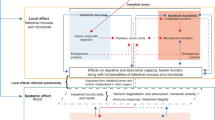
Abstract
A trial was conceived to evaluate the effects of the dietary inclusion of defatted BSF larvae meal on growth performance, breast meat quality traits, cecal microbiota composition and metabolomics profile of turkeys. A total of 1512 female turkeys (B.U.T. 6) were divided into two groups (9 replicate pens) fed either a basal diet (CON group) or CON diet with 5% BSF meal from 65 days to slaughtering (105 days; INS group). The administration of BSF meal improved final body weight (10.17 vs. 10.06 kg/bird, respectively for INS and CON; P = 0.04) as well as daily weight gain and feed conversion ratio in the rearing cycle (96.22 vs. 95.23 g/bird/day and 2.127 vs. 2.141, respectively; P = 0.03). Breast meat quality traits and cecal microbiota were only slightly affected by the treatment. The cecal concentration of tyramine was significantly lower in INS turkeys, which showed higher levels of glucose and malonate (P = 0.03). The use of BSF meal tended (0.05 < P < 0.10) to increase the cecal content of isoleucine, betaine and butyrate, and to reduce the amount of 3-phenylpropionate. Overall, the dietary inclusion of 5% BSF meal from 65 to 105 days improved the growth performances of female turkeys mainly through the modulation of the gut metabolomics profile.
Similar content being viewed by others
Introduction
Whilst world population is increasing and protein demand expanding, enormous food losses still occur in the food supply chain with approximately 30% of the food produced for human consumption that is lost or wasted from harvesting to the consumer plate1,2. The reduction of food losses has thus become a main target of environmental and food security policies aimed at promoting a more sustainable and efficient use of natural resources3. Within this context, the adoption of circular economy approaches in which wastes can be reintroduced into the production process to close nutrient and energy cycles has gained popularity in recent years for optimizing resources utilization while minimizing environmental impacts4,5.
Insects farming represents a quintessential example of circularity as some insect species are able to recover and transform nutrients from low-value side streams of the food industry generating high-quality products that could return into the food or feed chains6,7. Indeed, while the direct human consumption of insects could face some limitations especially in countries where insects are still an unusual food8,9, the utilization of insect-derived products has been largely proposed in livestock, thus promoting an indirect entomophagy. In particular, the use of insects in poultry feeding seems to be well accepted by consumers9,10,11, likely because insects represent part of the “natural” diet of wild birds12 or farmed poultry with access to outdoor areas13. In the poultry sector, protein meals obtained from farmed insect species can be considered as a valid alternative to traditional protein feedstuffs, in particular soybean meal (SBM) whose trade and utilization patterns have generated global environmental concerns and socio-economic issues especially in importing countries such as Europe and China14,15. In Europe, the use of processed animal proteins (PAPs) deriving from farmed insects has been subjected for years to the so-called “feed ban” (i.e., Regulation 999/2001)16 and only recently authorized in poultry feeding (Regulation 1372/2021)17. Among the 8 insects species allowed by the EU legislation, the black soldier fly (BSF; Hermetia illucens L.) has been widely studied as a promising source of insect PAPs mostly because of its valuable nutritional profile and its capacity of growing with high feed efficiency and short rearing cycles on a different range of organic substrates18,19,20. As a result, the available literature on the use of BSF meal as alternative protein source in meat-type chicken diets has rapidly expanded in the past ten years18,19,20. Taken together, the results show that BSF larvae meal can effectively replace part of the conventional protein sources in broiler diets, with dosages up to 10%21 or 15%22 that should not compromise growth performances provided that insect-based diets present similar metabolizable energy content and amino acid supply than conventional diets22.
Despite the abundance of information on broiler chickens, only a couple of studies focused on the use of BSF meals in the feeding of modern turkey hybrids, which however present a remarkably high protein and amino acid intake to satisfy their elevated nutritional requirements. Full-fat BSF larvae meal has been tested in 28-day-old turkeys either as feed additive (0.3% inclusion dosage) to enhance anti-inflammatory, immunological and antioxidant traits23 or as a dietary source of proteins and fats24. In the latter study, the authors concluded that full-fat BSF meal inclusion up to 15% can improve feed conversion efficiency as well as the immunological status of the small intestine and cecal fermentation processes24. To the best of our knowledge, only Lalev et al.25 investigated the use of BSF meals (both defatted and whole larvae meal) as protein source in growing-finishing turkeys. Despite some limitations in the experimental design, especially as concern the number of animals used for the evaluation of growth performances (i.e., 15 birds/dietary treatment), the results indicate that the inclusion of 10% BSF meals from 56 to 130 days can be well tolerated by turkeys without detriments of productive performance, meat quality traits and physiological parameters, yet highlighting the need for more robust and comprehensive evaluations of this nutritional strategy in turkeys. In addition, as also recommended by Biasato et al.26, the application of multi-omics approaches can be a valuable tool to shed light on the effects of BSF meal-based diets on the gut health conditions of commercial poultry species such as turkeys, in which this information is particularly scant. To expand the knowledge on these topics, this trial was conducted to evaluate the effects of the dietary inclusion of defatted BSF larvae meal on the growth performance, breast meat quality traits, cecal microbiota composition and metabolomics profile of female turkeys.
Results
Growth performance
The growth performance results are summarized in Table 1. As expected, turkeys reached a similar body weight (BW) at 64 days of age (4.796 vs. 4.804 kg/bird, respectively for CON and INS; P = 0.78) as a result of the administration of the same basal diet and a balanced experimental design. The dietary inclusion of BSF larvae meal had no relevant effects on performance traits from 65 to 78 days of age. Conversely, it tended to improve daily weight gain (DWG) and feed conversion ratio (FCR) from 79 to 93 days (141.3 vs. 144.7 g/bird/day and 2.501 vs. 2.434, respectively for CON and INS; P = 0.09 and P = 0.07), resulting in higher BW at the end of the phase (8.756 vs. 8.821 kg/bird, respectively; P = 0.04). In the finisher phase (94—105 days), INS turkeys consumed more feed per day than the CON counterpart (343.7 vs. 353.7 g/bird/day, respectively for CON and INS; P = 0.04), which contributed to achieving a higher final BW (10.06 vs. 10.17 kg/bird, respectively for CON and INS; P = 0.04). Analyzing the performance in the overall trial period (0–105 days), the inclusion of BSF larvae meal significantly increased DWG and reduced FCR (95.23 vs. 96.22 g/bird/day and 2.141 vs. 2.127, respectively for CON and INS; P = 0.03). Mortality was remarkably low in both experimental groups, highlighting no significant variation in each feeding phase and in the overall trial period. Eviscerated carcass as well as breast and thigh yields (expressed as % of eviscerated carcass weight) were 75.4 vs. 74.5%, 33.7 vs. 33.7% and 31.6 vs. 31.5%, respectively for CON and INS.
Breast meat quality
The results concerning meat quality analysis are reported in Table 2. No significant effect of the administration of the INS diet was observed on the proximate composition of breast meat, including moisture, crude protein, ash and total fat content. As for the technological properties, breasts from CON and INS groups achieved a similar ultimate pH value. In turn, meat water holding capacity did not differ between groups as indicated by the comparable drip and cooking losses. Regarding the color profile, lightness (L*) and redness (a*) values did not present significant variations, while yellowness (b*) showed a lower value in the INS group (2.83 vs. 1.95, respectively for CON and INS; P = 0.03). Shear force was not affected by the dietary treatment.
Cecal microbiota composition
No significant difference in terms of relative abundance was observed between CON and INS groups at both phylum and genus level (Table 3). Moreover, alpha and beta diversity did not change as a result of the dietary treatment. Firmicutes and Bacteroidetes were the most abundant phyla in all analyzed samples. At the genus level, the relative abundance Faecalibacterium and Megamonas tended to be reduced in INS group (12.2 vs. 8.08% and 0.47 vs. 0.17%, respectively for CON and INS; P = 0.07).
Cecal metabolomics profile
A total of 71 metabolites were identified through the proton nuclear magnetic resonance (1H-NMR) spectometry analysis of the cecal content samples. The 7 metabolites whose concentration was either significantly different (P < 0.05) or tended to be different (0.05 < P < 0.10) between CON and INS groups are listed in Table 4. The concentrations of the remaining 64 metabolites are reported in Supplementary Table S1 online. Compared to CON, the concentration of tyramine was significantly lower in the ceca of INS turkeys (8.27 × 10–4 vs. 5.48 × 10–4 mmol/L, respectively for CON and INS; P = 0.03), which on the opposite showed higher levels of glucose and malonate (1.02 × 10–3 vs. 1.53 × 10–3 and 7.41 × 10–4 vs. 1.01 × 10–3 mmol/L, respectively; P = 0.03). Furthermore, the administration of the BSF larvae meal tended to increase the content of isoleucine (P = 0.06), betaine (P = 0.07) and butyrate (P = 0.10), and to reduce the amount of 3-phenylpropionate (P = 0.10).
Discussion
The scientific knowledge about the use of BSF meal in turkey nutrition is very limited. On the one hand, most of the studies conducted so far focused on the potential role of BSF meal as feed additive (i.e., dietary dosage < 1%) to enhance gut health conditions because of the nutraceutical properties of this product. On the other hand, when BSF meal has been considered as a dietary protein source, only few studies taken into account the grower-finisher phase which is, however, particularly important from a sustainability standpoint as the largest part of feed consumption occurs in the last stages of the rearing cycle. The results of this trial indicate that the dietary administration of 5% BSF meal from 65 days to slaughter (105 days of age) improved the productive performance of female turkeys, which showed higher final BW as well as greater DWG and lower FCR compared to those fed a conventional corn-wheat-soybean diet. To our knowledge, only one study25 investigated the use of defatted BSF meal in growing-finishing turkeys. Despite the aforementioned limitations in the experimental design (i.e., number of replications and animals used in the study), the Authors concluded that 10% defatted BSF meal inclusion can have beneficial effects on the growth performance of the birds. Other studies, such as that of Jankowski et al.24, indicated that full-fat BSF larvae meal inclusion up to 15% can improve feed conversion efficiency in young turkeys (28 days of age). Overall, our results provide updated and robust information regarding the use of BSF meal as protein source in growing-finishing turkeys. In this context, the adoption of more comprehensive experimental designs (e.g., dose–response studies) can help to identify optimal BSF meal dosages in turkey diets.
As for the meat quality, the dietary inclusion of 5% BSF meal did not substantially affect the proximate composition and technological traits of turkey breast meat. Our results corroborate those of Lalev et al.27, who found a similar protein and fat content in breast muscles from female turkeys fed diets with 10% BSF meal. Moreover, the Authors revealed that the treatment did not influence the color profile of breast meat while reduced water holding capacity and cooking loss. In our study, the only slight but still statistically significant difference was related to the color profile, with breasts from INS group showing lower yellowness compared to the CON counterpart. This variation could be potentially ascribed to the slight reduction in the amount of corn—a rich source of carotenoids—in the INS diet, which was necessary to allow the inclusion of BSF meal while maintaining the same energy content of the CON diet. However, such difference in meat yellowness has minimal practical implications being not detectable to the naked-eye and thus not able to influence consumers’ perception. Overall, the results obtained in this study support the conclusion of Dalle Zotte28, who pointed out that the dietary use of BSF meal has either no or limited influence on the physicochemical traits of poultry meat, with the only exception being the fatty acid profile which generally presents greater concentrations of saturated fatty acids.
In addition, the administration of 5% BSF meal exerted minimal effects on the cecal microbiota composition, as demonstrated by the similar values of alpha and beta diversity at both phylum and genus level between CON and INS groups. Overall, the cecal microbiota of both groups was characterized by a predominance of Firmicutes and Bacteroidetes, as generally observed in this section of the gastrointestinal tract of turkeys29. Both these phyla, representing more than 90% of the cecal microbiota in both tested groups, include genera involved in the fermentation of dietary polysaccharides producing short-chain fatty acids (SCFAs), such as butyrate, which are beneficial for energy metabolism and gut health. At the genus level, the relative abundance of only two genera, Faecalibacterium and Megamonas, tended to be reduced in the INS group. Some species belonging to the genus Faecalibacterium, particularly Faecalibacterium prausnitzii, are typically associated with a healthy microbiota in both humans and animals because of their capacity of producing SCFAs and improving intestinal mucosa conditions30. The genus Megamonas was previously detected in the ceca of growing turkeys and its presence was positively correlated with the abundance of Campylobacter spp.29. Moreover, an increased abundance of Megamonas has been associated with some metabolic diseases in humans31. In other studies, Megamonas hypermegale has been identified as a significant component of the cecal microbiota in wild turkeys and as a producer of propionic and acetic acids inhibiting Salmonella colonization in the gut32. Biasato et al.26 highlighted that insect meals administration can influence the gut health conditions of farmed poultry, pigs and fish because of the presence of some nutraceutical components such as chitin, antimicrobial peptides, and lauric acid. These insect-derived compounds are thought to increase microbiota alpha diversity, promoting the growth of bacterial strains able to produce SCFAs while reducing pathogens abundance26. However, the dietary inclusion of BSF meal has led to inconsistent results as concern alpha-diversity in poultry species, with studies showing contrasting outcomes in response to this dietary strategy33,34,35. On the other hand, beta-diversity changes have been frequently observed in monogastric animals fed insects-based diets, even though with some specie-specific differences and not always consistently even within the same species26. Biasato et al.26 hypothesized that the genetic selection carried out in the last decades on meat- and egg-type chickens might have reduced the capacity of modern genotypes to utilize insects as part of their feeding regimens especially when provided at high dosages (e.g., > 10–15%). Accordingly, it can be speculated that the low dosage of BSF meal tested in this study can be well tolerated by growing female turkeys, resulting in improved productive performance with only minimal changes in the cecal microbiota composition. To as concerns other insect products, some studies applying a fluorescence in situ hybridization technique revealed that the use of BSF fat as alternative to soybean oil in turkey nutrition can exert some beneficial effects on Enterobacteriaceae spp. and Bacteroides-Prevotella cluster counts, while other parameters were substantially unaffected by the dietary treatment36,37. Overall, further studies are needed to better understand the potential modulating effect of BSF meal on the turkey microbiota. In future studies, shotgun metagenomic can be used instead of amplicon sequencing as the latter is able to detect only part of the gut microbiota community revealed by shotgun sequencing38.
The 1H-NMR analysis showed interesting changes in the cecal metabolomics profile of female turkeys in response to the dietary administration of BSF meal. For instance, tyramine is a biogenic amine deriving from the decarboxylation of tyrosine by means of bacterial breakdown in the gut39. In humans, high fecal concentrations of tyramine have been detected in patients with inflammatory bowel disease40 and other in-vitro studies confirmed the role of tyramine in stimulating intestinal inflammatory processes, particularly by disrupting tight junction proteins and promoting prostanoid release41,42. Pretorius and Smith43, using a zebrafish larval model, found that tyramine can negatively affect gut health by triggering and exacerbating intestinal inflammation. A decrease in the cecal levels of biogenic amines, including tyramine, has been associated with improved gut health conditions and growth performances in yellow-feathered broilers fed diets supplemented with citrus extract44. In our study, the reduction of tyramine concentration in the cecal content of INS birds might be considered as a potential indicator of enhanced gut health conditions that, in turn, can have contributed to the performance improvements observed in the turkeys fed the insect-based diet. In addition, considering that tyrosine content tended to be higher in the INS group (3.88 × 10–4 vs. 4.93 × 10–4 mmol/L, respectively for CON and INS; P = 0.11; see Supplementary Table S1 online), it can be hypothesized that the microbial population of INS birds might have a lower capacity of fermenting tyrosine compared to the CON counterpart, even though no remarkable differences emerged from the microbiota analysis. Indeed, some metabolites are the end-product of the metabolism of a variety of microbial species that, although showing relatively small differences in their abundance when considered singularly, can contribute to the overall variations observed at the metabolomics level.
The cecal content of INS group was also characterized by higher levels of glucose, a well-known sugar involved in energy metabolism. Rzeznitzeck et al.45 found that the amount of glucose decreases steadily along the intestinal tract of turkeys, with the lowest concentration detected in the ceca. Differences in cecal glucose levels can be attributed to variations in the absorption rate of this sugar in the previous intestinal segments and/or to changes in the fermentation processes occurring in the ceca. BSF meal contains chitin, a polymer of (1 → 4)-β-linked N-acetyl-d-glucosamine, which represents the main component of the insect exoskeleton46. From a metabolic perspective, chitin is generally considered as an indigestible compound for poultry species even if Tabata et al.47,48 documented acid chitinase activity in the chicken gastrointestinal tract. Much attention has been reserved to the role of chitin as substrate for bacterial fermentations in the hindgut, where it can act as a prebiotic and modulator of the microbiome26. During chitin degradation, chitosan or even cellulose-like molecules can be produced as a result of deacetylation and deamination processes, potentially generating glucose as end product of these reactions49. Previously, Jankowski et al.24 revealed a higher activity of microbial enzymes involved in chitin degradation in BSF-fed turkeys, which could be consistent with the hypothesis of an increased metabolism of insect chitin in the ceca of INS birds. Therefore, the higher amount of free glucose detected in the ceca of INS turkeys could originate from the bacteria fermentation processes carried out on chitin, which could have determined a greater production of such sugar as final product of bacteria metabolic pathways.
Finally, betaine and butyrate concentrations tended to be higher in the cecal content of INS birds. Betaine, the trimethyl derivative of the amino acid glycine, has emerged as a valuable molecule able to exert gut health benefits in both humans and farmed animals, playing positive effects not only on intestinal structure and functionality but also on nutrient digestibility and microbiota composition50,51. In chickens, the beneficial effects of betaine have been demonstrated in both physiological and challenging conditions such as coccidiosis infection or heat stress exposure52,53. In terms of systemic metabolism, betaine can improve the availability of methionine and choline and can interact with several metabolic factors regulating body growth as well as protein and lipid metabolism, thereby enhancing productive performance54. On the other hand, butyrate is a well-known SCFA deriving from bacterial fermentations that can exert a multitude of beneficial effects on gut conditions and, in turn, on animal performance and health55. Besides its trophic role on enterocytes, the presence of butyrate in the intestinal tract of poultry has been associated with improved epithelial barrier structure and functionality, increased production of mucin and antimicrobial peptides as well as reduced pathogens colonization and inflammation55,56. In turkeys, the increase of cecal concentration of butyrate as a result of the dietary supplementation of different forms of this SCFA was associated with enhanced growth performance, greater protein digestibility, better gut health conditions, and reduced fecal pathogens content57. In line with our results, Jankowski et al.24 observed higher butyrate concentrations in the ceca of 28-day-old turkeys fed on diets with 5, 10 and 15% full-fat BSF meal. As stated before, the Authors also reported an increased activity of microbial enzymes involved in chitin degradation, such as β-glucosidase and β-glucuronidase, in BSF-fed turkeys. Similar findings concerning cecal butyrate production have also been observed in laying hens and broiler chickens receiving diets with defatted BSF and Tenebrio molitor larvae meal as feed protein source33,58. According to these results, it can be hypothesized that the chitin contained in BSF meal could have stimulated the metabolic activity of the cecal microbiota resulting in a greater production of butyrate, which, in turn, can have contributed in improving gut health conditions and growth performances in INS turkeys. Taken together, the cecal metabolomics profile seems to indicate that BSF meal administration could have determined positive effects in terms of gut health conditions, which can reasonably be considered the main factor behind the growth performance improvements observed in INS turkeys.
Overall, the results of this study show that the inclusion of 5% BSF meal as partial replacement for soybean meal from 65 to 105 days (grower-finisher phases) improved the growth performances of female turkeys in the overall rearing cycle while having negligible effects on breast meat quality traits. Even though microbiota analysis revealed limited variations, the cecal metabolomics profile suggests that the performance improvements observed in INS turkeys might be related to changes in the concentration of available gut metabolites (i.e. tyramine, glucose, butyrate and betaine) playing important roles in animal metabolism and health.
Methods
Ethic statement
This research was authorized by the Ethical Committee of the University of Bologna (ID: 1145/2020) and all activities were in compliance with the European Legislation and guidelines on the protection of animals kept for farming and scientific purposes as well as on the protection of animals at slaughtering59,60,61. All methods are reported in accordance with the ARRIVE guidelines.
Animals, housing, and management conditions
A total of 1512 one-day-old female turkey poults (genotype: B.U.T. 6), obtained from the same breeder flock and hatching batch, were used in the trial. All turkeys were vaccinated at the hatchery following routine practices and transferred under the same conditions to an environmentally controlled poultry facility equipped with 18 identical floor pens (18 m2/each). Each pen presented two pan feeders and a bell-type drinker. A labelled bin was provided for each pen and the feed contained in it was manually transferred to the feeder on a daily basis. The concrete floor of the pens was covered with bedding material, specifically wood shavings. Management and husbandry conditions were representative of current commercial practices in Europe and temperature and lighting programs were defined according to the recommendations of the breeding company.
Dietary treatments
Each pen was assigned, according to a randomized block design, to one of the following experimental groups (9 replicate pens of 84 turkeys each): CON group, receiving a corn-wheat-soybean basal diet in all feeding phases, and INS group, which was fed the CON diet up to 64 days and then CON diet with 5% defatted BSF larvae meal in partial substitution for SBM until slaughter (105 days). The analyzed chemical composition and the amino acid profile of the BSF larvae meal (Mutatec, Cavaillon, France) are summarized in Table 5. All experimental diets (Table 6) were formulated to meet current nutritional recommendations following a 6-phase feeding program: starter, 0–22 days; grower I, 23–50 days; grower II, 51–64 days; grower III, 65–78 days; grower IV, 79–93 days; and finisher, 94–105 days. In each feeding phase, CON and INS diets were isoenergetic and with a similar amino acid profile, which was optimized on a digestible basis. The dietary content of SBM was reduced in INS diets to allow the inclusion of BSF meal, resulting in an 11% decrease of soybean use compared to CON in each feeding phase. Feed and water were offered for ad-libitum consumption. As for the feed physical form, starter diet was crumbled while pellet was used afterwards (grower I: diameter 2.5 mm; grower II—slaughter: diameter 3.5 mm). The coccidiostat monensin was included into the feed up to the end of the of grower III phase (i.e., 78 days) as usually done in commercial conditions.
Growth performance and slaughtering measurement
On a replicate basis, turkeys were weighed and counted at housing (0 day), at each feeding phase switch (22, 50, 64, 78 and 93 days), and at processing (105 days) to compute average body weight (BW) and daily weight gain (DWG). Feed residuals at the end of each feeding phase were weighed pen-wise to calculate feed intake (FI) and daily feed intake (DFI). Feed conversion ratio (FCR) was determined according to these parameters and results reported for each feeding phase and for the overall trial period. Dead or culled turkeys were daily recorded and their BW was considered to adjust performance parameters. At 105 days, all turkeys were processed into a commercial abattoir according to standard procedures and in accordance with the EU legislation. A controlled atmosphere stunning systems (multi-stage, carbon dioxide atmosphere process) was used in the slaughterhouse according to the Reg. EU 1099/200961. Slaughtering yields, including eviscerated carcass, boneless and skinless breast, thighs, were obtained after air-chilling (in-line air tunnel system) on all processed birds on a group basis.
Breast meat quality evaluation
At 24 h post-mortem, 12 breast muscles (Pectoralis major) without visible macroscopic defects were selected from each experimental group to assess the main meat quality traits. Proximate composition analysis was conducted according to the procedures described by the Association of Official Analytical Chemists62. Briefly, moisture and ash were calculated after drying (105 °C for 18 h) and incinerating (550 °C for 8 h) meat samples, respectively. Crude protein and total fat content was determined through the Kjeldahl copper catalyst method62 and the chloroform–methanol extraction defined by Folch et al.63, respectively. Meat ultimate pH (pHu) was measured 48 h post-mortem by means of a portable pH-meter equipped with a stainless-steel blade tip for meat penetration and temperature compensation (HI98163, Hanna Instruments Inc., USA). A reflectance colorimeter (illuminant source C; Minolta Chroma Meter CR-400, Minolta Italia S.p.A., Milan, Italy) was utilized to determine the color profile (L*—lightness, a*—redness, b*—yellowness; CIE, 1978) of breast meat samples. Such evaluation was done in triplicate on the cross-section of each fillet. Drip and cooking losses were measured to evaluate meat water holding capacity. Drip loss (%) was calculated as the weight lost from a parallelepiped meat cut (8 × 4 × 2 cm; weight about 80 g) obtained from the same cranial portion of each breast muscle after storage at 4 ± 1 °C for 48 h in plastic boxes over sieved plastic racks. The cooking loss assessment was conducted using the same samples, which were vacuum packed and cooked in water bath (set at 75 °C) until reaching an inner core temperature of 72 °C. After being chilled at room temperature, samples were gently blotted and weighed to calculate cooking loss (expressed as % of the initial weight). Shear force (kg) was measured on subsamples (4 × 1 × 1 cm) obtained from the cooked meat samples by means of a TA.HDi Heavy Duty texture analyzer (Stable Micro Systems Ltd., Godalming, Surrey, UK) equipped with a 5 kg load cell and a Warner Bratzler shear probe.
Cecal microbiota and metabolome evaluation
At slaughtering, the cecal content was obtained from 15 birds/group selected according to the average body weight of each experimental group and clearly identified with plastic leg bands. Samples were collected into 15 ml sterile falcons and immediately snap-frozen in liquid nitrogen. The falcons were kept at -80 °C until microbiota and metabolomics analyses.
For the microbiota, approximately 2 ml of cecal content were used for DNA extraction through a bead-beating procedure followed by the use4 of the QIAmp® DNA Stool Mini Kit (Qiagen, Milan, Italy). Total DNA was fragmented and tagged with sequencing indexes and adapters following the 16S Metagenomic Sequencing Library Preparation protocol (Illumina, San Diego, CA, USA), set to amplify the V3 and V4 hypervariable regions of the 16S rRNA gene in order to obtain a single amplicon of approximately 460 bp. Amplicon sequencing was performed with MiSeq System (Illumina) in paired-end mode (2 × 251 bp) with MiSeq Reagent kit v2 500 cycles (Illumina), characterized by a maximum output of 8.5 Gb. Raw sequences were analyzed using the QIIME2 bioinformatics pipeline. First, the sequences were imported as paired-end reads and processed with the dada2 algorithm with the aim to denoise and to merge forward and reverse sequences per each pair. The taxonomic classification of cleaned data was performed by applying the VSEARCH-based classifier and adopting the Greengenes 13_8 97% OUT dataset as reference.
As for samples preparation for the metabolome analysis, a D2O solution containing 10 mmol/L of TSP (3-(trimethylsilyl)-propionic-2,2,3,3-d4 acid sodium salt) and 2 mmol/L of NaN3 was produced. The two solutes were employed, respectively, as a 1H-NMR chemical shift reference and as a blocking agent for microorganisms. Each plasma sample was centrifuged at 18,630 × g and 4 °C for 900 s, to obtain 0.65 mL of supernatant, which was then mixed with 0.1 mL of the D2O solution and centrifuged again as described above. 1H-NMR spectra were recorded with an AVANCE™ III spectrometer (Bruker, Milan, Italy) operated by the software Topspin (v.3.5). The spectra were registered at 298 K and at a frequency of 600.13 MHz. Water residual signal was reduced by presaturation, while broad resonances due to large molecules were minimized with a CPMG-filter (total filter of 330 ms, made by 400 echoes with a τ of 400 µs and a 180° pulse of 24 µs). The total number of scans and relaxation delay for each spectrum were set to 256 and 5 s, respectively. The number of data points was 32,000, while the spectral window was 7184 Hz. The subsequent phase-adjustment was performed in Topspin v3.5. Spectra were then exported to ASCII format and imported into the R software environment with the script convbin2asc and scripts developed in house, respectively. The Human Metabolome Database64 and Chenomx software libraries (Chenomx Inc., Edmonton, AB, Canada, v10) were used for signals’ assignment, by comparing multiplicity, shape and position through the routine implemented in Chenomx. The quantification of molecules was done by relying on TSP as internal standard and potentially different water contents from sample to samples were considered by PQN (probabilistic quotient normalization)65. Molecules concentration was obtained from the area of one of their signals, measured by GSD (global spectra deconvolution) implemented in MestReNova software (v14.2.0–26256; Mestrelab research S.L., Santiago De Compostela, Spain) with 5 as limit of quantification (LOQ). To do so, a 0.3 line broadening and a baseline adjustment were performed, the latter by Whittaker Smoother procedure.
Statistical analysis
The dietary treatment was considered as the experimental factor for all the parameters (dependent variables) considered in this study. Data were screened using the Shapiro–Wilk test, which confirmed a normal distribution for all investigated parameters. Growth performance data were evaluated through a one-way blocked ANOVA with the replicate pen as experimental unit. No statistical insights were conducted on carcass and cut-up yields data as these parameters were recorded on a group basis without replicates. As no blocking factor was defined for the other traits, meat quality and metabolomics data were analyzed with a two-tailed Student’s t-test with the breast and cecal content samples considered as experimental unit, respectively. For the cecal microbiota, the comparative analysis between taxa abundances was done by applying a two-tailed Student’s t-test, while alpha and beta diversity were calculated in QIIME2. A P-value threshold of 0.05 was defined to declare differences as statistically significant. When the P-value was from 0.05 to 0.10, differences between groups were considered as tendencies.
Data availability
The datasets generated and/or analysed during the current study are available in the NCBI-SRA repository under the bioproject PRJNA1237329 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA1237329?reviewer=f4gj7g748mest5ni09jric3poc).
Abbreviations
- AME:
-
Apparent metabolizable energy
- BSF:
-
Black soldier fly
- BW:
-
Body weight
- CON:
-
Experimental group fed a corn-wheat-soybean diet in all feeding phases
- DFI:
-
Daily feed intake
- DWG:
-
Daily weight gain
- FCR:
-
Feed conversion ratio
- FI:
-
Feed intake
- GSD:
-
Global spectra deconvolution
- INS:
-
Experimental group fed the CON diet up to 64 days and then CON diet with 5% defatted BSF larvae meal in partial substitution for SBM until slaughter (105 days).
- LOQ:
-
Limit of quantification
- PAPs:
-
Processed animal proteins
- PQN:
-
Probabilistic quotient normalization
- SBM:
-
Soybean meal
- SCFAs:
-
Short-chain fatty acids
- SID:
-
Standardized ileal digestibility
- TSP:
-
3-(Trimethylsilyl)-propionic-2,2,3,3-d4 acid sodium salt
References
Food and Agriculture Organization of the United Nations. The State of Food and Agriculture. Moving forward on food loss and waste reduction. https://www.fao.org/interactive/state-of-food-agriculture/2019/en/ (2019).
United Nations Environment Programme. Food Waste Index Report. https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (2021).
Corrado, S. & Sala, S. Food waste accounting along global and European food supply chains: State of the art and outlook. Waste Manag. 79, 120–131 (2018).
Ghisellini, P., Cialani, C. & Ulgiati, S. A review on circular economy: the expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 114, 11–32 (2016).
De Oliveira, M. M., Lago, A. & Dal’Magro, G. P. Food loss and waste in the context of the circular economy: a systematic review. J. Clean. Prod. 294, 126284. https://doi.org/10.1016/j.jclepro.2021.126284 (2021).
Ojha, S., Bußler, S. & Schlüter, O. K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 118, 600–609 (2020).
Gasco, L., Biancarosa, I. & Liland, N. S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Curr. Opin. Green Sust. Chem. 23, 67–79 (2020).
Moruzzo, R., Mancini, S., Boncinelli, F. & Riccioli, F. Exploring the acceptance of entomophagy: A survey of Italian consumers. Insects 12, 123. https://doi.org/10.3390/insects12020123 (2021).
Mina, G., Peira, G. & Bonadonna, A. The potential future of insects in the European food system: A systematic review based on the consumer point of view. Foods 12, 646. https://doi.org/10.3390/foods12030646 (2023).
Verbeke, W. et al. Insects in animal feed: Acceptance and its determinants among farmers, agriculture sector stakeholders and citizens. Anim. Feed Sci. Technol. 204, 72–87 (2015).
La Barbera, F., Amato, M., Fasanelli, R. & Verneau, F. Perceived Risk of insect-based foods: an assessment of the entomophagy attitude questionnaire predictive validity. Insects 12, 403. https://doi.org/10.3390/insects12050403 (2021).
Klasing, K. C. Poultry nutrition: A comparative approach. J. Appl. Poult. Res. 14, 426–436 (2005).
Józefiak, D. et al. Insects—A natural nutrient source for poultry—A review. Ann. Anim. Sci. 16, 297–313 (2016).
Boerema, A. et al. Soybean trade: Balancing environmental and socio-economic impacts of an intercontinental market. PLoS ONE 11, e0155222. https://doi.org/10.1371/journal.pone.0155222 (2016).
Wang, M., Liu, D., Wang, Z. & Li, Y. Structural evolution of global soybean trade network and the implications to China. Foods 12, 1550. https://doi.org/10.3390/foods12071550 (2023).
European Union. Regulation (EC) n. 999/2001 of the European parliament and of the council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Off. J. L147, 1–40 (2021).
European Union. Regulation (EU) n. 1372/2021 of European Commission of 17 August 2021 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards the prohibition to feed non-ruminant farmed animals, other than fur animals, with protein derived from animals. Off. J. L295, 1–17 (2021).
Gasco, L., Biasato, I., Dabbou, S., Schiavone, A. & Gai, F. Animals fed insect-based diets: state-of-the-art on digestibility, performance and product quality. Animals 9, 170. https://doi.org/10.3390/ani9040170 (2019).
Barragan-Fonseca, K. B., Dicke, M. & Van Loon, J. J. A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed. 3, 105–120 (2017).
Wang, Y. S. & Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 6, 91. https://doi.org/10.3390/foods6100091 (2017).
Dalmoro, Y. K., Franceschi, C. H. & Stefanello, C. A systematic review and metanalysis on the use of Hermetia illucens and Tenebrio molitor in diets for poultry. Vet. Sci. 10, 702. https://doi.org/10.3390/vetsci10120702 (2023).
Martínez Marín, A. L., Gariglio, M., Biasato, I., Gasco, L. & Schiavone, A. Meta-analysis of the effect of black soldier fly larvae meal in diet on broiler performance and prediction of its metabolisable energy value. Ital. J. Anim. Sci. 22, 379–387 (2023).
Kozłowski, K. et al. Growth performance, immune status and intestinal fermentative processes of young turkeys fed diet with additive of full fat meals from Tenebrio molitor and Hermetia illucens. Anim. Feed Sci. Technol. 278, 114994. https://doi.org/10.1016/j.anifeedsci.2021.114994 (2021).
Jankowski, J. et al. The effect of dietary full-fat Hermetia illucens larvae meal on gut physiology and growth performance in young turkeys. Anim. Feed Sci. Technol. 275, 114879. https://doi.org/10.1016/j.anifeedsci.2021.114879 (2021).
Lalev, M. et al. Effects of insect- and probiotic-based diets on turkeys’ production, health, and immune parameters. Bulg. J. Agric. Sci. 26, 1254–1265 (2020).
Biasato, I., Gasco, L., Schiavone, A., Capucchio, M. T. & Ferrocino, I. Gut microbiota changes in insect-fed monogastric species: State-of-the-art and future perspectives. Anim. Front. 13, 72–80 (2023).
Lalev, M. et al. Insect-based diets effects on turkey meat quality. Bulg. J. Agric. Sci. 27, 980–989 (2021).
Dalle Zotte, A. Meat quality of poultry fed with diets supplemented with insects: A review. IOP Conf. Ser. Earth Environ. Sci. 854, 012019. https://doi.org/10.1088/1755-1315/854/1/012019 (2021).
Wilkinson, T. J. et al. Characterization of the microbiome along the gastrointestinal tract of growing turkeys. Front. Microbiol. 8, 1089. https://doi.org/10.3389/fmicb.2017.01089 (2017).
Miquel, S. et al. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 16, 255–261 (2013).
Zhou, J. et al. The relationship of Megamonas species with nonalcoholic fatty liver disease in children and adolescents revealed by metagenomics of gut microbiota. Sci. Rep. 12, 22001. https://doi.org/10.1038/s41598-022-25140-2 (2022).
Scupham, A. J., Patton, T. G., Bent, E. & Bayles, D. O. Comparison of the cecal microbiota of domestic and wild turkeys. Microb. Ecol. 56, 322–331 (2008).
Borrelli, L. et al. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 7, 16269. https://doi.org/10.1038/s41598-017-16560-6 (2017).
Kawasaki, K. et al. Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals 9, 98. https://doi.org/10.3390/ani9030098 (2019).
Biasato, I. et al. Black soldier fly and gut health in broiler chickens: insights into the relationship between cecal microbiota and intestinal mucin composition. J. Anim. Sci. Biotechnol. 11, 11. https://doi.org/10.1186/s40104-019-0413-y (2020).
Sypniewski, J. et al. Replacement of soybean oil by Hermetia illucens fat in turkey nutrition: effect on performance, digestibility, microbial community, immune and physiological status and final product quality. Br. Poult. Sci. 61, 294–302 (2020).
Kierończyk, B. et al. Replacement of soybean oil with cold-extracted fat from Hermetia illucens in young turkey diets: Effects on performance, nutrient digestibility, selected organ measurements, meat and liver tissue traits, intestinal microbiota modulation, and physiological and immunological status. Anim. Feed Sci. Technol. 286, 115210. https://doi.org/10.1016/j.anifeedsci.2022.115210 (2022).
Durazzi, F. et al. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 11, 3030. https://doi.org/10.1038/s41598-021-82726-y (2021).
Santos, M. H. S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 29, 213–231 (1996).
Santoru, M. L. et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 7, 9523. https://doi.org/10.1038/s41598-017-10034-5 (2017).
Pretorius, L. & Smith, C. Aspalathus linearis (Rooibos) and agmatine may act synergistically to beneficially modulate intestinal tight junction integrity and inflammatory profile. Pharmaceuticals 15, 1097. https://doi.org/10.3390/ph15091097 (2022).
Pretorius, L., Van Staden, A. D. P., Van Der Merwe, J. J., Henning, N. & Smith, C. Alterations to microbial secretome by estrogen may contribute to sex bias in irritable bowel syndrome. Inflammopharmacol 30, 267–281 (2022).
Pretorius, L. & Smith, C. Tyramine-induced gastrointestinal dysregulation is attenuated via estradiol associated mechanisms in a zebrafish larval model. Toxicol. Appl. Pharmacol. 461, 116399. https://doi.org/10.1016/j.taap.2023.116399 (2023).
Yu, M. et al. Dietary supplementation with citrus extract altered the intestinal microbiota and microbial metabolite profiles and enhanced the mucosal immune homeostasis in yellow-feathered broilers. Front. Microbiol. 10, 2662. https://doi.org/10.3389/fmicb.2019.02662 (2019).
Rzeznitzeck, J. et al. Morphology, microbiota, and metabolome along the intestinal tract of female turkeys. Poult. Sci. 101, 102046. https://doi.org/10.1016/j.psj.2022.102046 (2022).
Khoushab, F. & Yamabhai, M. Chitin research revisited. Mar. Drugs 8, 1988–2012 (2010).
Tabata, E. et al. Gastric and intestinal proteases resistance of chicken acidic chitinase nominates chitin-containing organisms for alternative whole edible diets for poultry. Sci. Rep. 7, 6662. https://doi.org/10.1038/s41598-017-07146-3 (2017).
Tabata, E. et al. Chitin digestibility is dependent on feeding behaviors, which determine acidic chitinase mRNA levels in mammalian and poultry stomachs. Sci. Rep. 8, 1461. https://doi.org/10.1038/s41598-018-19940-8 (2018).
Beier, S. & Bertilsson, S. Bacterial chitin degradation—Mechanisms and ecophysiological strategies. Front. Microbiol. 4, 149. https://doi.org/10.3389/fmicb.2013.00149 (2013).
Eklund, M., Bauer, E., Wamatu, J. & Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 18, 31–48 (2005).
Arumugam, M. K. et al. Beneficial effects of betaine: A comprehensive review. Biology 10, 456. https://doi.org/10.3390/biology10060456 (2021).
Metzler-Zebeli, B. U., Eklund, M. & Mosenthin, R. Impact of osmoregulatory and methyl donor functions of betaine on intestinal health and performance in poultry. World’s Poult. Sci. J. 65, 419–442 (2009).
Alhotan, R. A., Al Sulaiman, A. R., Alharthi, A. S. & Abudabos, A. M. Protective influence of betaine on intestinal health by regulating inflammation and improving barrier function in broilers under heat stress. Poult. Sci. 100, 101337. https://doi.org/10.1016/j.psj.2021.101337 (2021).
Shakeri, M. & Le, H. H. Deleterious effects of heat stress on poultry production: Unveiling the benefits of betaine and polyphenols. Poultry 1, 147–156 (2022).
Bedford, A. & Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 4, 151–159 (2018).
Onrust, L. et al. steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2, 75. https://doi.org/10.3389/fvets.2015.00075 (2015).
Makowski, Z., Lipiński, K. & Mazur-Kuśnirek, M. The effects of sodium butyrate, coated sodium butyrate, and butyric acid glycerides on nutrient digestibility, gastrointestinal function, and fecal microbiota in turkeys. Animals 12, 1836. https://doi.org/10.3390/ani12141836 (2022).
Loponte, R. et al. Fatty acid profile of lipids and caeca volatile fatty acid production of broilers fed a full fat meal from Tenebrio molitor larvae. Ital. J. Anim. Sci. 18, 168–173 (2019).
European Union. Council Directive (EC) n. 58/1998 of 20 July 1998 concerning the protection of animals kept for farming purposes. Off. J. L221, 23–27 (1998).
European Union. Directive (EU) n. 63/2010 of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. L276, 33–79 (2010).
European Union. Council Regulation (EC) n. 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. L303, 1–30 (2009).
Association of Official Analytical Chemists. Official methods of analysis, 15th ed. (1990).
Folch, J., Lees, M. & Stanley, G. H. S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Wishart, D. S. et al. HMDB: The human metabolome database. Nucleic Acids Res. 35, 521–526 (2007).
Dieterle, F., Ross, A., Schlotterbeck, G. & Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H-NMR metabonomics. Anal. Chem. 78, 4281–4290 (2006).
Acknowledgements
The authors sincerely appreciate the technical assistance provided by Stefano Pignata and Raffaela Piscitelli (Department of Agricultural and Food Sciences, Alma Mater Studiorum—University of Bologna, Ozzano dell’Emilia, Italy) and by Filiberto Ceccaroni, Francesco Biguzzi and Thomas Valentini (Amadori—Gesco S.C.A., San Vittore di Cesena, Forlì-Cesena, Italy) for their assistance in diets formulation and birds processing.
Funding
This research was undertaken under the NextGenProteins (Transformation of Biomass into Next Generation Proteins for Food and Feed) project, which has received funding from the European Union’s Horizon 2020 Research and Innovation Programme, Call H2020-LC-SFS-17-2019, grant agreement no. 862704 (https://nextgenproteins.eu/). Part of the study was implemented within the Agritech National Research Center and the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—Next-GenerationEU, Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP J33C22001190001, Project title “National Biodiversity Future Center—NBFC”. The funding sources had no role in study design, collection, analysis and interpretation of data, writing of the report, and decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
M.Z. analyzed growth performance data, interpreted the results and wrote the manuscript. A.D.C. and V.I. analyzed the microbiota data and revised the manuscript. L.L. analyzed the metabolomics data and revised the manuscript. F.So. and M.P. analyzed the meat quality data and revised the manuscript. C.T. provided the BSF meal as well as information regarding its chemical composition analysis and application in poultry diets. E.B. and J.D. provided support in biological samples collection and analysis as well as manuscript editing and formatting. F.Si. conceived and supervised the study. All authors revised the manuscript, and read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This research was authorized by the Ethical Committee of the University of Bologna (ID: 1145/2020) and all activities were in compliance with the European Legislation and guidelines on the protection of animals kept for farming and scientific purposes and on the protection of animals at slaughtering59,60,61. All methods are reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zampiga, M., De Cesare, A., Laghi, L. et al. Growth performance, meat quality, cecal microbiota and metabolomics profile of turkeys fed diets containing black soldier fly (Hermetia illucens) meal. Sci Rep 15, 22040 (2025). https://doi.org/10.1038/s41598-025-05624-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05624-7