Abstract
Supplementary irrigation with plasma-activated water (PAW) has been shown to boost seed germination and seedling vigor, and it has the potential to induce host plant resistance against pest populations, such as, two-spotted spider mites (TSSM) (Tetranychus urticae Koch). However, there is limited knowledge about the relative susceptibility of TSSM life stages to supplementary PAW irrigation. Here, we used age two-sex life table analysis to examine demographic parameters on leaf discs from control tomato plants (no PAW irrigation) and from plants receiving supplementary PAW irrigation, PAW1 and PAW2 (treatment of water for 6.0 and 9.4 min with atmospheric plasma jet respectively). Immature TSSM mortality was significantly higher on PAW1 (52%) and PAW2 (26%) treatments compared to control (6%). Immature developmental duration, adult pre-oviposition period and total pre-oviposition periods, adult longevity, fecundity, and sex ratio were all significantly reduced in response to PAW irrigation. Life table analyses showed that intrinsic rate of increase (r), net reproductive rate (R0), and finite rate of increase (λ) were significantly reduced on leaf discs from PAW-irrigated plants compared to control. Population modeling over a 60-day time showed a 10-11-fold reduction in TSSM populations on PAW-irrigated plants compared to control. These findings confirm the suppressive effects of supplementary PAW irrigation on TSSM population dynamics. Furthermore, results support the hypothesis that early-stage susceptibility, prolonged developmental times of individual life stages, and reduced fecundity are key factors driving PAW-based suppression of TSSM population dynamics. Thus, we conclude that supplementary PAW irrigation should be considered a potential component of long-term and sustainable pest management against TSSM and other major crop pests.
Similar content being viewed by others
Introduction
Two-spotted spider mites (TSSM) [Tetranychus urticae Koch (Acari: Tetranychidae)] are highly destructive agricultural pests1,2 with a host range of 1,100 known plant species3. In greenhouse tomato (Solanum lycopersicum L.) production, yield losses may be as high as 50% due to feeding causing chlorosis, reduced photosynthesis, and stunted growth4,5. Under severe infestations, TSSM produce silk-like webbing on host plants6. Additionally, TSSM infestations may induce stress, which increases susceptibility to secondary pests and diseases7. Traditional control methods often rely on frequent applications of synthetic acaricides8. However, rapid development of resistance in TSSM populations due to their short life cycle and high reproductive rate has rendered many acaricides ineffective8,9. Resistance development of this herbivore to 96 active ingredients has been reported (APRD, www.pesticideresistance.org/). Furthermore, the overuse of chemical agents poses serious environmental and health risks, highlighting the need for alternative, eco-friendly pest management strategies.
Releases of predatory mites, such as, Phytoseiulus persimilis [Athias-Henriot (Acari: Phytoseiidae)], have shown promise in management of TSSM populations2,10. However, the adoption of biological control programs in commercial tomato production has been limited due to inconsistent performance and due to practical constraints associated with needs for extensive pest population monitoring11. In certain tomato cultivars, this inconsistency is attributed to dense glandular trichomes, which can hinder the movement, establishment, and effectiveness of predatory mites12,13. Integrated Pest Management (IPM) strategies combining soft miticides with biological control agents have been recommended14,15,16. However, they may not be effective under high TSSM-pressure conditions, necessitating the development of preventive management tactics to minimize risks of high TSSM population densities.
High-voltage discharge to ionize gases at low or atmospheric pressure converting them into plasma has been known since the early 1900s17,18,19,20. This technology has been widely applied in surface sterilization of electronics, medical sterilization, fusion energy research, spacecraft propulsion, and biomaterials/food treatment21,22. Within the last decade, its applications have expanded, as it has been used to generate Plasma-Activated Water (PAW)23,24. PAW can be produced by introducing cold plasma ions from atmospheric air or gases like argon, oxygen, helium, or nitrogen into water25,26,27. PAW is rich in reactive oxygen and nitrogen species (RONS), including hydrogen peroxide (H₂O₂), nitrate (NO₃⁻), and nitrite (NO₂⁻), which offer multiple benefits, including in crop production. For instance, PAW has been shown to enhance seed germination, plant growth, nutrient uptake, and nutrient solubility26,28,29,30,31,32,33. PAW has also been shown to increase antioxidant enzyme activity and improve plant defense responses34. Additionally, it has been shown to suppress plant pathogenic viruses, fungi, and bacteria35,36,37. Finally, there is report of PAW possessing insecticidal activity against citrus mealybugs [Planococcus citri (Risso) (Hemiptera: Pseudococcidae)]38.
Our recent study investigated effects of PAW when applied as supplementary irrigation of tomato plants and its potential impact on TSSM populations32. Results demonstrated that supplementary PAW irrigation induced significant changes in leaf element composition and both non-glandular and glandular trichome densities, key components of plant resistance mechanisms against arthropod pests. These trichomes act as physical and chemical barriers, secreting high-viscosity allelochemicals such as acyl sugars, methyl-ketones, and sesquiterpenes that deter arthropod settling and feeding39,40. PAW-irrigated plants also caused a significant reduction in TSSM settling and overall population dynamics32.
These findings suggest that PAW can enhance plant defenses and indirectly suppress pest populations, offering a novel and eco-friendly approach to long-term and sustainable management of this and other important greenhouse pests. However, limited knowledge is available about effects of PAW irrigation of host plants on relative susceptibility of different pest life stages. A comprehensive breakdown of population structure, including sex and stage differentiation, is essential for informed decision-making in TSSM management programs41,42. Life table studies, which are powerful tools in population ecology, provide valuable insights by analyzing survival, development, and reproductive rates across specific life stages, linking individual-level responses to population-level outcomes43,44,45. Such approaches have been widely used to identify life stages or population structures that drive overall population dynamics under various stressors, including environmental conditions46,47 host plant quality48,49,50, and chemical treatments51,52. The age-stage, two-sex life table theory by Chi and Liu53 takes into account both sexes and stage-specific developmental rates among individuals. In so doing, it can provide comprehensive insight into population characteristics and be used to forecast temporal TSSM population dynamics in response to PAW irrigation42,54,55.
As outlined in Fig. 1, we examined the hypothesis that PAW irrigation of host plants has life-stage-specific effects on TSSM population dynamics. Results from this study are based on an analytical approach that has broad relevance to mechanistic analyses of demographic responses by arthropod populations to specific stressors. More specifically, in-depth insight into suppressive effects of supplementary PAW irrigation on TSSM population dynamics. Thus, we conclude that PAW irrigation should be considered a potential component of long-term and sustainable pest management tactics against TSSM and other major crop pests.
Materials and methods
Production and characterization of PAW
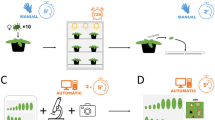
Two PAW regimes were used, where UC Davis tap water (control) was treated for either 6.0 min (PAW 1) or 9.4 min (PAW 2) and left for 24 h after treatment for further characterization. These specific durations were selected based on their distinct responsiveness in promoting plant growth observed in preliminary trials, including Savi, et al.32 which tested a broader range of PAW treatments. Physicochemical properties of PAW (pH, electrical conductivity, and oxidation-reduction potential) were measured using pH/EC(OHAUS-AB331M-F) and ORP meters (Hanna HI2202-01). Reactive species nitrate (NO₃⁻), nitrite (NO₂⁻), and hydrogen peroxide (H₂O₂) were quantified using a benchtop multiparameter photometer (HI83399-01). Measurements were collected following a completely randomized design with eight replicates per treatment, ensuring reliable data. PAWs were generated using an Openair™ Plasma System from Plasmatreat USA, Inc., as described in Savi, et al.32. For each treatment, 1 L of water was placed in a 2.5-liter Pyrex glass beaker and continuously stirred on a magnetic stirrer during plasma exposure (Fig. 1a). The setup ensured uniform treatment and prevented spillage with a corrosion-resistant steel cover. PAW samples used for supplementary irrigation of tomato plants were stored at 5 °C and were not older than four days at the time of application. Although literature reports that key reactive species such as H₂O₂, NO₃⁻, and NO₂⁻ can remain stable under cold storage for up to 21 days56 we deliberately limited the storage duration to four days to ensure the consistent use of freshly generated throughout the plant production cycle.
Plants
Tomato cultivar Micro-Tom (S. lycopersicum L.) plants were used in the experiments. Micro Tom is widely recognized as a model cultivar in Solanaceae research due to its relatively small size and its short determinate life cycle57. This cultivar is also susceptible to herbivores including TSSM13 making it suitable for evaluating PAW effects on plant responses to TSSM. Micro-Tom seeds obtained from Seeds Buck (Augusta, California, USA) were grown in tray cells with a sterile, homogeneous substrate composed of pumice, sphagnum peat moss, sand, redwood sawdust, and dolomite. The growing medium was autoclaved at 121 °C for 1 h. Trays were placed in greenhouse facilities at the University of California, Davis, and an automated sprinkling irrigation system (Mix Rite injector, model 2502) provided water and fertilizer four times a day at 7 am, 10 am, 2 pm, and 5 pm. Fertilizer solution had the following composition (values in ppm): N = 150, P = 50, K = 200, Ca = 175, Mg = 55, S = 120, Fe = 2.5, Cu = 0.02, B = 0.5, Mn = 0.5, Mo = 0.01, and Zn = 0.05. Four weeks after sowing, seedlings were individually transplanted to 1.5-L pots 80% filled with a homogeneous mixture of soil, sand, and tanned bovine manure (1:1:1) autoclaved at 121 °C for 1 h. A 35 ml solution of water and fertilizer (prepared at the previously mentioned rates) was delivered to the pot twice daily (at 7 am and 6 pm) using an automated drip irrigation system. In addition, 50 ml of either PAW1, PAW2, or tap water was applied once a day, between 11 am and 12 noon, to each plant until the conclusion of the experiments (Fig. 1b). As a result, PAW treatments or control water accounted for 42% of the total water supplied to individual tomato plants.
Given that performance of arthropod herbivores is influenced by parental host plant (Maternal Effects Compensation Hypothesis) and that multiple generations are needed for physiological adjustment to a new host plant (Host Adaptation Hypothesis)58,59 TSSM colonies originally collected from soybean plants (Glycine max L. Merr.) at the Department of Entomology and Nematology, UC Davis, USA, were reared for several generations on control and PAW-irrigated plants before being used in experiments (Fig. 1c). Colonies were maintained in a greenhouse under controlled conditions: 25.2 ± 1.0 °C, 77 ± 10% RH, and a 12:12 L: D photoperiod.
Experimental procedure
Experimental units consisted of plastic deli containers (50 mm diameter, 2 cm height) covered with a nylon foam layer (10 mm thick) lined with moistened cotton wool (Fig. 1d). A 30 mm diameter leaf disc from each treatment was placed abaxial side up in the center. Individual gravid TSSM females were transferred to leaf discs. After six hours, females and excess eggs were removed, leaving only one per disc. Using only individual eggs in experimental units is important to avoid bias induced by cannibalism and competition. Units were maintained under controlled conditions matching the TSSM colonies. Upon adulthood, TSSM individuals were sexed and paired in new units, with males from the stock colony used if needed. Units were examined daily under a stereomicroscope to record developmental stages, survivorship, size, mobility, and molting (via exuviae presence). Parameters recorded included pre-oviposition period (APOP: adult emergence to first oviposition), total pre-oviposition period (TPOP: first oviposition of parent to offspring), oviposition days, longevity, sex ratio, and fecundity. Males dying before females were replaced. Eggs laid were removed after every observation period, and leaf discs were replaced every three days to maintain leaf quality. Data on males from colonies and TSSM who died on the cotton wool strip while attempting to escape were excluded from statistical analyses.
Data analysis
All data analyses were performed in R v3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria). Physicochemical properties of PAW did not meet homoscedasticity and normality assumptions, even after transformations; they were analyzed based on nonparametric Kruskal–Wallis’ comparison of averages of treatments (library ‘AgroR’). Raw data on development, reproduction, and population parameters, and population parameters were estimated using an age-stage two-sex life table model53 developed by Chi60 and available at http://140.120.197.173/ecology/Download/Twosex-MSChart.rar. Parameters estimated (Table 1) included age-stage-specific survival rate (sxj), age-specific survival rate (lx), age-specific fecundity (mx), age-stage-specific fecundity (fxj), net reproduction rate (R₀), intrinsic rate of increase (r), finite rate of increase (λ), and average generation time (T). Standard errors (SE) of development, fecundity, reproduction period, and population parameters were calculated using a bootstrap procedure with 100,000 resamplings. Treatment differences were assessed using paired bootstrap tests (B = 100,000) at a 95% confidence interval61,62. TSSM population was projected using life table rate data based on the age-stage, two-sex life table theory53,54. Population growth over 60 days was simulated using the TIMING-MSChart software42with an initial population of 10 newly laid eggs across the three treatments.
Results
Characteristics of PAW used for plant growing
Compared to control, which had a pH of 8.8 ± 0.1, both PAW1 and PAW2 treatments showed significant reductions in pH (χ² = 23.19, df = 2, p < 0.001), with values dropping to 3.1 ± 0.1 and 2.9 ± 0.0, respectively (Fig. 2a). Electrical conductivity (EC) increased significantly (χ² = 23.14, df = 2, p < 0.001), with the highest value observed in PAW2 (633.8 ± 7.1 µS/cm), followed by PAW1 (454.1 ± 11.0 µS/cm), and the lowest in control water (214.9 ± 8.1 µS/cm) (Fig. 2b). A similar trend was seen in oxidation-reduction potential (ORP) (χ² = 23.16, df = 2, p < 0.001), with PAW2 having the highest value (563.2 ± 0.7 mV), PAW1 intermediate (553.8 ± 1.7 mV), and the control the lowest (281.4 ± 3.9 mV) (Fig. 2c). Nitrite (NO₂⁻) and nitrate (NO₃⁻) concentrations also increased significantly (NO₂⁻: χ² = 23.67, df = 2, p < 0.001; NO₃⁻: χ² = 23.15, df = 2, p < 0.001), with the highest levels in PAW2 (116.44 ± 5.38 mg/L and 716.55 ± 66.61 mg/L, respectively), followed by PAW1 (102.44 ± 4.87 mg/L and 551.55 ± 39.79 mg/L), and the lowest in the control (0.00 ± 0.00 mg/L and 0.77 ± 0.49 mg/L) (Fig. 2d and e). Hydrogen peroxide (H₂O₂) concentrations increased significantly in both PAW1 and PAW2 treatments, reaching 583.3 ± 98.3 mg/L and 666.7 ± 121.1 mg/L, respectively (χ² = 12.3, df = 2, p = 0.002), compared to the control (0.4 ± 0.3 mg/L) (Fig. 2f).
Developmental stages, reproduction, and life table parameters
TSSM immature mortality rate was significantly higher on leaf discs from both PAW1- (52%) and PAW2- (26%) irrigated plants than control (6%). PAW1 led to the highest mortality rate (Table 2). Mortality rates varied across developmental stages. In PAW1 treatment, the highest mortality was observed in the egg (20%), larva (14%), and deutonymph (8%) stages. In PAW2, the highest mortality rate was observed in both egg and larval stages (12% for each stage). In contrast, the control group did not show mortality above 2% in any immature stage. No significant differences were observed in development times (in days) across treatments: eggs (8.0–8.1), larvae (3.1), and deutonymphs (3.1–3.4). Protonymph life stage varied significantly across treatments, with control showing the shortest duration (2.1), followed by PAW1 treatment (2.2 days), and PAW2 treatment eliciting the longest duration (2.4 days). TSSM female pre-adult time varied significantly among treatments, with PAW1 (17.0) having the most extended duration followed by PAW2 (16.7), while the control (16.4) had the shortest development time. TSSM male pre-adult time was significantly longer on leaf discs from PAW2-irrigated plants (16.8) than on leaf discs from PAW1-irrigated plants (16.0) and control (15.5).
Adult pre-oviposition period (APOP) was significantly longer on leaf discs from PAW1 (1.6 days) and PAW2 (1.7 days) irrigated plants compared to control (1.5 days) (Table 3). Similarly, total pre-oviposition period (TPOP) was significantly extended on leaf discs from PAW1- (18.6 days) and PAW2- (18.3 days) irrigated plants than on leaf discs from control (17.8 days). Oviposition days were significantly reduced in PAW2 treatment (9.9 days) compared to the control (13.0 days), with PAW1 treatment (10.5 days) showing an intermediate effect. Female adult longevity was significantly shorter on leaf discs from PAW1 (14.8 days) and PAW2 (13.6 days) irrigated plants than on control (17.3 days). Similarly, male adult longevity was significantly reduced on leaf discs from PAW1 (9.1 days) and PAW2 (8.0 days) compared to the control (14.2 days). Sex ratio (♀/♂+♀) was significantly lower on leaf discs from PAW1- (0.42) and PAW2- (0.50) irrigated plants than on control (0.82). Fecundity followed the same trend, being significantly reduced on leaf discs from PAW1- (37.14 eggs/female) and PAW2- (32.52 eggs/female) irrigated plants compared to leaf discs from control plants (49.93 eggs/female).
PAW treatments significantly affected life table parameters (Table 4). The net reproductive rate (R0) was significantly lower when plants were treated with PAW1 (15.60 offspring/individual) and PAW2 (16.26 offspring/individual) compared to the control (40.94 offspring/individual). Similarly, the intrinsic rate of increase (r) and finite rate of increase (λ) were significantly reduced in PAW1 (r = 0.113 day⁻¹; λ = 1.10 day⁻¹) and PAW2 (r = 0.115 day⁻¹; λ = 1.12 day⁻¹) relative to the control (r = 0.156 day⁻¹; λ = 1.16 day⁻¹). In contrast, the mean generation time (T) did not differ significantly among treatments (Control: 23.8 days; PAW1: 23.8 days; PAW2: 24.2 days).
Age-survival rate and fecundity of the specific stage
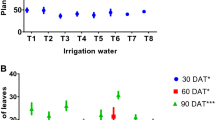
Age-stage survival (sxj) curves in Fig. 3a illustrate TSSM proportion at each developmental stage relative to the initial number of eggs (50) across different treatments. The observed overlapping proportions of distinct stages are due to variations in developmental rates among individuals. The proportions reached their maximum value, dropping as a function of molting to the next stage or mortality. The probability of a freshly laid TSSM egg surviving to the female adult stage was significantly lower on leaf discs from plants irrigated with PAW1 (0.42) and PAW2 (0.50) compared to the control (0.82) (Fig. 3a). In contrast, the probability of survival to the male adult stage was significantly higher on leaf discs from PAW1 (0.16) and PAW2 (0.20) treatments than on the control (0.12).
The age-specific survival rate (lx) represents the probability of a TSSM surviving up to age x across all developmental stages (Fig. 3b). Results showed that 80% of TSSM individuals maintained on leaf discs from PAW1- and PAW2-irrigated plants died before reaching 10 and 12 days of age, respectively. In contrast, those on control plants only experienced this level of mortality after 27 days. The age-specific fecundity (mx), defined as the average daily fecundity per individual at age x (Fig. 3b), showed the lowest peaks for PAW1 (3.07 eggs) and PAW2 (3.80 eggs) treatments on the 22nd and 20th days, respectively. In comparison, the control treatment exhibited the highest peak (4.12 eggs) on the 23rd day. Age-stage-specific fecundity fx5 (Fig. 3b), representing the daily number of eggs produced per female at age x, showed higher egg-laying peaks on control (4.73 eggs) on day 22 of their age. In contrast, peak fecundity fx5 was lower on PAW1 (3.80 eggs) and PAW2 (3.60 eggs) treatments on day 22 and day 35 of their age, respectively.
TSSM population growth projection
Figure 4 shows TSSM population projection over 60 days on control plants, PAW1- and PAW2-irrigated plants, starting from an initial cohort of 10 eggs. During the first 10 days, population size remained stable across treatments, as most individuals were still in the egg stage, resulting in minimal changes in population size. Between days 10 and 15, a decline was observed across all groups, likely due to molting events or early-stage mortality. From day 20 onward, the majority of TSSM reached the adult stage, leading to exponential population growth. By the end of 60 days, control treatment revealed the highest population size (2633.3 individuals). In contrast, PAW 1 (249.5 individuals) and PAW 2 (238.4 individuals) resulted in significantly lower population size, corresponding to an approximate 10.56- to 11-fold reduction in TSSM population compared to the control.
Discussion
In a recent study, we demonstrated that supplementary PAW irrigation has potential to elicit significant TSSM avoidance and suppress population dynamics on tomato plants32. Pest population dynamics are largely driven by behavioral responses and relative susceptibility of individual pest life stages63,64so further insight into effects of supplementary PAW irrigation on individual life stages is needed. Accordingly, we performed two-sex life table analysis of TSSM when reared on plants subjected to supplementary PAW irrigation, and life table data were compared with those obtained from control plants. Our results revealed that supplementary PAW irrigation significantly increased mortality of immature TSSM life stages. Additionally, developmental durations and pre-reproductive phases were significantly prolonged in TSSM maintained on leaf discs from PAW-irrigated plants. We also demonstrated that supplementary PAW irrigation significantly reduced the proportion of females relative to males in a population (sex ratio), adult longevity, and fecundity compared to control. The intrinsic rate of increase (r), net reproductive rate (R0), and finite rate of increase (λ), which collectively reflect developmental, survival, and reproductive parameters, were significantly lower in TSSM populations maintained on leaf discs from PAW-irrigated plants. Moreover, a 60-day TSSM population projection showed a 10- to 11-fold reduction in PAW-irrigated plants compared to the control. These findings confirm the suppressive effects of PAW irrigation on TSSM population dynamics and support the hypothesis that early-stage susceptibility, developmental delays, and reduced fecundity are key factors driving this population dynamic. Consistent with our results, Dilip, et al.65 reported that rice plants grown from atmospheric cold plasma-treated seeds and irrigated with PAW elicited altered plant traits that negatively affected fall armyworm (FAW) [Spodoptera frugiperda (Lepidoptera: Noctuidae)]. They observed a significant reduction in larval mass gain and prolonged pupation periods, leading to a 25% increase in FAW mortality. These results suggest that PAW may broadly impact pest-plant interactions across various pest species, making it a valuable eco-friendly component of IPM strategies.
A major driver of PAW-driven suppression of TSSM population dynamics in PAW-irrigated plants appears to be the significant increase in immature mortality, which peaked at 52% in the PAW1 treatment and 26% in PAW2, in contrast to just 6% in the control. This suggests that significant physicochemical differences (i.e. pH, NO₂⁻, NO₃⁻, and H₂O₂) induced plant defense responses increased TSSM mortality. Previous studies have reported that leaf trichomes, when present in high densities in tomato plants, can elicit strong negative impacts on pest immature survival. These trichomes, categorized as non-glandular (types II, III, and V) and glandular (types I, IV, VI, and VII), act as physical and chemical barriers by secreting toxic exudates such as acyl sugars, methyl-ketones, and sesquiterpenes to impair pest movement and reduce survival15,40,50,66,67. Similarly, PAW irrigation has been reported to increase both glandular and non-glandular trichome densities, which may account for the higher mortality rates observed in PAW-irrigated plants2,39. This increase in trichome density is likely mediated by the high levels of ROS and RNS present in PAW. These ROS and RNS act as signaling molecules that influence hormone signaling pathways such as jasmonic acid (JA) and gibberellin (GA) known to trigger structural defense responses such as trichome proliferation and the activation of defense-related genes68 by modulating JAZ repressors or activating mitogen-activated protein kinase (MAPK) cascades29,69. At the molecular level, PAW irrigation also triggers oxidative stress responses, as reflected in the upregulation of miRNAs such as miR159, miR395, and miR398 each known to respond to H₂O₂ accumulation30,70. These miRNAs regulate key antioxidant enzymes and stress-related genes, contributing to sustained ROS signaling and enhanced defense readiness, including increased callose deposition and altered redox homeostasis30. Moreover, differences in H₂O₂ concentrations across PAW treatments likely influenced plant defense responses, contributing to variations in TSSM juvenile mortality. Although PAW2 contained a significantly higher H₂O₂ concentration than PAW1, it did not result in higher juvenile mortality (26% vs. 52%, respectively). This unexpected trend suggests a potential “optimal concentration effect,” in which moderate levels of H₂O₂ may be more effective at enhancing plant defenses than higher concentrations. Similar non-linear, dose-dependent responses have been reported in study by Adhikari, et al.29where excessive reactive oxygen species can lead to reduced efficacy due to compensatory detoxification mechanisms or hormonal imbalances.
A significant increase in development time was observed on leaf discs from PAW-irrigated plants. PAW1 treatment extended the development of individuals that matured into females, whereas PAW2 treatment caused a delay in those that matured into males. These findings suggest that PAW may interfere with TSSM molting and maturation depending on the quality of PAW applied. Furthermore, our results showed that PAW irrigation significantly reduced the proportion of females relative to males, adult longevity, and fecundity in TSSM populations, suggesting that supplementary PAW irrigation alters host plant quality in a way that affects sex allocation and reproductive success. Previous studies have shown that TSSM populations on tomato plants are typically female-biased, but this balance can be altered by extrinsic factors such as temperature71host plant quality72,73which is consistent with our findings. The shift toward a male-biased population on PAW-irrigated plants (PAW1: 0.42♀; PAW2: 0.50♀) compared to control (0.82♀) may be associated with reduced fertilization success, impaired sperm storage, or altered male reproductive behavior due to the high density of glandular trichomes and associated compounds, as previously mentioned32. Additionally, our visual observations indicated that eggs laid on leaf discs from PAW-irrigated plants appeared smaller than those on control plants, suggesting nutritional stress or limited resource availability. This is consistent with the mechanism described by Macke, et al.74who demonstrated that in TSSM, smaller eggs are less likely to be fertilized and more often develop into male offspring, whereas larger eggs are more likely to be fertilized and produce females. Thus, the observed reduction in female-to-male sex ratio, may be directly linked to egg size differences resulting from altered host plant quality. Therefore, the combined effects of a reduced female-to-male sex ratio and delayed reproduction (APOP) could contribute to reducing fecundity and slowing TSSM population expansion.
Life table parameters are widely recognized as reliable tools for assessing the bottom-up effects of host plants on pest populations42,50,53,62 Among these, the intrinsic rate of increase (r) is the most informative measure, as it integrates development, survival, fecundity, and sex ratio, serving as a key indicator of population performance45,75,76. In this study, r ranged from 0.156 day⁻¹ in control to 0.115 day⁻¹ in PAW-irrigated plants, falling within the reported range for TSSM populations on tomato varieties (0.060 to 0.295 day⁻¹)72,77,78. The significantly lower r in PAW-irrigated plants confirms its suppressive effect on TSSM population dynamics, making PAW irrigation a promising component for enhancing IPM strategies.
The 60-day population projection revealed around 11-fold reduction in TSSM populations on PAW-treated plants compared to the control. These results are consistent with recent findings under greenhouse conditions, where PAW-irrigated plants maintained consistently lower TSSM populations over three weeks32.This significant suppression suggests that PAW irrigation can serve as a long-term component for mitigating TSSM outbreaks, particularly in greenhouse environments where TSSM can rapidly reach economic injury levels. Additionally, delayed population growth has important IPM implications, as it can create a window of opportunity for natural enemies, such as predatory mites, to establish and control TSSM populations before they reach damaging levels.
In conclusion, this study provides strong evidence that increased immature mortality, delayed development, and reduced reproductive success are key drivers of the suppressive effects of supplementary PAW irrigation on overall TSSM population dynamics. Life table analyses have shown to be a crucial tool in assessing these impacts, reinforcing PAW’s potential as an effective component of IPM strategies. The ability of supplementary PAW irrigation to disrupt TSSM population dynamics suggests its potential to complement existing IPM tactics by reducing initial pest infestations and may favor predatory mites as biocontrol agents to suppress the TSSM population efficiently. Although studies of PAW-irrigated plant compatibility with biocontrol agents are still scarce, a significant increase in both non-glandular and glandular trichome densities by PAW in tomato plants32 may hinder the establishment of predatory mites. Additionally, a recent study investigated PAW direct effects on three species of entomopathogenic nematodes (EPNs) such as Steinernema feltiae Filipjev, S. carpocapsae Weiser, and Heterorhabditis bacteriophora Poinar79. Their results revealed PAW compatibility with S. carpocapsae but significant harmful effects on other two species. Therefore, further research is needed to evaluate PAW’s effects on a broader range of non-target organisms before its widespread implementation as a component of IPM programs. Further research is necessary to validate these findings on other crops and evaluate the long-term feasibility of PAW irrigation in diverse cropping systems. This will contribute to a deeper understanding of PAW-irrigated plant-pest interactions and support the development of sustainable pest management practices that enhance crop resilience while promoting ecological balance in agricultural systems.
Overview of experimental setup and workflow. (a) Atmospheric plasma jet (APJ) used to generate PAW (b) Tomato plants irrigated with tap water (control) and with PAW1 or PAW2 (water treated for 6.0 and 9.4 min with APJ, respectively) (c) Colonies of two-spotted spider mite (TSSM) Tetranychus urticae on different experimental tomato plants (d) Experimental setup to evaluate effects of PAW-irrigated plants on TSSM life stages.
(a) pH, (b) Conductivity, (c) Oxidation–Reduction Potential (ORP), (d) Concentrations of Nitrate (NO2-, e) Nitrite (NO3-, and f) concentration of Hydrogen Peroxide (H2O2), in untreated tap water (control) and 6 min (PAW1) and 9.4 min (PAW2) treated plasma-activated water. Bars followed by different letters are significantly different (Kruskal–Wallis’s test, p < 0.05).
Variation in survival rates and fecundity of two-sppoted spider mite Tetranychus urticae across life stages on leaves from untreated and PAW-supplemented- tomato plants; (a) age-stage specific survival rates (sxj), and (b) age-specific survival rate (lx), age-specific fecundity (mx) and age-stage-specific fecundity (fx5). The two PAWs corresponded to plasma activation of water for 6.0 (PAW 1) and 9.4 min (PAW 2).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jakubowska, M., Dobosz, R., Zawada, D. & Kowalska, J. A. Review of crop protection methods against the two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) with special reference to alternative methods. Agriculture 12, 898 (2022).
Savi, P. et al. Effects of timed LED regimes on tomato plant traits, performance of two-spotted spider mites, and predatory mites. Pest Manag Sci. 81, 2300–2311. https://doi.org/10.1002/ps.8630 (2025).
Grbic, M. et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479, 487–492 (2011).
Knapp, M., Palevsky, E. & Rapisarda, C. in In Integrated Pest and Disease Management in Greenhouse Crops. 101–146 (eds Gullino, M. L.) (Springer International Publishing, 2020). Philippe C. Nicot.
Jayasinghe, G. & Mallik, B. Growth stage based economic injury levels for two spotted spider mite, Tetranychus urticae Koch (Acari, Tetranychidae) on tomato, Lycopersicon esculentum mill. Trop Agric 22, 54-65 (2011).
Gerson, U. & Webbing Spider mites: their biology, natural enemies and control. Elsevier, Amsterdam, 223–232 (1985).
Santamaria, M. E. et al. Plant defenses against Tetranychus urticae: Mind the gaps. Plants 9, 464 (2020).
Savi, P. J. et al. Performance of Tetranychus urticae (Acari: Tetranychidae) on three hop cultivars (Humulus lupulus). Exp. Appl. Acarol. 84, 733–753. https://doi.org/10.1007/s10493-021-00643-1 (2021).
Van Leeuwen, T., Vontas, J., Tsagkarakou, A., Dermauw, W. & Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important acari: a review. Insect Biochem. Mol. Biol. 40, 563–572. https://doi.org/10.1016/j.ibmb.2010.05.008 (2010).
McMurtry, J., de Moraes, G. J. & Sourassou, N. F. Revision of the lifestyles of phytoseiid mites (Acari:Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 18, 297–320. https://doi.org/10.11158/saa.18.4.1 (2013).
Weinblum, N. et al. Tomato cultivars resistant or susceptible to spider mites differ in their biosynthesis and metabolic profile of the monoterpenoid pathway. Front. Plant. Sci. 12 https://doi.org/10.3389/fpls.2021.630155 (2021).
Savi, P. J., De Moraes, G. J. & De Andrade, D. J. Effect of tomato genotypes with varying levels of susceptibility to Tetranychus evansi on performance and predation capacity of Phytoseiulus longipes. BioControl 66, 687–700 (2021). https://doi.org/10.1007/s10526-021-10096-5
Tabary, L., Navajas, M., Tixier, M. S. & Navia, D. Tomato trichomes: trade-off between plant defenses against pests and benefits for biological control agents. Acarologia 64, 1232–1253 (2024).
Tiftikçi, P., Kök, Ş. & Kasap, İ. The effect of host plant on the biological control efficacy of the predatory mite, Phytoseiulus persimilis Athias-Henriot against two-spotted spidermites, Tetranychus urticae Koch on field-grown vegetables. Crop Prot. 158, 106012. https://doi.org/10.1016/j.cropro.2022.106012 (2022).
Lucini, T., Faria, M. V., Rohde, C., Resende, J. T. V. & De Oliveira, J. R. F. Acylsugar and the role of trichomes in tomato genotypes resistance to Tetranychus urticae. Arthropod Plant. Interact. 9, 45–53. https://doi.org/10.1007/s11829-014-9347-7 (2015).
Savi, P. J., Martins, M. B., De Moraes, G. J., Hountondji, F. C. C. & De Andrade, D. J. Bioactivity of oxymatrine and Azadirachtin against Tetranychus evansi (Acari: Tetranychidae) and their compatibility with the predator Phytoseiulus longipes (Acari: Phytoseiidae) on tomato. Syst. Appl. Acarol. 26, 1264–1279 (2021).
Raizer, Y. P. & Allen, J. E. Gas Discharge PhysicsVol. 2 (Springer, 1997).
Fridman, A. Plasma Chemistry (Cambridge University Press, 2008).
Monell, S. H. High Frequency Electric Currents in Medicine and Dentistry: their Nature and Actions and Simplified Uses in External Treatments (WR Jenkins Company, 1910).
Mayhan, K. Plasma-Formed polymers for biomedical applications. NBS Spec. Pub. 415, 1–12 (1975).
Montie, T. C., Kelly-Wintenberg, K. & Roth, J. R. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 28, 41–50 (2000).
Gomathi, N., Sureshkumar, A. & Neogi, S. RF plasma-treated polymers for biomedical applications. Curr Sci. 94, 1478–1486 (2008).
Gao, Y., Francis, K. & Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 157, 111246 (2022).
Thirumdas, R. et al. Plasma activated water (PAW): chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 77, 21–31 (2018).
Guo, D., Liu, H., Zhou, L., Xie, J. & He, C. Plasma-activated water production and its application in agriculture. J. Sci. Food Agric. 101, 4891–4899. https://doi.org/10.1002/jsfa.11258 (2021).
Han, Q. Y., Wen, X., Gao, J. Y., Zhong, C. S. & Ni, Y. Y. Application of plasma-activated water in the food industry: A review of recent research developments. Food Chem. 405, 134797. https://doi.org/10.1016/j.foodchem.2022.134797 (2023).
Montalbetti, R., Machala, Z., Gherardi, M. & Laurita, R. Production and chemical composition of plasma activated water: a systematic review and meta-analysis. Plasma Process. Polym. 22, 2400249. https://doi.org/10.1002/ppap.202400249 (2025).
Pankaj, S. K. & Keener, K. M. Cold plasma: background, applications and current trends. Curr. Opin. Food Sci. 16, 49–52. https://doi.org/10.1016/j.cofs.2017.07.008 (2017).
Adhikari, B., Adhikari, M., Ghimire, B., Park, G. & Choi, E. H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 9 https://doi.org/10.1038/s41598-019-52646-z (2019).
Zambon, Y. et al. Plasma activated water triggers plant defence responses. Sci. Rep. 10 https://doi.org/10.1038/s41598-020-76247-3 (2020).
Priatama, R. A., Beak, H. K., Song, I., Park, S. J. & Lee, Y. K. Long-term plasma-activated-water Irrigation Improves Fruit Yield in Tomato (Research Square Platform LLC, 2023).
Savi, P. J. et al. Indirect effects of plasma-activated water irrigation on Tetranychus urticae populations. J. Pest Sci. https://doi.org/10.1007/s10340-024-01791-0 (2024).
Aceto, D. et al. Assessing plasma activated water irrigation effects on tomato seedlings. Front. Phys. 12 https://doi.org/10.3389/fphy.2024.1399910 (2024).
Kučerová, K., Henselová, M., Slováková, Ľ. & Hensel, K. Effects of plasma activated water on wheat: germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 16, 1800131. https://doi.org/10.1002/ppap.201800131 (2019).
Lee, G., Choi, S. W., Yoo, M., Chang, H. J. & Lee, N. Effects of plasma-activated water treatment on the inactivation of microorganisms present on Cherry tomatoes and in used wash solution. Foods 12, 2461. https://doi.org/10.3390/foods12132461 (2023).
Ambrico, P. F. et al. Surface dielectric barrier discharge plasma: a suitable measure against fungal plant pathogens. Sci. Rep. 10 https://doi.org/10.1038/s41598-020-60461-0 (2020).
Filipić, A. et al. Cold atmospheric plasma as a novel method for inactivation of potato virus Y in water samples. Food Environ. Virol. 11, 220–228. https://doi.org/10.1007/s12560-019-09388-y (2019).
Ten Bosch, L., Köhler, R., Ortmann, R., Wieneke, S. & Viöl, W. Insecticidal effects of plasma treated water. Int. J. Environ. Res. Public. Health. 14, 1460. https://doi.org/10.3390/ijerph14121460 (2017).
Savi, P. J. et al. Impact of leaflet trichomes on settlement and oviposition of Tetranychus evansi (Acari: Tetranychidae) in African and South American tomatoes. Syst. Appl. Acarol. 24, 2559–2576. https://doi.org/10.11158/saa.24.12.19.short (2019).
Simmons, A. T. & Gurr, G. M. Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric. Entomol. 7, 265–276. https://doi.org/10.1111/j.1461-9555.2005.00271.x (2005).
Rossini, L. et al. Life tables in entomology: A discussion on tables’ parameters and the importance of Raw data. PLoS One. 19, e0299598. https://doi.org/10.1371/journal.pone.0299598 (2024).
Chi, H. et al. Age-Stage, two-sex life table: an introduction to theory, data analysis, and application. Entomol Gen. 40, 103-124 (2020).
Kareithi, D., Salifu, D., Owuor, N., Subramanian, S. & Tonnang, E. An algorithm for data reconstruction from published articles–application on insect life tables. Cogent Math. Stat. 6, 1701377 (2019).
Pinto, J. R. L., Fernandes, O. A., Higley, L. G. & Peterson, R. K. D. Do patterns of insect mortality in temperate and tropical zones have broader implications for insect ecology and pest management? PeerJ 10, e13340. https://doi.org/10.7717/peerj.13340 (2022).
Savi, P. J., de Moraes, G. J., Melville, C. C. & Andrade, D. J. Population performance of Tetranychus evansi (Acari: Tetranychidae) on African tomato varieties and wild tomato genotypes. Exp. Appl. Acarol. 77, 555–570. https://doi.org/10.1007/s10493-019-00364-6 (2019).
Khodayari, S., Nematollahi, N., Abedini, F. & Rasouli, F. The response of common bean (Phaseolus vulgaris L.) to salinity and drought stresses and life table parameters of Tetranychus urticae Koch reared on it. Syst. Appl. Acarol. 26, 62–74 (2021).
Li, G. Y. & Zhang, Z. Q. Sex dimorphism of life-history traits and their response to environmental factors in spider mites. Exp. Appl. Acarol. 84, 497–527. https://doi.org/10.1007/s10493-021-00632-4 (2021).
Miyazaki, J., Wilson, L. J. & Stiller, W. N. Fitness of twospotted spider mites is more affected by constitutive than induced resistance traits in cotton (Gossypium spp). Pest Manag Sci. 69, 1187–1197. https://doi.org/10.1002/ps.3546 (2013).
Montazersaheb, H., Zamani, A. A., Sharifi, R. & Darbemamieh, M. Effects of plant probiotic bacteria and herbivore-induced plant volatiles on life table parameters of Tetranychus urticae (Acari: Tetranychidae) on kidney bean’s attached leaves. Int. J. Acarol. 47, 520–527. https://doi.org/10.1080/01647954.2021.1957012 (2021).
Savi, P. J., de Moraes, G. J., Carvalho, R. F. & Andrade, D. J. Bottom-up effects of breeding tomato genotypes on behavioural responses and performance of Tetranychus evansi population. J. Pest Sci. 95, 1287–1301. https://doi.org/10.1007/s10340-021-01437-5 (2022).
Savi, P. J., de Moraes, G. J., Hountondji, F. C. C., Nansen, C. & de Andrade, D. J. Compatibility of synthetic and biological pesticides with a biocontrol agent Phytoseiulus longipes (Acari: Phytoseiidae). Exp. Appl. Acarol. 93, 273–295. https://doi.org/10.1007/s10493-024-00926-3 (2024).
Duso, C., Van Leeuwen, T. & Pozzebon, A. Improving the compatibility of pesticides and predatory mites: recent findings on physiological and ecological selectivity. Curr. Opin. Insect Sci. 39, 63–68. https://doi.org/10.1016/j.cois.2020.03.005 (2020).
Chi, H. & Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24, 225–240 (1985).
Chi, H. Timing of control based on the stage structure of pest populations: a simulation approach. J. Econ. Entomol. 83, 1143–1150. https://doi.org/10.1093/jee/83.4.1143 (1990).
Akköprü, E. P., Atlıhan, R., Okut, H. & Chi, H. Demographic assessment of plant cultivar resistance to insect pests: a case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 108, 378–387 (2015).
Vlad, I. E. & Anghel, S. D. Time stability of water activated by different on-liquid atmospheric pressure plasmas. J. Electrost. 87, 284–292. https://doi.org/10.1016/j.elstat.2017.06.002 (2017). https://doi.org:.
Nagasaki, H. et al. Genomic variation across distribution of Micro-Tom, a model cultivar of tomato (Solanum lycopersicum). DNA Res. 31 https://doi.org/10.1093/dnares/dsae016 (2024).
Cahenzli, F. & Erhardt, A. Transgenerational acclimatization in an herbivore–host plant relationship. Proc. Biol. Sci. 280, 20122856 (2013). https://doi.org/10.1098/rspb.2012.2856
Newcombe, D., Moore, P. J. & Moore, A. J. The role of maternal effects in adaptation to different diets. Biol. J. Linn. Soc. 114, 202–211. https://doi.org/10.1111/bij.12408 (2015).
.61.Chi, H. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis., (2025). http://140.120.197.173/Ecology/prod02.htm>
Efron, B. & Tibshirani, R. An Introduction To the Bootstrap, Monographs on Statistics and Applied Probability (Chapman and Hall/CRC, 1994).
Wei, M. et al. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis Pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 113, 2343–2353. https://doi.org/10.1093/jee/toaa149 (2020).
Hentschel, R., Möller, K., Wenning, A., Degenhardt, A. & Schröder, J. Importance of ecological variables in explaining population dynamics of three important pine pest insects. Front. Plant. Sci. 9, 1667 (2018).
Duan, J. J., Abell, K. J., Bauer, L. S., Gould, J. & Van Driesche, R. Natural enemies implicated in the regulation of an invasive pest: a life table analysis of the population dynamics of the Emerald Ash borer. Agric. Entomol. 16, 406–416. https://doi.org/10.1111/afe.12070 (2014).
Dilip, D., Modupalli, N., Rahman, M. M. & Kariyat, R. Atmospheric cold plasma alters plant traits and negatively affects the growth and development of fall armyworm in rice. Sci. Rep. 15, 3680. https://doi.org/10.1038/s41598-025-87560-0 (2025).
Savi, P. et al. Impact of leaflet trichomes on settlement and oviposition of Tetranychus evansi (Acari: Tetranychidae) in African and South American tomatoes. Syst. Appl. Acarol. 24, 2559–2576 (2019).
Yang, F. et al. Flavonoid-producing tomato plants have a direct negative effect on the zoophytophagous biological control agent Orius sauteri. Insect Sci. 30, 173–184. https://doi.org/10.1111/1744-7917.13085 (2023).
Traw, M. B. & Bergelson, J. Interactive effects of jasmonic acid, Salicylic acid, and Gibberellin on induction of trichomes in Arabidopsis. Plant. Physiol. 133, 1367–1375. https://doi.org/10.1104/pp.103.027086 (2003).
Chou, Y. J. & Ting, Y. Plasma-activated water regulated transcriptome gene expression leading to high germination and growth of mung beans. Chem. Biol. Technol. Agric. 10, 146. https://doi.org/10.1186/s40538-023-00497-2 (2023).
Alakhdar, H. H. & Shoala, T. Exogenous application of hydrogen peroxide in different resistant bean cultivars of Phaseolus vulgaris to Tetranychus urticae (Acari: Tetranychidae). Arthropod-Plant Interact. 15, 439–445. https://doi.org/10.1007/s11829-021-09829-1 (2021).
Roy, M., Brodeur, J. & Cloutier, C. Temperature and sex allocation in a spider mite. Oecologia 135, 322–326. https://doi.org/10.1007/s00442-002-1160-9 (2003).
Keskin, N. & Kumral, N. A. Screening tomato varietal resistance against the two-spotted spider mite [Tetranychus urticae (Koch)]. Int. J. Acarol. 41, 300–309. https://doi.org/10.1080/01647954.2015.1028440 (2015).
Nawar, M. Temperature effect study on fecundity and development of Tetranychus urticae, Koch.(Acari-Tetranychidae) on different host plants. J. Plant. Prot. Pathol. 10, 553–556 (2019).
Macke, E. et al. Sex allocation in haplodiploids is mediated by egg size: evidence in the spider mite Tetranychus urticae Koch. Proc. Biol. Sci. 278, 1054–1063. https://doi.org/10.1098/rspb.2010.1706 (2011). https://doi.org
Krips, O., Witul, A., Willems, P. & Dicke, M. Intrinsic rate of population increase of the spider mite Tetranychus urticae on the ornamental crop gerbera: intraspecific variation in host plant and herbivore. Entomol. Exp. Appl. 89, 159–168 (1998).
Noorinahad, S., Shakarami, J. & Bazgir, F. Demographic parameters of Tetranychus Turkestani (Trombidiformes: Tetranychidae) on different cultivars of greenhouse cucumber. Int. J. Acarol. 50, 189–197. https://doi.org/10.1080/01647954.2024.2311659 (2024).
Castagnoli, M. et al. Tomato Transgenic lines and Tetranychus urticae: changes in plant suitability and susceptibility. Exp. Appl. Acarol. 31, 177–189 (2003).
Atalay, E. & Kumral, N. A. Tetranychus urticae (Koch)(Acari: Tetranychidae)’nin farklı sofralık domates çeşitlerinde biyolojik özellikleri ve yaşam çizelgeleri. Turk. Entomoloji Derg. 37, 329–341 (2013).
Doshi, P., Klas, M., Kyzek, S., Zahoranová, A. & Šerá, B. Investigating the effect of plasma activated water on entomopathogenic nematodes under laboratory conditions. Heliyon 11 https://doi.org/10.1016/j.heliyon.2025.e42038 (2025).
Acknowledgements
This research was partially funded by the USDA/ARS Floriculture, Nursery Research Initiative, the American Floral Endowment, the USDA/Specialty Crop Multi-State Program (grant#21-0732-001-SF), Western Sustainable Agriculture Research and Education (WSARE Project #SW24-012), and the NIFA/Organic Agriculture Program (grant# 2023-04746).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology were performed by PJS; investigation by PJS, SR and AM; analysis by PJS; writing draft by PJS, BAC, GA, and CN. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Savi, P.J., Robertson, S., Mantri, A. et al. Plasma-activated water irrigation increases mortality of immature spider mites (Tetranychus urticae) on tomato plants. Sci Rep 15, 22118 (2025). https://doi.org/10.1038/s41598-025-05629-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05629-2