Abstract
Diabetic foot problems pose a substantial global health challenge as a major complication of diabetes mellitus. These issues encompass a spectrum of conditions affecting the feet of individuals with diabetes, including neuropathy (nerve damage), peripheral artery disease (impaired blood circulation), foot ulcers, infections, and, in critical situations, amputation. To mitigate the burden of lower extremity amputations, the development of innovative wound dressings and drug delivery systems is crucial. One such advancement is the “lyophilized wafer formulation,” a recently developed medicated dressing material known for its rapid healing properties and high exudate absorption capacity in chronic wounds. Following successful completion of assessment tests for lymphatic fluid retention, viscosity, in vitro drug release, FTIR, DSC, and XRD, the exudate absorption capacity of calcium alginate was evaluated in the current study. Notably, the wafer formulation composed of 2% calcium alginate, 1% gelatin (bloom 150), and water as the solvent demonstrated excellent performance across exudate management properties, as well as in FTIR, DSC, XRD, and in vitro drug release assays using a dialysis membrane. This optimal formulation achieved a significant drug release of 96.9 ± 0.1% after 24 h. The Linezolid-loaded calcium alginate wafer formulation exhibited promising outcomes concerning antimicrobial properties, exudate handling, and drug release. Furthermore, the animal study indicated the absence of adverse effects, underscoring the formulation’s potential as a safe and effective treatment for diabetic foot ulcers without inducing skin irritation. To fully realize its therapeutic value, comprehensive investigations into its stability profile and detailed pharmacological effects are strongly recommended.
Similar content being viewed by others
Introduction
Diabetic foot ulcers (DFUs) are serious wounds linked to diabetes that can lead to major physical problems like losing a limb. Beyond the physical impact, they also cause significant social, emotional, and economic difficulties for those affected1. A foot ulcer, which manifests as a layer of deteriorated skin on the lower leg or feet, can affect anyone. Foot ulcers can be caused by either type 1 or type 2 diabetes2. DFUs are frequently colonized by methicillin-resistant S. aureus (MRSA) bacteria, such as Enterococcus, Streptococcus group B, Clostridium perfringens, and other harmful organisms3. Anyone infected with MRSA and other germs should avoid developing osteomyelitis, a significant DFU outcome1. Generally, nerve damage may hinder the ability of skin wounds to heal and/or mend themselves when blood sugar levels are high or unstable4. Even after a minor cut, a foot ulcer may form, and the pH of the area will be between 6.0 and 8.05. Records show that people with severe foot ulcers release 5000 mg of exudates per 1000 mm2/24 hours3. Because insufficient amounts of nutrients, blood cells, and oxygen are delivered to the wound site, chronic wounds like diabetic foot ulcers take longer than 84 to 98 days to heal4,6,7.
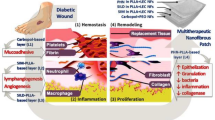
Wounds are broadly classified as either acute or chronic, and while the healing duration differs based on the wound type, both progress through four distinct phases. For chronic injuries4,8, the initial phase, hemostasis, involves the formation of a fibrin network from fibrinogen to limit bleeding and initiate blood clotting, a process that can last from a few minutes to three days6,8,9. Following this, the inflammation phase occurs, during which hemostasis continues, and this stage can extend from one day to two weeks9,10. The subsequent phase, proliferation, is characterized by tissue rebuilding, including angiogenesis, collagen deposition, epithelialization, and wound contraction8,9. The final stage, maturation or remodeling, entails the complete restoration of tissue at the wound site, culminating in scar formation, a process that can span two to twenty-four months8,9. A significant factor in Diabetic Limb Amputation is ischemia, caused by the constriction of blood vessels. In such instances, oral or intravenous antibiotic therapy often proves less effective due to the reduced delivery of oxygen and nutrients to the tissues, ultimately necessitating surgical removal of the leg. Doctors utilize a metric known as the Wagner Grade to classify the severity of wound infections. Specifically, diabetic foot ulcers are categorized using this system, which encompasses six distinct grades, ranging from 0 to 52,611,12,13.
The traditional absorbent pad of lint or non-fibrous materials used in wound dressing is replaced by a revolutionary drug-loaded dressing material termed lyophilised wafer formulation14. By providing a barrier against outside pollutants, the dressing seeks to let air into the wound and eliminate additional moisture5,15,16,17. Pharmaceutically active chemicals can be used to inhibit bacterial growth and promote wound healing. A wafer is created using the freeze-drying technique known as lyophilization. A polymer substrate, a surfactant, and a wound-healing agent can be combined to form a solution that can be used to make a wafer3.
To learn how to use Linezolid in critical situations, a review of the drug’s pharmacological properties and functions was undertaken on patients admitted to critical care. This review study discusses drug interactions, Linezolid patents, comparisons to other antibiotics, and the mechanism of resistance against diseases. There are few treatment options for infections caused by common bacteria in the intensive care unit. According to the researchers, the inclusion of Linezolid in a number of clinical practice guidelines suggests that the medicine can be a beneficial adjunct to the antibiotic toolkit in the fight against MRSA and VRE18. Haemostasis, the process of creating microparticles by the engraftment of lymphatic and microvascular veins, is an inevitable element of this cycle. This can take anywhere from a week to five weeks9,19.
The Linezolid wafer was designed to fight infections in diabetic foot ulcers caused by aerobic bacteria, like Staphylococcus aureus. When this wafer is placed on a wound, it will soak up fluids and turn into a moist gel. This moist environment helps the wound heal. Unlike some other dressings like foam or gauze, this gel form will allow for easy and pain-free removal, which should make it easier for patients to stick to their treatment. Since systemic antibiotic therapy frequently fails, direct wound site delivery is required. This can be accomplished with a wafer, and the drug can be released in a regulated manner. Potential advantages of wafer formulation include a lower frequency of dosing and the ability to stop the treatment by just taking the wafer out of the wound. Self-administration is feasible. Systemic adverse effects are less likely to occur because the medication will be applied topically. Furthermore, the use of biodegradable polymers aims to enhance wound healing and provide a continuous release of medication.
Materials and methods
Calcium Alginate [CAS No.: 9005-35-0] was sourced from Shidong fan tai, while Linezolid [CAS No.: 165800-03-3] was obtained from Mylan Pharmaceuticals. The remaining materials, including Gelatin 150 bloom [CAS No.: 9000-70-8] from Nitta Gelatin, Sodium Chloride [CAS No.: 7647-14-5], Sodium carbonate [CAS No.: 497-19-8], Ethanol [CAS No.: 64-17-5], and Tween-80 [CAS No.: 9005-65-6], were procured from Chemdyes Corporation. The Gram-positive bacterium, Methicillin-Resistant Staphylococcus aureus, was obtained from the Microbiology Lab at Parul Institute of Applied Science.
Preparation of gelatin solution
First, to make a 1% gelatin solution, gelatin was mixed into 70% of the total amount of Milli-Q water needed for the whole process and heated to 50 °C until it dissolved. Then, calcium alginate was slowly added to this gelatin solution to create a 2% calcium alginate and gelatin mixture (CA-Gelatin Solution), and the temperature was allowed to cool down to room temperature. In a different container, the active pharmaceutical ingredient (API) was mixed with 10% of the total Milli-Q water, and then more water was added to bring the total volume up to 100%. After this, the API solution was added to the CA-Gelatin Solution and thoroughly mixed. This gel mixture was then poured into 6-well culture plates. Finally, these plates were placed in a lyophilizer, a machine that freeze-dries materials, for 48 h to create the final product6.
Method of solution stability study
The stability of the Linezolid drug in solution was assessed using three different phosphate buffer solutions with pH values of 6.8, 7.0, and 7.4. The drug was dissolved in each of these buffer solutions and then stored at a temperature of 40 °C for 24 h. Following this period, the concentration (Assay) of the drug remaining in each solution was determined using High-Performance Liquid Chromatography (HPLC) at a wavelength of 251 nm to measure the absorbance18,20,21,22.
Method of preparation of linezolid loaded wafers of batches L1–L9
Wafer formulations containing Linezolid were prepared using different concentrations of gelatin. The polymer (CA) weight was maintained at a constant 400 mg, while the gelatin content was altered to 200 mg, 500 mg, and 900 mg. Table S1 displayed the precisely weighed quantity of Linezolid, 100 mg, as per an IV dosage. Using a magnetic stirrer, the drug solution was continuously introduced and stirred into the polymeric gel solution. To achieve a uniform gel, the mixture was subsequently poured into a 6-well culture plate and left to subside for 10 min at room temperature. After that, the plates were lyophilized for 48 h to form wafers. Finally, the prepared wafers were removed from the Petri plate, packed in an aluminum pouch, and then stored in desiccators for further evaluation studies.
Fourier transforms infrared spectroscopy (FTIR)
Fourier Transform Infrared (FTIR) spectroscopy, utilizing KBr pellets, was employed to identify the Active Pharmaceutical Ingredient (API) and excipients, as well as to investigate drug-excipient compatibility. For this analysis, FTIR spectra of the pure drug and its mixtures with excipients were acquired using an FTIR spectrophotometer. KBr pellets were prepared via a press pellet technique. In this method, spectroscopic-grade KBr was compressed, followed by a 2-hour drying period in a hot air oven to eliminate any residual moisture. Subsequently, these KBr pellets were utilized to prepare sample pellets containing the drug and selected excipients. The prepared pellets were then introduced into the FTIR spectrophotometer’s sample holder to record the characteristic infrared absorption peaks14,23,24,25.
Differential scanning calorimetry (DSC)
Through endothermic peaks fading, the emergence of new peaks, changes in peak shapes and onsets, peak temperatures, or melting points, DSC is a method that can reveal an interaction. Linezolid-calcium alginate inclusion complex DSC curves of the Linezolid, calcium alginate physical combination determined using the DSC instrument (Shimadzu instrument, Japan). Samples were precisely weighed in a DSC aluminium pan, crimped, and heated from 40 °C to 300 °C at a scanning rate of 10 °C/min while in an inert nitrogen environment22,24,25,26,27.
X-ray diffraction (XRD)
X-ray Diffraction (XRD) was employed to ascertain the crystalline or amorphous nature of the pure drug, the polymer, the blank wafer (BLK), and the drug-loaded (DL) wafers. For the analysis, the wafers were carefully adhered to the sample cells using translucent cling film and a glass slide, ensuring they covered the round tiles of the holder. The testing was conducted in transmission mode using a Bruker D8 Advance X-ray diffractometer. The equipment was set at a power supply of 40,000 V and 0.04 A with a major solar slit of 4° and a supplemental solar slit of 2.5°. The exit slit was 0.6 mm. The Lynx Eye silicon band position-sensitive sensor was set up with an orifice of 3°, and the Lynx Iris was adjusted at 6.5°mm. At a rotational speed of 15 rpm, the samples were scanned on two theta scales between 10° and 50°. The step size was 0.02°, and each step took 0.1s to count [3, 24, 25, 27 ] .
Calibration curve of linezolid in simulated wound fluid (SWF)
Preparation of simulated wound fluid
Potassium dihydrogen phosphate (2.7 g) and sodium hydrogen phosphate (17.8 g) were weighed precisely. These were then added to a 1000 mL volumetric flask and mixed with distilled water.
Preparation of stock solution
A precisely weighed 100 mg aliquot of Linezolid was transferred to a 100 mL volumetric flask. The drug was subsequently dispersed in 1000 mL of SWF to yield a stock solution with a concentration of 100 parts per million (ppm). This stock solution was further serially diluted to generate working solutions with concentrations of 75 ppm, 50 ppm, 25 ppm, and 10 ppm20.
Preparation of calibration curve in simulated wound fluid
The stock solution’s peak intensity was assessed via High-Performance Liquid Chromatography (HPLC) utilizing a Shimadzu Prominence-i LC-2030 C Plus system. The maximum absorbance wavelength (λmax) of linezolid in simulated wound fluid was established at 250 nm. Following this determination, the initial stock solution (100 µg/mL) underwent subsequent dilution to yield a working concentration range of 5–20 µg/mL. Absorbance measurements were then conducted using a UV-visible spectrophotometer, with simulated wound fluid serving as the reference standard. All analyses were carried out in triplicate, and a calibration curve spanning the entire concentration range was constructed by averaging the triplicate absorbance data and computing the standard deviation.
HPLC method for analysis of wafer formulation
The HPLC method was developed in accordance with the standard procedure outlined in the Indian Pharmacopeia 2022. Overlay Chromatogram with different concentrations of 10 ppm, 25 ppm, 50 ppm, 75 ppm and 100 ppm with areas of 5,10,460 to 50,56,689 and Initial sample analysis of Wafer formulation with 50 ppm concentration was prepared and analysis performed, area observed was 26,11,252.
-
Column oven temperature: was maintained at 40 °C.
-
Autosampler temperature: was set at 25 °C.
-
Mobile phase system: Employed Gradient Elution.
-
Mobile phase A: An aqueous buffer solution (prepared by dissolving 1.0 mL of triethylamine in 1000 mL of ultrapure water and adjusting the pH to 3.0 with orthophosphoric acid) was mixed with Methanol in a 90:10 (v/v) ratio.
-
Mobile phase B: The same aqueous buffer solution was mixed with Methanol in a 10:90 (v/v) ratio.
-
Flow rate: was maintained at 1.0 mL min−1.
-
Detection wavelength: was set at 250 nm.
-
Injection volume: was 50 µL.
Sample processing:
-
1.
A representative portion of the Linezolid wafer formulation was accurately weighed.
-
2.
The analyte(s) were dissolved or extracted by adding the weighed sample to a known volume of Methanol.
-
3.
The resulting suspension/solution was subjected to sonication for 25 min to ensure complete dissolution or extraction.
-
4.
The sample solution was clarified by filtration through a 0.45 μm Polyvinylidene Fluoride (PVDF) syringe filter.
-
5.
The filtered sample aliquot was introduced into the High-Performance Liquid Chromatography system for quantitative analysis.
Buffer solution preparation:
-
1.
mL of triethylamine was dissolved in 1000 mL of ultrapure water.
-
2.
The pH of the aqueous solution was adjusted to 3.0 through the careful addition of orthophosphoric acid.
Viscosity
The viscosity of the wafer formulation is determined by using a Labman viscometer with spindle no. S-2 at 60 rpm and viscosity are measured in Pa/s (pascal per second)25,28.
Weight variation
Ten wafers of each formulation, each equally drug-loaded, were weighed on a digital balance to determine weight variance. The average weights are computed and the observed result for weight fluctuation as the standard deviation from the moderate effects16,25 .
Moisture content
After being precisely weighed, samples of placebo and drug-loaded wafers were warmed to 212 °F till mass remained stable (Wf = Final Weight). The moisture content has been computed as a percent weight loss in the following Eqs.23,29.
\(\% {\text{ Weight}}\;{\text{loss }}={\text{ }}\left[ {\left( {{\text{Wi}} - {\text{Wf}}} \right)/{\text{Wi}} \times {\text{1}}00} \right]\)
Water uptake test
According to the wafer’s water uptake capacity, the wafer formulation may be able to digest and hold a sizable quantity of water (WUC). The more excellent intrinsic hygroscopicity of the polymer will determine its capacity to absorb water23.
\({\text{Water}}\;{\text{ uptake }}\% {\text{ }}={\text{ }}\left[ {\left( {{\text{Ws}} - {\text{Wi}}} \right)/{\text{Wi}} \times {\text{1}}00} \right]\)
Drug content
A wafer was selected at random and cut into small 2 × 2 cm² pieces. These pieces were then dissolved and diluted with Simulated Wound Fluid (SWF) to achieve the correct concentration in a 100 mL amber-colored volumetric flask. This solution was stirred overnight and then filtered. Next, a 1 mL sample of this filtered solution was transferred to a 10 mL amber-colored volumetric flask and further diluted with SWF to the appropriate final concentration. The amount of drug in each wafer was then measured using a UV-visible spectrophotometer at a wavelength of 251 nm, and the results were recorded29,30.
Measurement of thickness
The brittleness of the wafer necessitated the thickness of the wafer. A micrometre screw gauge was used to evaluate each wafer’s thickness at five different positions (centre and four corners), and the mean value of the five places was used as the wafer thickness16.
\(\begin{aligned} & {\text{Main}}\,{\text{ Scale }}\,{\text{Reading}}\,{\text{ }}\left( {\text{A}} \right),{\text{ Circular}}\,{\text{ scale }}\,{\text{reading }} \times {\text{ Least }}\,{\text{Count }}\left( {\text{B}} \right) \\ & {\text{Thickness}}\,=\,{\text{A}}\,+\,{\text{B}}. \\ \end{aligned}\)
Exudate absorption capacity
To determine the exudate absorption capacity of the wafer composition, we added 5 g of Simulated Wound Fluid (SWF) to a Petri dish positioned on a weighing scale. We then submerged the wafer composition in this 5 g of SWF for 180 min to assess the wafer’s ability to handle effluent. To check the wafer formulation’s water-holding capacity and the rate of water absorption, we incrementally added more SWF until the mixture formed a liquid gel, and then measured the total amount of SWF absorbed31.
\({\text{Exudate}}\;{\text{ absorption }}\;{\text{capacity }}={\text{ }}\left( {\left( {{\text{Wf}} - {\text{Wi}}} \right)} \right)/{\text{Wi}} \times {\text{1}}00\)
Determination of porosity
The porosity of the wafers was evaluated using the solvent displacement approach3. The sample’s thickness and diameter were measured using a Vernier calliper micrometre gauge, and the total pore volume (V0) was calculated. After that, samples were weighed (W0), and to achieve saturation, they were submerged for 180 min in 10 mL of ethanol. The blank region on the wafers was filled with ethanol. The samples were then carefully removed from the solvent, blotted with tissue paper to remove excess solvent, and measured back quickly to prevent loss of ethanol due to its volatile nature (W1). Using the equation shown below, it was possible to determine the dressing’s porosity.
\(\begin{aligned} & {\text{Porosity }}\left( \% \right){\text{ }}={\text{ }}\left( {{\text{W1}} - {\text{W}}0} \right)/(\uprho {\text{ethV}}0){\text{ }} \times {\text{ 1}}00 \\ & \uprho {\text{eth}}:{\text{ density}}\;{\text{ of }}\;{\text{Ethanol}}\,=\,{\text{789 kg}}/{{\text{m}}^{\text{3}}} \\ \end{aligned}\)
In vitro studies
In present study, a Franz Diffusion cell was used to analyze the pharmaco-kinetic profile of an antibiotic wafer formulation employing a cellulose membrane that has already been soaked in SWF. The wafer of appropriate size containing 100 mg drug is placed in the donor chamber, and the recipient chamber is filled with SWF with cellulose membrane placed on it. Later, aliquots were taken at a specific time and replaced with SWF to maintain sink conditions3.
In vivo studies
The mice were obtained from animal facility centre of Parul University, Vadodara, Gujarat, India. All procedures involving the mice were performed in compliance with Institutional Ethical Guidelines and with prior approval from the IAEC (984/2020-10 CPCSEA). Any skin irritation or rash formation on the rat is studied after placing a wafer in this study. In this study, the rat is anaesthetized and shaved with a hair clipper to get rid of dorsal surface hair. The wafer of 1 × 1 cm2 is afterwards cut and paper taped to the rat’s dorsal surface. For 14 days, the rat was examined for any skin rashes or irritation23,32.
Antimicrobial test
In this test, the anti-microbial properties of the wafer and the formulation are determined and compared with the standard drug. Two Agar plates were prepared and sterilized under UV laminar airflow for 15 min and wafer formulation and standard drug were in this test. Later, agar plates were streaked with a loop full of Staphylococcus aureus on each plate, and on one plate formulation, cut into 1 × 1 cm2 places, and in another plate, the pure drug was placed boring in the centre of the plate with the help of borer. Plates were thereafter placed in an incubator and kept at 37 °C for 24 h3.
Ethical statement
All experimental procedures adhered strictly to the relevant Institutional guidelines and regulations. The in vivo experiments involving mice were conducted with full ethical approval from the Institutional Animal Ethics Committee (IAEC) under protocol number 984/2020-10 CPCSEA. Furthermore, we confirm that the design, execution, and reporting of this study are in full compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines, ensuring transparency and rigor in our animal research practices.
Results
Calibration of linezolid in phosphate buffer pH 7.4
The calibration curve for Linezolid in a phosphate buffer solution with a pH of 7.4 was evaluated over a concentration range of 2 to 18 micrograms per milliliter (µg/mL). This assessment confirmed that the absorbance of Linezolid was proportional to its concentration within this interval, demonstrating linearity. The regression coefficient (R²) at the measurement wavelength of 251 nanometers (nm) was found to be 0.9975, signifying a robust linear association. The equation representing this calibration curve was established as y = 0.0545x + 0.0024, where ‘y’ denotes the absorbance recorded by the UV spectrometer and ‘x’ signifies the concentration of Linezolid. The data points for this standard calibration curve are listed in Table 1, and the corresponding graph is illustrated in Fig. 1. The calibration curve was produced using a UV spectrometer.
HPLC analysis of wafer formulation
Linearity of the Linezolid drug was performed with different concentrations of 10 ppm, 25 ppm, 50 ppm, 75 ppm, and 100 ppm with an area range of 510,460, 1,352,954, 2,611,635, 3,982,621, and 5,056,689 observed respectively. Moreover, the calculation and Linearity graph of the same was calculated as shown in Figs. 2 and 3. Furthermore, Stability analysis of Initial, 3-month and 6-month accelerated condition sample analysis of wafer formulation with 50 ppm concentration was prepared and analysis performed, Calculation given in Tables 2, 3 and 4 and chromatographs as shown in Figs. 4 and 5. Solution stability of Linezolid drug in Phosphate buffer in different pH 6.8, 7.0 and 7.4 was performed and chromatographs of same was shown in Fig. 5 and calculation in Table 3.
Absorbance capacity of wafer formulation
The wafer formulation was placed on a weighing balance, and its initial weight was recorded. Water was then gradually added until the wafer formulation completely transformed into a gel. The maximum amount of water absorbed by the wafer formulation before gelling was 5 mL, as illustrated in Fig. 6. The absorption capacity of the wafer was calculated by using formulae, Percentage Weight Absorption = (Wet weight – Dry weight/ Dry weight) x 100, which was found to be 3220% more than the weight of the wafer formulation.
Viscosity
To determine the gel’s viscosity, we used Labman Viscometer by using two different spindles Sp – 2 and Sp- 3. The Gel’s consistency of the final L1 formulation was selected, and a graph is plotted as demonstrated in Fig. 7 and readings are presented in Table 5.
Weight variation
Five wafers of each formulation, each with equally drug-loaded wafers, were weighed on a digital balance to determine weight variance. The average weights were computed, and Table 6 shows a plot of the standard deviation from the mild effects because of weight fluctuation.
Moisture content
The percentage of weight loss drug-loaded wafers is demonstrated in Table 7.
Measurement of thickness
Table 8 presents the thickness measurements for all wafer batches, L1 through L9. While most batches exhibited a consistent thickness with minor variations, batches L6 and L8 showed a slightly greater thickness. This marginal increase in thickness for L6 and L8 could potentially be attributed to a lower degree of water entrapment within their matrices.
Fourier transforms infrared spectroscopy (FTIR)
While the detected frequency was discovered to be 3359.48 cm−1,1670.08 cm−1, stretching and 1442.42 cm−1 bending respectively, the prominent peaks of N-H aliphatic primary amine, C=O stretching and O–H bending in the pure drug are between 3300 and 3400 cm−1, 1400 –1000 cm−1, and 1740 –1720 cm−1, respectively. Moreover, the principal frequency of N-H aliphatic primary amine stretching in pure is 3360.11 cm−1, and the observed frequency was 3359.48 cm−1 as shown in the given Fig. 8 – (A- FTIR of Calcium alginate, B- FTIR of Linezolid) and C- FTIR of Physical Mixture) i.e. Calcium alginate and Linezolid.
The FTIR results for pure drug, calcium alginate, and drug-loaded wafer
The predominant frequencies of the stretching of the polymer CA O–H bending, N–H aliphatic primary amine stretching, and C=O are respectively, between 1740 –1720 cm−1 and 3300–3400 cm−1, 1400 –1000 cm−1, observed peaks were 3359.48 cm-1, 1670.08 cm−1, and 1442.42 cm−1, respectively. No interaction was found between drug and polymer when formulated in the form of a wafer i.e., Drug-loaded wafer formulation as shown in Fig. 8.
Differential scanning calorimetry (DSC)
Linezolid has a single clear endothermic peak at 180 °C, which is also the drug’s melting point. The DSC curves demonstrate that water molecules were released between 80 and 231 degrees Celsius since the calcium alginate thermogram revealed a significant thermal peak between these temperatures. In the formulation of the Linezolid-loaded wafer, the endothermic peaks of free Linezolid and calcium alginate were just overlaid, showing that there is no interaction between the host and guest molecules. The DSC (Differential Scanning Calorimetry) curve for the Calcium Alginate-Linezolid Inclusion Complex, as shown in Fig. 9, did not display an endothermic peak at the temperature where Linezolid typically melts. This absence of the characteristic melting peak supports our finding that the Linezolid in the complex is in an amorphous (non-crystalline) form. In simpler terms, the heat analysis didn’t show Linezolid melting like a crystal, confirming it’s present in a non-structured state within the complex. These phenomena might be caused by forming stable complexes by embedding the Linezolid molecule into calcium alginate cavities.
X-ray diffraction (XRD)
The XRD analysis (Fig. 10) revealed that the mixture of calcium alginate and Linezolid was highly crystalline, exhibiting several distinct sharp peaks at 16.8, 22.4, and 29.4 degrees (2θ). The disappearance or weakening of these characteristic peaks in the drug-loaded (DL) wafer suggests that the drug was likely dispersed within the polymeric matrix, potentially influencing its release rate. Furthermore, slight shifts of the dressing peaks in the DL wafer to higher diffraction angles hint at a possible interaction between the drug and the polymer.
Determination of porosity
Table 9; Fig. 11 illustrate the porosity of different wafer batches (L1 and others), with L1 exhibiting a favorable pore size. The 3D and contour plots in Fig. 11, considering gelatin and water content as dependent variables, reveal a trend: higher quantities of gelatin and water in the formulation correlate with increased porosity or water uptake of the wafer. Conversely, lower amounts of gelatin and water result in a higher porosity of the wafer formulation.
In vitro drug release
An in vitro drug release evaluation was carried out utilizing a Franz diffusion cell. Samples (aliquots) were gathered at predetermined intervals: every 30 min during the initial 4 h, then hourly for the succeeding 2 h, and additional samples were taken at 6 h and 12 h (resulting in a total duration of 24 h). The cumulative drug release (% CDR) of Linezolid from the wafer formulations was determined using the linear regression equation ‘y = mx + ( Table 10; Fig. 12).
Among all the batches from L1-L9, the L1 and L2 batches showed the highest drug release for 24 h, i.e., 96.9% and 86.7%, respectively. The use of a low quantity of gelatin and calcium alginate during formulation increased the porosity, which helps absorb adequate exudates and eventually enhance drug release.
Initially, drug release was low as the wafer absorbed the buffer solution of the receiver compartment and it took a long time to wet the wafer to release the bound water and API.
In vivo studies
Following approval from the Institutional Animal Ethics Committee (IAEC), protocol number 984/2020-10, this animal study was conducted in accordance with the committee’s ethical guidelines. Male Sprague-Dawley rats weighing 280 g were used for a duration of 14 days. For the study, drug-loaded wafers measuring 1 × 1 cm² were prepared and adhered to the shaved dorsal skin of the rats, as illustrated in Fig. 13.
As shown in Fig. 14, a 1 × 1 cm² piece of the wafer was cut and adhered to paper tape. Subsequently, this was attached to the previously shaved dorsal side of the rat. Over the 14-day observation period, no signs of skin irritation or rashes were noted on the rat.
Anti-microbial test
The Zone of Inhibition (ZOI), the area where bacterial growth was inhibited, was determined by measuring the diameter (in millimeters) of the clear zone around discs containing the test samples placed on agar plates (Table 11). Compared to a standard wafer formulation, our coated wafers demonstrated significantly reduced ZOIs. Specifically, at Linezolid concentrations of 0.25 mg, 0.5 mg, and 25.0 mg, our wafers yielded ZOIs of 52.7 mm, 61.6 mm, and 68.1 mm, respectively. These values represent only 12.4%, 7.25%, and 0.16% of the much larger ZOIs observed with the standard wafer formulation, which were 425 mm, 850 mm, and 42,500 mm for the corresponding Linezolid concentrations.
Zone of Inhibition in Petri-plate containing drug-loaded wafer was found to be 52.7, 61.6 and 68.1. mm, for 0.25 mg/mL, 0.5 mg/mL and 25.0 mg/mL respectively, whereas Petri-plate containing standard drug showed a zone of inhibition of 9.4, 11.6, 13.8, 19.4, 23.6 and 27.2 mm for 0.5, 1,2,4,8 and 16µµ/mL concentration which is more than the drug-loaded wafer but sufficient to inhibit the bacterial growth as shown in Fig. 14.
Discussion
The development of a Linezolid-loaded calcium alginate wafer offers a dual-action approach for treating diabetic foot ulcers (DFUs), functioning both as an exudate absorber and delivering antibacterial effects, particularly against infections caused by methicillin-resistant Staphylococcus aureus (MRSA)3. The high prevalence of DFUs among diabetic patients, coupled with the alarming rate of lower extremity amputations, underscores the urgent need for innovative wound care solutions. This study successfully demonstrates the potential of a lyophilized wafer formulation to address these challenges of exudate absorption and sustained manner of drug release for 24 h as an antibacterial effect33.
The development of a localized antibiotic delivery system, such as the Linezolid-loaded calcium alginate wafer, directly addresses the challenges posed by DFUs. Traditional treatments often fail to provide adequate concentrations of antibiotics at the site of infection, especially in the context of resistant organisms like MRSA34. The targeted delivery mechanism of the wafer ensures that Linezolid is released directly into the wound environment, potentially enhancing its effectiveness against resistant strains and reducing the risk of systemic side effects. This localized approach not only improves the therapeutic outcome but also aligns with the principles of antimicrobial stewardship, which advocate for minimizing unnecessary systemic antibiotic use. Calcium alginate serves as an excellent matrix for drug delivery due to its biocompatibility and ability to form hydrogels that retain moisture35. The incorporation of Linezolid into this matrix allows for sustained release, which is crucial for maintaining therapeutic levels of the antibiotic over time. The ability of the wafer to create a moist wound environment can further support the healing process by facilitating cellular migration and proliferation, essential steps in wound healing. This dual action antimicrobial activity combined with a conducive healing environment positions the wafer as a superior alternative to conventional dressings36.
Fourier Transform Infrared Spectroscopy (FTIR) is a valuable tool for investigating potential interactions between drugs and polymers in pharmaceutical formulations by identifying characteristic functional group vibrations37. Our observation of characteristic peaks in the drug-loaded wafer, closely matching those of the pure drug and calcium alginate, suggests that the principal functional groups of both the drug and the polymer are present in the final formulation38,39. Our results indicate that the L1 batch of the wafer, composed of 2% calcium alginate and 0.5% glycerin, exhibited superior exudate handling properties and a remarkable in-vitro drug release rate of 96.9% within 24 h40. This rapid release profile is crucial for maintaining effective local antibiotic concentrations at the wound site, thereby enhancing the therapeutic efficacy against MRSA. The ability of the wafer to absorb exudates and transform into a hydrogel creates a moist wound environment, which is known to facilitate healing and reduce pain during dressing changes41.
The antimicrobial assays conducted in this study demonstrated that the drug-loaded wafers exhibited substantial inhibition of bacterial growth, with effectiveness levels closely approaching those of standard Linezolid. These Zone of Inhibition (ZOI) findings strongly suggest that the wafers possess the capability to deliver Linezolid directly to the site of infection. This localized delivery mechanism holds the potential to circumvent the inherent limitations often associated with systemic antibiotic therapy in diabetic patients, where compromised blood circulation (poor tissue perfusion) can significantly impede drug penetration to the affected area42. The direct application of the drug via the wafer not only enhances local concentrations but also minimizes systemic side effects, making it a safer alternative for patients.
The in vivo studies conducted on Sprague-Dawley rats showed no signs of skin irritation or adverse reactions over a 14-day period. This finding is critical, as it indicates the biocompatibility of the wafer formulation, which is essential for patient compliance and overall treatment success43. The absence of irritation suggests that the materials used in the wafer are well-tolerated, further supporting the potential for clinical application.
The introduction of the Linezolid-loaded calcium alginate wafer is particularly relevant in the context of rising antibiotic resistance, especially among common pathogens like MRSA44. The ability to deliver antibiotics directly to the wound site can significantly enhance treatment efficacy while minimizing systemic exposure, which is crucial for diabetic patients who often have compromised immune responses45.
The mechanism by which the wafer formulation promotes wound healing can be further explored. The hydrogel formation upon contact with wound exudates not only provides a moist environment conducive to healing but also facilitates the controlled release of Linezolid. This dual action can help in reducing the bacterial load while simultaneously promoting tissue regeneration14.
A comparative analysis of the Linezolid wafer with existing wound dressings could provide valuable insights. For instance, traditional dressings often require frequent changes, which can be painful and disruptive for patients. The sustained release and biocompatibility of the wafer may offer a more patient-friendly alternative, potentially improving adherence to treatment protocols46. The stability of the wafer formulation over time and under various storage conditions is a critical factor for its practical application. Investigating the effects of temperature, humidity, and light exposure on the drug’s stability and the physical integrity of the wafer could inform best practices for storage and handling in clinical settings47.The impact of the wafer on patient compliance and overall quality of life should be considered. By reducing the frequency of dressing changes and minimizing pain during removal, the wafer could enhance patient comfort and adherence to treatment regimens, ultimately leading to better health outcomes48.
The Linezolid wafer addresses the discomfort and infection risks associated with frequent dressing changes, reducing the frequency of dressing changes, enhancing biocompatibility, and patient comfort49. The stability of the Linezolid wafer formulation is critical for its practical application in clinical settings. Various factors can influence the stability of the drug and the physical integrity of the wafer50. The Linezolid wafer has the potential to improve patient compliance and quality of life by reducing discomfort and frequency of dressing changes.
The economic implications of using the Linezolid-loaded wafer in clinical practice should also be discussed. While the initial cost of developing and producing such advanced dressings may be higher, the potential for reduced hospital stays, fewer complications, and lower rates of amputation could lead to significant cost savings in the long run51. While the results are promising, there are limitations to this study that warrant consideration. The in vitro and in vivo studies, although indicative of the formulation’s potential, should be complemented by larger-scale clinical trials to validate efficacy and safety in human subjects3. Additionally, further investigations into the long-term stability of the wafers and their performance in various wound environments are necessary to optimize their formulation.
Future research could focus on optimizing the formulation further by exploring different ratios of calcium alginate and gelatin, or incorporating additional bioactive compounds that could enhance healing. Additionally, studies could investigate the wafer’s effectiveness against a broader range of pathogens, including those that are less common but still pose a risk to diabetic patients. Finally, the regulatory pathway for the approval of the Linezolid wafer as a medical device or drug delivery system should be addressed. Understanding the requirements for clinical trials, safety assessments, and efficacy evaluations will be essential for bringing this innovative product to market.
The discussion highlights the potential impact of Linezolid-loaded calcium alginate wafer in diabetic foot ulcer management, suggesting that optimizing formulation and expanding antimicrobial spectrum could improve patient outcomes. Efficient regulatory navigation will ensure timely access to innovative treatment for diabetic patients, reducing complications and surgical interventions. This could lower healthcare costs, improve resource utilization, and enhance patient comfort, ultimately improving quality of life.
In conclusion, the Linezolid-loaded calcium alginate wafer represents a significant advancement in the treatment of diabetic foot ulcers. Future research should focus on optimizing the formulation, expanding its antimicrobial efficacy, and understanding the regulatory requirements for approval. By addressing these areas, researchers can enhance the wafer’s clinical utility and solidify its role in improving outcomes for patients with DFUs. This innovative approach to wound management has the potential to transform care practices, offering a more effective and patient-centred solution to a challenging and prevalent health issue.
(A) Drug-loaded wafer cut into 1 × 1cm2 piece, (B) drug Loaded wafer cut into 1 × 1cm2 and backed with paper tape, (C) Dorsal side of the rat was shaved using trimmer, (D) Day 1 Drug-loaded wafer attached on shaved Dorsal side of the rat, (E) Day 7 Rat was evaluated for any skin rashes and discomfort., (F) On day 14 Rat was evaluated for any rashes or itchy skin.
(A) Culture plates containing 25 mg strength wafer formulation, (B) culture plate containing 0.5 mg strength wafer formulation, (C) culture plate containing 0.25 mg strength wafer formulation disc and (D) Culture plates containing drug loaded disc with 0.25 µL, 0.5 µL, 2 µL, 4 µL, 8 µL, 16 µL concentration and VC (vehicle control) disc.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and can be avail from the corresponding author on reasonable request.
References
Ramirez-Acuña, J. M. et al. Diabetic foot ulcers: current advances in antimicrobial therapies and emerging treatments. Antibiotics 8, 193 (2019).
Boulton, A. J. M. The diabetic foot. Med. (United Kingdom). 47, 69–74 (2019).
Ahmed, A. Developing a Single Dressing Containing both Antifungal and Antibacterial Drugs for Treating Mixed Infections in Diabetic Foot Ulcers (Doctoral dissertation, University of Nottingham, 2018).
Boateng, J. S. et al. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 97, 2892–2923 (2008).
Pawar, H. V. et al. Multifunctional medicated lyophilised wafer dressing for effective chronic wound healing. J. Pharm. Sci. 103, 1720–1733 (2014).
Jani, R. K., Puri, G. K. & Ubana, S. A. Review of diabetic foot ulcer infections and lyophilized wafer formulation. J. Pharm. Res. Int. 33, 13–25 (2021).
Bhatt, P., Miraghajani, M. S., Pathak, S. & Pathak, Y. Nutraceuticals for Prenatal, Maternal and Offspring’s Nutritional Health (CRC, 2019).
Garraud, O., Hozzein, W. N. & Badr, G. Wound healing: Time to look for intelligent, natural immunological approaches? BMC Immunol. 18, 5 (2017).
Zontangos, G. & Anderson, A. R. OpenAIR @ RGU the open access institutional repository at Robert Gordon University. Qual. Market Res. Int. J. 7, 228–236 (2004).
Armstrong, D. G., Boulton, A. J. M. & Bus, S. A. Diabetic foot ulcers and their recurrence. N Engl. J. Med. 376, 2367–2375 (2017).
Clayton, W. & Elasy, T. A. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin. Diabetes. 27, 52–58 (2009).
Jeffcoate, W. J. et al. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 41, 645–652 (2018).
Barchielli, M. et al. Clinical pharmacokinetics of lercanidipine. J. Cardiovasc. Pharmacol. 29, 306–309 (1997).
Jani, R. K. et al. Nanosponges as a biocatalyst carrier — An innovative drug delivery technology for enzymes, proteins, vaccines, and antibodies. Biocatal. Agric. Biotechnol. 42, 102329 (2022).
Boateng, J. S. et al. In vitro drug release studies of polymeric freeze-dried wafers and solvent-cast films using Paracetamol as a model soluble drug. Int. J. Pharm. 378, 66–72 (2009).
Rezvanian, M., Amin, M. C. I. M. & Ng, S. F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 137, 295–304 (2016).
Verma, G. Transdermal drug delivery system, advance development and evaluation-a review. Int. J. Pharm. Sci. Res. 8, 385–400 (2017).
Teaima, M. H. et al. Eco-friendly synthesis of functionalized chitosan-based nanoantibiotic system for potential delivery of linezolid as antimicrobial agents. Saudi Pharm. J. 28, 859–868 (2020).
Bandyk, D. F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin Vasc Surg. 31, 43–48 (2018).
Saviano, A. M. & Lourenço, F. R. Uncertainty evaluation for determining linezolid in injectable solution by UV spectrophotometry. Meas. (Lond). 46, 3924–3928 (2013).
Shah, S. et al. Pulmonary delivery of linezolid nanoparticles for treatment of tuberculosis: design, development, and optimization. J. Pharm. Innov. 15, 707–717 (2020).
Zheng, S. et al. Determination and correlation of solubility of linezolid form II in different pure and binary solvents. Fluid Phase Equilib. 432, 18–27 (2017).
Atia, N. M. et al. Diosmin nanocrystal–loaded wafers for treatment of diabetic ulcer: in vitro and in vivo evaluation. J. Pharm. Sci. 108, 1857–1871 (2019).
Chakraborty, P. et al. Mathematical optimization and characterisation of pharmaceutically developed novel mucoadhesive wafers for rapid bioactive delivery of Loratadine. J. Pharm. Investig. 43, 133–143 (2013).
Kianfar, F. et al. Lyophilized wafers comprising carrageenan and pluronic acid for buccal drug delivery using model soluble and insoluble drugs. Colloids Surf. B Biointerfaces 103, 99–106 (2013).
Khatri, S. & Rathnanand, M. Formulation and evaluation of wound healing activity of linezolid topical preparations on diabetic rats. Int. J. Appl. Pharm. 8, 30–36 (2016).
Maccaroni, E. et al. Polymorphism of linezolid: A combined single-crystal, powder diffraction and NMR study. Int. J. Pharm. 351, 144–151 (2008).
Tabassum, A., Furtado, S. C. & Bharath, S. Development of antimicrobial colloidal silver incorporated lyophilized biopolymer wafers for wound care. Wound Med. 21, 1–7 (2018).
Patel, N. & Jani, R. Development and optimization of controlled release formulation and process of levetiracetam with hot melt coating technology. J. Pharm. Res. Int. 33, 80–102 (2021).
Ahmed, A. & Boateng, J. Calcium alginate-based antimicrobial film dressings for potential healing of infected foot ulcers. Ther. Deliv. 9, 185–204 (2018).
Ahmed, A., Getti, G. & Boateng, J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv Transl Res. 8, 1751–1768 (2018).
Bitto, A. et al. Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol. Res. 57, 159–169 (2008).
Jani, R., Puri, G. K. & Ubana, S. A. Review of diabetic foot ulcer infections and lyophilized wafer formulation. J. Pharm. Res. 40, 1–13 (2021).
Liao, T. Y. et al. HarDNet-DFUS: An enhanced harmonically-connected network for diabetic foot ulcer image segmentation and colonoscopy polyp segmentation. arXiv Preprint at (2022). https://doi.org/10.48550/arXiv.2203.14974
Sukhum, K. V. et al. Antibiotic-resistant organisms establish reservoirs in new hospital built environments and are related to patient blood infection isolates. Commun. Med. 2, 118 (2022).
Claeys, K. & D Johnson, M. Leveraging diagnostic stewardship within antimicrobial stewardship programmes. Drugs Context 12, 2023–2025 (2023).
Nandiyanto, A. B. D., Fadhlulloh, M. A., Rahman, T. & Mudzakir, A. Synthesis of carbon nanoparticles from commercially available liquified petroleum gas. IOP Conf. Series: Mater. Sci. Eng. 128, 012042 (2016).
Madni, A. et al. Liposomal drug delivery: A versatile platform for challenging clinical applications. J. Pharm. Pharm. Sci. 17, 401–426 (2014).
El-Houssiny, A. S. et al. Drug–polymer interaction between glucosamine sulfate and alginate nanoparticles: FTIR, DSC and dielectric spectroscopy studies. Adv. Nat. Sci. NanoSci. NanoTechnol. 7, 025014 (2016).
Lei, L. et al. Current understanding of hydrogel for drug release and tissue engineering. Gels 8, 230 (2022).
Liang, Y., He, J. & Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 15, 12779–12793 (2021).
Razek, A. M. A. E. et al. Metoclopramide hydrochloride loaded oral wafers for postoperative care of children: In vitro and in vivo evaluation. Am. J. PharmTech Res. 9, 1–15 (2019).
Warmink, K. et al. Sprague Dawley rats show more severe bone loss, osteophytosis and inflammation compared to wistar han rats in a high-fat, high-sucrose diet model of joint damage. Int. J. Mol. Sci. 23, 12345 (2022).
Shoaib, M. et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 13, 1073757 (2023).
Prompetchara, E., Ketloy, C. & Palaga, T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 38, 145–154 (2020).
Huang, H. et al. Intra-articular drug delivery systems for osteoarthritis therapy: Shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 29, 767–791 (2022).
Kafetzis, K. et al. The effect of cryoprotectants and storage conditions on the transfection efficiency, stability, and safety of Lipid-Based nanoparticles for mRNA and DNA delivery. Adv. Healthc. Mater. 12, 2202388 (2023).
Lu, X. The effects of patient health information seeking in online health communities on patient compliance in china: Social perspective. J. Med. Internet Res. 25, e35815 (2023).
Ye, Z. et al. Emerging MoS2 wafer-scale technique for integrated circuits. Nano-Micro Lett. 15, 121 (2023).
van Doremalen, N. et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl. J. Med. 382, 1564–1567 (2020).
Nicola, M. et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 78, 185–193 (2020).
Author information
Authors and Affiliations
Contributions
Conceptualization: G.K.P., R.K.J., and S.T.H.; Investigation and formal analysis: G.K.P., R.K.J., S.M.S.H.; Writing original draft: M.C.S. and V.U.; Writing, review, and editing: S.T.H. and O.A.A.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Puri, G.K., Jania, R.K., Upadhye, V. et al. Calcium alginate medicated dressing for enhanced absorption in diabetic foot ulcer management. Sci Rep 15, 23335 (2025). https://doi.org/10.1038/s41598-025-05982-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05982-2