Abstract
Efficient utilization of cellulose resources is of great significance to environmental protection and to solve the crisis of global energy shortage. In the present work, a polysaccharide (CGP-TS) was successfully produced by Chaetomium globosum CGMCC 6882 from waste tobacco stalk via submerged fermentation. Chemical composition analysis showed that the carbohydrate and protein contents in CGP-TS were 92.55% ± 3.16% and 4.73% ± 1.04%, respectively. Structural characterization indicated that CGP-TS was not pectin and composed of rhamnose, arabinose, galactose, glucose and xylose in a molar ratio of 22.2166: 9.8269: 36.2455: 3.3111: 0.416, and its molecular weight was 613.235 kDa. In vitro bioactivity and potential application investigation showed that antioxidant activities of CGP-TS against DPPH, ABTS, hydroxyl and superoxide radicals were positively correlated to its concentration from 0.5 mg/mL to 2.5 mg/mL. Meanwhile, CGP-TS exhibited immunomodulatory activity via enhancing the phagocytic activity and promoting the cytokines (TNF-α, IL-1β, IL-6 and NO) release of RAW 264.7 macrophages.
Similar content being viewed by others
Introduction
Immunity maintains the physiological balance and the health of body by resisting and defending against microorganisms or parasites infections, especially in facing infectious diseases, immune system plays the role of the first defense1. However, the increase of life pressure, changes in eating habits, irregular work and rest, and other factors have led to decreased immunity and frequent occurrence of diseases2. Apart from reasonable rest and exercise3, the most important way to improve body immunity is to eat a regular diet, especially foods rich in immune-active compounds4. Therefore, many compounds with potential immunomodulatory activity have been explored and developed by scholars. Bushmeleva et al.5 suggested that Aronia melanocarpa flavonols had immunomodulatory effect and could significantly attenuate the injury induced by cyclophosphamide during tumor therapy. Mamun et al.6 reviewed and found that polyphenols might be a potential candidate for regulating obesity via their immunomodulatory functions. Grazul et al.7 indicated that essential oils could be used as natural immune boosters to regulate the immunomodulatory functions of body during inflammation. Verhaeghe et al.8 suggested that alkaloids could be used to attenuate the ulcerative colitis symptoms via enhancing the immune function. Meanwhile, Giampazolias et al.9 found that Vitamin D could be used to improve the anti-tumor immunities. Furthermore, Hashemi et al.10 suggested that probiotics might be a good choice for improving the body immunity and preventing diseases.
Polysaccharide is biopolymer connected by monosaccharides via glycosidic bonds, which not only has antioxidant11,12, anti-inflammatory13,14, antibacterial15,16, probiotic17 and anti-tumor18 activities, but also has good immunomodulatory activities19,20. Apart from plant and animal-based polysaccharides21,22,23, microbial polysaccharides also have potential immunomodulatory activities and applications. Zhong et al.24 suggested that microbial β-glucan had significant immunomodulatory activities and applications. Liu et al.25 indicated that a heteropolysaccharide extracted from Inonotus hispidus fruiting body had excellent immunomodulatory activity. Previously, we found that an alkaline-precipitated polysaccharide produced by Chaetomium globosum CGMCC 6882 had immunoregulatory activity and could be used as a potential ingredient of immunomodulator19. Combined with the green and high efficiency characteristics of microbial fermentation, immune exopolysaccharides produced by microorganisms have good development and application prospects.
Based on the large amounts of cellulose resources, rich organic and mineral substances in tobacco materials26, as well as the properties of C. globosum CGMCC 6882 could use cellulose resources to produce bioactive polysaccharides13,27. Present work tested the utilization feasibility of waste tobacco stalk by C. globosum CGMCC 6882 on plating medium in the first place. Secondly, a polysaccharide (CGP-TS) was extracted, purified and obtained via submerged fermentation with waste tobacco stalk as the sole carbon source. Thirdly, the chemical composition and structural features of CGP-TS were characterized. Furthermore, in vitro antioxidant activity of CGP-TS against DPPH, ABTS, hydroxyl and superoxide radicals and its immunomodulatory activity was analyzed for exploring its potential applications.
Materials and methods
Materials and microorganisms
Waste tobacco stalk was provided by Zhengzhou Tobacco Research Institute of CNTC (Zhengzhou, China) and pretreated into silk, Chaetomium globosum CGMCC 6882 used for waste tobacco stalk utilization and polysaccharide production was isolated from isolated from Gynostemma pentaphyllum herb and stored in China General Microbiological Culture Collection Center (Beijing, China)28,29. Vitamin C (Vc), salicylic acid, and ABTS were bought from Beijing Solarbio Science &Technology Co., Ltd. (Beijing, China). Commercial pectin (P8030) was bought from Beijing Solarbio Science &Technology Co., Ltd. (Beijing, China) and its galacturonic acid content was ≥ 68.5%. Enzyme-linked immunosorbent assay (ELISA) kits for detecting cell viability, nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) factors were bought from Beyotime Biotechnology (Shanghai, China). Other chemical reagents used in present work were analytical grade and purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China).
Production and extraction of CGP-TS
Plating medium used for detecting the utilization feasibility of waste tobacco stalk by C. globosum CGMCC 6882 contained waste tobacco stalk (20 g/L), peptone (1.0 g/L), yeast extract (1.0 g/L) and agar (15 g/L); pH of the plating medium was controlled to 7.0 ± 0.2 with 1 mol/L NaOH and 1 mol/L HCl, culture temperature was set to 28 °C. The seed medium was similar to those of plating medium but without agar, 500 mL flask was filled with 50 mL seed liquid, the shaking speed was 150 r/min, culture temperature and incubation time were 28 °C and 72 h, respectively. Fermentation medium was composed of waste tobacco stalk (40 g/L), peptone (1.0 g/L), yeast extract (1.0 g/L), MgSO4 (1.0 g/L), KH2PO4 (1.0 g/L) and K2HPO4 (1.0 g/L). Fermentation was conducted in a 7.0 L fermenter (BioFlo 115, New Brunswick, USA) with 4.0 L liquid volume, inoculation volume was 10% (v/v), cultivation temperature and time were 28 °C and 7 d, agitation and aeration were 120 rpm and 0.8 vvm, respectively.
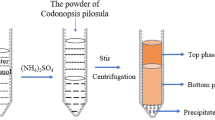
After fermentation, the fermentation broth was filtered through eight layers gauze to remove mycelia and the residual waste tobacco stalk, and the filtrate was centrifuged at 8000 × g for 10 min to remove small insoluble matters. Then, the filtrate was collected and concentrated to one-fifth at 60 °C and 0.1 MPa. After that, three volumes Sevag solution were added and shaken vigorously, the mixture was centrifuged at 10,000 × g for 10 min to collect the supernatant. After which, four volumes ethanol were added and placed at 4 °C overnight for precipitating polysaccharide. Subsequently, the precipitated polysaccharide was re-dissolved in water, rotary evaporated at 60 °C and 0.1 MPa. Polysaccharide concentrate was purified and de-salted by dialysis (molecular weight cut-off was 10.0 kDa) against deionized water. Finally, the dialytic solution was collected, lyophilized and the obtained polysaccharide was termed as CGP-TS.
Chemical composition analysis
Carbohydrate content in CGP-TS was detected using anthrone-sulfonic acid colorimetric method with glucose as standard30, protein content in CGP-TS was measured using Coomassie Brilliant Blue method with bovine serum albumin as standard31.
Monosaccharides and molecular weight detection
Monosaccharides and molecular weight of CGP-TS were determined by Shanghai Sanshu Biotechnology Co., Ltd (Shanghai, China), and the detailed detection processes are as follows. For monosaccharide composition and proportion detection, CGP-TS was dissolved in 2 mol/L trifluoroacetic acid (TFA) and hydrolyzed at 120 °C for 2 h, then the hydrolysate was washed three times with methanol and evaporated to dryness for removing the residual TFA. Finally, the hydrolyzed material was transferred to a 25 mL volumetric flask, diluted to 25 mL by deionized water and subjected to Dionex ICS5000 system (Dionex, USA) equipped with CarboPac PA20 column (ID 3 mm × 150 mm). The mobile phase was deionized water (A), 0.25 mol/L NaOH (B), 1 mol/L NaAc (C) and eluted as follows (A%, B%, C%): 0 min: 99.2, 0.8, 0; 30 min: 99.2, 0.8, 0; 40 min: 79.2, 0.8, 20; 40.1 min: 20, 80, 0; 60 min: 99.2, 0.8, 0. The flow rate was set as 0.45 mL/min and the injection volume was 25 µL, column temperature was 30 °C and detected by a pulsed ampere detector, Au electrode, Ag/AgCl reference electrode. The standard monosaccharides used presently were fucose, rhamnose, arabinose, glucosamine, galactose, glucose, xylose, mannose, fructose, ribose, galacturonic acid, mannuronic acid, guluronic acid and glucuronic acid.
For molecular weight detection, CGP-TS was dissolved in distilled water to a concentration of 2 mg/mL, detection system consisted Waters 2695 HPLC system equipped with multiple detectors: refractive index detector (RI) and a UV detector for concentration determination, multiple angle laser light scattering detector (MALLS, DAWNHELEOS, Wyatt Technology, USA) for direct molecular weight determination and differential pressure viscometer (DP) for viscosity determination. TSK PWXL 6000 and 3000 gel filtration columns were eluted with PB buffer (0.15 mol/L NaNO3 and 0.05 mol/L NaH2PO4, pH = 7) at flow rate of 0.5 mL/min. Calibration of laser photometer was performed with ultrapure toluene. Normalization was conducted with a bovine serum albumin globular protein (Mw = 66.7 kDa, Rg = 2.9 nm). A value of 0.146 mL/g was used as refractive index increment (dn/dc) for molecular weight calculation. Astra software (Version 6.1.1) was utilized for data acquisition and analysis. Column temperature and RI detector temperature were maintained at 35 °C.
Fourier transform infrared (FT-IR) spectroscopy analysis
Approximately 1 mg CGP-TS sample was added into mortar with 100 mg potassium bromide with commercial pectin as the control, ground thoroughly and pressed to tablet for detection. Sample was scanned in infrared spectroscopy from 500 cm− 1 to 4000 cm− 1 with a Nexus 470 Fourier-transform infrared (FT-IR) spectrophotometer (Nicolet, USA).
In vitro antioxidant activity of CGP-TS
CGP-TS was dissolved in deionized water to concentrations of 0.5, 1.0, 1.5, 2.0 and 2.5 mg/mL, respectively. Then, CGP-TS solutions were filtrated through 0.22 μm aqueous membrane and the in vitro antioxidant activity of which against DPPH, ABTS, hydroxyl and superoxide radicals were detected according to the methods reported previously with Vc as control32.
Toxicity analysis
RAW 264.7 cell line was cultured in dulbecco’s modified eagle medium (DMEM) containing 10% (v/v) fetal bovine serum, 100 µg/mL streptomycin and 100 U/mL penicillin at 37 °C and 5% (v/v) CO2. The cultured RAW 264.7 cells were digested by 0.25% (w/v) trypsin EDTA continuously, then RAW 264.7 cells in logarithmic growth phase were used for cell viability assay. RAW 264.7 cells at concentration of 2 × 105 cells/mL were transferred into 96-well plates, and incubated at 37 °C and 5% CO2 (v/v) for 24 h. Then, the RAW 264.7 cells were treated with 100 µL different concentrations (5.0, 2.5, 1.25, 0.625 and 0.3125 mg/mL) CGP-TS and incubated for another 24 h. After which, the cell viability was detected using CCK-8 kit according to the kit instructions. Absorbance of each 96-well at 450 nm was determined by a microplate reader, and the cell viability was calculated as follow: cell viability (%) = Ai/A0 × 100%. Experimental group at absorbance of 450 nm was set as Ai and absorbance of DMEM medium without CGP-TS dissolution was set as A0.
Phagocytic activity analysis
Effect of CGP-TS on the phagocytic activity of RAW 264.7 cells was detected according to the methods reported by Zhang et al.33 with some modifications. RAW 264.7 cells in logarithmic growth phase with the concentration of 2 × 105 cells/mL were transferred into 96-well plates, and incubated at 37 °C and 5% CO2 (v/v) for 24 h. Then, RAW 264.7 cells were treated with 100 µL different concentrations (2.5, 1.25, 0.625 and 0.3125 mg/mL) CGP-TS solutions and incubated at 37 °C and 5% CO2 (v/v) for another 24 h. After which, the culture medium was discarded and the collected RAW 264.7 cells were washed twice with phosphate buffered solution (pH = 7.2–7.4). Then, 100 µL neutral red (0.1%, w/w) DMEM solution was added into each well and continued to incubate for another 1 h. Thus, the neutral red solution was discarded and RAW 264.7 cells were collected and washed twice with phosphate buffered solution. The collected RAW 264.7 cells were lysed for 2 h by adding 200 µL cell lysis buffer (acetic acid: ethanol = 1: 1, v: v) and absorbance of the supernatant at 490 nm was determined by a microplate reader and translated into phagocytic activity by comparing with untreated RAW 264.7 cells. Meanwhile, LPS (1 µg/mL) DMEM medium was used as the positive control.
In vitro immune activity analysis
In vitro immunomodulatory activity of CGP-TS on RAW 264.7 cells was detected according to the methods reported previously with slight modifications19. CGP-TS was dissolved in DMEM medium to concentrations of 2.5, 1.25, 0.625 and 0.3125 mg/mL. Meanwhile, the RAW 264.7 cells in logarithmic growth phase were regulated to 2 × 105 cell/mL with 0.25% (w/v) trypsin EDTA, then 500 µL RAW 264.7 cells were seeded into 12-well plates and incubated for 24 h. After which, each 12-well was separately added 100 µL CGP-TS solution with different concentrations and incubated for another 24 h, and LPS (1 µg/mL) DMEM medium was used as the positive control. Then, the culture supernatant of RAW 264.7 cells was collected for detecting the levels of TNF-α, IL-1β, IL-6 and NO according to the manufacturer’s protocols.
Statistical analysis
All data were expressed as the mean ± SD after three repeats. Data were analyzed by analysis of the variance (ANOVA) using Origin software (Origin Pro 8.5).
Results and discussion
Chemical composition analysis
Large amounts of cellulose resources, organic and inorganic compounds in tobacco materials make them a potential substrate for microorganism growth26. As can be seen from Fig. 1A, Chaetomium globosum CGMCC 6882 could grow on the plating medium with waste tobacco stalk as the sole carbon source, which indicated that the heavy metals and nicotine in waste tobacco stalk had almost no inhibitory effects on C. globosum CGMCC 6882 growth34,35. This might due to the high tolerance of C. globosum CGMCC 6882 and it was verified in our previous work28. After submerged fermentation, extraction and purification, a polysaccharide of CGP-TS was obtained (Fig. 1B). Meanwhile, we previously found that C. globosum CGMCC 6882 could use cellulose resources (wheat straw, corn stover and waste distillers’ grain) to produce bioactive polysaccharides13,27. Due to the utilization difficulty of cellulose resources induced by their tight structure and composition36, the yield of CGP-TS produced from waste tobacco stalk was only 1.17 ± 0.26 g/L and similar results were observed previously. With wheat straw was used as the sole carbon source, the yield of polysaccharide produced by C. globosum CGMCC 6882 was 1.73 ± 0.18 g/L27. Polysaccharide yield was 2.07 ± 0.18 g/L when C. globosum CGMCC 6882 used waste distillers’ grain as the sole carbon source13. Meanwhile, Table 1 showed that the carbohydrate and protein contents in CGP-TS were 92.55% ± 3.16% and 4.73% ± 1.04%, respectively. Microbial exopolysaccharides may contain relatively higher protein due to a large number of enzymes involved in the growth and metabolism of microorganisms.
Monosaccharide analysis
Tobacco materials contain large amounts of pectin26, so the monosaccharides of CGP-TS were firstly detected to verify that if it contained the typical monosaccharide of galacturonic acid in pectin37. As can be seen from Table 1, CGP-TS was composed of rhamnose, arabinose, galactose, glucose and xylose in a molar ratio of 22.2166: 9.8269: 36.2455: 3.3111: 0.416, preliminarily indicating that CGP-TS was not pectin but a new polysaccharide produced by C. globosum CGMCC 6882 from waste tobacco stalk. Due to the different metabolic pathways, fermentation conditions and extraction methods, microbial exopolysaccharides may have different monosaccharides, thus showing various bioactivities and applications38,39. Chen et al.40 found that the contents of fucose and galactose affected the anti-inflammatory activity of Saccharina japonica sulfated galactofucan. Song et al.41 suggested that the antioxidant and hepatoprotective effects of polysaccharides isolated from the intracellular mycelium of Pleurotus geesteranus were influenced by their fucose content. Xiang et al.42 found that mannose content affected the antioxidant activity of exopolysaccharides produced by Inonotus obliquus from corn stover. Cescutti et al.43 found that fucose content influenced the anti-cancer and anti-inflammatory activities of Enterobacter amnigenus exopolysaccharide. Effect of monosaccharides on the bioactivity and application of CGP-TS will be investigated in the future works.
Molecular weight analysis
Apart from regulating the molecular weight of microbial exopolysaccharides via physical, chemical and biological treatments44,45, molecular weight of them will also be radically affected by the metabolic pathways in microorganisms46. As can be seen from Table 1, weight-average molecular weight and number-average molecular weight of CGP-TS were 613.235 kDa and 388.859 kDa, and its polydispersity was 1.577. Molecular weights of exopolysaccharides produced by C. globosum CGMCC 6882 from waste distillers’ grain13 and wheat straw27 were 52.37 kDa and 35.38 kDa, and which were lower than that of CGP-TS. Xiang et al.42 found that molecular weights of polysaccharides produced by Inonotus obliquus from corn stover were between 44 kDa and 29 kDa, which was also lower than that of CGP-SL. Molecular weight may affect the bioactivity and application of polysaccharides by influencing their water solubility, morphology, size and spatial configuration16,44. Li et al.47 found that sulfated algal polysaccharide with lower molecular weight had higher probiotic activity. Hu et al.48 suggested that higher molecular weight Sargassum fusiforme polysaccharide had better anti-oxidation and anti-photoaging activities. Abu-Sbeih et al.49 found that only appropriate molecular weight chitosan oligosaccharides had antibacterial activity. However, other researchers suggested that bioactivity of polysaccharides were affected mainly by their glycoside bond type and spatial configuration, and not their molecular weights50,51.
FT-IR spectra
It is well known that the typical monosaccharide in pectin was galacturonic acid with corresponding absorption peak37, so the functional groups in CGP-TS were future analyzed by FT-IR. As can be seen from Fig. 2, the peaks between 3400 cm− 1 and 3200 cm− 1 might relate to intermolecular H-bridge of OH groups and OH stretching, the peaks between 3000 cm− 1 and 2900 cm− 1 might relate to CH2 antisymmetric stretch, they were characteristic absorption peaks of polysaccharides32. The peaks between 1800 cm− 1 and 1700 cm− 1 might attribute to COOH groups or C = O stretch from acetyl, and the peaks between 1600 cm− 1 and 1400 cm− 1 might correspond to CH2 symmetric ring stretching or CH2 scissors vibration in CGP-TS52. The peaks between 1400 cm− 1 and 1100 cm− 1 might relate to OH in-plane deformation, C-O-C antisymmetric stretching and C-O stretching13, and the peaks between 900 cm− 1 and 500 cm− 1 might relate to C-anomeric groups stretching and pyran ring stretching11. Although Fig. 2 showed that CGP-TS had similar FT-IR spectra to those of commercial pectin, commercial pectin had typical COOH groups at around 1800 cm− 1 but CGP-TS did not. Based on the results of monosaccharide (Table 1) and FT-IR spectra (Fig. 2), we speculated that CGP-TS was not pectin but a new heteropolysaccharide produced by C. globosum CGMCC 6882 from waste tobacco stalk.
In vitro antioxidant activity analysis
Antioxidant activity of compounds usually affected their immune activity53, so we detected the antioxidant activity of CGP-TS. As can be seen from Fig. 3, in vitro antioxidant activities of CGP-TS were positively correlated to its concentration but lower than those of Vc. At the concentration of 0.5 mg/mL, scavenging effects of CGP-TS against DPPH, ABTS, hydroxyl and superoxide radicals were 25.55% ± 2.73%, 36.29% ± 3.18%, 28.32% ± 2.39% and 24.9% ± 2.81%, and which increased to 74.27% ± 2.91%, 83.06% ± 2.52%, 63.93% ± 1.64% and 55.28% ± 3.26% with increase of CGP-TS concentration to 2.5 mg/mL. Carbonyl and ester groups in polysaccharides endow them have excellent antioxidant activity32. Tao et al.54 found that exopolysaccharides produced by endophytic fungus Cyclocarya paliurus had in vitro antioxidant activity against DPPH and ABTS radicals. Xiang et al.42 and Xu et al.55 found that exopolysaccharides produced by Inonotus obliquus from cellulose resources had scavenging effects against DPPH and hydroxyl radicals. Previously, we also found that exopolysaccharides produced by C. globosum CGMCC 6882 from wheat straw27 and waste distillers’ grain13 had scavenging effects against DPPH, ABTS, hydroxyl and superoxide radicals. Meanwhile, polysaccharides isolated from plants and animals also showed good antioxidant activity in vitro56,57.
Toxicity analysis
Macrophage is a common model for investigating the immune activity of polysaccharides58, so the toxicity of CGP-TS on RAW 264.7 cells were analyzed firstly. As can be seen from Fig. 4, during the concentration of CGP-TS from 0.3125 mg/mL to 2.5 mg/mL, the cell viability of RAW 264.7 macrophages increased from 103.69% ± 4.16–110.49% ± 3.37%, indicating that CGP-TS is safe to RAW 264.7 cells. However, the cell viability decreased to 94.82% ± 6.32% when CGP-TS concentration increased to 5.0 mg/mL. Therefore, the concentration of CGP-TS was selected between 0.3125 mg/mL and 2.5 mg/mL in the following work. Many scholars obtained similar results to present work. Chen et al.58 found that Erythronium sibiricum bulb polysaccharides could increase the cell viability of RAW 264.7 macrophages at low concentrations, but their proliferative effects decreased with increasing concentration. Wang et al.59 found that the cell viability of RAW 264.7 macrophages was increased at low concentrations of Asparagus officinalis L. polysaccharide fractions, and the cell proliferation rate was decreased at high concentration. While, some scholars obtained different results to present work. Dong et al.60 and Choi et al.61 suggested that Pueraria lobata and fermented Morinda citrifolia L. polysaccharides did not affect the cell viability of RAW 264.7 macrophages and the cell survival rate maintained at almost 100% with different polysaccharide concentrations. Li et al.62 and You et al.63 found that Aspergillus aculeatus polysaccharides and Lactobacillus pentosus LZ-R-17 exopolysaccharide could increase the cell viability of RAW 264.7 macrophages in a dose-dependent manner.
Phagocytic activity analysis
Phagocytic activity, a defense mechanism of organisms, has been widely used as an indicator to evaluate the immunomodulatory activity of macrophages64. As can be seen from Fig. 5, based on the uptake test of neutral red by macrophages, phagocytic activity of CGP-TS on RAW 264.7 cells increased in a dose-dependent manner from 105.78% ± 6.43–120.86% ± 7.15% with its concentration increased from 0.3125 mg/mL to 2.5 mg/mL. Many scholars obtained similar results to present work that polysaccharides could improve the phagocytic function of macrophages. Li et al.62 and You et al.63 found that Aspergillus aculeatus polysaccharides and Lactobacillus pentosus LZ-R-17 exopolysaccharide could increase the phagocytic activity of RAW 264.7 macrophages in a dose-dependent manner. However, other scholars obtained different results to present work. Zhang et al.33 found that low concentration of Coriolus versicolor polysaccharide had phagocytic activity on RAW264.7 cells, but the phagocytic activity of high polysaccharide concentration was relatively poor. Chen et al.64 and Wang et al.65 found that the phagocytic activity of exopolysaccharides produced by Lacticaseibacillus rhamnosus ZFM216 and Lactobacillus plantarum JLK0142 increased first and then decreased with the increase of their concentrations.
In vitro immune activity analysis
Immune regulation plays an important role in maintaining the stability of body internal environment, and polysaccharides can mediate immunomodulatory activity of body by promoting the release of various cytokines58,64. As can be seen from Fig. 6, CGP-TS could promote the release of immunomodulatory cytokines in RAW 264.7 macrophages in a dose-dependent manner. The levels of TNF-α, IL-1β, IL-6 and NO were 130.15 ± 6.27 pg/mL, 35.09 ± 2.13 pg/mL, 52.06 ± 3.26 pg/mL and 14.47 ± 1.52 µM when the concentration of CGP-TS was 0.3125 mg/mL, but which increased to 271.48 ± 9.36 pg/mL, 55.31 ± 0.98 pg/mL, 103.94 ± 4.07 pg/mL and 22.45 ± 2.98 µM with 2.5 mg/mL CGP-TS. Many scholars obtained similar results to present work and verified that polysaccharides could promote the release of immunomodulatory cytokines. Li et al.62 found that Aspergillus aculeatus polysaccharides could increase the releases of TNF-α, IL-6 and NO by RAW264.7 cells in a dose-dependent manner. Wang et al.65 found that Lactobacillus plantarum JLK0142 exopolysaccharide increased the serum cytokines levels of IL-2 and TNF-α in immunosuppressed mice with a dose-dependent manner. Although You et al.63 found that Lactobacillus pentosus LZ-R-17 exopolysaccharide could promote the releases of NO and IL-10 by RAW264.7 cells in a dose-dependent manner, cytokines levels of TNF-α, IL-1β and IL-6 increased first and then decreased with the increase of polysaccharide concentration. Chen et al.64 found that Lacticaseibacillus rhamnosus ZFM216 exopolysaccharide increased the cytokines levels of TNF-α, IL-1β, IL-6 and NO released by RAW264.7 cells first and then decreased with the increase of its concentration. The different effects of polysaccharides on the cytokines release of RAW264.7 cells might be related to their structural difference, and which will be investigated in the future work.
Conclusion
Presently, waste tobacco stalk was tested as the sole carbon source to produce polysaccharide (CGP-TS) by Chaetomium globosum CGMCC 6882 via submerged fermentation. Structural analysis showed that CGP-TS was not pectin but a new heteropolysaccharide. Bioactive results indicated that CGP-TS could not only scavenge DPPH, ABTS, hydroxyl and superoxide radicals, but also promote the phagocytic activity and cytokines (TNF-α, IL-1β, IL-6 and NO) release of RAW 264.7 macrophages. However, the yield of CGP-TS was only 1.17 ± 0.26 g/L, metabolic engineering and fermentation regulation strategies will be adopted to improve CGP-TS yield. Furthermore, immunologic mechanism of CGP-TS will be investigated in the future work.
Data availability
The original data presented in the study can be directed to the corresponding author.
References
Carpenter, S. & O’Neill, L. A. J. From periphery to center stage: 50 years of advancements in innate immunity. Cell 187, 2030–2051 (2024).
Dufrasne, F. Immune diseases: challenges, hopes and recent achievements. Pharmaceuticals 17, 97 (2024).
Fiuza-Luces, C. et al. The effect of physical exercise on anticancer immunity. Nat. Rev. Immunol. 24, 282–293 (2024).
Medzhitov, R. & Iwasaki, A. Exploring new perspectives in immunology. Cell 187, 2079–2094 (2024).
Bushmeleva, K. et al. Effect of flavonols of Aronia melanocarpa fruits on morphofunctional state of immunocompetent organs of rats under cyclophosphamide-induced immunosuppression. Biomolecules 14, 578 (2024).
Mamun, M. A. A. et al. Polyphenols: role in modulating immune function and obesity. Biomolecules 14, 221 (2024).
Grazul, M., Kwiatkowski, P., Hartman, K., Kilanowicz, A. & Sienkiewicz, M. How to naturally support the immune system in inflammation—essential oils as immune boosters. Biomedicines 11, 2381 (2023).
Verhaeghe, C. et al. Tobacco alkaloid assessment in a DSS-induced colitis mouse model with a fully humanized immune system. Int. J. Mol. Sci. 24, 6419 (2023).
Giampazolias, E. et al. Reis e Sousa C Vitamin D regulates microbiome-dependent cancer immunity. Science 384, 428–437. (2024).
Hashemi, B. et al. The effect of probiotics on immune responses and their therapeutic application: A new treatment option for multiple sclerosis. Biomed. Pharmacother. 159, 114195 (2023).
Wang, Z. et al. Antioxidant protection of a polysaccharide produced by Chaetomium globosum CGMCC 6882 on H2O2-challenged HepG2 cells. Carbohydr. Polym. Technol. Appl. 8, 100530 (2024).
Wang, Z. et al. Effect of Saccharomyces cerevisiae CICC 32883 fermentation on the structural features and antioxidant protection effect of Chinese Yam polysaccharide. Foods 14, 564 (2025).
Wang, Z. et al. Characterization and anti-inflammation of a polysaccharide produced by Chaetomium globosum CGMCC 6882 on LPS-induced RAW 264.7 cells. Carbohydr. Polym. 251, 117129 (2021).
Wang, Z. et al. Antioxidant and anti-inflammatory activities of an anti-diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. Int. J. Biol. Macromol. 145, 484–491 (2020).
Wang, Z. et al. Improvement of antibacterial activity of polysaccharides via chemical modification: A review. Int. J. Biol. Macromol. 269, 132163 (2024).
Wang, Z. et al. Insight into antibacterial mechanism of polysaccharides: A review. LWT 150, 111929 (2021).
Sun, X. et al. Effect of an antibacterial polysaccharide produced by Chaetomium globosum CGMCC 6882 on the gut microbiota of mice. Foods 10, 1084 (2021).
Li, S. et al. Effect of structural features on the antitumor activity of plant and microbial polysaccharides: A review. Food Bioscience, 61, 104648 (2024).
Wang, S. et al. Immunoregulatory activity of an alkaline-precipitated polysaccharide CGP-AP produced by Chaetomium globosum CGMCC 6882. Carbohydr. Polym. Technol. Appl. 7, 100430 (2024).
Yang, Y. et al. Structural characterization and combined Immunomodulatory activity of fermented Chinese Yam polysaccharides with probiotics. Int. J. Biol. Macromol. 307, 142290 (2025).
Duymaz, D., Kebabci, A. O. & Kizilel, S. Harnessing the Immunomodulatory potential of Chitosan and its derivatives for advanced biomedical applications. Int. J. Biol. Macromol. 307, 142055 (2025).
Liu, X. et al. Exploring the Immunomodulatory mechanisms of Osmunda Japonica thunb polysaccharides: activation of MAPK and NF-κB signaling pathways. Food Bioscience. 63, 105695 (2025).
Wang, Z. et al. Effect of probiotic fermentation on the extraction rate and bioactivity of plant-based polysaccharides: A review. Innovative Food Sci. Emerg. Technol. 98, 103863 (2024).
Zhong, X. et al. Immunomodulatory effect and biological significance of β-glucans. Pharmaceutics 15, 1615 (2023).
Liu, X. et al. Structural characterization, chain conformation and Immunomodulatory activity of a heteropolysaccharide from Inonotus hispidus. Int. J. Biol. Macromol. 260, 129187 (2024).
Banožić, M., Babić, J. & Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Ind. Crops Prod. 144, 112009 (2020).
Wang, Z. et al. Antioxidant activity of a polysaccharide produced by Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 141, 955–960 (2019).
Wang, Z. et al. Anticancer activity of polysaccharides produced from glycerol and crude glycerol by an endophytic fungus Chaetomium globosum CGMCC 6882 on human lung cancer A549 cells. Biomolecules 8, 171 (2018).
Wang, Z. et al. Efficient production of polysaccharide by Chaetomium globosum CGMCC 6882 through co-culture with host plant Gynostemma pentaphyllum. Bioprocess Biosyst. Eng. 42, 1731–1738 (2019).
Wang, Z. et al. Antioxidant analysis of flavonoids extracted from Artemisia argyi leaf and their antibacterial activities against food-borne pathogens Escherichia coli and Staphylococcus aureus. Biologia 79, 975–983 (2024).
Wang, Z. et al. In vitro antioxidant analysis of flavonoids extracted from Artemisia argyi stem and their anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. 407, 135198 (2023).
Wang, Z. et al. Antioxidant and antibacterial activities of a polysaccharide produced by Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 233, 123628 (2023).
Zhang, X., Cai, Z., Mao, H., Hu, P. & Li, X. Isolation and structure Elucidation of polysaccharides from fruiting bodies of mushroom Coriolus versicolor and evaluation of their Immunomodulatory effects. Int. J. Biol. Macromol. 166, 1387–1395 (2021).
Lin, Y. et al. Transformation of tobacco biomass into value-added carbohydrate, aromatics, and Biochar. Biomass Convers. Biorefinery. 14, 11697–11705 (2024).
Wang, Z. et al. Research advances on endophytic fungi and their bioactive metabolites. Bioprocess Biosyst. Eng. 46, 165–170 (2023).
Wang, Z. et al. Enhancing enzymatic hydrolysis of corn Stover by twin-screw extrusion pretreatment. Ind. Crops Prod. 143, 111960 (2020).
Du, Y. et al. High-intensity pulsed electric field-assisted acidic extraction of pectin from citrus peel: physicochemical characteristics and emulsifying properties. Food Hydrocoll. 146, 109291 (2024).
Wang, Z. et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int. J. Biol. Macromol. 254, 127955 (2024).
Wang, Z. et al. Effect of polysaccharide addition on food physical properties: A review. Food Chem. 431, 137099 (2024).
Chen, X. et al. Molecular mechanism of anti-inflammatory activities of a novel sulfated Galactofucan from Saccharina Japonica. Mar. Drugs. 19, 430 (2021).
Song, X. et al. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus against alcoholic liver diseases. Int. J. Biol. Macromol. 114, 979–988 (2018).
Xiang, Y., Xu, X. & Li, J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn Stover medium. Food Chem. 134, 1899–1905 (2012).
Cescutti, P. et al. Structure of the exopolysaccharide produced by Enterobacter amnigenus. Carbohydr. Res. 340, 439–447 (2005).
Wang, Z. et al. Effect of ultrasonic degradation on the structural feature, physicochemical property and bioactivity of plant and microbial polysaccharides: A review. Int. J. Biol. Macromol. 236, 123924 (2023).
Wang, Z. et al. Effect of Lactobacillus fermentation on the structural feature, physicochemical property, and bioactivity of plant and fungal polysaccharides: A review. Trends Food Sci. Technol. 148, 104492 (2024).
Wang, Z. et al. Regulation strategy, bioactivity, and physical property of plant and microbial polysaccharides based on molecular weight. Int. J. Biol. Macromol. 244, 125360 (2023).
Li, X. et al. Depolymerized non-digestible sulfated algal polysaccharides produced by hydrothermal treatment with enhanced bacterial fermentation characteristics. Food Hydrocoll. 130, 107687 (2022).
Hu, J. et al. Structural characterization and anti-photoaging activity of a polysaccharide from Sargassum fusiforme. Food Res. Int. 157, 111267 (2022).
Abu-Sbeih, K. A., Al-Mazaideh, G. M. & Al-Zereini, W. A. Production of medium-sized Chitosan oligomers using molecular sieves and their antibacterial activity. Carbohydr. Polym. 295, 119889 (2022).
Liu, W. et al. Leukemia cells apoptosis by a newly discovered heterogeneous polysaccharide from Angelica sinensis (Oliv.) diels. Carbohydr. Polym. 241, 116279 (2020).
Ferreira-Lazarte, A., Kachrimanidou, V., Villamiel, M., Rastall, R. A. & Moreno, F. J. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr. Polym. 199, 482–491 (2018).
Wang, Q. et al. Antibacterial activity of a polysaccharide isolated from Artemisia argyi leaf against Staphylococcus aureus and mechanism investigation. Int. J. Biol. Macromol. 253, 126636 (2023).
Gasmi, A. et al. Natural ingredients to improve immunity. Pharmaceuticals 16, 528 (2023).
Tao, X. et al. Characterization and antioxidant properties of three exopolysaccharides produced by the Cyclocarya paliurus endophytic fungus. Int. J. Biol. Macromol. 271, 132110 (2024).
Xu, X., Hu, Y. & Quan, L. Production of bioactive polysaccharides by Inonotus obliquus under submerged fermentation supplemented with lignocellulosic biomass and their antioxidant activity. Bioprocess Biosyst. Eng. 37, 2483–2492 (2014).
Akshatha, S., Gnanesh Kumar, B. S., Mazumder, K. & Eligar, S. M. Structural characterization and bioactivities of maize Bran feruloylated arabinoxylan and oligosaccharides obtained by a combined hydrothermal and enzymatic pre-treatment. Food Bioscience. 60, 104417 (2024).
Yang, J. et al. Physicochemical, structural characterization, and antioxidant activities of chondroitin sulfate from Oreochromis niloticus bones. Food Sci. Hum. Wellness. 12, 1102–1108 (2023).
Chen, C., Xie, X. & Li, X. Immunomodulatory effects of four polysaccharides purified from Erythronium sibiricum bulb on macrophages. Glycoconj. J. 38, 517–525 (2021).
Wang, N. et al. Fractionation, structural characteristics and Immunomodulatory activity of polysaccharide fractions from asparagus (Asparagus officinalis L.) skin. Carbohydr. Polym. 256, 117514 (2021).
Dong, Z. et al. Structural characterization and Immunomodulatory activity of a novel polysaccharide from Pueraria lobata (Willd.) Ohwi root. Int. J. Biol. Macromol. 154, 1556–1564 (2020).
Choi, S. I. et al. Immunomodulatory effect of polysaccharide from fermented Morinda citrifolia L. (Noni) on RAW 264.7 macrophage and Balb/c mice. Foods 11, 1925. (2022).
Li, H., Xie, W., Sun, H., Cao, K. & Yang, X. Effect of the structural characterization of the fungal polysaccharides on their Immunomodulatory activity. Int. J. Biol. Macromol. 164, 3603–3610 (2020).
You, X. et al. Isolation, purification, characterization and immunostimulatory activity of an exopolysaccharide produced by Lactobacillus pentosus LZ-R-17 isolated from Tibetan Kefir. Int. J. Biol. Macromol. 158, 408–419 (2020).
Chen, L., Gu, Q. & Zhou, T. Statistical optimization of novel medium to maximize the yield of exopolysaccharide from Lacticaseibacillus rhamnosus ZFM216 and its Immunomodulatory activity. Frontiers Nutrition 9, 2022 (2022).
Wang, J., Wu, T., Fang, X., Min, W. & Yang, Z. Characterization and Immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy Tofu. Int. J. Biol. Macromol. 115, 985–993 (2018).
Acknowledgements
We thanked Shanghai Sanshu Biotechnology Co., Ltd (Shanghai, China) for polysaccharide detection.
Funding
This work is funded by Project of Basic Research Fund of Henan Provincial Institute of Medical and Pharmacological Sciences (2024BP0204).
Author information
Authors and Affiliations
Contributions
Conceptualization: Shuaiyang Wang; Methodology: He Chang; Software: Na Li; Resources: Danye Zhu; Writing-original draft preparation: Xueyi Qiao and Shaobo Duan; Writing-review and editing: Zichao Wang.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Chang, H., Li, N. et al. Structural characterization and immunomodulatory activity of a polysaccharide produced by chaetomium globosum CGMCC 6882. Sci Rep 15, 19992 (2025). https://doi.org/10.1038/s41598-025-05693-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05693-8
Keywords
This article is cited by
-
Structural Characterization and Anti-inflammatory Activity of a Polysaccharide Produced by Endophytic Fungus Talaromyces Sp. CCTCC M 2025051
Journal of Polymers and the Environment (2025)