Abstract
This study aims to investigate the clinical relevance of GOLT1A in papillary thyroid carcinoma (PTC) and evaluate its significance in prognosis of PTC. In this study, 527 data downloaded from the TCGA database and 280 data downloaded from the GTEx database were used for differential gene expression analysis. Univariate and multivariate statistical analyses were performed on the data of 499 PTC patients from the TCGA database and the data of 305 PTC patients subjected to fine-needle aspiration biopsy (FNAB) from the Department of General Surgery of the First Affiliated Hospital of Soochow University and the First Affiliated Hospital of Wenzhou Medical University. In the gene expression of 527 normal tissues and thyroid carcinoma tissues data from the TCGA database and 280 normal tissues from the GTEx database, the expression of GOLT1A was significantly higher in thyroid carcinoma tissues than in normal thyroid tissues (P < 0.001), validated by GEO databases (P < 0.001) and immunohistochemical (IHC) (P < 0.05). Univariate and multivariate analyses of 499 PTC patients and 309 FNAB PTC patients in the TCGA database showed that high expression of GOLT1A was closely related to the poor prognosis of PTC. Specifically, GOLT1A is associated with differentiation risk (P = 0.005), sex (P = 0.006), capsular invasion (P = 0.038) and lateral lymph node metastasis (P < 0.001). In summary, the expression of GOLT1A in thyroid carcinoma tissues was significantly higher than that in normal tissues, and high GOLT1A expression was probably related to the poor prognosis of PTC. GOLT1A is a promising prognostic genetic marker and pathogenic target of PTC.
Similar content being viewed by others
Introduction
The incidence of thyroid carcinoma has been steadily increasing, as evidenced by the latest cancer statistics from the United States. In 2022, the National Comprehensive Cancer Network (NCCN) estimated that 43,800 new cases of thyroid carcinoma would be diagnosed in the USA1. By 2024, approximately 44,020 new cases of thyroid carcinoma were projected in the USA2. A similar upward trend has been observed in China, where the incidence of thyroid carcinoma has increased by 20.1% annually3. The underlying causes of this rise remain controversial and continue to fuel scholarly debate. Thyroid carcinoma encompasses a spectrum of pathological types, including well-differentiated forms such as papillary, follicular and eosinophilic types which originate from thyroid follicular cells, as well as poorly differentiated and undifferentiated form, which are notably more aggressive. Additionally, medullary thyroid carcinoma arises from parafollicular C cells. Among them, papillary thyroid carcinoma (PTC) accounts for the majority, representing approximately 90% of all cases3. According to the World Health Organization (WHO), the main variants of PTC include classical, follicular and microcarcinoma (≤ 1 cm), alongside rarer subtypes such as tall cell, diffuse sclerosing, oncocytic, among others4.
Some researchers attribute the rising incidence of thyroid carcinoma to the phenomenon of “overdiagnosis”, particularly of papillary thyroid microcarcinoma (PTMC), which has been facilitated by the widespread use of high-resolution ultrasound. For instance, Xu et al.5 argue that although the incidence is increasing annually, the mortality rate has remained relatively stable. They caution that such overdiagnosis and subsequent aggressive treatment may fail to reduce cancer-related mortality, yet significantly compromise patients’ quality of life and increase healthcare expenditures. Current treatment options for thyroid carcinoma include total thyroidectomy, subtotal thyroidectomy, radioiodine therapy, and molecular targeted therapy for advanced metastatic thyroid carcinoma, such as tyrosine kinase receptor inhibitors, including sorafenib, lenvantinib, vandetanib, and cabozantinib6. Among these, total thyroidectomy is associated with considerable risks, with hypoparathyroidism emerging as one of the most challenging complications, contributing to prolonged hospital stays and increased costs due to hypocalcemia-related symptoms7. Therefore, Xu et al.5 advocate for addressing overdiagnosis to mitigate the harm caused by overtreatment. However, this perspective is subject to ongoing debate. Hajeer et al.4 argue that while overdiagnosis might explain part of the increase in incidence, it is not the sole contributor, and the true rise in PTC cases, especially those involving aggressive subtypes must not be overlooked. As they emphasize, we should not adopt a simplistic view that attributes the increased incidence of thyroid carcinoma solely to overdiagnosis, especially considering the emergence of aggressive PTC subtypes such as tall cell, columnar, and hobnail variants, which exhibit aggressive clinical behavior and are associated with significant mortality8.
Therefore, the rapidly increasing incidence of thyroid carcinoma requires a nuanced and dialectical approach. In this context, accurate prognostication is particularly important, and there is an urgent need for a predictive method that can not only exclude indolent thyroid cancer that does not require surgical treatment, but also screen out aggressive subtypes that require early surgical treatment. Recent advancements in genetic marker research have shown considerable promise in improving thyroid carcinoma prognosis prediction. RET, BRAF, RAS gene mutations are the most widely studied and clinically used gene markers. Among these, mutations in the RET, BRAF, and RAS genes are the most extensively studied and widely applied in clinical practice. In addition to their diagnostic utility, these genetic alterations also serve as the basis for the molecular classification of thyroid carcinoma9. Beyond gene mutations, the detection of mRNA and microRNA (miRNA) marker expression in thyroid fine-needle aspiration biopsy (FNAB) specimens has also been found to be of prognostic value in several studies10. For instance, genes such as MET and LGALS3 have been found to be upregulated in well-differentiated thyroid carcinoma, while MT1G and DIO1 are significantly downregulated. These distinct molecular alterations provide potential biomarkers for clinical application11. Regarding miRNA, molecules such as miR-222, -214 and − 181b have been shown to be significantly overexpressed in PTC and are associated with malignancy. However, it is worth noting that a study by Zarkesh et al. found that certain miRNAs, such as miR-137, were upregulated in tumor tissues and cell lines but not in FNAB specimens12. In addition, circular RNAs (circRNAs) have also been reported to exhibit clinicopathological relevance and prognostic significance in thyroid carcinoma13. The integration of genetic markers into traditional pathological diagnostic frameworks offers new avenues for precise prognostic assessment, helping to minimize overdiagnosis and facilitate the identification of novel therapeutic targets.
As a member of the Golgi transport 1 (GOLT1) family, GOLT1A encodes a vesicle transport protein thought to mediate the fusion of endoplasmic reticulum-derived vesicles with the Golgi complex14. GOLT1A is predominantly localized in the Golgi compartment, endoplasmic reticulum, and nuclear envelope, and exhibits highest expression levels in the liver, duodenum, and small intestine. The protein encoded by the GOLT1A gene is associated with lipase activity, indicating a potential role in endoplasmic reticulum-to-Golgi vesicle transport15.
Although studies on GOLT1A remain limited, existing research has linked its dysregulation to a variety of malignancies, including breast cancer15 lung carcinoma16,17 gastrointestinal goblet cell adenocarcinomas18 adenoid cystic carcinomas19 and melanoma20. Upregulation of GOLT1A has been reported the first three tumors, while GOLT1A-KISS1 fusions has been observed in highly metastatic adenoid cystic carcinomas, along with high GOLT1A expression in hypoxic melanomas. It’s believed that the upregulation of GOLT1A is associated with the poor prognosis of breast cancer15 lung carcinoma16,17 and adenoid cystic carcinomas19. An important homolog of this gene, GOLT1B, has also received limited research attention over the past decade. Existing studies have mainly focused on its role in promoting hepatitis C virus replication21 facilitating colorectal cancer metastasis22 and its impact on breast cancer prognosis23. However, to date, no research has explored the potential role of the GOLT1 family in the development or prognosis of thyroid carcinoma.
Based on the above background, this study aims to investigate the clinical relevance of GOLT1A in PTC and to evaluate its potential as a prognostic biomarker.
Materials and methods
The cancer genome atlas database(TCGA)
Gene expression data of 527 thyroid carcinoma tissues and normal thyroid tissue, and clinical information of 499 PTC patients were downloaded from The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/) for analysis. The data in the TCGA database were derived from primary thyroid carcinoma tissues, and we also normalized the expression of GOLT1A with reference to normal tissues and used this data to verify the relationship between GOLT1A expression and clinical features of thyroid carcinoma.
The genotype-tissue expression project (GTEx)
Gene expression data for 280 normal thyroid tissue were downloaded from the Genotype-Tissue Expression Project (GTEx) database available on the UCSC Xena website (https://xena.ucsc.edu/). These data were used to supplement the normal tissue in TCGA database, thus participating in the validation of GOLT1A expression differences between thyroid carcinoma and normal tissues.
Gene expression omnibus (GEO)
“Thyroid papillary carcinoma” as keywords in Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) to retrieve the Gene Expression profile. Dataset inclusion criteria: (1) Gene expression analysis must include both case and control groups; (2) The tissue source of sequencing was human thyroid tissue. (2) The expression data were complete for transformation and analysis. Finally, three datasets (GSE33630, GSE6004, GSE29265) were ultimately downloaded. GSE33630 (PTC: 49 cases, normal control: 45 cases), GSE6004 (PTC: 14 cases, normal control: 4 cases) and GSE29265 (PTC: 20 cases, normal control: 20 cases) were executed on the GPL570 platform. Firstly, the matrix files in the gene expression profile were subjected to log2 transformation, and then the corresponding annotation files were used to match the probes with their respective gene symbols to generate a gene matrix with gene column names and sample row names for subsequent analysis.
Patients and clinicopathologic parameters
In this study, 164 patients with postoperative pathologically diagnosed PTC in the First Affiliated Hospital of Soochow University and 141 PTC patients at the First Affiliated Hospital of Wenzhou Medical University from January 2023 to July 2024 were selected to be retrospectively analyzed. Patients were excluded from this research on the condition that they owned any of the elements below: (1) previous history of thyroid-related surgery; (2) long-term use of thyroid hormone drugs; and (3) signs of metastasis in preoperative ultrasound of cervical lymph nodes (Fig. 1).
After excluding 175 patients who did not meet the criteria, 305 patients were included, of which 75 were males and 230 were females, with an age distribution of 18–72 years. After resection, the fine-needle aspiration biopsy (FNAB) specimens were flash frozen in liquid nitrogen and then stored at − 80 °C for subsequent RNA extraction studies. This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Informed consent was obtained from all patients for the use of their biological materials.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from FNAB samples using TRIzol Reagent (Thermo Fisher Scientific, USA) and reverse-transcribed into cDNA using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara, Japan), following the manufacturer’s protocols. qRT-PCR was performed in triplicate on an ABI Prism 7500 detection system (Thermo Fisher Scientific) with TB Green® Premix Ex Taq™ II (Takara, Japan). Reaction conditions adhered to the kit specifications. GAPDH mRNA level was used for normalization. Primer sequences were as follows: GOLT1A : 5′- GGGCCTGTCCCTCATCATT − 3′ and 5′- TTTGTGCCGTTGGAAGAAGAA − 3′; GAPDH: 5′-GGTCGGAGTCAACGGATTTG-3′ and 5′-ATGAGCCCCAGCCTTCTCCAT-3′.
Immunohistochemistry (IHC)
Paraffin-embedded tissue sections were dewaxed in xylene and rehydrated through a graded alcohol series. Endogenous peroxidase activity was quenched by incubation with 3% H₂O₂ in deionized water for 15 min. Antigen retrieval was performed by heating the sections in citrate buffer (pH 6.0) at 95 °C for 15 min, followed by cooling to room temperature. Non-specific binding sites were blocked with 5% bovine serum albumin (BSA) for 30 min at room temperature. The sections were then incubated with primary antibody against GOLT1A (Sigma-Aldrich) overnight at 4 °C. After three washes with PBS, a ready-to-use secondary antibody (Beyotime) was applied and incubated for 60 min at 37 °C. Following additional PBS washes, immunoreactivity was visualized using 3,3’-diaminobenzidine (DAB) chromogen, followed by counterstaining with hematoxylin. Finally, the sections were dehydrated, cleared, and mounted for microscopic examination. Known positive tissue sections served as positive controls, while negative controls were prepared by replacing the primary antibody with PBS. All IHC-stained sections were examined and imaged under a light microscope. GOLT1A expression was scored by multiplying the percentage of positive cells (0: <5%; 1: 5–25%; 2:26–50%; 3: 51–75%; 4: >75%) by staining intensity (0: none; 1: light yellow; 2: brown-yellow; 3: dark brown), yielding a range of 0–12. Two blinded pathologists independently assessed slides, with final scores averaged and categorized as low (0–4), normal (5–8), or high (9–12) expression.
Statistical analysis
In gene expression differential analysis, the normal group was composed of the data of normal thyroid tissues in TCGA database and GTEx database, and the batch correction was performed using the combat R package. The tumor group was derived from the tumor tissues in the TCGA database, and then the limma R package was used to analyze the expression difference of GOLT1A gene between the two groups. Univariate analysis was performed on the clinical data in TCGA database and clinical samples. For clinical characteristics of patients with different GOLT1A expression, categorical variables were expressed as percentages and analyzed using the χ2 test or Fisher’s exact test, as appropriate. Independent sample t test was used for normally distributed measurement data, and Wilcoxon-Mann-Whitney test was used for non-normally distributed measurement data. P < 0.05 was considered statistically significant. The statistically significant univariate variables were used as covariates for multivariate analysis, and logistic regression was used to analyze the odds ratio (OR) of these variables. SPSS 26.0 and R Studio (R.4.4.1) software were used for data analysis and mapping.
Results
The expression of GOLT1A in thyroid carcinoma
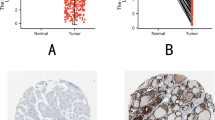
To explore the differential expression of GOLT1A between thyroid carcinoma tissues and normal tissues, we selected 527 thyroid carcinoma tissues and normal tissues downloaded from TCGA and 280 normal thyroid tissues downloaded from GTEx database to analyze the differential expression of GOLT1A. As shown in Fig. 2, GOLT1A expression was significantly higher in thyroid carcinoma tissues than in normal thyroid tissues (P < 0.001).
To validate findings above, we analyzed GOLT1A expression in PTC using GEO datasets (GSE33630, GSE6004, GSE29265). As shown in Fig. 3, GOLT1A was significantly upregulated in PTC tissues compared to normal controls across all datasets (***P < 0.001).
Immunohistochemistry of papillary thyroid carcinoma
As illustrated in Fig. 4, immunohistochemical (IHC) staining revealed distinct patterns of GOLT1A expression in PTC tissues (Figure 4A) compared with adjacent normal thyroid tissues (Figure 4B). GOLT1A immunoreactivity was predominantly localized in the cytoplasm, exhibiting characteristic brownish-yellow granular staining. Notably, while adjacent normal thyroid tissues consistently showed low expression (mean intensity score: 0.33 ± 0.47), PTC tissues exhibited markedly high expression (mean intensity score: 10.3 ± 2.4) in 66.67% of cases (4/6). A comparative analysis of six randomly selected paired samples demonstrated significantly higher GOLT1A expression levels in PTC tissues relative to matched normal counterparts (P<0.05). Detailed IHC scoring results for individual cases are provided in Supplementary Table 1.
Relationship between GOLT1A expression and clinical characteristics
In order to analyze whether GOLT1A plays a role in the prognosis of PTC, we selected 499 PTC patients in the TCGA database and investigated the relationship between the expression of GOLT1A and their clinical characteristics. Based on the median of GOLT1A expression, patients were divided into high and low expression groups. High GOLT1A expression was found to be associated with extrathyroidal extension (ETE) (P = 0.01), lymph node metastasis (LNM) (P = 0.0029), histological type (P < 0.001), histological risk factors (P = 0.01), BRAF mutation (P < 0.001), subtype (P < 0.001), differentiation risk (P < 0.001), and lower thyroid differentiation score (TDS) (P < 0.001; Table 1).
In multivariate analysis, high GOLT1A expression was significantly associated with differentiation risk (odds ratio [OR] = 2.400, 95% confidence interval [CI] 1.300-4.429, P = 0.005; Table 2), which was an independent factor affecting differentiation risk. Therefore, we can know from the above results that the high GOLT1A expression has a certain correlation with the differentiation risk of PTC.
To further explore whether the high GOLT1A expression was associated with special subtype, we investigated the relationship between GOLT1A and clinical characteristics in FNAB specimens from 305 PTC patients. The patients were divided into two groups according to the median of GOLT1A expression. Univariate analysis showed that high GOLT1A expression was associated with sex (P = 0.018), high and low risk level (P < 0.001), capsular invasion (P = 0.024) and lateral lymph node metastasis (P = 0.002; Table 3).
At the same time, We found that high GOLT1A expression was associated with sex (OR = 0.429, 95%CI 0.234–0.786, P = 0.006), capsular invasion (OR = 9.631, 95%CI 1.130-82.121, P = 0.038), lateral lymph node metastasis (OR = 5.405, 95%CI 1.130-82.121, P = 0.038). 95%CI 2.338–12.496, P < 0.001; Table 4). Therefore, it’s not difficult to conclude that the high GOLT1A expression has a certain correlation with sex, capsular invasion and lateral lymph node metastasis.
Discussion
Thyroid carcinoma is the most prevalent malignant tumor of the endocrine system, contributing significantly to the global medical burden as its incidence continues to rise worldwide. According to GLOBOCAN updated statistics in 2022, thyroid carcinoma ranks as the seventh most common cancer globally26. This epidemiological trend exhibits marked geographic variation, with East Asia demonstrating incidence rates twice as high as those in North America. Particular attention has been drawn to the exceptionally high incidence in China, where 466,000 new cases accounted for approximately half of all new thyroid carcinoma cases globally26. Moreover, the rising trend is evident in both high-income and less affluent countries27. For instance, projections for thyroid carcinoma diagnosis in the United States indicate a significant increase from 2022 to 20241,2, mirroring trends observed in China5.
The debate surrounding this epidemic primarily centers on the “overdiagnosis” hypothesis versus true biological increase26. Overdiagnosis, defined as detection of indolent disease that would not manifest clinically, has been particularly implicated in papillary thyroid carcinoma (PTC) due to widespread imaging utilization27. In essence, overdiagnosis refers to the identification of a “disease” that may never impact the patient’s health or survival. Interestingly, Li et al.27 observed that overdiagnosis was more prevalent in women, likely due to the combined effects of hormonal fluctuations during puberty and the heightened susceptibility of women to healthcare interventions. This sex difference was further corroborated by Hu et al.28. Notably, the increased incidence is accompanied by stable mortality rates5 lending further support to the overdiagnosis hypothesis. However, overdiagnosis is not without consequences. Firstly, it does not necessarily reduce cancer mortality5. Instead, aggressive treatments associated with overdiagnosis often lead to complications, exacerbating patients’ physical and psychological distress while causing unnecessary suffering27. Second, overdiagnosis places a substantial financial burden on healthcare systems26,27. However, attributing the entire increase in thyroid carcinoma incidence to overdiagnosis is overly simplistic. Hajeer et al.4 argue that, although overdiagnosis is a major contributor, a true biological increase—particularly in aggressive subtypes—cannot be dismissed. For example, the incidence of aggressive type has also been on the rise. Thus, it is essential to adopt a balanced perspective, neither abandoning screening efforts due to concerns over overdiagnosis, nor neglecting the increasing prevalence of biologically aggressive subtypes that carry significant clinical and prognostic implications8. In this context, the development of accurate diagnostic tools for PTC is urgently needed. Genetic markers provide a promising solution, offering high specificity to mitigate overdiagnosis and overtreatment while identifying novel targets for PTC diagnosis and therapy.
The Golgi complex is a central hub of an endomembrane system and an active signaling hub involved in the regulation of diverse cellular processes. Structural and functional disorganization of the Golgi apparatus has been observed in cancer cells, contributing to malignant transformation and progression. Specific Golgi-resident proteins and associated signaling molecules are implicated in oncogenesis by participating in multiple signaling cascades29. GOLT1A, a member of the Golgi transport 1 (GOLT1) family, encodes a vesicle transport protein and encodes a protein associated with lipase activity. It is hypothesized to mediate the transport and fusion of endoplasmic reticulum-derived vesicles with the Golgi apparatus14,15. Although studies on GOLT1A remain limited, its dysregulation has been linked to the development of various cancers. Since Ikeda et al.15 first identified GOLT1A as a target of miR-378a-3p in breast cancer and demonstrated that low GOLT1A expression correlated with better survival and reduced tamoxifen resistance in 2015. its role has been further explored in gastrointestinal goblet cell adenocarcinomas, melanoma, lung carcinoma, and adenoid cystic carcinomas. The upregulation of GOLT1A was also observed in gastrointestinal goblet cell adenocarcinomas18 and hypoxic melanoma20 although its association with clinical characteristics remains largely underexplored. In our study, higher expression of GOLT1A was also found in thyroid carcinoma tissues. This finding was further validated at the protein level using immunohistochemistry, which revealed a consistent expression pattern between mRNA and protein levels. Similarly, studies by Zi et al.17 and Zhang et al.16 reported high GOLT1A expression in lung carcinoma, with Zhang et al. further demonstrating that GOLT1A overexpression was associated with poor prognosis and high malignancy in lung adenocarcinoma. This effect was proposed to result from GOLT1A-mediated promotion of tumor cell proliferation via cell cycle regulation. A comparable phenomenon was observed in thyroid carcinoma in our study. Univariate and multivariate analyses using TCGA data revealed that GOLT1A is an independent risk factor for differentiation and is strongly associated with sex, capsular invasion, and lateral lymph node metastasis in FNAB specimens. Notably, PTC patients with lateral lymph node metastasis were reported by Gao et al.30 to exhibit relatively poor prognosis, while capsular invasion has similarly been linked to worse long-term outcomes31. These findings suggest that high GOLT1A expression correlates with aggressive clinical characteristics and poor prognosis. In terms of mechanistic insight, Ke et al.32 demonstrated that knockdown of GOLT1A in thyroid cancer cell lines inhibited proliferation, migration, and invasion, whereas its overexpression enhanced these malignant properties, as shown by CCK-8, colony formation, and Transwell assays. In addition, their study also demonstrated that GOLT1A promoted epithelial-mesenchymal transition (EMT), which is regarded as a key cause of malignant tumor invasion and metastasis, in thyroid cancer cells by observing the expression of EMT-related genes.
To further investigate this association, we hypothesized that GOLT1A overexpression may be linked to the tall cell variant (TCV) of PTC. TCV is histologically characterized by cells at least two to three times taller than their width and is considered a more aggressive PTC subtype with poor prognosis33. Although TCV accounts for only 3–19% of PTC cases34 several studies suggest its incidence is increasing alongside overall PTC rates35. Our univariate analysis of data from the TCGA database showed that thyroid carcinoma tissues with high GOLT1A expression were more likely to be associated with TCV compared to those with low expression. Notably, prior studies have demonstrated that TCV presents with more aggressive clinical features, including multifocality, ETE, LNM, and distant metastasis compared, to classical PTC35. This was further corroborated by Morris et al.34 who additionally suggested that TCV is associated with poorer 5-year disease-specific survival. Consistent with these findings, our study also found that high GOLT1A expression was closely related to some malignant clinical characteristics. Based on these results, we hypothesized that the cause for the poor prognosis of PTC caused by high expression of GOLT1A may be related to TCV. However, further studies are required to validate this conjecture.
In recent years, an increasing number of studies have demonstrated that peripheral blood can serve as a non-invasive diagnostic tool for various cancers, including breast cancer36,37 colorectal cancer38 lung cancer39 and bladder cancer40. In the context of thyroid cancer, Hong et al.41 utilized six PTC predictive markers in peripheral blood cell-free DNA to distinguish papillary thyroid carcinoma (PTC) from benign thyroid nodules. Additionally, overexpression of Bone Morphogenetic Protein 8 A (BMP8A) has been reported to be abnormally elevated in thyroid cancer, promoting tumor progression42. Furthermore, Wang et al.43 further revealed that DNA methylation in peripheral-blood lymphocytes could effectively differentiate malignant from benign thyroid nodules. Peripheral blood testing offers significant advantages over traditional methods such as ultrasonography and fine-needle aspiration biopsy (FNAB). Unlike ultrasound, which has high sensitivity but low specificity, peripheral blood biomarkers may help reduce overdiagnosis by improving diagnostic accuracy. Moreover, FNAB has technical limitations for nodules smaller than 1 cm, whereas peripheral blood analysis is not constrained by nodule size, making it a promising complementary approach. Although GOLT1A has not yet been extensively explored as a peripheral blood biomarker for thyroid cancer, the findings from our study highlight its potential clinical significance. Given the growing interest in non-invasive molecular markers for early cancer detection, further research is warranted to determine whether GOLT1A is detectable and differentially expressed in peripheral blood samples. Such investigations could provide valuable insights into its applicability for non-invasive diagnosis and monitoring of thyroid carcinoma.
Given these findings, FNAB remains essential for the evaluation of thyroid nodules, especially when imaging or clinical suspicion suggests malignancy. This approach not only aids in understanding the clinical characteristics of thyroid nodules but also facilitates more accurate prognostic assessments. Specifically, GOLT1A expression profiling could assist surgeons in identifying patients who would benefit from early surgical intervention, while sparing others from unnecessary procedures. Moreover, given the prognostic significance of elevated GOLT1A expression, it may serve as a novel therapeutic target, offering new avenues for targeted treatment strategies in thyroid carcinoma.
However, our study has several limitations. Firstly, the FNAB specimens included in this study were all from China and did not involve other regions, and the sample size was small. A larger, more diverse sample size is needed for validation. Secondly, this study is a retrospective study, and more prospective studies are needed to further verify our conclusions. Future research could focus on elucidating the specific mechanisms and signaling pathways by which high GOLT1A expression drives poor prognosis of PTC. Thirdly, The short follow-up period limited our analysis of GOLT1A’s prognostic role, as no recurrence or death events occurred. Extended follow-up is warranted for further validation. Moving forward, research efforts should prioritize verification of the hypothesized GOLT1A-TCV association while exploring its translational potential in two key directions: the development of peripheral blood-based noninvasive detection methods and novel targeted therapeutic strategies for thyroid carcinoma.
Conclusion
In this study, 527 cases of thyroid cancer tissues and normal thyroid tissues in TCGA database and 280 cases of normal thyroid tissues in GTEx database were selected for differential gene expression analysis. The expression of GOLT1A in thyroid cancer tissues was higher than that in normal thyroid tissues, and we also selected three GEO databases for verification. Secondly, we confirmed the high expression of GOLT1A in PTC tissues at protein level by IHC and confirmed its cytoplasmic localization. Thirdly, univariate and multivariate analyses of 499 PTC patients and 309 FNAB PTC patients in the TCGA database showed that high expression of GOLT1A was closely related to the malignant clinical characteristics and poor prognosis of PTC. Specifically, GOLT1A is associated with differentiation risk, sex, capsular invasion and lateral lymph node metastasis. Therefore, we propose that GOLT1A is a promising thyroid carcinoma prognostic genetic marker and a pathogenic target affecting the prognosis of PTC.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Haddad, R. I. et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 20, 925–951 (2022).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Boucai, L., Zafereo, M. & Cabanillas, M. E. Thyroid cancer. Rev. JAMA 331, 425–435 (2024).
Hajeer, M. H., Awad, H. A., Abdullah, N. I., Almuhaisen, G. H. & Abudalu, L. E. The rising trend in papillary thyroid carcinoma. True increase or over diagnosis? Saudi Med. J. 39, 147–153 (2018).
Xu, S. & Han, Y. The overdiagnosis of thyroid micropapillary carcinoma: the rising incidence, inert biological behavior, and countermeasures. J. Oncol. 2021, 5544232 (2021).
Raue, F. & Frank-Raue, K. Thyroid cancer: Risk-Stratified management and individualized therapy. Clin. Cancer Res. 22, 5012–5021 (2016).
Testini, M., Gurrado, A., Lissidini, G. & Nacchiero, M. Hypoparathyroidism after total thyroidectomy. Minerva Chir. 62, 409–415 (2007).
Nath, M. C. & Erickson, L. A. Aggressive variants of papillary thyroid carcinoma: hobnail, tall cell, columnar, and solid. Adv. Anat. Pathol. 25, 172–179 (2018).
Giordano, T. J. et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene 24, 6646–6656 (2005).
Ye, N. & Mn, N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 7 (2011).
Abdullah, M. I. et al. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int. J. Med. Sci. 16, 450–460 (2019).
Zarkesh, M. et al. BRAF V600E mutation and MicroRNAs are helpful in distinguishing papillary thyroid malignant lesions: tissues and fine needle aspiration cytology cases. Life Sci. 223, 166–173 (2019).
Yang, C., Cao, Z. G., Zhou, Z. W. & Han, S. J. Circ0005654 as a new biomarker of thyroid cancer interacting with SP1 to influence the prognosis: A case-control study. Medicine 102, e32853 (2023).
Conchon, S., Cao, X., Barlowe, C. & Pelham, H. R. Got1p and Sft2p: membrane proteins involved in traffic to the golgi complex. EMBO J. 18, 3934–3946 (1999).
Ikeda, K. et al. miR-378a-3p modulates Tamoxifen sensitivity in breast cancer MCF-7 cells through targeting GOLT1A. Sci. Rep. 5, 13170 (2015).
Zhang, L. et al. Lidocaine inhibits the proliferation of lung cancer by regulating the expression of GOLT1A. Cell. Prolif. 50, e12364 (2017).
Zi, H., Chen, L. & Ruan, Q. Lidocaine represses the malignant behavior of lung carcinoma cells via the circ_PDZD8/miR-516b-5p/GOLT1A axis. Histol. Histopathol. 37, 461–474 (2022).
Lin, D. L. et al. Gastrointestinal goblet cell adenocarcinomas harbor distinctive clinicopathological, immune, and genomic landscape. Front. Oncol. 11, 758643 (2021).
Zhang, L. et al. GOLT1A-KISS1 fusion is associated with metastasis in adenoid cystic carcinomas. Biochem. Biophys. Res. Commun. 526, 70–77 (2020).
Xu, H. et al. BIRC7 is beneficial for melanoma progression and hypoxic response. Clin. Cosmet. Investig Dermatol. 15, 1109–1117 (2022).
Butterworth, J. et al. GOLT1B activation in hepatitis C Virus-Infected hepatocytes links ER trafficking and viral replication. Pathogens 11, 46 (2021).
Liu, T. et al. Vesicle transporter GOLT1B mediates the cell membrane localization of DVL2 and PD-L2 and promotes colorectal cancer metastasis. Cancer Cell. Int. 21, 287 (2021).
J, L. et al. Multi-Omics analyses revealed GOLT1B as a potential prognostic gene in breast Cancer probably regulating the immune microenvironment. Front. Oncol. 11 (2022).
Varghese, F., Bukhari, A. B., Malhotra, R. & De, A. IHC profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 9, e96801 (2014).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to imagej: 25 years of image analysis. Nat. Methods. 9, 671–675 (2012).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. https://doi.org/10.3322/caac.21834
Li, M., Maso, D., Vaccarella, S. & L. & Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 8, 468–470 (2020).
Hu, S., Wu, X. & Jiang, H. Trends and projections of the global burden of thyroid cancer from 1990 to 2030. J. Glob Health. 14, 04084 (2024).
Spano, D. & Colanzi, A. Golgi complex: A signaling hub in Cancer. Cells 11, 1990 (2022).
Gao, L. et al. Large-Volume lateral lymph node metastasis predicts worse prognosis in papillary thyroid carcinoma patients with N1b. Front. Endocrinol. (Lausanne). 12, 815207 (2021).
Gubbiotti, M. A. et al. Does the presence of capsule influence prognosis in poorly differentiated thyroid carcinoma? Hum. Pathol. 136, 96–104 (2023).
Ke, S., Xu, Z., Zhou, Y. & Zheng, C. Golgi transport 1A promotes cell proliferation and metastasis of papillary thyroid carcinoma. zggx 45, 69–75 (2024).
Ghossein, R. & Livolsi, V. A. Papillary thyroid carcinoma tall cell variant. Thyroid 18, 1179–1181 (2008).
Morris, L. G. T., Shaha, A. R., Tuttle, R. M., Sikora, A. G. & Ganly, I. Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid 20, 153–158 (2010).
Wang, X., Cheng, W., Liu, C. & Li, J. Tall cell variant of papillary thyroid carcinoma: current evidence on clinicopathologic features and molecular biology. Oncotarget 7, 40792–40799 (2016).
Darvishvand, R. et al. Natural killer cell subsets and their functional molecules in peripheral blood of the patients with breast cancer. Immun. Inflamm. Dis. 12, e1255 (2024).
Dumeaux, V. et al. Peripheral blood cells inform on the presence of breast cancer: A population-based case–control study. Int. J. Cancer. 136, 656–667 (2015).
Han, M. et al. Novel blood-based, five-gene biomarker set for the detection of colorectal cancer. Clin. Cancer Res. 14, 455–460 (2008).
Zhang, Q. et al. Peripheral blood transcriptome heterogeneity and prognostic potential in lung cancer revealed by RNA-Seq. J. Cell. Mol. Med. 25, 8271–8284 (2021).
Osman, I. et al. Novel blood biomarkers of human urinary bladder cancer. Clin. Cancer Res. 12, 3374–3380 (2006).
Hong, S. et al. Cell-free DNA methylation biomarker for the diagnosis of papillary thyroid carcinoma. eBioMedicine 90, 104497 (2023).
Liu, K., Gao, M., Qin, D., Wang, H. & Lu, Q. Serous BMP8A has clinical significance in the ultrasonic diagnosis of thyroid cancer and promotes thyroid cancer cell progression. Endocr. Metab. Immune Disord Drug Targets. 20, 591–598 (2020).
Wang, F. et al. Blood leukocytes as a non-invasive diagnostic tool for thyroid nodules: a prospective cohort study. BMC Med. 22, 147 (2024).
Acknowledgements
We thank all participants for their help, as well as the reviewers for their work.
Funding
This work was supported by grants from the Civilian-Oriented Horizontal Research Project (H220971).
Author information
Authors and Affiliations
Contributions
TJD wrote the main manuscript text, YYZ and HJZ prepared figures and tables, ZHZ and ELG edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First Affiliated Hospital of Soochow University (No.2025009) and the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (No. KY2024-004).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publish
The authors affirm that human research participants provided informed consent for publication of the contents of Tables 3 and 4.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, T., Zhu, Y., Zhou, H. et al. High GOLT1A expression induces poor prognosis in papillary thyroid carcinoma. Sci Rep 15, 20979 (2025). https://doi.org/10.1038/s41598-025-05747-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05747-x