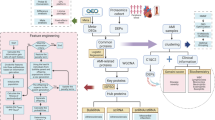
Abstract
Acute myocardial infarction (AMI) poses a significant global mortality burden. Utilizing bio informatics, this study explored cellular aging-related genes (CARGs) and mitochondrial-related genes (MRGs). in AMI Public AMI datasets were analyzed using differential expression and weighted gene co-expression network analysis (WGCNA) to identify 1,373 differentially expressed genes (DEGs), of which 643 were upregulated and 730 downregulated. A turquoise module related to cellular aging was identified by gene set variation analysis (WGCNA). Venn analysis revealed 37 candidate genes intersecting DEGs, key module genes, and MRGs. Machine learning, receiver operating characteristic (ROC) analysis, and reverse transcription quantitative polymerase chain reaction (RT-qPCR) validated Isopentenyl-diphosphate delta-isomerase 1 (IDI1) and phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1) as key AMI genes. A comprehensive lncRNA-miRNA-mRNA network was constructed, and Cyclosporine showed strong binding affinities with IDI1 (-6.2 kcal/mol) and PMAIP1 (-4.8 kcal/mol), indicating therapeutic potential. This study may provide insights into AMI pathogenesis and highlight potential drug targets for further investigation.
Similar content being viewed by others
Introduction
Acute myocardial infarction (AMI) is a leading cause of morbidity and mortality worldwide1, representing a critical manifestation of coronary artery disease2. AMI occurs when there is a sudden blockage of blood flow to a part of the heart muscle, resulting in ischemia and subsequent necrosis of myocardial tissue3. Clinically, AMI presents with symptoms such as chest pain, shortness of breath, diaphoresis4, and in severe cases, such as heart failure, recurrent myocardial infarction, or stroke5. Epidemiologically, AMI remains a significant public health challenge, with incidence rates influenced by factors like age, sex, lifestyle, and genetic predisposition6. Current therapeutic strategies for AMI include reperfusion therapies like percutaneous coronary intervention (PCI) and thrombolytic agents7, alongside pharmacological treatments such as antiplatelet agents, beta-blockers, and statins8. Despite advancements in therapeutic interventions9, numerous limitations persist in the management of AMI, encompassing myocardial reperfusion injury10, adverse pharmacological effects, and the irreversible nature of myocardial damage, among others. Molecular therapy has emerged as a focal point of research aimed at enhancing therapeutic outcomes following myocardial infarction through the modulation of molecular mechanisms, offering renewed hope in the field of AMI treatment. Notably, the regulation of genes involving 5-methylcytosine (5mC), which participates in the progression and prognosis of cardiovascular diseases, presents a novel direction for early AMI detection and personalized therapy through its distinctive modification patterns11. Therefore, this study aimed to explore new key genes for improved diagnosis and treatment of AMI.
Cellular aging, characterized by progressive functional decline and increased susceptibility to stressors, plays a pivotal role in cardiovascular diseases, including AMI12. The fundamental mechanisms of cellular aging encompass DNA damage accumulation, telomere shortening, and the generation of reactive oxygen species (ROS), all of which contribute to cellular senescence and dysfunction13. Some research reports have shown that the decline in cellular function leads to an increase in pro-inflammatory factors (such as IL-6, TNF-α) in the blood, which accelerates the senescence of vascular endothelial cells and the development of atherosclerosis14. Blood plays multiple roles in the pathological process of acute myocardial infarction (AMI): it serves as a medium for thrombus formation (through platelet activation and the coagulation cascade), a carrier of the inflammatory response, and a source of biomarkers for myocardial injury simultaneously15. Mitochondria, as the powerhouse of the cell, are crucial for maintaining energy metabolism and cellular homeostasis16. Mitochondrial dysfunction, often resulting from excessive ROS production and metabolic imbalance, can accelerate cellular aging and exacerbate cardiovascular pathology17. Studies have shown that the production and function of blood cells are highly dependent on the energy metabolism and redox balance of mitochondria. Among them, the self-renewal of hematopoietic stem cells requires precisely regulated levels of mitochondrial reactive oxygen species (ROS)18. Meanwhile, mitochondrial components can be released into the blood as signaling molecules and participate in the systemic inflammatory response and metabolic regulation by activating receptors such as TLR919,20. Under pathological conditions, mitochondrial dysfunction can lead to hematological diseases, and the inflammatory factors in the blood can, in turn, feedback-inhibit the function of tissue mitochondria, forming a vicious cycle21. The interplay between cellular aging and mitochondrial-related genes is increasingly recognized as a significant factor in the pathogenesis of AMI. These interactions may potentiate the occurrence and progression of AMI by impairing cellular resilience and promoting adverse remodeling of myocardial tissue22. However, to date, no studies have reported the involvement of common genes associated with cellular senescence and mitochondria in the progression of AMI, nor have they elucidated the underlying mechanisms. Therefore, this study aimed to elucidate the key common genes and their mechanisms of action related to cellular senescence and mitochondria in the pathogenesis of AMI.
Blood serves as a reservoir of biomarkers, carrying circulating miRNAs and epigenetic modifications with diagnostic value23. Building on this foundation, our study employed integrated bioinformatics approaches to systematically investigate the critical roles of cellular senescence pathways and mitochondria-related gene networks in AMI pathogenesis using blood-derived datasets. By employing differential gene expression analysis, gene set variation analysis (WGCNA), and machine learning techniques, we aimed to identify key genes and regulatory networks associated with these processes in AMI. Furthermore, drug-gene interaction analyses will facilitate the identification of potential therapeutic targets and strategies. The findings of this study are expected to enhance our understanding of the molecular mechanisms underlying AMI and pave the way for the development of novel diagnostic and therapeutic interventions.
Results
Identification of DEGs and key modules gene in AMI
In order to identify the genes with significant differences in expression between samples, in the comparison of AMI with control samples, 1,373 DEGs were identified, comprising 643 genes with significantly increased expression and 730 genes with significantly decreased expression (Fig. 1a and b). Separately, 112 cellular aging-related genes (CARGs) with significant expression variations were identified in the AMI and control groups (P < 0.05). Additionally, the differentially expressed CARGs single-sample Gene Set Enrichment Analysis (ssGSEA) scores were significantly different between the AMI and control groups (Fig. 1c). WGCNA facilitated the identification of the most relevant modules, and the clustering analysis of the samples revealed no outliers; therefore, all samples were retained for analysis (Fig. 1d). A soft threshold was set at R²= 0.85, with a β value of 12 selected as it exceeded the red cut-off line, ensuring a mean connectivity close to zero (Fig. 1e). WGCNA identified seven co-expression modules and one unclustered gray module (Fig. 1f), among which the turquoise module had the most significant connection with CARGs scores (R = 0.86, P < 0.001). This module, which contained 5,824 genes, was identified as the key module associated with cellular aging (Fig. 1g) and used for later stages of analysis.
Identification of DEGs and key modules gene between AMI and control group. (a) Volcano map of differentially expressed genes. (b) Differentially expressed gene heat map. (c) Differences in ssGSEA scores for CARGs. (d) After introducing sample traits, sample hierarchical clustering. (e) Soft threshold screening. (f) Identification of co-expression module. (g) ssGSEA Score Correlation Heat Map for Key Modules and CARGs.
Discovery and functional enrichment exploration of 37 candidate genes
To obtain candidate genes in AMI, 37 overlapping genes were identified by intersecting 1373 DEGs, 5824 key modular genes, and 1136 MRGs and designated as candidate genes, highlighting their potential importance in disease mechanisms (Fig. 2a). Gene Ontology (GO) enrichment analysis identified 255 terms, including 177 biological process (BP) related to mitochondrial gene expression, translation, membrane organization, transport, and targeted proteins. Within the cellular component (CC) category, 32 terms were significantly enriched, including mitochondrial matrix, inner mitochondrial membrane, mitochondrial protein complexes, ribosomes, and mitochondrial-ribosomal associations. Additionally, 46 molecular function (MF) terms were identified, including ribosomal structural components, nucleotide transmembrane transporter activity, organic phosphate transmembrane transporter activity, and RNA binding (Fig. 2b, Table S2). Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis identified nine significantly enriched pathways, including ribosome, mitophagy-animal, p53 signaling pathway, platinum drug resistance, colorectal cancer, apoptosis-multiple species, primary bile acid biosynthesis, apoptosis, and lipoic acid metabolism (Fig. 2c). These pathways collectively regulate cellular homeostasis, the stress response, apoptosis, and metabolism, which are crucial for disease progression. The protein-protein interactions (PPI) network comprised of 27 nodes and 41 edges. For example, BCL2 is associated with BNIP3L and phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1), indicating interactions between these proteins. Another cluster included MRPL35, which interacted with multiple ribosomal proteins such as MRPL18, MRPS5, and MRPS28 (Fig. 2d).
Isopentenyl-diphosphate delta-isomerase 1 (IDI1) and PMAIP1 as key genes for AMI through LASSO, SVM-RFE, and ROC analysis
Screening key diagnostic markers for acute myocardial infarction (AMI) by machine learning algorithms. Least absolute shrinkage and selection operator (LASSO) regression analysis was first performed on the candidate genes, and nine characterized genes were obtained when the Lambda was 0.05296 (PMAIP1, IDI1, SLC25A33, TOMM40L, CRYZ, MRPL18, LIPT1, FARS2, THEM4)(Fig. 3a). Then support vector machine recursive feature elimination (SVM-RFE) analysis, the SVM-RFE analysis showed that the model achieved the highest prediction accuracy when 25 signature genes were used. These genes included IDI1, PMAIP1, BNIP3L, MRPS28, BCL2, MRPL18, FARS2, CRYZ, THEM4, METTL5, MRPS6, MRPS14, TOMM5, PNPLA8, MRPS5, CLPX, TIMMDC1, SLC25A33, AKAP1, GLS, SLC25A42, OMA1, PREPL, LIPT1, and OSGEPL1 (Fig. 3b). The intersection of genes in LASSO and SVM-RFE analyses identified eight genes: PMAIP1, IDI1, SLC25A33, CRYZ, MRPL18, LIPT1, FARS2, and THEM4 (Fig. 3c). Wilcoxon test showed that IDI1 and PMAIP1 had significantly different expression levels in the AMI and control groups (p < 0.05), showing stable expression patterns in both sets (Fig. 3d). Additionally, receiver operating characteristic (ROC) curve unveiled that area under the curve (AUC) values for two genes were both greater than 0.7 in the training and testing sets, underscoring their high diagnostic value for AMI (Fig. 3e). These findings identified IDI1 and PMAIP1 as key genes.
Development and confirmation of a high-accuracy nomogram incorporating IDI1 and PMAIP1
A column-line graphical model for AMI risk prediction based on the key genes IDI1 and PMAIP1. Leveraging the expression levels of IDI1 and PMAIP1, a nomogram was created to assess AMI risk (Fig. 4a). The model demonstrated robust predictive performance, with an MAE of less than 0.1, indicating strong predictive performance (Fig. 4b). The ROC curve confirmed this with AUC values exceeding 0.7, further validating the diagnostic capability of the model (Fig. 4c). Furthermore, the net benefit of the decision curve for the nomogram was higher than that of the individual genes, highlighting its superior predictive value for AMI risk compared to single genes (Fig. 4d).
Key signaling pathways linked to IDI1, PMAIP1 in AMI
To analyze the molecular mechanisms of IDI1 and PMAlP1 in the pathogenesis of acute myocardial infarction (AMI). GeneMANIA was used to predict the key functions associated with the conduct of IDI1 and PMAIP1, including secondary alcohol biosynthesis process, regulation of sterol biosynthesis process, sterol biosynthesis process, cholesterol metabolism process, cholesterol metabolism process, sterol metabolism process and cholesterol biosynthesis process (Figure S1a). Additionally, Gene set enrichment analysis (GSEA) revealed the functions of IDI1, showing its involvement in 73 signaling pathways. These pathways spanned various metabolic and immune processes, including the B-cell receptor and mTOR signaling pathways (Figure S1b). Additionally, PMAIP1 was enriched in 18 signaling pathways, predominantly related to metabolic regulation, cell cycle, and cancer-related pathways (Figure S1c). Moreover, gene set variation analysis (GSVA) of Hallmark pathways showed 29 differential pathways in AMI versus the control group, with 5 downregulated and 24 upregulated pathways. The top 10 GSVA results highlighted significant pathways, such as MYC targets V1 and TNFα signaling via NFKB, IL6 JAK STAT3 signaling, inflammatory response, hypoxia, KRAS signaling upregulation, oxidative phosphorylation, peroxisome, E2F-targets, and MYC targets V2 (Figure S1d). These differential pathways reflect significant changes in metabolism and signaling during the development of AMI.
Marked variations in immune cell infiltration and subcellular localization of IDI1 and PMAIP1 in AMI
For the analysis of acute myocardial infarction and immune cell correlation, the enrichment score obtained using the ssGSEA algorithm was 28 immune cells (Fig. 5a). The AMI and control groups showed significant differences in enrichment scores for 25 immune cell types (P < 0.05), including activated dendritic cells, activated B cells, CD56dim natural killer cells, activated CD8 T cells, activated CD4 T cells, and CD56bright natural killer cells. Compared with the control group, AMI manifested lower infiltration levels in four immune cell types: activated B cells, activated CD8 T cells, type 17 T helper cells, and central memory CD4 T cells (Fig. 5b). Additionally, key genes were significantly correlated with the 25 differentially enriched immune cells. Among them, IDI1 showed the strongest positive association with central memory CD8 + T cells (r = 0.515, P < 0.05), whereas PMAIP1 exhibited the sharpest opposite correlation with type 17 T helper cells (r = −0.366) (Fig. 5c, Table S3). In terms of subcellular localization, IDI1 was predominantly located in the nucleus, with the highest score observed in this region. In contrast, PMAIP1 showed the highest score in the cytoplasm, indicating its primary localization (Fig. 5d).
Marked variations in immune cell infiltration and subcellular localization of IDI1 and PMAIP1 in AMI. (a) Immune infiltration cell enrichment fraction based on heat map between AMI group and control group. (b) Box plot of the difference in enrichment fraction of 28 immunoinfiltrating cells between AMI group and Control group. (c) Heat map of correlation between biomarkers and differential immune cells. (d) Biomarker subcellular localization.
Molecular regulatory network revealed key interactions for IDI1 and PMAIP1
To construct the transcriptional regulatory networks of IDI1 and PMAlP1 and reveal their multilevel regulatory AMI. A regulatory network was constructed to investigate the mechanisms that control IDI1 and PMAIP1. This network included 18 nodes and 16 edges, consisting of IDI1, PMAIP1, and 16 transcription factors (TFs). Among these, 11 TFs were found to regulate IDI1, including YY1, JUN, and ATF1, whereas 5 TFs regulated PMAIP1, including E2F1, TP53, and ZBTB16 (Fig. 6a). Using the miRNet and NetworkAnalyst databases, 149 miRNAs were predicted to target these genes. In addition, 204 overlapping lncRNAs associated with these miRNAs were identified by further analysis using the miRNA and StarBase databases. Consequently, an lncRNA-miRNA-mRNA network containing 278 nodes and 1,306 edges was constructed. In this network, genes such as PMAIP1 were regulated by LINC00271 and UCA1 via hsa-mir-106b-5p (Table S4), whereas IDI1 was regulated by LINC01876 and LINC01126 via hsa-mir-3617-5p (Fig. 6b). This visualization highlighted the joint regulation of these key genes by miRNAs and lncRNAs, revealing complex regulatory interactions.
Drug prediction and molecular Docking reveal strong binding affinity of cyclosporine with IDI1 and PMAIP1
Screening potential therapeutic agents targeting IDI1 and PMAIP1 and assessing their binding activities through drug-gene interaction networks and molecular docking analysis to inform targeted therapeutic strategies. This study further explored potential therapeutic strategies targeting IDI1 and PMAIP1. A drug-gene interaction network was visualized using Cytoscape software, revealing 31 nodes (two key genes and 29 drugs) and 32 edges. The analysis revealed that PMAIP1 was associated with the greatest amount of drugs, with Cyclosporine, Valproic Acid, and Cisplatin regulating both the target genes (Fig. 7a). Based on these findings, molecular docking analysis was conducted for the three drugs and key genes. The results showed that IDI1 exhibited the highest binding strength with cyclosporine, measured at −6.2 kcal/mol (Table S5). These results suggested that cyclosporine demonstrated strong binding capabilities with both genes, particularly IDI1 (Fig. 7b).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The expression levels of IDI1 and PMAIP1 in the control and AMI groups were detected to validate the experimental results. The expression levels of IDI1 and PMAIP1 reached 2.6 and 5.18, respectively, and all P values were less than 0.05 (Table S6, Fig. 8a-b). These results are consistent with those of the previous analyses, indicating that the previous results were reliable.
Discussion
AMI is the main clinical cause of death and cardiovascular disease; thus, it is associated with high rates of morbidity and mortality. Cell senescence and mitochondrial dysfunction are hallmarks of aging and are closely interconnected24. CARGs plays an important role in AMI25. Given the central role of the mitochondria in cellular energy metabolism, mitochondrial dysfunction may be a significant driver of AMI26. However, the relationship among cell senescence, mitochondrial damage, and AMI remains unclear.
In this study, we performed a comprehensive analysis to investigate the molecular mechanisms underlying AMI. Using differential gene expression analysis, WGCNA, and machine learning algorithms, we identified two key genes associated with cellular aging and mitochondria in AMI, IDI1 and PMAIP1, explored immune infiltration patterns, predicted potential drug candidates, and validated the findings through molecular docking and RT-qPCR. The findings offer novel perspectives on the pathological mechanisms and potential therapeutic targets associated with AMI.
IDI1 is essential for cholesterol biosynthesis27 and regulates isomerization, which is crucial for steroid molecule production28. Cholesterol metabolism29, closely tied to atherosclerosis, is the primary cause of AMI30-indicates IDI1’s indirect role in influencing AMI risk. In addition, studies have shown that IDI1 plays a regulatory role in atrial lipotoxic myopathy associated with atrial enlargement28. IDI1 plays an important role in cell senescence and mitochondrial dysfunction, and abnormal expression of IDI1 is closely associated with AMI (PMID: IDI1 plays an important role in cell senescence and mitochondrial dysfunction, and abnormal expression of IDI1 is closely associated with AMI31. IDI1 gene plays a key role in the pathway of isopenedioic acid metabolism, mainly catalyzes the synthesis of farnesyl diphosphate, and then participates in cholesterol biosynthesis28. Because isoprenoid metabolism is essential for maintaining mitochondrial membrane stability and function32, there is currently a lack of research on IDI1 directly related to cell aging, so abnormal expression of IDI1 may affect isoprenoid metabolism, thereby destroying the stability of mitochondrial membrane, leading to mitochondrial dysfunction, and ultimately promoting cell aging.
PMAIP1, which encodes NOXA protein, is vital for regulating apoptosis33. Apoptosis is a key mechanism for clearing damaged cells, particularly under hypoxic and oxidative stress conditions seen in AMI34. Excessive activation of apoptotic pathways can lead to widespread cardiomyocyte death, exacerbating AMI severity of AMI35. In terms of mitochondrial function, PMAIP1 is directly involved in apoptotic regulation of mitochondrial pathways36. IDI1 and PMAP1 may be involved in AMI development by affecting cholesterol metabolism and apoptosis. Recent studies have shown that mitochondrial dysfunction is a key factor driving cell senescence, and is closely related to the occurrence and development of cardiovascular diseases. Miwa et al. revealed the central role of mitochondrial dysfunction in cellular senescence24, while Camacho-Encina et al. further demonstrated the synergistic effect of mitochondrial dysfunction and cellular senescence in cardiovascular disease37. Together, these findings underscore the importance of mitochondrial dysfunction in age-related diseases. It is worth noting that this study is the first to discover the important role of IDI1 in AMI, but the biological processes involved require further study. RT-qPCR results were aligned with bioinformatic predictions, confirming the upregulation of IDI1 and PMAIP1 in clinical AMI samples. These findings reinforce the reliability of our computational approach and suggest that IDI1 and PMAIP1 are key AMI genes. In cases where RT-qPCR results diverged from bioinformatics data, technical variables, such as sample selection or assay sensitivity, as well as biological differences between datasets, were considered. These discrepancies may reflect variations in the disease stages or patient populations, warranting further investigation.
This study focuses on cellular senescence and mitochondria-related genes in acute myocardial infarction (AMI), but fails to distinguish the differences among the infarct area, the border zone, and the remote area. In fact, there is significant spatial heterogeneity in gene expression and cellular states among these regions, and this spatial heterogeneity is of great value for elucidating the pathological mechanisms of AMI and formulating precise treatment strategies.
The infarct zone is the most intensively studied part in AMI. Myocardial cells in this zone suffer irreversible damage and death due to prolonged ischemia and hypoxia38, and thus cellular senescence and mitochondrial dysfunction are likely to be more severe39. Although the myocardial tissue in the area remote from the infarct has not been directly damaged by the infarction, it may be affected by a certain degree of ischemia and is in a sub-healthy state40. The cellular senescence and mitochondrial function in this region may be between those of the infarct area and the non-infarct area41. Understanding the gene expression characteristics of the area remote from the infarct is helpful for evaluating the overall functional status of the myocardium and the potential risks of the disease.
The myocardial tissue in the non-infarct area is relatively normal in AMI patients, but it may be affected by systemic pathophysiological changes42. In the non-infarct area, cellular senescence and mitochondrial function may maintain a relatively stable state, but they will also be influenced by the overall pathological environment of AMI43.
The changes in cellular senescence and mitochondria-related genes in AMI, and the differences in different infarct regions and pathological microenvironments show a gradient change from severe damage to a relatively stable state. This is crucial for mechanistic research and precise treatment.
Pathway analysis using GeneMANIA and GSEA revealed the significance of IDI1 and PMAIP1 in cholesterol metabolism and steroid biosynthesis, which are dysregulated in AMI. Additionally, GSVA pathway analysis revealed significant differences in inflammation and oxidative phosphorylation between AMI patients and controls. These pathways contribute to metabolic imbalances and inflammatory responses, worsening the myocardial damage44. Understanding how IDI1 and PMAIP1 regulate these processes could guide the development of targeted therapies.
Molecular docking and drug prediction analyses identified Cyclosporine, Valproic Acid (VPA), and cisplatin as potential therapeutic agents. Recent studies have shown that cyclosporine, as a classical immunosuppressant, exhibits multiple protective effects in acute myocardial infarction (AMI) treatment45. Studies have shown that cyclosporine significantly reduces cardiomyocyte apoptosis and necrosis in myocardial ischemia reperfusion injury by inhibiting the opening of mitochondrial permeability transition pore (mPTP)39. At the same time, the drug effectively inhibits inflammatory response by blocking calcineurin signaling pathway and reduces myocardial tissue damage after AMI46. Molecular docking analysis further revealed that IDI1 and PMAIP1 have strong binding affinity, which provides a new perspective for elucidating their molecular mechanism in AMI. Tian et al. showed that VPA treatment significantly reduced cardiac damage after MI, and that the cardioprotective effect of VPA was likely mediated via the Foxm1 pathway. Further clinical trials are necessary to validate the efficacy and safety of these compounds in AMI47.
Additionally, we constructed TF-mRNA regulatory networks (TP53, ZBTB16 et al.) and mRNA-miRNA-lncRNA networks (hsa-mir-106b-5p, hsa-mir-3617-5p, UCA1 et al.). Studies by Deng Liang et al.48 have shown that TP53 is closely related to the molecular mechanism of Artemisia annua in treating AMI, indicating that regulating the expression of TP53 is of great significance for the treatment of AMI.The results of Yongpan Liu et al.49 and Youyou Yan et al.23 showed that circulating miR-1/UCA1 is a novel biomarker for the diagnosis and prognosis of acute myocardial infarction. Long non-coding RNA UCA1 relieves cardiomyocyte H9c2 injury induced by oxygen-glucose deprivation by decreasing miR-12250. These regulatory relationships are closely related to the progression of acute myocardial infarction and its associated diseases and may be potentially effective targets for the treatment of acute myocardial infarction.
This study identified IDI1 and PMAIP1 as critical genes in AMI through integrated bioinformatic analyses, WGCNA, and machine learning. Construction of the lncRNA-miRNA-mRNA regulatory network revealed intricate mechanisms that control these genes. Molecular docking studies have highlighted CsA as a promising therapeutic candidate. These findings provide important insights into AMI’s molecular basis of AMI, and suggest potential therapeutic targets for mitochondrial dysfunction, cellular senescence, and cholesterol metabolism.
In this study, we identified two critical genes (IDI1 and PMAIP1) associated with cellular senescence and mitochondrial dysfunction in acute myocardial infarction (AMI) through comprehensive bioinformatics analysis. However, several limitations should be acknowledged. First, the research primarily relied on public dataset analysis without functional validation through cellular or animal models. Since our samples were derived only from blood samples, we failed to specifically sort CECs, possibly missing endothelium-specific signals, and the molecular mechanisms remain incompletely elucidated. Furthermore, drug predictions were exclusively based on computational simulations. The binding energy parameters predicted by molecular docking could not distinguish between specific and nonspecific binding. Second, the study did not thoroughly differentiate molecular distinctions between infarct core, border zones, and remote non-injured regions, which might compromise the comprehensive understanding of gene expression patterns and cellular states. Additionally, the investigation omitted analysis of fibroblasts and other non-cardiomyocyte populations that potentially contribute to AMI pathophysiology.
IDI1 and PMAIP1 were established as pivotal regulatory genes in AMI, yet to fully elucidate their molecular mechanisms and advance clinical translation. Subsequent investigations should be conducted through multidimensional strategies. Molecular-level analyses incorporating spatial transcriptomics to delineate region-specific expression profiles across infarct core, border zones, and remote myocardium, complemented by PRM or Olink proteomic platforms to enhance low-abundance protein detection sensitivity. Cellular-level validations employing CRISPR-engineered cellular models and genetically modified animal systems to interrogate gene functions in cellular senescence, mitochondrial homeostasis, and fibrotic remodeling, alongside establishing endothelial-cardiomyocyte coculture systems with real-time metabolic tracking to decipher intercellular crosstalk mechanisms. Therapeutic development utilizing surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC) for precise quantification of ligand binding kinetics/thermodynamics, supplemented by competitive binding assays for site specificity validation and X-ray crystallography for structural resolution of molecular complexes; and clinical translation integrating single-cell sequencing with vascularized organoid technology to model circulating endothelial cell (CEC) dynamics and engineer liquid biopsy-based diagnostic platforms. These integrated multi-omics and multiscale approaches will rigorously validate endothelial specificity of existing biomarkers, facilitate discovery of novel vasculoprotective targets, and pioneer mechanism-driven precision therapeutics for AMI management.
Materials and methods
Data source
The GSE66360 (GPL570) dataset, procured from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), including 50 controls and 49 patients with AMI, was adopted as the training set. The GSE29532 (GPL5175) dataset consisted of 49 healthy controls and 6 AMI patients, also obtained from the GEO database, and was designated as the testing set. The sample type for both the GSE66360 and GSE29532 datasets are blood samples, see Table S7-8 for detailed dataset clinical information. In addition, a set of 307 CARGs was extracted using the CellAge database (https://genomics.senescence.info/download.html#cellage), while 1,136 mitochondrial-related genes (MRGs) MRGs were retrieved from the MitoCarta3.0 database (https://www.broadinstitute.org/mitocarta)51. These datasets were further leveraged for functional analyses and model development.
Differential expression analysis
Once the data were collected, GSE66360 was processed by “limma” package (v 3.54.052) to uncover DEGs in AMI versus control, with thresholds set at p adj. < 0.05 and (|log2FoldChange (FC)| ≥ 0.5). Visualization of DEGs was performed through a volcano plot generated using “ggplot2” package (v 3.4.153). Additionally, a heatmap was constructed by “ComplexHeatmap” package (v 2.14.054).
Weighted gene co-expression network analysis (WGCNA)
After identifying DEGs, Wilcoxon test (P < 0.05), implemented by “rstatix” package (v 0.7.2, https://rpkgs.datanovia.com/rstatix/), was employed to scrutinize the differential expression of CARGs. Subsequently, ssGSEA algorithm from “GSVA” package (v 1.42.055) was adopted to perform calculations of CARGs scores for the differentially expressed CARGs across all samples from training set. The Wilcoxon test was executed again to compare the CARG ssGSEA scores between the AMI and control groups (P < 0.05). The expression matrix from all samples in the training set was subjected to WGCNA using the WGCNA” package (v. 1.7156). Initially, all samples underwent hierarchical clustering, guided by Euclidean distance, to detect potential outliers, which were subsequently removed. To model a scale-free network, an appropriate soft-thresholding power was chosen to optimize the fit, based on the highest R² value. By applying the selected soft threshold, a scale-free network was established with a minimum module size of 100 genes and merging threshold (mergeCutHeight) set at 0.25. Next, the CARGs scores derived from the previous analysis were used as phenotypes to explore their association with the identified gene modules. The relationships between the gene modules and phenotypes were analyzed, and the correlation coefficients and p-values were calculated. A heatmap by “ComplexHeatmap” package (v 2.14.0) was then generated to visually represent the strength of these associations. Next, the module with the strongest correlation to CARGs was selected for further analysis, and key module genes were extracted.
Identification and functional assessment of candidate genes
A Venn diagram was constructed using the “VennDiagram” package (v 1.7.157), which intersected three gene sets: DEGs, key module genes from the WGCNA analysis, and MRGs. The overlapping genes among these three sets were selected as candidate genes. Then, the candidate genes were put through GO and KEGG pathway analyses58,59,60 (P < 0.05) by “clusterProfiler” package (v 4.7.1.00361). GO enrichment analysis was performed across BP, CC, and MF, and 15 most significantly enriched pathways were illustrated via “enrichplot” package (v 1.18.362). Similarly, KEGG enrichment analysis results were also visualized with “enrichplot” package (v 1.18.3). To explore PPI, candidate genes were entered into the STRING database (https://cn.string-db.org/) and annotated with an interaction confidence score greater than 0.4 were retained. The resulting data were imported into Cytoscape software (v. 3.8.263) to visualize the PPI network and highlight the key interactions.
Uncovering key genes via machine learning
Two machine learning approaches, LASSO regression and SVM-RFE, facilitate further selection of feature genes. LASSO regression was executed using “glmnet” package (v 4.1–864), while SVM-RFE analysis was carried out via “caret” package (v 6.0–9365). Shared feature genes from LASSO and SVM-RFE were captured and a Venn diagram was generated using the “VennDiagram” package (v 1.2.2) to visualize the overlap. In both sets, intersected feature genes were analyzed between AMI and control samples via Wilcoxon test (p < 0.05), implemented via “rstatix” (v 0.7.2). Additionally, intersected feature genes were appraised by plotting receiver operating characteristic (ROC) curves via “pROC” package (v 1.18.066). Both sets had ROC curves generated, and the area under the curve (AUC) values were assessed for each gene, with an AUC greater than 0.7 considered to have good diagnostic performance. Genes that showed significant differential expression between the AMI and control groups and consistent expression trends in both sets were identified as key genes.
Development and assessment of nomogram
The expression levels of key genes were used to design a nomogram model in the AMI training set, which was considered the outcome event. The “rms” package (v 6.5-167) was used to develop this nomogram. To gauge the model’s predictive effectiveness, calibration curves were generated using “ResourceSelection” package (v 0.3–568), with better alignment between the curve and the diagonal line indicating stronger predictive precision. A mean absolute error (MAE) of < 0.1 suggests minimal discrepancy between predicted and actual disease risk, revealing the high precision of the nomogram model in forecasting AMI. ROC curves were plotted using the “pROC” (v. 1.18.0) to assess the diagnostic value of the nomogram. Decision curve analysis (DCA) was performed to compare the effectiveness of forecasting individual genes and nomograms. Decision curves were plotted via “rmda” package (v 1.6, https://cran.r-project.org/web/packages/rmda/index.html) was employed to plot the decision curves, further illustrating the net benefit of the nomogram in clinical decision-making.
Functional gene and pathway analysis
Genes and pathways functionally related to the key genes were predicted using GeneMANIA (http://www.genemania.org/). To assess the biological roles of genes in disease progression, correlations linking each gene to the rest were measured using Spearman coefficients in the training set using “psych” package (v 2.1.669). Genes were ranked in descending order of correlation coefficients, generating a ranked gene list for each gene. Next, GSEA was executed by means of “c2.cp.kegg.v2023.1. Hs.symbols.gmt” from the Molecular Signatures Database (MSigDB) as a backdrop gene set. A false discovery rate (FDR) < 0.05 and |normalized enrichment score (NES)| > 1 were labeled as significantly enriched pathways. The top 10 enriched pathways were visualized via “enrichplot” package (v 1.18.3). GSVA was then directed on the training set via “h.all.v2023.1. Hs.symbols.gmt” gene set to investigate pathway variations across AMI and control groups. Pathway enrichment scores were compared via “limma” package (v 3.54.0), P < 0.05 considered significant. Pathways with t-values > 0 were classified as upregulated, and those with t-values < 0 were classified as downregulated.
Immune infiltration analysis and subcellular localization
In the training set, “GSVA” package (v 1.44.0) was used to measure enrichment scores of 28 immune cells70 in each sample based on the ssGSEA algorithm, and the difference in these scores from AMI group was assessed in comparison to control group. Next, derived from the enrichment scores of significantly different immune infiltrating cells, “psych” package (v 2.1.6) was used to perform Spearman correlation analysis, calculating r values and p values between per cell type and the key genes. Specifically, |r| ≥ 0.3 and P < 0.05, indicated a correlation. Subsequently, FASTA sequences of key genes were retrieved from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/) and input into the mRNALocater database (http://bio-bigdata.cn/mRNALocater) for intracellular mRNA localization prediction. The database employs machine learning algorithms, including Categorical Boosting (CatBoost), extreme Gradient Boosting (XGBoost), and Light Gradient Boosting Machine (LightGBM), combined with optimal feature subsets to build stable models for projecting the subcellular placement of eukaryotic mRNA.
Construction of regulatory network
To explore TFs and miRNAs that govern key genes, the miRNet database (ENCODE, https://www.mirnet.ca/) was first used to anticipate TFs that may regulate key genes. The TF-mRNA (key gene) network was visualized using the Cytoscape software. Additionally, the miRNet and NetworkAnalyst databases (https://www.networkanalyst.ca/) were used to identify miRNAs associated with key genes, and the intersection of the predicted miRNAs from both databases was used to identify those consistently associated with key genes. Next, the miRNet and StarBase databases (https://starbase.sysu.edu.cn/) were used to identify lncRNAs corresponding to miRNAs, and intersecting lncRNAs were obtained from both databases. The lncRNA-miRNA-mRNA regulatory network was constructed and mapped using the Cytoscape software (v 3.8.2).
Drug screening and molecular docking investigations
In the training set, key genes were extracted from the Comparative Toxicogenomics Database (CTD, https://ctdbase.org/) to predict the corresponding targeted drugs. Drugs reported in at least two published studies were selected as the final targeted drugs. The prediction results were then imported into Cytoscape software to visualize the relationships between the genes and the targeted drugs. Molecular docking was performed between the key genes and drugs identified in the previous analysis. First, the 3D structures of the active compounds were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Protein crystal structures corresponding to these genes were retrieved from Protein Data Bank PDB (https://www.rcsb.org/). Molecular docking was performed using the CB-Dock database (http://clab.labshare.cn/cb-dock/php/blinddock. php/). A binding energy below − 5 kcal/mol is typically considered indicative of a strong binding affinity between genes and drugs. The docking results between the active compounds and gene were visualized using PyMOL software (v. 3.1.171).
RT-qPCR
In this study, bioinformatics analysis was carried out based on the GSE66360 and GSE29532 blood sample datasets, and in order to maintain the consistency of the research strategy, peripheral blood samples were also selected for key gene expression detection in the subsequent experimental validation phase. RT-qPCR was used to quantitatively validate the screened differentially expressed genes. Five pairs of frozen whole blood samples were collected from Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, with 5 samples five control samples and five AMI samples. This study was approved by the Ethics Committee of Xiangyang City Central Hospital (NO: 2024 − 178). All the patients signed an informed consent form. Subsequently, TRIzol (Ambion, Austin, USA) was used to extract total RNA according to the manufacturer’s instructions, and 1ul RNA was used to detect the concentration of RNA using a NanoPhotometer N50. Reverse transcription was performed to synthesize cDNA using the SweScript First Strand cDNA synthesis kit (Servicebio, Wuhan, China). RT-qPCR was then performed using the CFX96 real-time quantitative fluorescence PCR instrument, with the following reaction program: 1 min at 95 °C, 40 cycles at 95 °C for 20 s, 55 °C for 20 s, and 30 s at 72 °C. The primers used for RT-qPCR are listed in Table S1. GAPDH was used as an internal reference gene. The gene expression levels were calculated using the 2−△△Ct method72. The results were visualized using GraphPad Prism 5 software (GraphPad Prism 5 Software, Inc., USA).
Statistical analysis
Bioinformatic analyses were performed using the R programming language (v. 4.2.2). In addition, differences in RT-qPCR results between the groups were assessed using t-tests. Statistical significance was set at P < 0.05.
Data availability
The datasets generated during and/or analysed during the current study are available in the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/), the CellAge database (https://genomics.senescence.info/download.html#cellage) and the MitoCarta3.0 database (https://www.broadinstitute.org/mitocarta).
Abbreviations
- AMI:
-
Acute myocardial infarction
- CARGs:
-
Cellular aging-related genes
- MRGs:
-
Mitochondrial-Related Genes
- WGCNA:
-
Weighted gene co-expression network analysis
- ROC:
-
Receiver operating characteristic
- TFs:
-
Transcription factors
- RT-qPCR:
-
Reverse transcription quantitative polymerase chain reaction
- IDI1:
-
Isopentenyl-diphosphate delta-isomerase 1
- PMAIP1:
-
Phorbol-12-myristate-13-acetate-induced protein 1
- DEGs:
-
Differentially expressed genes
- PCI:
-
Percutaneous coronary intervention
- 5mC:
-
5-methylcytosine
- ROS:
-
Reactive oxygen species
- ssGSEA:
-
Single-sample Gene Set Enrichment Analysis
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- BP:
-
Biological process
- CC:
-
Cellular component
- MF:
-
Molecular function
- PPI:
-
Protein-protein interactions
- LASSO:
-
Least absolute shrinkage and selection operator
- SVM-RFE:
-
Support vector machine-recursive feature elimination
- AUC:
-
Area under the curve
- MAE:
-
Mean absolute error
- DCA:
-
Decision curve analysis
- GSEA:
-
Gene set enrichment analysis
- MSigDB:
-
Molecular Signatures Database
- FDR:
-
False discovery rate
- NES:
-
Normalized enrichment score
- GSVA:
-
Gene set variation analysis
- NCBI:
-
National Center for Biotechnology Information
- CatBoost:
-
Categorical Boosting
- LightGBM:
-
Light Gradient Boosting Machine
- CTD:
-
Comparative Toxicogenomics Database
- PDB:
-
Protein Data Bank
- SPR:
-
Surface plasmon resonance
- ITC:
-
Isothermal titration calorimetry
- mPTP:
-
mitochondrial permeability transition pore
References
Peikert, A. & Vaduganathan, M. Sodium glucose Co-Transporter 2 Inhibition following acute myocardial infarction: the DAPA-MI and EMPACT-MI trials. JACC Heart Fail. 12, 949–953. https://doi.org/10.1016/j.jchf.2024.01.019 (2024).
Song, Z. et al. Identification of five hub genes based on Single-Cell RNA sequencing data and network Pharmacology in patients with acute myocardial infarction. Front. Public. Health. 10, 894129. https://doi.org/10.3389/fpubh.2022.894129 (2022).
Galli, M. et al. Coronary microvascular obstruction and dysfunction in patients with acute myocardial infarction. Nat. Rev. Cardiol. 21, 283–298. https://doi.org/10.1038/s41569-023-00953-4 (2024).
Moreno, P. R. & Fuster, V. J. A. C. C. Focus seminar on mechanical complications of acute myocardial infarction. J. Am. Coll. Cardiol. 83, 1775–1778. https://doi.org/10.1016/j.jacc.2024.03.414 (2024).
Marfella, R. et al. GLP-1 receptor agonists-SGLT-2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: an observational study in patients with type 2 diabetes. Cardiovasc. Diabetol. 23, 10. https://doi.org/10.1186/s12933-023-02118-6 (2024).
De Luca, L. et al. Impact of age, gender and heart failure on mortality trends after acute myocardial infarction in Italy. Int. J. Cardiol. 348, 147–151. https://doi.org/10.1016/j.ijcard.2021.12.023 (2022).
Chamié, D. & Pfau, S. Complete revascularization in acute myocardial infarction: the clock is ticking. Circ. Cardiovasc. Interv. 17, e014284. https://doi.org/10.1161/circinterventions.124.014284 (2024).
Akhtar, K. H. et al. The spectrum of post-myocardial infarction care: from acute ischemia to heart failure. Prog Cardiovasc. Dis. 82, 15–25. https://doi.org/10.1016/j.pcad.2024.01.017 (2024).
Barrère-Lemaire, S. et al. Mesenchymal stromal cells for improvement of cardiac function following acute myocardial infarction: a matter of timing. Physiol. Rev. 104, 659–725. https://doi.org/10.1152/physrev.00009.2023 (2024).
Welt, F. G. P. et al. Reperfusion injury in patients with acute myocardial infarction: JACC scientific statement. J. Am. Coll. Cardiol. 83, 2196–2213. https://doi.org/10.1016/j.jacc.2024.02.056 (2024).
Guo, Y. et al. 5mC modification patterns provide novel direction for early acute myocardial infarction detection and personalized therapy. Front. Cardiovasc. Med. 9, 1053697. https://doi.org/10.3389/fcvm.2022.1053697 (2022).
Hu, C., Zhang, X., Teng, T., Ma, Z. G. & Tang, Q. Z. Cellular senescence in cardiovascular diseases: A systematic review. Aging Dis. 13, 103–128. https://doi.org/10.14336/ad.2021.0927 (2022).
de Magalhães, J. P. Cellular senescence in normal physiology. Science 384, 1300–1301. https://doi.org/10.1126/science.adj7050 (2024).
Geiger, H., de Haan, G. & Florian, M. C. The ageing Haematopoietic stem cell compartment. Nat. Rev. Immunol. 13, 376–389. https://doi.org/10.1038/nri3433 (2013).
Goriki, Y. et al. Simple risk-score model for in-hospital major bleeding based on multiple blood variables in patients with acute myocardial infarction. Int. J. Cardiol. 346, 1–7. https://doi.org/10.1016/j.ijcard.2021.11.046 (2022).
Yaribeygi, H., Maleki, M., Butler, A. E., Jamialahmadi, T. & Sahebkar, A. Sodium-glucose cotransporter 2 inhibitors and mitochondrial functions: state of the Art. Excli J. 22, 53–66. https://doi.org/10.17179/excli2022-5482 (2023).
Cui, M. et al. HKDC1, a target of TFEB, is essential to maintain both mitochondrial and lysosomal homeostasis, preventing cellular senescence. Proc. Natl. Acad. Sci. U S A. 121, e2306454120. https://doi.org/10.1073/pnas.2306454120 (2024).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of Haematopoietic stem cells. Nature 431, 997–1002. https://doi.org/10.1038/nature02989 (2004).
Zhang, Q. et al. Circulating mitochondrial damps cause inflammatory responses to injury. Nature 464, 104–107. https://doi.org/10.1038/nature08780 (2010).
Lee, C. et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell. Metab. 21, 443–454. https://doi.org/10.1016/j.cmet.2015.02.009 (2015).
Chen, Y. et al. Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ. Res. 125, 1087–1102. https://doi.org/10.1161/circresaha.119.315833 (2019).
Camacho-Encina, M. et al. Cellular senescence, mitochondrial dysfunction, and their link to cardiovascular disease. Cells 13 https://doi.org/10.3390/cells13040353 (2024).
Yan, Y. et al. Circulating long noncoding RNA UCA1 as a novel biomarker of acute myocardial infarction. Biomed. Res. Int. 2016 (8079372). https://doi.org/10.1155/2016/8079372 (2016).
Miwa, S., Kashyap, S., Chini, E. & von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Invest. 132 https://doi.org/10.1172/jci158447 (2022).
Xiang, J., Shen, J., Zhang, L. & Tang, B. Identification and validation of senescence-related genes in Circulating endothelial cells of patients with acute myocardial infarction. Front. Cardiovasc. Med. 9, 1057985. https://doi.org/10.3389/fcvm.2022.1057985 (2022).
Anzai, A., Ko, S. & Fukuda, K. Immune and inflammatory networks in myocardial infarction: current research and its potential implications for the clinic. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23095214 (2022).
Chen, W. et al. The potential role and mechanism of circrna/mirna axis in cholesterol synthesis. Int. J. Biol. Sci. 19, 2879–2896. https://doi.org/10.7150/ijbs.84994 (2023).
Fang, C. Y. et al. Idi1 and Hmgcs2 are affected by stretch in HL-1 atrial myocytes. Int. J. Mol. Sci. 19 https://doi.org/10.3390/ijms19124094 (2018).
Zhang, Y. et al. SULT2B1b inhibits reverse cholesterol transport and promotes cholesterol accumulation and inflammation in lymphocytes from AMI patients with low LDL-C levels. Clin. Sci. (Lond). 134, 273–287. https://doi.org/10.1042/cs20190459 (2020).
Ouyang, J., Wang, H. & Huang, J. The role of lactate in cardiovascular diseases. Cell. Commun. Signal. 21, 317. https://doi.org/10.1186/s12964-023-01350-7 (2023).
Xu, J. et al. A transcriptomic analysis of dental pulp stem cell senescence in vitro. Biomed. Eng. Online. 23, 102. https://doi.org/10.1186/s12938-024-01298-w (2024).
Phillips, M. A., D’Auria, J. C., Gershenzon, J. & Pichersky, E. The Arabidopsis thaliana type I isopentenyl diphosphate isomerases are targeted to multiple subcellular compartments and have overlapping functions in isoprenoid biosynthesis. Plant. Cell. 20, 677–696. https://doi.org/10.1105/tpc.107.053926 (2008).
Guo, Y. et al. Cannabidiol protects against acute aortic dissection by inhibiting macrophage infiltration and PMAIP1-induced vascular smooth muscle cell apoptosis. J. Mol. Cell. Cardiol. 189, 38–51. https://doi.org/10.1016/j.yjmcc.2024.02.006 (2024).
Datta Chaudhuri, R., Banik, A., Mandal, B. & Sarkar, S. Cardiac-specific overexpression of HIF-1α during acute myocardial infarction ameliorates cardiomyocyte apoptosis via differential regulation of hypoxia-inducible pro-apoptotic and anti-oxidative genes. Biochem. Biophys. Res. Commun. 537, 100–108. https://doi.org/10.1016/j.bbrc.2020.12.084 (2021).
Liao, B. et al. LncRNA Kcnq1ot1 renders cardiomyocytes apoptosis in acute myocardial infarction model by up-regulating Tead1. Life Sci. 256, 117811. https://doi.org/10.1016/j.lfs.2020.117811 (2020).
Liu, G. et al. Identification of mitochondria-related gene biomarkers associated with immune infiltration in acute myocardial infarction. iScience 27, 110275. https://doi.org/10.1016/j.isci.2024.110275 (2024).
Booth, L. K. et al. Heart disease and ageing: the roles of senescence, mitochondria, and telomerase in cardiovascular disease. Subcell. Biochem. 103, 45–78. https://doi.org/10.1007/978-3-031-26576-1_4 (2023).
Frangogiannis, N. G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 11, 255–265. https://doi.org/10.1038/nrcardio.2014.28 (2014).
Hausenloy, D. J. & Yellon, D. M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100. https://doi.org/10.1172/jci62874 (2013).
Sivaraman, V., Pickard, J. M. & Hausenloy, D. J. Remote ischaemic conditioning: cardiac protection from Afar. Anaesthesia 70, 732–748. https://doi.org/10.1111/anae.12973 (2015).
Hong, Y. X. et al. Cardiac senescence is alleviated by the natural flavone Acacetin via enhancing mitophagy. Aging (Albany NY). 13, 16381–16403. https://doi.org/10.18632/aging.203163 (2021).
Walaszczyk, A. et al. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell. 18, e12945. https://doi.org/10.1111/acel.12945 (2019).
Prabhu, S. D. & Frangogiannis, N. G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112. https://doi.org/10.1161/circresaha.116.303577 (2016).
Vona, R., Iessi, E. & Matarrese, P. Role of cholesterol and lipid rafts in Cancer signaling: A promising therapeutic opportunity?? Front. Cell. Dev. Biol. 9, 622908. https://doi.org/10.3389/fcell.2021.622908 (2021).
Rahman, F. A. et al. Efficacy and safety of cyclosporine in acute myocardial infarction: A systematic review and Meta-Analysis. Front. Pharmacol. 9, 238. https://doi.org/10.3389/fphar.2018.00238 (2018).
Monassier, L., Ayme-Dietrich, E., Aubertin-Kirch, G. & Pathak, A. Targeting myocardial reperfusion injuries with cyclosporine in the CIRCUS Trial - pharmacological reasons for failure. Fundam Clin. Pharmacol. 30, 191–193. https://doi.org/10.1111/fcp.12177 (2016).
Kholmukhamedov, A. et al. Cyclosporin A in left ventricular remodeling after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 306, H53–59. https://doi.org/10.1152/ajpheart.00079.2013 (2014).
Liang, D. et al. Mechanism of Artemisia annua L. in the treatment of acute myocardial infarction: network pharmacology, molecular Docking and in vivo validation. Mol. Divers. https://doi.org/10.1007/s11030-023-10750-3 (2023).
Liu, Y., Mao, S., Luo, X., Wang, Y. & Circulating miR-1/UCA1 is a novel biomarker for the diagnosis and prognosis of acute myocardial infarction. Int. J. Cardiol. 310, 137. https://doi.org/10.1016/j.ijcard.2020.01.005 (2020).
Zhang, Z. et al. Long non-coding RNA UCA1 relieves cardiomyocytes H9c2 injury aroused by oxygen-glucose deprivation via declining miR-122. Artif. Cells Nanomed. Biotechnol. 47, 3492–3499. https://doi.org/10.1080/21691401.2019.1652630 (2019).
Chen, B. et al. Comprehensive analysis of mitochondrial dysfunction and necroptosis in intracranial aneurysms from the perspective of predictive, preventative, and personalized medicine. Apoptosis 28, 1452–1468. https://doi.org/10.1007/s10495-023-01865-x (2023).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47. https://doi.org/10.1093/nar/gkv007 (2015).
Gustavsson, E. K., Zhang, D., Reynolds, R. H., Garcia-Ruiz, S. & Ryten, M. ggtranscript: an R package for the visualization and interpretation of transcript isoforms using ggplot2. Bioinformatics 38, 3844–3846 (2022). https://doi.org/10.1093/bioinformatics/btac409
Gu, Z. & Hübschmann, D. Make interactive complex heatmaps in R. Bioinformatics 38, 1460–1462. https://doi.org/10.1093/bioinformatics/btab806 (2022).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7. https://doi.org/10.1186/1471-2105-14-7 (2013).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 9, 559. https://doi.org/10.1186/1471-2105-9-559 (2008).
Chen, H. & Boutros, P. C. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 12, 35. https://doi.org/10.1186/1471-2105-12-35 (2011).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–d677. https://doi.org/10.1093/nar/gkae909 (2025).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Ogata, H. et al. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 27, 29–34. https://doi.org/10.1093/nar/27.1.29 (1999).
Wu, T. et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. (Camb). 2, 100141. https://doi.org/10.1016/j.xinn.2021.100141 (2021).
Wang, L. et al. Cuproptosis related genes associated with Jab1 shapes tumor microenvironment and Pharmacological profile in nasopharyngeal carcinoma. Front. Immunol. 13, 989286. https://doi.org/10.3389/fimmu.2022.989286 (2022).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. https://doi.org/10.1101/gr.1239303 (2003).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Zhang, Z., Zhao, Y., Canes, A., Steinberg, D. & Lyashevska, O. Predictive analytics with gradient boosting in clinical medicine. Ann. Transl Med. 7, 152. https://doi.org/10.21037/atm.2019.03.29 (2019).
Robin, X. et al. pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinform. 12, 77. https://doi.org/10.1186/1471-2105-12-77 (2011).
Sachs, M. C. & plotROC A tool for plotting ROC curves. J. Stat. Softw. 79 https://doi.org/10.18637/jss.v079.c02 (2017).
Lele, P. S. a. S. R. Revisiting resource selection probability functions and single-visit methods: clarification and extensions. Methodsin Ecol. Evolution, 7, 196–205 (2016).
Correction to Lancet Psych. ; 9: 938. Lancet Psychiatry 10, e10 (2023). (2022). https://doi.org/10.1016/s2215-0366(22)00411-4
Charoentong, P. et al. Pan-cancer Immunogenomic analyses reveal Genotype-Immunophenotype relationships and predictors of response to checkpoint Blockade. Cell. Rep. 18, 248–262. https://doi.org/10.1016/j.celrep.2016.12.019 (2017).
Seeliger, D. & de Groot, B. L. Ligand Docking and binding site analysis with PyMOL and autodock/vina. J. Comput. Aided Mol. Des. 24, 417–422. https://doi.org/10.1007/s10822-010-9352-6 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Funding
This study was supported by Sanming Project of Medicine in Shenzhen, No. SZSM20221107 and Science, Technology & Innovation Project of Xiongan New Area, No. 2023XAGG0070.
Author information
Authors and Affiliations
Contributions
Dehong Chen, Hanlin Ding, Lingling Yao, and Zhengyuan Xia : wrote the main manuscript text. Ke Mo, Zhiheng Liu and Linan Li: prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and informed consent
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Xiangyang City Central Hospital (NO: 2024 − 178). All the patients signed an informed consent form.
Consent to participate
All participants signed an informed written consent form to participate in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, D., Yao, L., Mo, K. et al. Identification of key genes associated with cellular aging and mitochondria in acute myocardial infarction. Sci Rep 15, 21384 (2025). https://doi.org/10.1038/s41598-025-05849-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05849-6