. & Wang, C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. 14, 627–638 (2019).
Cheng, L., Cord-Ruwisch, R. & Shahin, M. A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 50, 81–90 (2013).
Mortazavi Bak, H., Noorbakhsh, M., Halabian, A. M., Rowshanzamir, M. & Hashemolhosseini, H. Application of the Taguchi method to enhance bearing capacity in geotechnical engineering: case studies. Int. J. Geomech. 21, 04021167 (2021).
Taguchi, G. System of experimental design; engineering methods to optimize quality and minimize costs. (1987).
Tan, Ö. Investigation of soil parameters affecting the stability of homogeneous slopes using the Taguchi method. Eurasian Soil. Sci. 39, 1248–1254 (2006).
Kolivand, F. & Rahmannejad, R. Estimation of geotechnical parameters using taguchi’s design of experiment (DOE) and back analysis methods based on field measurement data: case study: Tehran metro line 7. Bull. Eng. Geol. Environ. 77, 1763–1779 (2018).
Khayati, G. & Barati, M. Bioremediation of petroleum hydrocarbon contaminated soil: optimization strategy using Taguchi design of experimental (DOE) methodology. Environ. Processes. 4, 451–461 (2017).
Kariminia, T. et al. Soil microbial improvement using enriched Vinasse as a new abundant waste. Sci. Rep. 13, 22279 (2023).
Mortazavi Bak, H. et al. Effect of sample Preparation on the reliability of Large-Scale physical modeling in geotechnical systems: ACase study. Geotech. Geol. Eng. 42, 2693–2707 (2024).
Van Paassen, L. et al. in Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering (Volumes 1, 2, 3 and 4). 2328–2333 (IOS Press).
Gu, J., Suleiman, M. T., Bastola, H., Brown, D. G. & Zouari, N. in IFCEE 2018 155–164 (2018).
Behzadipour, H., Pakbaz, M. S. & Ghezelbash, G. R. Effects of biocementation on strength parameters of silty and clayey sands. Bioinspired Biomim. Nanobiomaterials. 9, 24–32 (2019).
DeJong, J. T., Mortensen, B. M., Martinez, B. C. & Nelson, D. C. Bio-mediated soil improvement. Ecol. Eng. 36, 197–210 (2010).
Ghasemzadeh, H., Mehrpajouh, A. & Pishvaei, M. Compressive strength of acrylic polymer-stabilized kaolinite clay modified with different additives. ACS Omega. 7, 19204–19215 (2022).
Adabi, M., Darvishan, E., Eyvazi, G. & Jahanbaksh Motlagh, H. Geoenvironmental application of novel Persian gum biopolymer in sandy soil stabilization. Arab. J. Sci. Eng. 47, 12915–12929 (2022).
Ashraf, M. S., Azahar, S. B. & Yusof, N. Z. in IOP Conference Series: Materials Science and Engineering. 012058 (IOP Publishing).
Soldo, A. & Miletić, M. Study on shear strength of Xanthan gum-amended soil. Sustainability 11, 6142 (2019).
Fu, T., Saracho, A. C. & Haigh, S. K. Microbially induced carbonate precipitation (MICP) for soil strengthening: a comprehensive review. Biogeotechnics 1, 100002 (2023).
Kontoyannis, C. G. & Vagenas, N. V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 125, 251–255 (2000).
Falini, G., Albeck, S., Weiner, S. & Addadi, L. Control of Aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271, 67–69 (1996).
Omoregie, A. I., Palombo, E. A., Ong, D. E. & Nissom, P. M. Biocementation of sand by Sporosarcina pasteurii strain and technical-grade cementation reagents through surface percolation treatment method. Constr. Build. Mater. 228, 116828 (2019).
Author information
Authors and Affiliations
Contributions
T.K.: Conceptualization, Formal analysis, Investigation, Methodology, Writing - original draft. M.A.R: Methodology, review & editing. S.M.A.: Conceptualization, Methodology. H.M: Conceptualization, Methodology, review & editing. (A) Kh, (B) Sh, S.S.and A.B. :Methodology, review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kariminia, T., Rowshanzamir, M.A., Bak, H.M. et al. Enhancing the efficiency and sustainability of soil Bio-Cementation improvement using Persian gum. Sci Rep 15, 22456 (2025). https://doi.org/10.1038/s41598-025-05607-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05607-8