Abstract
We investigated the association between glycemic status and pancreatic cancer risk in individuals with nonalcoholic fatty liver disease (NAFLD). This study included 1,093,832 individuals with NAFLD who underwent the Korean national health screening in 2009. NAFLD was defined as fatty liver index ≥ 30 after excluding heavy alcohol use and viral hepatitis. Multivariable Cox proportional hazards regression assessed the risk of pancreatic cancer according to glycemic status (normoglycemia, impaired fasting glucose [IFG], and diabetes mellitus [DM]). During a median follow-up of 10.3 years, 4124 (0.38%) developed pancreatic cancer. Compared to normoglycemic controls, the risk of pancreatic cancer was significantly higher in those with IFG (adjusted hazard ratio [aHR] 1.16; 95% confidence interval [CI] 1.08–1.25) and DM (aHR 1.48; 95% CI 1.37–1.60). The increased risk of pancreatic cancer with advanced hyperglycemia was consistent across subgroups, including obesity, smoking, and alcohol use. People without regular exercise showed a stronger association between hyperglycemia and pancreatic cancer compared to regular exercisers. In conclusion, hyperglycemia was associated with a higher risk of incident pancreatic cancer among people with NAFLD, independent of obesity and health behaviors. This suggests that hyperglycemia, even in IFG status, is an important modifiable risk factor for pancreatic cancer in NAFLD.
Similar content being viewed by others
Background
Despite advances in early diagnosis and treatment, pancreatic cancer remains a deadly disease1. It is the sixth most common cause of cancer death in men and women combined, with pancreatic cancer mortality accounting for nearly 5% of all cancer deaths worldwide1. The global annual incidence of pancreatic cancer has increased 2.3-fold over the past 25 years and is expected to continue to rise as the world’s population ages and the prevalence of environmental risk factors increases2. The lethality and healthcare burden of pancreatic cancer make prevention and early detection imperative.
Nonalcoholic fatty liver disease (NAFLD) is the most dominant cause of chronic liver disease, ranging from simple liver steatosis to fibrosis and cirrhosis3. The prevalence of NAFLD is estimated to be 32% in the general population worldwide and 30.3% in South Korea3,4. As the link between NAFLD and various complications is studied, evidence of heightened risks for extrahepatic malignancies, including pancreatic cancer, has emerged5,6. Over a median follow-up of 13.8 years, Swedish individuals with histologically confirmed NAFLD had a 2.2-fold increased risk of new-onset pancreatic cancer compared to controls without NAFLD5. Moreover, NAFLD serves as a significant prognostic marker for poor survival in people with pancreatic cancer6, which may be explained by dysregulated cytokine profiles in NAFLD—such as reduced adiponectin and altered IGF-1 signaling—that contribute to enhanced inflammation and tumor progression7,8,9.
Although modifiable risk factors for pancreatic cancer are poorly understood, hyperglycemia is recognized as widespread contributor10. Furthermore, diabetes mellitus (DM) is highly correlated with NAFLD, with both conditions sharing a common pivotal pathophysiology of insulin resistance11. Our analysis of the population with NAFLD found that 26.5% of those who developed pancreatic cancer had DM at baseline, which was significantly higher than the 14.1% of those without pancreatic cancer (Table 1; P-value < 0.001). Given the close relationship of hyperglycemia with both pancreatic cancer and NAFLD, which is supported by our preliminary findings, this longitudinal cohort study aimed to examine the association of glycemic status with incident pancreatic cancer in a population-based cohort of Korean people with NAFLD.
Methods
Data source
Data for this study were obtained from the Korean National Health Insurance Service (NHIS), a nationwide government-administered health insurance system covering 97% of the total population. The NHIS database contains sociodemographic, anthropometric, laboratory, and claims data, including diagnoses, medical procedures, and prescriptions. Previous studies have demonstrated the robustness of the NHIS dataset with comprehensive information and minimal attrition12. This study was approved by the Institutional Review Board (IRB) of Soongsil University (IRB No. SSU-202007-HR-236-01). The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Because the NHIS database was anonymized by strict confidentiality protocols, the requirement for written informed consent was waived by the IRB of Soongsil University.
Study population
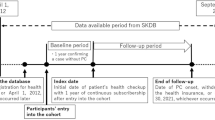
A total of 4,234,412 adults aged ≥ 20 years who underwent a health examination in 2009 were identified (Fig. 1). We excluded individuals with heavy alcohol use (n = 354,376) and viral hepatitis (n = 404,588) based on the definition of NAFLD13. Hepatitis was defined using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes B15–B1914. To reduce the influence of pre-existing advanced liver disease, participants with cirrhosis (n = 14,835) or hepatocellular carcinoma (n = 679) at baseline, as defined by ICD-10-CM codes K70.3 and C22, respectively, were excluded14. Participants diagnosed with pancreatic cancer before or within 1 year of cohort entry were excluded to minimize concerns about reverse causation (n = 57,295)14. After excluding those with missing variables (n = 177,904) and a fatty liver index (FLI) of < 30 (n = 2,130,903), 1,093,832 participants with NAFLD were included in the final cohort. Participants were stratified into groups by glycemic status, which included normoglycemia, impaired fasting glucose (IFG), and DM. They were followed up until the date of the incident pancreatic cancer, death, or December 31, 2020, whichever came first.
Data collection
Health behavior and sociodemographic information were collected using standardized, self-administered questionnaires. Smoking status was classified as never, former, or current smoker. The study determined the average daily alcohol intake by multiplying the frequency of drinking per week by the amount of alcohol consumed each time. None, light-to-moderate, and heavy drinking were defined as consuming 0 g, < 30 g, and ≥ 30 g of alcohol per day for men and 0 g, < 20 g, and ≥ 20 g per day for women, respectively14. Regular exercise was defined as engaging in at least 20 min of vigorous-intensity exercise for at least three days a week or at least 30 min of moderate-intensity exercise for at least five days a week14. Low income was defined as the lowest quartile of insurance fee requirements or eligibility for free healthcare.
A qualified health professional recorded the height, weight, and waist circumference (WC) of the participants while wearing light clothing. The WC was obtained at the midpoint between the lower ribs and the iliac crest in a standing position. Body mass index (BMI) was calculated by dividing the person’s weight in kilograms by the square of their height in meters. Blood pressure was measured while the participants were seated after resting for a minimum of 5 min. Blood glucose, creatinine, lipid, and γ-glutamyl transferase (GGT) levels were measured after overnight fasting. The Modification of Diet in Renal Disease study equation was used to calculate the estimated glomerular filtration rate (eGFR).
Definition of NAFLD
International guidelines from the European Association for the Study of the Liver recommend well-studied non-invasive biomarkers as the preferred alternative for evaluating liver steatosis in large-scale epidemiologic studies, among which the FLI is one of the best-established markers15,16. The performance of the FLI in detecting NAFLD has been externally validated with liver ultrasonography in the general population in Korea and with gold standard liver biopsy17,18,19. We used the FLI to diagnose NAFLD because imaging tools or liver biopsies were not available for the general population in this nationwide cohort. The calculation of the FLI is expressed as follows, resulting in values ranging from 0 to 100: (e0.953*Ln (triglycerides) + 0.139 × BMI + 0.718 × Ln (GGT) + 0.053 × waist circumference ± 15.745) / (1 + e0.953 × Ln (triglycerides) + 0.139 × BMI + 0.718 × Ln (GGT) + 0.053 × waist circumference ± 15.745) x 10020. The FLI cut-off for defining NAFLD was set at ≥ 30, which has been validated as the ideal threshold for identifying sonographically diagnosed NAFLD in the Korean population17.
Definitions of pancreatic cancer and other disorders
The primary outcome was newly diagnosed pancreatic cancer, based on the ICD-10-CM code (C25) for inpatient treatment and the reimbursement code from the National Cancer Registry (V193). All Korean individuals with physician-confirmed cancer are enrolled in a cancer registry administered by the NHIS to receive reimbursement for cancer-related medical services and procedures. Fasting glucose levels of < 100 and 100–125 mg/dL were defined as normoglycemia and IFG, respectively. DM was defined by either a fasting glucose level ≥ 126 mg/dL or a prescription of antidiabetic medication with ICD-10-CM codes E10–E14. Hypertension was defined based on systolic/diastolic blood pressure ≥ 140/90 mmHg or prescription of antihypertensive medication with ICD-10-CM codes I10–I13 and I15. Dyslipidemia was defined based on total cholesterol levels ≥ 240 mg/dL or the prescription of lipid-lowering medications with ICD-10-CM codes E78. Chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73 m2. Pancreatitis was defined using ICD-10-CM codes K85, K86.0, and K86.1, and cholangitis by K83.01 and K83.09. Obesity was defined according to the World Health Organization Asia-Pacific criteria as a BMI ≥ 25 kg/m[221. Abdominal obesity was defined by WC as ≥ 90 cm for men and ≥ 85 cm for women based on the Korean Society for the Study of Obesity guidelines22.
Statistical analysis
Baseline characteristics of participants were compared by development of pancreatic cancer during follow-up using an independent t-test for continuous variables or the chi-squared test for categorical variables. The incidence rates of pancreatic cancer were determined by dividing the number of new cases by the total number of person-years followed, and the results were expressed as incidence per 10,000 person-years. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the development of pancreatic cancer were calculated for the IFG and DM groups relative to the normoglycemic group using multivariable Cox proportional hazards regression models. Three adjustment models were used to assess the risk of pancreatic cancer: model 1 was an unadjusted model, model 2 was adjusted for age and sex, and model 3 was additionally adjusted for BMI, smoking status, alcohol consumption, regular exercise, income status, and presence of pancreatitis and cholangitis. As a sensitivity analysis, we also examined the association using a FLI cutoff of ≥ 60. Kaplan-Meier curves were used to plot the cumulative incidence probability of incident pancreatic cancer, and log-rank tests were used to compare the differences between the curves of the groups with each glycemic status. In addition, we estimated the risk of pancreatic cancer according to fasting plasma glucose categories (< 100, 100–125, 126–149, 150–179, and ≥ 180 mg/dL). Subgroup analyses for pancreatic cancer by glycemic status were performed after stratification for age (< 65 and ≥ 65 years), sex, obesity, abdominal obesity, hypertension, dyslipidemia, smoking, alcohol consumption (none and light-to-moderate), and regular exercise with interaction testing using the likelihood ratio test. Statistical significance was set at a two-sided P-value of < 0.05. All statistical analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of participants
Baseline characteristics of study participants according to the development of pancreatic cancer are shown in Table 1. After a median follow-up of 10.3 years (interquartile range 10.1–10.6), 4124 individuals developed pancreatic cancer. The mean age and proportion of men among the study participants were 49.2 years (standard deviation, 13.4) and 73.2%, respectively. Compared to individuals who did not develop pancreatic cancer, those with incident pancreatic cancer were older, more likely to be women, and had healthier lifestyles, including a higher proportion of non-smokers, non-drinkers, and regular exercisers, and a higher proportion of low-income individuals. They had lower BMI but higher WC, blood pressure, and fasting glucose, as well as lower low-density lipoprotein and total cholesterol, triglycerides, and eGFR. The prevalence of DM, hypertension, dyslipidemia, CKD, pancreatitis, and cholangitis was significantly higher in individuals who developed pancreatic cancer than in those who did not.
Association between glycemic status and pancreatic cancer
Newly diagnosed pancreatic cancer occurred in 1769 (0.28%), 1261 (0.40%), and 1094 (0.71%) individuals with normoglycemia, IFG, and DM, respectively (Table 2). The risk of incident pancreatic cancer was significantly higher in the IFG group than the reference risk in the normoglycemic group after adjusting for confounding variables (model 3: adjusted HR [aHR], 1.16; 95% CI, 1.08–1.25). Furthermore, individuals with DM exhibited a 1.5-fold and 1.3-fold increased likelihood of developing pancreatic cancer compared to those with normoglycemia (model 3: aHR, 1.48; 95% CI, 1.37–1.60) and IFG (model 3: aHR, 1.25; 95% CI, 1.15–1.35), respectively. In a sensitivity analysis using a FLI cutoff of ≥ 60, individuals with DM also showed a significantly higher risk compared to those with normoglycemia and IFG (Table 3). Similarly, the cumulative incidence of pancreatic cancer during follow-up was significantly higher in individuals with worse glycemic status (log-rank P < .001) (Fig. 2). A clear stepwise increase in pancreatic cancer risk was also observed with higher fasting glucose levels (Fig. 3). Compared to individuals with normal fasting glucose (< 100 mg/dL), those with fasting glucose levels ≥ 180 mg/dL had a 1.5-fold higher risk (model 3: aHR, 1.53; 95% CI, 1.31–1.79).
Subgroup analyses
To investigate whether the significant association between glycemic status and pancreatic cancer remained independent of various modifiable and non-modifiable factors, we performed subgroup analyses (Fig. 4). All subgroups consistently showed a progressively higher risk of pancreatic cancer in individuals with normoglycemia, IFG, and DM in a fully adjusted model. Increased risk of pancreatic cancer in people with DM compared to the normoglycemic group was more pronounced in individuals who did not exercise regularly (aHR, 1.56; 95% CI, 1.43–1.70) than in those who exercised regularly (aHR, 1.20; 95% CI, 1.01–1.42) (P for interaction = 0.02). No significant differences were observed in the association between the glycemic status and pancreatic cancer across other subgroups, including age, sex, comorbidities including obesity and abdominal obesity, smoking, and alcohol consumption.
Discussion
This population-based cohort study of people with NAFLD showed that, after a median follow-up of 10.3 years, both DM and IFG were significantly associated with an increased risk of developing pancreatic cancer, after adjusting for potential confounders including age, sex, BMI, health behaviors, and history of pancreatitis. This association remained robust when applying stricter definitions of hepatic steatosis, and a gradual increase in pancreatic cancer risk was observed with higher fasting glucose levels. Subgroup analyses consistently revealed a positive association between hyperglycemia and the risk of pancreatic cancer, independent of obesity and other metabolic risk factors. These findings highlight hyperglycemia as a significant modifiable risk factor for pancreatic cancer in populations with NAFLD, given the substantial prevalence of IFG and DM (43.2%) observed in our study.
Previous research has shown an increase in the incidence of pancreatic cancer with hyperglycemia in the general population. A meta-analysis of nine prospective studies on the association between fasting blood glucose and incident pancreatic cancer found a 14% increased risk of pancreatic cancer for every 10 mg/dL increase in glucose levels10. Meanwhile, a recent systematic review of cohort studies involving more than 10 million participants revealed a 1.3-fold higher risk of developing pancreatic cancer in people with NAFLD than in the general population23. With accumulating evidence supporting the role of hyperglycemia and NAFLD in the development of pancreatic cancer10,23,24, determining the risk of pancreatic cancer in people with both hyperglycemia and NAFLD is crucial. To the best of our knowledge, this study is novel to analyze the effect of glycemic status on pancreatic cancer only in people with NAFLD. In addition to the previously reported 17% increase in the risk of pancreatic cancer due to NAFLD in the Korean populations24, we demonstrated an additive risk of 48% in those with concurrent DM. Notably, this study suggests the possibility of a significant association between hyperglycemia and pancreatic cancer in the glycemic range of prediabetes.
Several potential mechanistic explanations underlie the development of pancreatic cancer in individuals with DM and NAFLD. Insulin resistance, a key pathogenesis of DM and NAFLD, is followed by compensatory hyperinsulinemia, which may activate pancreatic carcinogenesis through the augmented translation of digestive enzymes, induction of local inflammation, and formation of acinar-to-ductal metaplasia25. Chronic activation of inflammatory pathways in coexisting DM and NAFLD may also trigger tumor-promoting effects in the pancreas26,27. Nuclear factor-κB induces the transcription of proliferative, anti-apoptotic, and pro-inflammatory genes, leading to injury and fibrotic changes in pancreatic acinar cells28. Continuous activation of c-Jun N-terminal kinase drives pancreatic cancer cells toward invasion, metastasis, and survival29. Inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and transforming growth factor-β have also been implicated in pancreatic cell tumorigenesis30. Finally, gut dysbiosis associated with hepatic steatosis may contribute to the development of pancreatic cancer by promoting inflammation, immune suppression, and oncogenic microenvironment and metabolism31,32. Microbial disturbances in NAFLD may be exacerbated in DM, as hyperglycemia is associated with treatment-resistant pancreatic cancer due to the enrichment and alteration of bacterial colonization33.
Although NAFLD has classically been attributed to excessive weight gain, recognition of non-obese individuals with NAFLD has recently gained attention. A meta-analysis reported a remarkable global prevalence of non-obese NAFLD of 12.1% in the general population and 40.8% in the NAFLD population34. In our study, it is noteworthy that the association between hyperglycemia and the risk of developing pancreatic cancer did not differ between individuals with or without obesity or abdominal obesity. This finding underscores the need for vigilance in the development of pancreatic cancer in NAFLD populations with not only the typical obese phenotype, but also apparently normal weight. Meanwhile, subgroup analyses showed that the hyperglycemia-associated risk of pancreatic cancer was attenuated by regular exercise. Systematic review of observational studies of the effect of physical activity on pancreatic cancer found a 28% reduced risk of incident pancreatic cancer in physically active individuals35. Given the beneficial role of physical activity in improving insulin sensitivity and thus plausibly inhibiting pancreatic carcinogenesis25,35, regular exercise may prevent the development of pancreatic cancer in those with advanced hyperglycemia at baseline.
One of the strengths of our study was the use of a nationwide cohort that included the entire Korean population with a highly preserved follow-up period. Comprehensive medical data were collected from more than one million participants who were exclusively people with NAFLD. Our findings demonstrated a sustained and significant association between glycemic status and the incidence of pancreatic cancer over a 10-year cumulative follow-up period. To address potential confounding factors, we adjusted for various anthropometric, lifestyle, and medical parameters. Furthermore, we used reliable criteria for defining pancreatic cancer based on the National Cancer Registry, which is confirmed by an oncology expert and managed by the NHIS for financial support.
This study had several limitations. Firstly, due to its retrospective nature, establishing a causal relationship between hyperglycemia and pancreatic cancer was not possible. Although we excluded people who developed new pancreatic cancer within one year of enrollment, the possibility of reverse causation exists. Secondly, NAFLD diagnosis relied on the FLI without evaluation through imaging modalities or liver biopsy. However, performing expensive or invasive tests to identify NAFLD in all asymptomatic individuals undergoing routine health screening is impractical. The FLI is well suited for defining NAFLD on a population basis given its established role as a highly cost-effective and accessible biomarker in epidemiologic research15,16. Thirdly, the operational definition of NAFLD in this study did not completely incorporate exclusion criteria other than alcoholic liver disease and viral hepatitis36. Furthermore, we applied the terminology of NAFLD rather than adopting the recently introduced term metabolic dysfunction-associated steatotic liver disease37. However, the discrepancy between NAFLD and MASLD appears to be minimal, as only 2.3% of community-based and 0.2% of hospital-based NAFLD cases failed to meet MASLD criteria38. In addition, the NAFLD definition employed in our study has been widely accepted in recent high-quality studies based on the Korean NHIS database14,39. Another limitation is the unavailability of glycated hemoglobin levels for defining glycemic status, as these data are not included in the NHIS database. Alternatively, the definition of DM used in this study has been validated in previous research using the NHIS database14,40,41. Moreover, we did not assess the impact of changes in glycemic status on pancreatic cancer development, highlighting the need for further investigation into whether improvement in hyperglycemia mitigates the risk of pancreatic cancer in people with NAFLD. Of note, although we adjusted for a wide range of covariates, liver-related laboratory measurements, particularly alanine aminotransferase and aspartate aminotransferase, were not included in the analysis. The absence of these variables may introduce potential residual confounding related to liver disease severity. Finally, the generalizability of our findings to populations beyond the Korean demographic warrants confirmation. Nevertheless, the large and nationally representative cohort in our study provides a basis for generalizing these results to people with NAFLD in other countries.
Conclusions
A nationwide cohort study involving over one million individuals with NAFLD revealed a progressive increase in the risk of incident pancreatic cancer over a 10-year follow-up period with IFG and DM than normoglycemia. In the growing population with NAFLD, hyperglycemia could serve as a clinically significant factor in the prevention of pancreatic cancer, a disease with limited modifiable risk factors and high mortality rates. Further exploration into the mechanistic interplay between hepatic steatosis and glycemic control in pancreatic carcinogenesis is warranted.
Data availability
The dataset supporting the conclusions of this article is available in the National Health Insurance Sharing Service repository, [https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do].
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. https://doi.org/10.3322/caac.21834 (2024).
Klein, A. P. Pancreatic cancer: A growing burden. Lancet Gastroenterol. Hepatol. 4, 895–896. https://doi.org/10.1016/s2468-1253(19)30323-1 (2019).
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7, 851–861. https://doi.org/10.1016/s2468-1253(22)00165-0 (2022).
Im, H. J., Ahn, Y. C., Wang, J. H., Lee, M. M. & Son, C. G. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin. Res. Hepatol. Gastroenterol. 45, 101526 (2021).
Simon, T. G. et al. Cancer risk in patients with Biopsy-Confirmed nonalcoholic fatty liver disease: A population-based cohort study. Hepatology 74, 2410–2423. https://doi.org/10.1002/hep.31845 (2021).
Chang, C. F. et al. Exploring the relationship between nonalcoholic fatty liver disease and pancreatic cancer by computed tomographic survey. Intern. Emerg. Med. 13, 191–197. https://doi.org/10.1007/s11739-017-1774-x (2018).
Pitt, H. A. Hepato-pancreato-biliary fat: The good, the bad and the ugly. HPB (Oxf.). 9, 92–97. https://doi.org/10.1080/13651820701286177 (2007).
Sanna, C., Rosso, C., Marietti, M. & Bugianesi, E. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int. J. Mol. Sci. 17 https://doi.org/10.3390/ijms17050717 (2016).
Tomizawa, M. et al. Insulin-like growth factor-I receptor in proliferation and motility of pancreatic cancer. World J. Gastroenterol. 16, 1854–1858. https://doi.org/10.3748/wjg.v16.i15.1854 (2010).
Liao, W. C. et al. Blood glucose concentration and risk of pancreatic cancer: Systematic review and dose-response meta-analysis. Bmj 350, g7371. https://doi.org/10.1136/bmj.g7371 (2015).
Tilg, H., Moschen, A. R. & Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 14, 32–42. https://doi.org/10.1038/nrgastro.2016.147 (2017).
Kim, H. K., Song, S. O., Noh, J., Jeong, I. K. & Lee, B. W. Data configuration and publication trends for the Korean National health insurance and health insurance review & assessment database. Diabetes Metab. J. 44, 671–678. https://doi.org/10.4093/dmj.2020.0207 (2020).
Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346, 1221–1231. https://doi.org/10.1056/NEJMra011775 (2002).
Park, J. H. et al. Increased risk of young-Onset digestive tract cancers among young adults age 20–39 years with nonalcoholic fatty liver disease: A nationwide cohort study. J. Clin. Oncol. 41, 3363–3373. https://doi.org/10.1200/jco.22.01740 (2023).
EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402. https://doi.org/10.1016/j.jhep.2015.11.004 (2016).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209. https://doi.org/10.1016/j.jhep.2020.03.039 (2020).
Cho, E. J. et al. Fatty liver index for predicting nonalcoholic fatty liver disease in an asymptomatic Korean population. Diagnostics (Basel) 11. (2021). https://doi.org/10.3390/diagnostics11122233
Kim, J. H., Kwon, S. Y., Lee, S. W. & Lee, C. H. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int. 31, 1600–1601. https://doi.org/10.1111/j.1478-3231.2011.02580.x (2011).
Fedchuk, L. et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 40, 1209–1222. https://doi.org/10.1111/apt.12963 (2014).
Bedogni, G. et al. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33. https://doi.org/10.1186/1471-230x-6-33 (2006).
Organization, W. H. The Asia-Pacific perspective: redefining obesity and its treatment. (2000).
Kim, K. K. et al. Evaluation and treatment of obesity and its comorbidities: 2022 update of clinical practice guidelines for obesity by the Korean society for the study of obesity. J. Obes. Metab. Syndr. 32, 1–24. https://doi.org/10.7570/jomes23016 (2023).
Zhang, Y., Zhou, B. G., Zhan, J. D. & Du, B. B. Association between metabolic dysfunction-associated steatotic liver disease and risk of incident pancreatic cancer: A systematic review and meta-analysis of cohort studies. Front. Oncol. 14, 1366195. https://doi.org/10.3389/fonc.2024.1366195 (2024).
Park, J. H., Hong, J. Y., Han, K., Kang, W. & Park, J. K. Increased risk of pancreatic cancer in individuals with non-alcoholic fatty liver disease. Sci. Rep. 12, 10681. https://doi.org/10.1038/s41598-022-14856-w (2022).
Zhang, A. M. Y. et al. Hyperinsulinemia acts via acinar insulin receptors to initiate pancreatic cancer by increasing digestive enzyme production and inflammation. Cell. Metab. 35, 2119–2135e2115. https://doi.org/10.1016/j.cmet.2023.10.003 (2023).
Gehrke, N. & Schattenberg, J. M. Metabolic inflammation-A Role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 158, 1929–1947.e (1926). https://doi.org/10.1053/j.gastro.2020.02.020 (2020).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107. https://doi.org/10.1038/nri2925 (2011).
Silke, J. & O’Reilly, L. A. NF-κB and pancreatic cancer; Chapter and verse. Cancers (Basel). 13. https://doi.org/10.3390/cancers13184510 (2021).
Sato, T. et al. c-Jun N-terminal kinase in pancreatic tumor stroma augments tumor development in mice. Cancer Sci. 108, 2156–2165. https://doi.org/10.1111/cas.13382 (2017).
Padoan, A., Plebani, M. & Basso, D. Inflammation and pancreatic cancer: Focus on metabolism, cytokines, and immunity. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20030676 (2019).
Leung, C., Rivera, L., Furness, J. B. & Angus, P. W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425. https://doi.org/10.1038/nrgastro.2016.85 (2016).
Wei, M. Y. et al. The microbiota and Microbiome in pancreatic cancer: More influential than expected. Mol. Cancer. 18, 97. https://doi.org/10.1186/s12943-019-1008-0 (2019).
Kesh, K., Mendez, R., Abdelrahman, L., Banerjee, S. & Banerjee, S. Type 2 diabetes induced Microbiome dysbiosis is associated with therapy resistance in pancreatic adenocarcinoma. Microb. Cell. Fact. 19, 75. https://doi.org/10.1186/s12934-020-01330-3 (2020).
Ye, Q. et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 5, 739–752. https://doi.org/10.1016/s2468-1253(20)30077-7 (2020).
O’Rorke, M. A., Cantwell, M. M., Cardwell, C. R., Mulholland, H. G. & Murray, L. J. Can physical activity modulate pancreatic cancer risk? A systematic review and meta-analysis. Int. J. Cancer. 126, 2957–2968. https://doi.org/10.1002/ijc.24997 (2010).
Hagström, H. et al. Administrative coding in electronic health care Record-Based research of NAFLD: An expert panel consensus statement. Hepatology 74, 474–482. https://doi.org/10.1002/hep.31726 (2021).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 78, 1966–1986. https://doi.org/10.1097/hep.0000000000000520 (2023).
Song, S. J., Lai, J. C., Wong, G. L., Wong, V. W. & Yip, T. C. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 80, e54–e56. https://doi.org/10.1016/j.jhep.2023.07.021 (2024).
Kwon, H. et al. Nonalcoholic fatty liver disease and the risk of thyroid Cancer among young adults in South Korea. J. Clin. Endocrinol. Metab. 109, e1095–e1104. https://doi.org/10.1210/clinem/dgad575 (2024).
Huh, J. H. et al. Remnant cholesterol is an independent predictor of type 2 diabetes: A nationwide population-based cohort study. Diabetes Care. 46, 305–312. https://doi.org/10.2337/dc22-1550 (2023).
Bae, J. H. et al. Diabetes fact sheet in Korea 2021. Diabetes Metab. J. 46, 417–426. https://doi.org/10.4093/dmj.2022.0106 (2022).
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing. We thank the staff of the Big Data Steering Department of the National Health Insurance Service and the participants in this study.
Author information
Authors and Affiliations
Contributions
HNJ interpreted the data and was a major contributor to writing the manuscript. JHH, ER, BJK, ML, JKK, JHK, BH, SJL, and SHI reviewed the research and edited the manuscript. KDH gathered resources and curated and analyzed the data. JGK conceptualized and managed the project and supervised the research. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jung, H.N., Huh, J.H., Roh, E. et al. Risk of pancreatic cancer according to glycemic status in nonalcoholic fatty liver disease: a nationwide cohort study. Sci Rep 15, 23308 (2025). https://doi.org/10.1038/s41598-025-05868-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05868-3