Abstract
The retinal pigment epithelium (RPE) is a fundamental monolayer of pigmented cells that supports visual function, situated between the neural retina and choroidal blood vessels. Radiotherapy is a common treatment for ocular tumors such as uveal melanoma; however, the RPE is inevitably exposed to radiation during treatment. FLASH radiotherapy, characterized by ultra-high dose-rates, has emerged as a promising approach to minimize normal tissue toxicity while maintaining tumor control. Despite its potential, few preclinical studies have explored the effects of FLASH irradiation on the RPE, and no studies have directly compared FLASH with conventional (CONV) radiotherapy in this context. Using a LINAC capable of switching between FLASH and CONV irradiation, we here address the radiobiological effects on ARPE-19 cells, an in vitro RPE model, and the RPE of living mice. FLASH treatment demonstrated protective effects on ARPE-19 cells, enhancing cell viability and modulating cytokine expression (e.g., IL-6, IL-8). Furthermore, RPE tissue exhibited dose-dependent radiation sensitivity, with FLASH-irradiated mice performing better than CONV-treated ones. These findings indicate that FLASH radiotherapy may protect the RPE during ocular tumor treatment and provide insights into tissue-specific radiation sensitivity in the eye, supporting further research into its clinical applications.
Similar content being viewed by others
Introduction
Radiation therapy is a cornerstone of cancer treatment, widely used to malignancies including breast, head, neck, brain, and ocular tumors. Despite its efficacy, the adverse effects on surrounding healthy tissues often limit its therapeutic potential1. Recent advancements in radiation delivery have sparked interest in novel approaches, including FLASH radiation therapy (FLASH-RT). The ‘FLASH effect’ refers to the normal tissue sparing response while maintaining the isoefficacy on the tumor. This effect is observed when radiation is delivered at ultra-high dose rates (UHDR), typically exceeding 40 Gy per second and a total irradiation time lower than 0.2 s2using different types of radiation beams (protons, electrons, etc.). A UHDR is 4,000 times faster than conventional (CONV) radiotherapy, and recent evidence suggests that it may activate unique biological mechanisms, such as reduced engagement of DNA damage response pathways, enhanced cellular repair, and differential immune activation, thereby minimizing toxicity to healthy tissues while preserving or even enhancing antitumor efficacy3. The hypothesis is that FLASH irradiation triggers distinct biological responses compared to CONV modalities, offering a safer alternative for preserving healthy tissues. This distinct biological response positions FLASH-RT as a safer alternative, particularly for treating central nervous system (CNS) tumors, including those at intraocular levels4.
Uveal melanoma, the most common primary intraocular malignant tumor, arises from melanocytes in the uveal tract. Historically, enucleation was the standard treatment for this tumor; however, the Collaborative Ocular Melanoma Study demonstrated that radiotherapy provides equivalent outcomes, leading to a paradigm shift toward eye-preserving approaches5. Today, radiation therapy, including proton beam radiotherapy, plaque brachytherapy (using isotopes like Iodine-125, Ruthenium-106, Palladium-103, or Cesium-131), and stereotactic techniques (e.g., Gamma Knife, CyberKnife, and LINAC-based radiosurgery), is the most widely adopted treatment6,7,8,9,10,11. Despite proven efficacy, radiotherapy can lead to vision-threatening complications, particularly radiation retinopathy and optic neuropathy, and the spatial relationship between the tumor and the optic nerve is a critical determinant of treatment-related morbidity12. FLASH-RT has the potential to overcome these limitations. In this context, we evaluated the differential effects of FLASH and CONV irradiation on the retinal pigment epithelium (RPE), a monolayer of pigmented cells situated between the neural retina and the choroidal vasculature. The RPE plays essential roles in retinal homeostasis, immune privilege, and visual function. RPE cells contain melanin granules that absorb excess light, reducing photo-oxidative stress. Their apical membrane interacts with photoreceptor outer segments (POS), which are regularly engulfed to allow renewal. Furthermore, through the visual cycle, the RPE regenerates 11-cis-retinal, necessary to light perception13. Tight junctions between RPE cells form the outer retinal blood barrier (oBRB), maintaining retinal homeostasis and protecting against xenobiotics14. Dysfunction of the RPE underpins various inherited and acquired blinding diseases, including Stargardt’s maculopathy, retinitis pigmentosa, and age-related macular degeneration15. Barrier disruptions and oxidative stress-induced tight junction impairments are commonly associated to diabetic retinopathy and uveitis, diseases characterized by major inflammation16. Given its critical role, minimizing RPE damage during radiotherapy is essential for preserving vision.
Using ARPE-19 cells (an in vitro model of human RPE17) and healthy laboratory mice, we employed a novel LINear ACcelerator (LINAC), ElectronFlash equipped with a triode gun, enabling precise delivery of both FLASH and CONV radiation under controlled and reproducible conditions18. This approach allowed us to correlate in vitro cellular outcomes with in vivo tissue responses, providing a comprehensive evaluation of radiation’s impact on the RPE. Our working hypothesis is that it is possible to achieve a FLASH, tissue-sparing effect in vitro and in vivo, with FLASH-irradiation reducing cellular damage and preserving RPE function compared to CONV irradiation. Our results, proving the working hypothesis, lay the groundwork for future functional studies in live animal models and highlight the potential of FLASH-RT to improve outcomes in ocular tumor treatment while minimizing collateral damage to surrounding healthy tissues.
Results
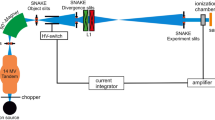
This study was performed using a novel LINear ACcelerator (LINAC) equipped with a triode gun, capable of irradiating in both FLASH and CONV modalities under controlled conditions. The specimen holder of the LINAC was designed to accommodate cell plates or whole rodents, choosing the specific body area of irradiation. The main components of the experimental setup are illustrated in Fig. 1, also showing the distribution of a specific radiation pattern over the mouse head and the following Montecarlo calculation. Table 1 summarizes fundamental parameters of irradiation modalities.
Configurations used for both in vitro and in vivo experiments. In vitro set-up: (A) EF vertical configuration; (B) flashDiamond (fD) reference position for beam output characterization; (C) cell plate positioning at the buildup region with fD on the side to monitor EF output. In vivo set-up: (D) 3D printed animal holder; red star indicates the mouth-fixing clamp at the end of the U-shaped compartment; arrow indicates the final place of U-shaped compartment in the animal holder; (E) scheme of EF oblique configuration with holder and mouse in position; (F) microCT, dose deposition simulation for a mouse positioned in the U-shaped compartment and resulting Dose-Volume Histogram (DVH) curve (G).
FLASH irradiation preserves cell viability and metabolic activity in ARPE-19 cells
ARPE-19 cells, a spontaneously immortalized cell line derived from human retinal pigment epithelium (RPE), exhibit several functional and morphological properties of native RPE cells under standard culture conditions. These cells form stable polygonal arrays and display morphological and functional polarity, expressing key RPE markers such as CRALBP, RPE-65, and tight junction proteins (e.g., ZO-1)17. Additionally, ARPE-19 cells retain critical RPE functions when differentiated, including the phagocytosis of photoreceptor outer segments and the secretion of growth factors and cytokines. They also form junctional complexes reminiscent of the oBRB19 and develop measurable trans-epithelial electrical resistance (TEER) after a minimum of two weeks in culture. Due to their ability to mimic native RPE behavior, ARPE-19 cells are widely used as an in vitro model to study retinal pathologies such as age-related macular degeneration and Retinitis Pigmentosa under stress conditions. In the context of ocular tumors, radiation therapy often impacts the RPE, necessitating a deeper understanding of its effects on this critical cell layer. Here, we investigate the effects of radiation on intact ARPE-19 cells to support the development of safer treatment protocols that minimize damage to healthy ocular tissues during radiotherapy for eye cancers. The metabolic activity ARPE-19 cells following various protocols of irradiation was assessed using a colorimetric MTT assay, which measures the reduction of 3-[4,5-dimethylthiazol-2-yl]-3,5-diphenyl-tetrazolium bromide (MTT) to formazan. Cell survival was evaluated through manual cell counting, as described in material and method section. Pyknotic cells, indicative of nuclear condensation and apoptosis, were detected and counted after Hoechst nuclear staining and fluorescence imaging. Assays were conducted three days post-irradiation at varying radiation doses. Results indicate that irradiation of fully differentiated ARPE-19 cells, whether in FLASH or CONV mode, reduces cell viability compared to non-irradiated controls. Both viability and metabolic activity exhibit a clear dose-dependent decline, particularly evident in FLASH mode (Fig. 2A, B, red bars). Pyknotic cells: (CTRL mean = 1,76 pyknotic cells/field; FLASH 4 Gy mean = 2,13 pyknotic cells/field; CONV 4 Gy mean = 2,63 pyknotic cells/field; FLASH 8 Gy mean = 2,47 pyknotic cells/field; CONV 8 Gy mean = 2,50 pyknotic cells/field; FLASH 16 Gy mean = 2,59 pyknotic cells/field; CONV 16 Gy mean = 2,65 pyknotic cells/field), (Fig. 2E, arrows). At higher doses (8 and 16 Gy), no significant differences in cell survival rates were observed between FLASH and CONV modalities 72 h post-exposure (Fig. 2B). These findings suggest that FLASH irradiation may offer a protective advantage at lower doses, preserving cell viability and metabolic activity more effectively than CONV irradiation.
FLASH irradiation preserves cell viability and metabolic activity in ARPE-19 cells. (A) MTT assay of ARPE-19 culture plates after exposures to 4, 8 and 16 Gy irradiation, in FLASH (red columns) and CONV (blue columns) modalities. Controls (gray columns) are age-matched culture plates, non-exposed to irradiation. Readings are done 72 h after exposure. Results of MTT assay were pooled from 12 replicate samples derived from 2 independent experiments and expressed as mean ± S.D. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. p values are indicated in the graphs. (B) Bar graphs illustrating the survival rate of ARPE-19 cells 72 h post irradiation of increasing intensities, in FLASH (red columns) or CONV (blue columns) modalities. Controls (gray columns) are age-matched culture plates, non-exposed to irradiation. The experiment was conducted on 3 biological replicates for each condition. Data are expressed as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. p values are indicated in the graphs. (C) ARPE-19 cells stained with Ab against ZO-1 (red signal) and α-Tubulin (green signal). Blue signal is referred to Hoechst nuclear staining. (D) Representative image of ARPE-19 cells stained with antibodies against ZO-1 (red signal) and α-Tubulin (green signal) at higher magnification. Blue signal is referred to Hoechst nuclear staining. (E) Representative ARPE-19 cell images for each condition of irradiation at 4 Gy labeled with Hoechst nuclear staining, with arrows pointing to pyknotic nuclei.
FLASH irradiation minimizes morphological alterations, senescence and cytokines production in ARPE-19 cells
ARPE-19 cells were cultured on coverslips for seven days and exposed to either FLASH or CONV radiation, with non-exposed cells serving as controls. 72 h post-irradiation, the cells were processed for high-resolution fluorescence microscopy to assess nuclear morphology. The most striking observation was the presence of numerous oversized nuclei (20–30 μm in diameter), 2–3 times larger than normal (Fig. 3A, B and C, arrows). These enlarged nuclei were associated with proportionally larger cells containing abundant cytoplasm. At 4 Gy, both FLASH and CONV treatments resulted in similar numbers of oversized nuclei. However, in CONV-treated cells, the number of oversized nuclei increased with dose, whereas in FLASH-treated cells, it plateaued at 8 Gy. Notably, at the highest dose (16 Gy), FLASH irradiation appeared less damaging to ARPE-19 cells compared to CONV radiation (p = 0.0019). Cell and nuclear enlargement indicate radiation-induced damage, senescence, and genomic instability. To determine whether these morphological changes were linked to senescence, we analyzed the expression of p21Cip1 and p16Ink4a, key regulators of cell cycle arrest. qRT-PCR revealed that p21Cip1 was upregulated in irradiated cells at all doses and time points (Fig. 3D). Notably, 16 Gy CONV-treated cells exhibited significantly higher p21Cip1 expression than FLASH-treated cells after three days, correlating with fewer oversized nuclei in FLASH group. In contrast, p16Ink4a expression remained unchanged across all conditions (Fig. 3E), suggesting the activation of a distinct cellular response pathway, potentially leading to senescence or apoptosis. These findings indicate that FLASH irradiation may mitigate radiation-induced senescence and preserve cell morphology more effectively than CONV radiation. We also analyzed the expression of IL-6 and IL-8 (Fig. 3F), cytokines involved in ocular homeostasis and cancer development. CONV radiation significantly increased IL-6 expression compared to controls (p = 0.0001) and FLASH-treated cells (p = 0.0034), while IL-8 remained unchanged. These results are coherent with the known role of IL-6 in inflammation and of IL-8 predominantly with acute immune responses.
FLASH irradiation minimizes morphological alterations, senescence and cytokines production in ARPE-19 cells. (A) Bar graphs of the percentage of oversized nuclei/field in ARPE-19 cells 72 h post irradiations of increasing intensities, in FLASH (red columns) or CONV (blue columns) modalities. Controls (gray columns) are age-matched culture plates, non-exposed to irradiation. The experiment was conducted on 3 biological replicates for each condition. Data are expressed as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. p values are indicated in the graphs. (B) Representative images of irradiated ARPE-19 cells immunostained with antibody against ZO-1 (green signal). Blue signal is referred to nuclear dye Hoechst. The arrow indicates an oversized nucleus. As shown in the image, the enlarged nuclear size is reflected in an overall increase in the cell’s size. (C) This panel shows representative ARPE-19 cells images for each conditions of irradiation at 4 Gy labeled with Hoechst nuclear staining with arrows pointing to oversized nuclei. (D, E, F) qRT-PCR TaqMan assay of ARPE-19 cells. Controls (black dotted line, set to 1) are age-matched culture plates, non-exposed to irradiation. In each graph, statistically significant results show comparisons between non exposed control cells and irradiated cells. The graphs also show results of comparisons between ARPE-19 irradiated in CONV and ARPE-19 irradiated in FLASH modalities. The experiment was conducted on 3 biological replicates for each condition. Data are expressed as mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. p values are indicated in the graphs. (D) Bar graphs illustrated expression level of p21 gene in ARPE-19 cells 24 h and 72 h post irradiation of increasing intensities, either in FLASH (red columns) or CONV (blue columns) modalities. (E) Bar graphs illustrated expression level of p16 gene in ARPE-19 cells 24 hand 72 h post irradiation of increasing intensities, either in FLASH (red columns) or CONV (blue columns) modalities. (F) Bar graphs illustrated expression levels of IL-6 and IL-8 in ARPE-19 cells 24 h post irradiation of increasing intensities, either in FLASH (red columns) or CONV (blue columns) modalities.
FLASH irradiation reduces intracellular ROS production in ARPE-19 cells compared to CONV irradiation
Radiation generates reactive oxygen species (ROS) within cells through the ionization of water molecules, leading to the formation of superoxide (O2-), hydroxyl radicals (OH•), hydrogen peroxide (H2O2), and other free radicals. These reactive species can cause oxidative damage to cellular components, contributing to the adverse effects of radiation on healthy tissues20,21,22. To compare ROS production following FLASH and CONV irradiation at different doses, ARPE-19 cells were incubated with 2’,7’-dichlorodihydrofluorescein diacetate (DCFDA), a fluorescent probe for detecting intracellular ROS. DCFDA diffuses passively into cells, where it is deacetylated to non-fluorescent DCFH (2’,7’-dichlorofluorescein). DCFH is then oxidized by ROS to form highly fluorescent DCF (2’,7’-dichlorofluorescein), with fluorescence intensity proportional to intracellular ROS levels.
Using a fluorescence plate-reader, we measured ROS levels in ARPE-19 cells exposed to three total irradiation doses (4, 8, and 16 Gy) in FLASH or CONV modalities, with non-irradiated, age-matched cells serving as controls. The results showed that CONV irradiation caused a significant increase in cellular ROS compared to controls, with a dose-dependent rise in fluorescence intensity. ROS levels were 3-fold higher at 4 and 8 Gy and 5-fold higher at 16 Gy compared to controls (Fig. 4A). In contrast, FLASH irradiation did not increase ROS levels at 4 Gy and resulted in only a moderate, steady increase at 8 and 16 Gy, approximately doubling control values (Fig. 4B). Importantly, ROS levels in FLASH-treated cells were significantly lower than those in CONV-treated cells at all doses (4 Gy p = 0.0070; 8 Gy p = 0.0032; 16 Gy p < 0.0001). This reduction in ROS production may contribute to the protective effects of FLASH irradiation on healthy tissues.
FLASH irradiation reduces intracellular ROS production in ARPE-19 cells compared to CONV radiation. (A) The curves show the formation of intracellular ROS over time in ARPE-19 cells after exposure to increasing doses of irradiation in both FLASH (red line) and CONV (blue line) modes. Controls (black line) are age-matched culture plates, non-exposed to irradiation. Results were pooled from 12 replicate samples and expressed as mean ± S.D. Data were analyzed using two-way ANOVA followed by Bonferroni’s multiple comparison test. p values are indicated in the graphs. (B) Bar graph shows the quantification of the initial peak of ROS fluorescence following irradiation (black arrow, IR) in both FLASH (red columns) and CONV (blue columns) modes. H2O2 (yellow columns) was used as positive control. Data are expressed as mean ± S.D. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. p values are indicated in the graphs.
FLASH irradiation preserves barrier integrity and enhances tight junction repair in ARPE-19 cells compared to CONV.
TEER measurements provide valuable insights into the integrity of tight junction barriers between cells. Using culture inserts, TEER is measured with a voltmeter to assess the ability of a cell monolayer to restrict ion and solute passage between apical and basolateral compartments, serving as an indicator of barrier function. High TEER values reflect intact and functional tight junctions with low permeability, while low TEER values suggest compromised barrier integrity. We measured TEER in ARPE-19 cells irradiated with 4, 8, and 16 Gy doses using FLASH or CONV modalities, comparing them to non-irradiated, age-matched controls at 3, 7, and 14 days post-irradiation. The extended time frame was chosen to allow ARPE-19 cells to develop sufficient TEER for accurate assessment. Results revealed a dose- and modality-dependent effect on TEER. FLASH irradiation at 4 Gy had negligible effects on TEER throughout the study period (Fig. 5A, red bars). However, FLASH doses of 8 and 16 Gy caused a transient drop in TEER 7 days post-exposure (Fig. 5A, red bars). In contrast, CONV irradiation was less protective: while 4 and 8 Gy doses were relatively safe, 16 Gy caused a significant decline in TEER 7 days post-irradiation (Fig. 5A, blue bars). Overall, FLASH irradiation better preserved TEER in ARPE-19 cells across all doses and time points compared to CONV irradiation. To further investigate the mechanisms underlying these observations, we analyzed the expression of ZO-1, a key tight junction protein that links transmembrane proteins (e.g., claudins, occludins) to the actin cytoskeleton and regulates paracellular permeability. ZO-1 mRNA levels were significantly upregulated in FLASH-irradiated ARPE-19 cells 24 h post-exposure compared to non-irradiated controls (Fig. 5B, red bars. 4 Gy p = 0.0198; 8 Gy p = 0.0015; 16 Gy p = 0.0084), whereas CONV irradiation did not elicit a similar response (Fig. 5B, blue bars). By 72 h, ZO-1 expression in both FLASH- and CONV-irradiated cells returned to baseline levels (Fig. 5B). Indeed, the disruption of ZO-1 arrangement observed upon irradiation was comparable to that described following exposure to oxidizing agents, such as H2O2 (Fig. 5C). These findings suggest that FLASH irradiation enhances acute-phase repair mechanisms in ARPE-19 cells, as evidenced by increased ZO-1 expression and higher TEER values. Collectively, the results highlight the potential of FLASH irradiation to better preserve barrier integrity and promote tight junction repair in ARPE-19 cells following radiation exposure.
FLASH irradiation preserves barrier integrity and enhances tight junction repair in ARPE-19 cells compared to CONV. (A) Bar graph shows Transepithelial Electrical Resistance (TEER) measurements of ARPE-19 cells 7 days post irradiation of increasing intensities, in FLASH (red columns) or CONV (blue columns) modalities. Controls (gray columns) are age-matched culture plates, non-exposed to irradiation. A minimum of 5 independent wells was used for measurements of TEER in each experimental condition. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as mean ± S.D. p values are indicated in the graphs. (B) Bar graph of qRT-PCR results of ZO-1 gene expression in ARPE-19 cells 24 h and 72 h post irradiation of increasing intensities, either in FLASH (red columns) or CONV (blue columns) modalities. Controls (black dotted line, set to 1) are age-matched culture plates, non-exposed to irradiation. The experiment was conducted on 3 biological replicates for each condition. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test and expressed as mean ± SD. Statistically significant results show comparisons between non exposed control cells and irradiated cells. The graphs also show results of comparisons between ARPE-19 irradiated in CONV and ARPE-19 irradiated in FLASH modalities. p values are indicated in the graphs. (C) Representative images of ARPE-19 cells immunostained with ZO-1 antibodies where the intact profile of the tight junctions can be observed in a control culture plate (left, CTRL), while their disruption is evident following oxidative stress (right).
FLASH and CONV irradiation effects on oBRB integrity in C57BL/6 mice
To investigate the physiological relevance of our in vitro results, we analyzed the structure and integrity of the retinal pigment epithelium (RPE) in healthy C57BL/6 mice exposed to radiation. A key role of the RPE is to form and maintain the outer blood-retinal barrier (oBRB), which relies on tight junctions (Fig. 6A). Disruption of the oBRB is a hallmark of inflammation and can leave the retina vulnerable to harmful agents.
To test whether FLASH irradiation preserves oBRB integrity, we used ZO-1 immunofluorescence to assess the condition of tight junctions. We began with a pilot study to determine optimal time points for detecting both acute and chronic RPE damage. Mice were sampled approximately 3 days (± 2 days) after irradiation to evaluate acute effects, and around 40 days (± 5 days) to assess chronic effects.
Subsequently, we focused on chronic damage assessment, as acute-phase damage was minimal at lower doses (10 and 15 Gy). We examined RPE damage at 10, 15, and 20 Gy doses (Fig. 6B). For comparison, we also measured RPE integrity 3 days after 20 Gy irradiation (Supplementary Fig. 1), where our pilot data showed minor early damage.
Our results revealed a small but significant disruption in RPE continuity 3 days post-irradiation, suggesting that radiation triggers a process that evolves over time. At the chronic time point, ZO-1 analysis showed a clear dose-dependent decline in tight junction integrity (Fig. 6B: 10 Gy, p = 0.0002 and p = 0.0001; 15 Gy, p = 0.0001 and p = 0.0010; 20 Gy, p < 0.0001. Figure 6C: 10 Gy, p = 0.0014 and p = 0.0010; 20 Gy, p < 0.0001), along with a reduced number of RPE cells (Fig. 6C: 10 Gy, p = 0.0377 and p = 0.0130; 20 Gy, p < 0.0001) compared to non-irradiated controls.
These findings demonstrate that radiation significantly disrupts RPE structure and oBRB barrier integrity23. Importantly, we did not observe any protective effect from FLASH irradiation at later time points, indicating that both FLASH and conventional (CONV) irradiation ultimately cause similar levels of oBRB disruption in this mouse model.
FLASH and CONV irradiation effects on oBRB integrity in C57BL/6 mice. (A) Representative images of mouse whole-mount RPE preparation immunostained with Ab against ZO-1. Preparations from irradiated animals clearly revealing disruptions in the ZO-1 pattern (arrow) compared to the regular and intact ZO-1 profile of control, non-irradiated mouse. (B, C) Bar graphs show comparison among ZO-1 density profiles in the RPE of C57BL6 mice irradiated at different doses, in FLASH (red columns) or CONV (blue columns) modalities. Controls (gray columns) are age-matched mice, non-exposed to irradiation. For 20 Gy long term experiments a total number of 9 mice were used (n = 3/treatment; n = 3 controls). For 15 Gy treatment a total number of 9 animals were used (n = 3/ treatment; n = 3 controls). For 10 Gy treatment a total number of 9 animals were employed (n = 3/ treatment; n = 3 controls). Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test and expressed as mean ± S.D. p values are indicated in the graphs. (B) Comparison of ZO-1 density profiles in the RPE of C57BL6 mice, quantified using a custom-designed grid. (C) Quantification of the ZO-1-positive pattern and RPE cell count per field was carried out using the custom-developed software hAPPythelium. This analysis validated the findings at both the highest and lowest irradiation doses explored in the study.
Behavioral and visual effects of FLASH and CONV irradiation in C57BL/6 mice
To evaluate the impact of FLASH and CONV irradiation on locomotion and visual depth perception, key indicators of brain function, we conducted behavioral assays in naïve C57BL/6 mice. First, we measured the total distance moved and mean velocity during spontaneous exploration of an empty arena. No differences were observed between 10 Gy FLASH- and CONV-treated animals. However, mice treated with 15 and 20 Gy exhibited reduced locomotor activity, covering shorter distances (CONV 15 Gy p = 0.0079; FLASH 20 Gy p = 0.0055, CONV 20 Gy p = 0.0027) and maintaining lower velocities (FLASH 15 Gy p = 0.0187, CONV 15 Gy p = 0.0187; FLASH 20 Gy p = 0.0060, CONV 20 Gy p = 0.0031) compared to untreated controls. Notably, 15 Gy FLASH-treated mice showed a milder reduction in locomotor capabilities than 15 Gy CONV-treated mice (p = 0.0249), suggesting a protective effect of FLASH irradiation at this dose (Fig. 7A). Next, we assessed depth perception in mice with normal locomotion (10 Gy-treated animals) by calculating their exploration index, which reflects their preference for the shallow side of a testing arena (Fig. 7B). No significant differences in depth perception were observed between 10 Gy FLASH- and CONV-treated mice compared to untreated controls (Fig. 7C). These findings indicate that while higher doses of radiation impair locomotion, FLASH irradiation may mitigate some of these effects at intermediate doses. However, neither FLASH nor CONV irradiation at 10 Gy affected visual depth perception in this model.
Behavioral and visual effects of FLASH and CONV irradiation in C57BL/6 mice. (A) The bar graphs illustrate the mean velocity and the total distance moved by mice 14 days before and 7 days after exposure to increasing irradiation doses in both FLASH (red bars) and CONV (blue bars) modes. Controls (gray bars) are age-matched mice non exposed to irradiation. For 20 Gy experiment, a total number of 9 mice were used (n = 3/treatment; n = 3 controls). For 15 Gy treatment a total number of 9 animals were used (n = 3/ treatment; n = 3 controls). For 10 Gy treatment a total number of 9 animals were employed (n = 3/ treatment; n = 3 controls). Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test and expressed as mean ± S.D. p values are indicated in the graphs. (B) Schematic illustration of the apparatus used for the visual cliff test; in the image, the two areas of the arena are visible: on the right, the “safe” or shallow side, and the “cliff” or deep side on the left. (C) The bar graph illustrates the time spent on the “cliff” side by mice 14 days before and 7 days after exposure to 10 Gy in both FLASH (red bars) and CONV (blue bars) modes. Controls (gray bars) are age-matched mice non exposed to irradiation. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test and expressed as mean ± S.D. p values are indicated in the graphs.
Discussion
Radiotherapy remains a cornerstone of cancer treatment, among others because of its efficacy and limited cost. Emerging modalities such as FLASH-RT have garnered considerable attention for their potential to spare normal tissues while maintaining tumor control. However, interpreting the “FLASH effect” presents challenges, primarily due to (i) the need for precise control over irradiation parameters and (ii) the lack of comprehensive studies bridging in vitro cellular responses with in vivo biological outcomes. To address these challenges, we utilized a state-of-the-art irradiation setup with rigorous dosimetry. Our EF LINAC ensured accurate dose delivery, and an in-house system enabled precise mouse positioning for in vivo studies24,25. Importantly, our setup allowed direct comparisons of FLASH and CONV irradiation under identical experimental conditions, minimizing errors.
We investigated the effects of FLASH and CONV irradiation on ARPE-19 cells and the native RPE in live mice to elucidate their differential impacts on healthy cells. Our findings demonstrate that: (i) FLASH irradiation induces unique biological effects in ARPE-19 cells under specific conditions; (ii) the native RPE exhibits a dose-dependent response to radiation; and (iii) FLASH-treated mice show superior behavioral outcomes compared to CONV-treated mice.
First, we assessed ARPE-19 cell viability three days post-irradiation. The MTT assay revealed that both FLASH and CONV irradiation reduced metabolic activity compared to controls. However, at 4 Gy, FLASH-treated cells exhibited significantly higher metabolic function than CONV-treated cells. DNA staining showed fewer pyknotic nuclei in FLASH-exposed cells at 4 Gy, indicating reduced apoptosis. At higher doses (8 and 16 Gy), no significant differences in cell survival or metabolic activity were observed between FLASH and CONV conditions. Morphological analysis revealed oversized nuclei, a hallmark of radiation-induced damage and genomic instability. At 8 Gy, FLASH-treated cells had a higher proportion of enlarged nuclei, whereas at 16 Gy, this effect was more pronounced in CONV-treated cells, suggesting that FLASH irradiation may be less damaging at higher doses.
Oversized nuclei and cells are indicative of radiation-induced senescence, often mediated by the p21Cip1 and p16Ink4a pathways26. We analyzed the expression of p21Cip1 and p16Ink4a mRNA in irradiated ARPE-19 cells (4, 8, and 16 Gy) at 24 and 72 h post-exposure. p21Cip1 was significantly upregulated, while p16Ink4a expression remained unchanged across all doses and modalities Combined with ROS data, these findings align with prior research indicating that p21Cip1-mediated senescence is driven by ROS accumulation in response to acute radiation damage27. p16Ink4a, however, might be associated with long-term cellular stress and aging28. Recent studies on genetically engineered animals demonstrated that radiation-induced osteoporosis is primarily mediated by p21Cip1, rather than p16Ink4a29. The identification of p21Cip1 as a regulator in the ARPE-19 cell radiation response provides valuable insights into the mechanisms underlying this complex biological process. We also analyzed the expression of IL-6 and IL-8, cytokines involved in ocular homeostasis and cancer progression30. CONV radiation significantly increased IL-6 expression compared to controls and FLASH-treated cells, while IL-8 levels remained unchanged across all conditions. These results are consistent with the established role of IL-6 in inflammation, whereas IL-8 predominantly associates with neutrophil chemotaxis and acute immune responses30. The ability of FLASH irradiation to limit IL-6 overexpression holds significant implications for radiotherapy. Elevated IL-6 levels can counteract radiation-induced cell death, reducing treatment efficacy31. In contrast, IL-6 inhibition sensitizes tumor cells to radiation32. FLASH irradiation maintained IL-6 levels comparable to non-irradiated controls, potentially enhancing radiotherapy effectiveness. Moreover, given that IL-6 is a secreted cytokine, its regulation may have broader implications in vivo, potentially affecting tissues adjacent to the RPE, such as the retina, contributing to bystander effects in radiation-exposed environments. Furthermore, in radiation biology, oxidative stress is a central concept, resulting from the generation of ROS upon radiation exposure. IL-6 plays a crucial role in mediating cellular responses to oxidative stress33. Our data demonstrated that ROS levels were significantly higher in CONV-treated cells compared to FLASH-treated cells at all doses and in a dose-dependent manner, supporting the notion that FLASH irradiation is less damaging to healthy RPE cells. These findings are consistent with viability assays, morphological analyses, and IL-6 expression data, altogether supporting the notion that FLASH radiation is less damaging to healthy RPE cells.
To gather functional data and assess the integrity of tight junctions, TEER analysis was performed in ARPE-19 cultures revealing that FLASH irradiation preserved tight junction integrity better than CONV radiation, particularly at 16 Gy. CONV radiation caused a substantial decrease in TEER amplitude, while FLASH-treated cells maintained higher intracellular resistance. This is relevant given the functional role of tight junctions in the native RPE, where they contribute the outer blood-retinal barrier integrity. Moreover, ZO-1 mRNA expression was transiently upregulated in FLASH-treated cells 24 h post-irradiation, suggesting a protective response that maintains tight junction integrity and adhesion proteins, thus protecting cells from further radiation-induced damage34. This transient ZO-1 increase may represent part of a broader cellular stress response, contributing to enhanced survival and homeostasis in FLASH-irradiated RPE cells.
In vitro data demonstrate a FLASH-sparing effect across various irradiation paradigms in differentiated ARPE-19 cells. While the FLASH effect does not manifest consistently at the same doses, it generally translates into higher cell survival compared to CONV irradiation. This protective effect is accompanied by a lower ROS production, maintenance of TEER amplitude, and a striking difference in IL-6 regulation. These findings provide a compelling rationale for future studies exploring whether FLASH radiation can extend to tissues connected to the RPE, and also indicate possible mechanisms underlying the occurrence of FLASH effect, sparing normal tissues. These might include limitation of ROS production, lower cytokine release, decreased DNA damage, leading to higher cell viability and metabolic activity.
To bridge the gap between in vitro and in vivo responses, we irradiated age-matched mice with single-fraction, whole-brain doses of 10, 15, and 20 Gy. ZO-1 antibody staining revealed progressive, dose-dependent damage to RPE barrier integrity and cell counts, with no significant FLASH-sparing effect observed.
ZO-1 antibody staining of RPE from irradiated animals demonstrated progressive, dose- and time-dependent adverse effects on barrier integrity and cell counts, observed both at 3 and 45 days post-irradiation. Notably, no protective effect of FLASH irradiation was detected in either acute or chronic conditions. However, these findings underscore two key aspects: (i) the resilience of the RPE in vivo and (ii) the dose-dependent impact over time and across different radiation doses. A striking discrepancy emerges between in vitro and in vivo results: while a sparing effect is evident in vitro, it is not observed in vivo. This difference may be attributed to the complex in vivo environment, where interactions with surrounding tissues, immune cells, and the extracellular matrix could influence the RPE response to radiation. Consequently, the protective effect seen with FLASH-RT in vitro may not translate in vivo due to these additional biological interactions.
The interpretation of the visual system effects observed here cannot be restricted to the native RPE, given the fact that, with our current apparatus, we could not limit irradiation to the eye region. However, a healthy retinal pigment epithelium is known to be essential for accurate performance on the visual cliff test. Our data demonstrate that RPE function remains intact in whole-brain-irradiated mice, indicating that the irradiation did not impair this critical cell population.
Given the critical role of the RPE in ocular health and its involvement in diseases such as macular degeneration, our findings offer valuable insights into its structural and functional responses to radiation. Additionally, as the RPE is secondarily exposed to radiation during uveal melanoma treatment, understanding its cellular-level response is crucial. These findings could contribute to the development of safer and more precise radiotherapy strategies, ultimately improving therapeutic outcomes for ocular conditions.
To gain a general idea of the animal well-being and a possible sparing FLASH effect, we assessed the impact of treatments on animal behavior and, for the first time, visual perception, using the same doses employed for assessing RPE responses. We observed that a dose of 10 Gy did not impair locomotion, regardless of whether FLASH or CONV irradiation was used. Likewise, mouse depth perception remained intact. Conversely, both 15 and 20 Gy reduced motor performance and the average speed and total distance travelled. Notably, in accordance with previous studies35among the 15 Gy-treated mice, those subjected to FLASH irradiation exhibited a milder decline in motor function compared to their CONV-treated counterparts, suggesting a potential sparing effect at this dose.
In conclusion, our study provides a comprehensive evaluation of FLASH and CONV irradiation effects on the RPE, combining in vitro and in vivo approaches. FLASH irradiation demonstrated protective effects on ARPE-19 cells, including reduced ROS production, preserved barrier integrity, and regulated cytokine expression. While no significant FLASH-sparing effect was observed in the native RPE, behavioral outcomes suggested potential benefits at intermediate doses. These findings underscore the importance of further research into FLASH irradiation as a safer and more effective treatment modality for ocular conditions, particularly in the context of uveal melanoma therapy, where eye irradiation inevitably creates secondary damage to surrounding organs, including the CNS.
Our results underscore the need for further investigation into adjunctive therapies or protective strategies that can be combined with radiotherapy to preserve RPE function and oBRB integrity. This could be particularly relevant for patients undergoing radiation treatment for uveal melanoma, orbital tumors, or in cases where the posterior eye may receive incidental exposure during head and neck cancer radiotherapy.
Overall, by characterizing the dose-dependent and time-evolving impact of both FLASH and CONV irradiation on the RPE, our study provides a foundational understanding that can inform future efforts to optimize radiotherapy protocols while minimizing vision-related side effects.
Methods
The linear accelerator (LINAC)
We used the ElectronFlash LINAC (EF) accelerator, installed in a dedicated bunker (Centro Pisano FLASH RadioTherapy, Pisa)18. The EF can deliver electronic bunches of 0.1–4 µs duration, with dose-per-pulse values ranging from 0.03 Gy up to 10 Gy with a beam size greater than 3 cm of diameter (with a uniformity dose distribution of 95%). The LINAC can also switch between two nominal energies: 9 MeV and 7 MeV, although for the experiments described here we opted for the 9 MeV beam to ensure the full coverage of the irradiated target (both in vitro and in vivo). Key feature of EF is a triode gun, which enables to vary the e-beam current (i.e., the dose output) while maintaining the same energy spectrum and thus the same irradiation setup for both CONV and FLASH treatments.
The dose rate chosen for the FLASH experiments was the maximum value achievable for all the doses set for that experiment. The difference in dose rate between the in vivo and in vitro experiments (240 Gy/s and 940 Gy/s respectively) is due to the different sizes of the collimator (100 mm in diameter for in vitro and 40 mm in diameter for in vivo), which affects the dose per pulse.
Experimental in vitro setup
For the in-vitro irradiations, EF was used in vertical position with the largest available PMMA applicator (∅ 120 mm; Fig. 1A). A PTW solid water slab, 12 mm thick was placed at the end of the applicator to reach the build-up uniform region for the 9 MeV beam (Fig. 1B, C). This configuration is optimal for in vitro experiments as it ensures irradiation under the most uniform and repeatable conditions. Table 1 shows the main relevant dosimetric quantities used for the CONV and FLASH irradiations. The main relevant dosimetric quantities were: 4-8-16 Gy, with a dose-rate of 240 Gy/s (FLASH) and 0.05 Gy/s (CONV). For dose monitoring, the protocol used included the intrinsic EF monitoring system36complemented by the reading of an absolute active dosimeter (FLASH Diamond – fD37) positioned next to the sample, allowing for a second accurate check at the measurement point.
Experimental in vivo setup
For the in vivo experiments, the EF was tilted at an oblique angle of 60°, using a smaller applicator compared to the in vitro counterpart (∅ 40 mm). This choice was made both to achieve more extreme beam parameter values and to better conform the beam to the target. The animals, placed in an ad hoc holder (Fig. 1D, E) received a single-fraction whole-brain irradiation, and the dose planning was carried out through Monte Carlo simulations (GATE) based on a micro-CT scan with an isotropic voxel size of 0.06 × 0.06 × 0.06 mm^3 (60-micron size) (Fig. 1F). This approach allowed for a prior evaluation of the irradiation plan quality through the creation of Dose-Volume Histograms (DVHs), enabling the selection of the best possible configuration to spare organs at risk while ensuring optimal target coverage (Fig. 1G).
The mouse holder (Fig. 1E) was specifically designed for the type of irradiation employed and to allows easy adjustments in both forward/backward and up/down directions. The mouse was placed in a U-shaped compartment angled at 60° relative to the ground, ensuring a 90° angle between the animal’s body and the applicator exit (i.e., the beam direction) (Fig. 1E). A mouth-fixing clamp securely held the mouse’s teeth, providing firm stabilization. The combination of the U-shaped structure, the 60° angle, and the force of gravity enhanced the stability of the restrained animal during the whole procedure. Micro-CT scans were performed with an IRIS PET/CT tomograph, at the Institute of Clinical Physiology, CNR-IFC, Pisa. A high resolution protocol was used, with the following settings: 65 kVp, 1 mA, 2000 projections, 70 ms/projection.
Cell cultures
The human RPE cell line ARPE-19 (CLR-2302; ATCC, Manassas, VA, USA) was cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 supplemented with 10% fetal bovine serum, 1% L-glutamine and 1% penicillin/streptomycin solution at 37 °C in 5% CO2. Chemicals used for cell cultures were purchased from Sigma–Aldrich (Merck, Darmstadt, Germany). Typically, cells were seeded at a density of 4 × 103 cells/cm2 and cultured for one week, until they reach confluence and are on the early phase of differentiation. The medium was changed 24 h before irradiation; analyses were performed 24 h, 48 h, 72 h and 14 days post radiation. ARPE-19 cells used for the experiments were between passages number 10 and passage number 20.
Cell metabolic activity assay
Cells metabolic activity was assessed using the CellTiter 96® Non-Radioactive Cell Proliferation, MTT Assay (Promega, Madison, WI, USA). ARPE-19 cells were seeded in a 96-well plate according to the protocol described above and cultured for one week. Seventy-two h (h) post-radiation the Dye Solution containing 3-(4,5-cimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide was added to medium. After 2 h of incubation, the cells were lysed using the Solubilization/Stop Solution provided with the kit and absorbance at 595 nm was measured using a fluorescence Biorad Marck Microplate reader (BioRad, Hercules, California, USA). Three consecutive measures were obtained for each plate and the readings were exported to Excel for statistics. Results of MTT assay were pooled from 12 replicate samples derived from 2 independent experiments and expressed as mean ± S.D.
Cell count, nuclear size assessment and Immunofluorescence
ARPE-19 cells were seeded on laminin-coated coverslips according to the protocols described above. Seventy-two h post exposure to FLASH or CONV radiation, cells were fixed in 4% paraformaldehyde for 15 min, then permeabilized with 0.5% Triton X-100 and blocked in 5% serum for 2 h and then incubated overnight at 4 °C with a monoclonal antibody against ZO-1 (Invitrogen, 61-7300, 1:500). The following day, after three 5 min washes with phosphate-buffered saline (PBS), cells were incubated with a fluorescent secondary antibody for 2 h at room temperature. Finally, cells were counterstained with 2 nM Hoechst, washed three times, and mounted on glass slides. Images were acquired using a Zeiss Imager.Z2 microscope equipped with an Apotome3 device (Carl Zeiss, Oberkochen, Germany) using a Plan Neofluar ×40/1.25 oil objective and processed with ImageJ software (Bethesda, MD, USA). For each condition of irradiation, 4 biological replicates were made. For each replicate, cells were counted in 5 fields regularly spaced along the 2 main meridians (horizontal and vertical) of the round coverslip. Counting areas were 223.8 × 167.6 μm fields. In each field, z stacks of adjacent images spaced 0,5 μm apart were systematically obtained. Stacks covered 15 μm along the z axis. Total number of cells/field was counted manually; pyknotic nuclei were subtracted to obtain the number of viable cells/field. For statistical analysis the mean number of viable cells for each biological replicate was compared. The diameters of all the nuclei in the field were measured. A normal nucleus measures on average 10 µM. Nuclei considered as «Large size» had a major diameter comprised between 20 and 30 µM. The number of Large-size nuclei was expressed as percentage referred to total number of nuclei of viable cells in each field. The mean number of large-size nuclei for each biological replicate was compared. The experiments were conducted on 4 independent biological replicates, each representing the average values obtained from 9 imaging fields per slide.
Intracellular ROS measurements
Reactive oxygen species were assayed using a DCFDA/H2DCFDA- Cellular ROS assay kit (ab113851, AbCam, Cambridge, UK) and a fluorescence spectroscopy detector. ARPE-19 cells were seeded in 96-well plates as described above. On the radiation day, cells were pre-incubated for 40 min at 37 °C and 5% CO2 with 20 µM DCFDA dissolved in Krebs-Henseleit Buffer enriched with 10 mM L-Glucose and 2,5 mM CaCl2. After 40’s, the solution containing DCFDA was removed and replaced with Krebs-Henseleit Buffer alone. A 5 min baseline was recorded using a Thermo Fisher Varioskan LUX Multimode microplate fluorescence reader (Thermo Fisher Scientific, Waltham, Massachusetts, USA) with excitation/emission at 485 nm/535 nm. The cells were then irradiated in either CONV or FLASH modality and DCF fluorescence measured continuously for 30 min after radiation exposure. The control plates contained age-matched cells not exposed to irradiation. In this experiment, H2O2 serves as positive control, as its ability to reliably induce oxidative stress validated the proper functioning of the instrument.
Gene expression assessment
Total RNAs from ARPE-19 cells were extracted using the RNeasy mini kit (74104; Qiagen, Germantown, MD, USA), according to the manufacturer’s protocol and quantified by using Nano Drop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Afterwards, cDNA was synthesized from isolated RNA (1000 ng) using QuantiTect Reverse Transcription Kit (205311; Qiagen, Germantown, MD, USA). mRNA expression was quantified via qRT-PCR using TaqMan Universal PCR Master Mix added to the specific TaqMan Gene expression assays (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The reaction was performed using the Thermal Cycler StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The assays used for the experiments were p21Cip1 (cdkn1a-Hs00355782_m1), p16Ink4a (cdkn2a-Hs00923894_m1), IL-6 (Hs00174131_m1), IL-8 (cxcl8-Hs00174103_m1), ZO-1 (Tjp1-Hs01551871_m1) and β-Actin (Hs01060665_g1) and were purchased from Thermo Fisher Scientific. Data were analyzed by the ΔΔCt method using β-Actin as housekeeping and compared by one-way ANOVA followed by Tukey’s multiple comparison test. The experiment was conducted on 3 biological replicates.
Trans epithelial electrical resistance (TEER) analysis
ARPE-19 cells were seeded as described above on Hanging Cell Culture Inserts (Millicell, Merck KGaA, Darmstadt, Germany) equipped with an Isopore Membrane (polycarbonate), having a 9.5 mm diameter and 0.4 μm pore size. The inserts were placed in 24-well plates, providing two-chamber compartments. Half of the plates containing the cells were irradiated in either CONV or FLASH mode, while the control plate consisted of age-matched cells, non-exposed to radiation. TEER was measured by The Millicell® ERS-2 System (Millipore, Billerica, MA) using STX3 chopstick electrodes. Measurements were obtained 72 h and 14 days after irradiation. The net TEER values (Ω) were calculated by subtracting the value of blank inserts (with no cells seeded) from the value recorded for each individual filter seeded with cells. A minimum of six biological replicates were used for each experimental condition.
Animals
All experimental procedures conformed to the European Communities Council Directive #86/609/EEC, were approved by the Italian Ministry of Health (1204/2020-PR, prot. B4BB8.30, 02/10/2020 and addendum prot.B4BB8.30.EXT.14 del 02-07-2024). Experimental procedures were in accordance with Italian and European institutional guidelines, and the experimental protocols were approved by the Italian Ministry of Health and by the intramural Ethical Committees. Protocols also adhered to ARRIVE guidelines. 57Bl6J mice were purchased from Charles River Laboratories (France) and were maintained in a local facility where they had free access to water and daily monitored food intake. Mice at 60 days of age (PN60) were used for experiments. This age corresponds to young adulthood, with a fully developed CNS. Controls (non-irradiated mice) were age-matched and maintained in the same conditions. Male and female mice were equally represented in all experimental groups. Mice were exposed to whole-brain irradiation at 20, 15 and 10 Gy doses using either CONV or FLASH delivery. The animals were observed daily following the entire post-radiation period; weight, hair loss and facial expressions were monitored to assess physical pain (Grimace scale), while the ability to create a nest and the presence of stereotyped behaviors were used as indicators of overall well-being. If pain levels were deemed too severe, the mice were humanely euthanized by decapitation as per guidelines. For 20 Gy acute experiments (3 days post radiation analysis) a total number of 12 animals were used (n = 4/each treatment; n = 4 controls). For 20 Gy chronic experiments (45 days post radiation analysis) a total number of 21 mice were used (n = 9/treatment; n = 3 controls). For 15 Gy treatment, a total number of 15 animals were used (n = 6/ treatment; n = 3 controls). For 10 Gy treatment, a total number of 9 animals were employed (n = 3/ treatment; n = 3 controls).
Motor activity and visual depth perception
The experimental setup consisted of a rectangular arena of 50 × 36 cm, constructed from poly (vinyl chloride) with opaque white walls and surrounded by black curtains. The arena was subdivided into two equal sections (25 × 36 cm each) with transparent Plexiglas plates forming the floors. Below each plate, a movable platform was positioned, whose depth could be adjusted using a mechanical scissor jack. The surface of each platform was covered with a 3-cm black-and-white checkered photographic pattern. Led lamps beneath the patterned platforms ensured uniform brightness across both sides of the arena. A camera mounted above the apparatus was connected to a computer, enabling the experimenter to observe and record the animals’ behavior in real-time. The experiments were conducted in a quiet room, with the arena divided into a shallow and a deep side. On the shallow side, the patterned platform was positioned directly beneath the Plexiglas plate, whereas on the deep side, the platform was lowered to 29 cm below the Plexiglas. Each animal was introduced to the shallow side, and the behavior was recorded for 5 min, during which the time spent exploring each side was measured. Locomotion patterns and velocity were recorded and analyzed using the Noldus EthoVision system (Wageningen, Netherlands). Between trials, the arena was cleaned with a 10% alcohol solution to eliminate scent cues. For all the animals that displayed normal locomotion, an exploration index was calculated to display a preference for the shallow side, interpreted as depth perception capability, as follows: (Time spent in the shallow side – Time spent in the deep side)/Total time.
Retinal pigment epithelium image analysis
For histological studies, mice were deeply anesthetized with intraperitoneal injections of 40 mg/kg of Zoletil, their eyes enucleated, and the animals humanely killed by decapitation. Eye cups were obtained and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 1 h at room temperature. Eye cups were washed in buffer, cryo-protected in 30% sucrose, frozen in cold isopentane, and stored at − 80 °C until use. Defrosted eye cups were submerged in buffer, 4 radial incisures were cut toward the optic nerve head to gently isolate the retina which was processed independently. The sclera-choroid and RPE were further cut radially with additional 4 to 8 incisions before being processed for whole mount immunocytochemistry (ICCH) using the following method: (1) block solution with 0.3% Triton X-100, 5% serum and 0.01 M PBS, overnight incubation; (2) incubation with rat polyclonal antibody against ZO-1 (MABT11, Merck-KGaA, Darmstadt, Germany) diluted 1:400, and rabbit polyclonal antibody against ionized calcium-binding adapter molecule 1 (Iba1) (FUJIFILM Wako Chemicals U.S.A. Corp) diluted 1:500, for 3 days at 4 °C; (3) incubation for 24 h in fluorescent secondary Ab (anti-rat IgG, conjugated with Alexa Fluor 488 and anti-rabbit IgG, conjugated with Alexa Fluor 568, all from Invitrogen, Termofisher Scientific). After repeated washing, the RPEs were counterstained with Hoechst (0.02 mg/mL), flat-mounted on glass slides and covered with Vectashield antifade mounting medium (Vector, Burlingame, USA). Images of RPE preparations were obtained with a Zeiss Imager.Z2 microscope as described above. Whole mount RPEs stained with ZO-1 were imaged regularly along 4 radial axes. Within each axis, 1 central and 2 mid-peripheral sampling fields were chosen in optimally flattened areas. This resulted in a total of 20 sampling fields, each measuring (224 × 168) µm, for each specimen. Images were captured as z stacks covering the entire width of the RPE leaflet, typically consisting of 10–15 evenly spaced sections. The Zeiss software ZEN®2 was used to adjust brightness and contrast of the pictures and to generate projection images which were saved as tiff files. Image J was used to count in each projection the number of intersections between ZO-1-positive profiles and an overlapping (custom-generated) grid, with 20 μm-spaced mashes. For additional quantification of ZO-1positive pattern, a software package developed ad hoc and called hAPPythelium was used to confirm the data. The app, devised by Raffaele Mazziotti, University of Florence, Italy, is available open source on GitHub at this link: https://github.com/raffaelemazziotti/hAPPytelium_code. Original description in Napoli et al., submitted.
Statistical analysis
Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test or by two-way ANOVA followed by Bonferroni’s multiple comparison test using Prism 8 (GraphPad, La Jolla, California, USA). A significant effect was indicated by a P-value < 0.05. Significance was indicated as follows: * p value < 0.05, ** p value < 0.01, *** p value < 0.001, **** p value < 0.0001. Data are expressed as mean ± SD.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
References
Kim, M. M. & Zou, W. Ultra-high dose rate FLASH radiation therapy for cancer. Med. Phys. 50, 58–61 (2023).
Chaikh, A. et al. Towards clinical application of ultra-high dose rate radiotherapy and the FLASH effect: challenges and current status. Cancer/Radiothérapie 28, 463–473 (2024).
Horst, F. et al. Dose and dose rate dependence of the tissue sparing effect at ultra-high dose rate studied for proton and electron beams using the zebrafish embryo model. Radiother. Oncol. 194, 110197 (2024).
Montay-Gruel, P. et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin. Cancer Res. 27, 775–784 (2021).
COLLABORATIVEOCULARMELANOMASTU. The collaborative ocular melanoma study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am. J. Ophthalmol. 138, 936–951 (2004).
Furdova, A. et al. Uveal melanoma survival rates after single dose stereotactic radiosurgery. Neoplasma 65, 965–971 (2018).
Foti, P. V. et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging—Part II: treatment indications and complications. Insights Imaging. 12, 67 (2021).
Sarici, A. M. & Pazarli, H. Gamma-knife-based stereotactic radiosurgery for medium- and large-sized posterior uveal melanoma. Graefe’s Archive Clin. Experimental Ophthalmol. 251, 285–294 (2013).
Verma, V. & Mehta, M. P. Clinical outcomes of proton radiotherapy for uveal melanoma. Clin. Oncol. 28, e17–e27 (2016).
Cicinelli, M. V. et al. Predictive factors of radio-induced complications in 194 eyes undergoing gamma knife radiosurgery for uveal melanoma. Acta Ophthalmol 99, (2021).
Joye, R. P. et al. Local control and results of Leksell gamma knife therapy for the treatment of uveal melanoma. Ophthalmic Surg. Lasers Imaging Retina. 45, 125–131 (2014).
Zemba, M. et al. Ocular complications of radiotherapy in uveal melanoma. Cancers (Basel). 15, 333 (2023).
Bok, D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci 189–195 (1993). (1993).
Hellinen, L. et al. RETRACTED: Hellinen et al. 12, 176. Pharmaceutics 14, 595 (2022). (2020).
Bird, A. Role of retinal pigment epithelium in age-related macular disease: a systematic review. Br. J. Ophthalmol. 105, 1469–1474 (2021).
Willermain, F. et al. Potential interplay between hyperosmolarity and inflammation on retinal pigmented epithelium in pathogenesis of diabetic retinopathy. Int. J. Mol. Sci. 19, 1056 (2018).
DUNN, K. C., AOTAKI-KEEN, A. E., PUTKEY, F. R. & HJELMELAND, L. M. ARPE-19, A human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 62, 155–170 (1996).
Di Martino, F. et al. Architecture, flexibility and performance of a special electron Linac dedicated to flash radiotherapy research: electronflash with a triode gun of the Centro Pisano flash radiotherapy (CPFR). Front Phys 11, (2023).
Mannermaa, E. et al. Filter-cultured ARPE-19 cells as outer blood–retinal barrier model. Eur. J. Pharm. Sci. 40, 289–296 (2010).
Olivares, A., Alcaraz-Saura, M., Achel, D. G., de Berná-Mestre, J., Alcaraz, M. & D. & Radiation-Induced bystander effect: loss of radioprotective capacity of Rosmarinic acid in vivo and in vitro. Antioxidants 10, 231 (2021).
Ariyoshi, K. et al. Radiation-Induced bystander effect is mediated by mitochondrial DNA in Exosome-Like vesicles. Sci. Rep. 9, 9103 (2019).
Feldman, T., Yakovleva, M., Utina, D. & Ostrovsky, M. Short-Term and Long-Term effects after exposure to ionizing radiation and visible light on retina and retinal pigment epithelium of mouse eye. Int. J. Mol. Sci. 24, 17049 (2023).
Georgiadis, A. et al. Correction: The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One. 18, e0295782 (2023).
Di Martino, F. et al. A new calculation method for the free electron fraction of an ionization chamber in the ultra-high-dose-per-pulse regimen. Physica Med. 103, 175–180 (2022).
Di Martino, F. et al. A new solution for UHDP and UHDR (Flash) measurements: theory and conceptual design of ALLS chamber. Physica Med. 102, 9–18 (2022).
Cohn, R. L., Gasek, N. S., Kuchel, G. A. & Xu, M. The heterogeneity of cellular senescence: insights at the single-cell level. Trends Cell. Biol. 33, 9–17 (2023).
Levine, A. J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997).
Ressler, S. et al. p16 INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 5, 379–389 (2006).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Holtkamp, G. M. et al. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin. Exp. Immunol. 112, 34–43 (2001).
Matsuoka, Y. et al. IL-6 controls resistance to radiation by suppressing oxidative stress via the Nrf2-antioxidant pathway in oral squamous cell carcinoma. Br. J. Cancer. 115, 1234–1244 (2016).
Wu, C. T., Chen, M. F., Chen, W. C. & Hsieh, C. C. The role of IL-6 in the radiation response of prostate cancer. Radiat. Oncol. 8, 159 (2013).
Bernardo-Colón, A., Lerner, M. & Becerra, S. P. Pigment epithelium-derived factor is an interleukin-6 antagonist in the RPE: insight of structure-function relationships. Front Physiol 13, (2022).
Kuo, W. T. et al. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 161, 1924–1939 (2021).
Montay-Gruel, P. et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. U S A. 116, 10943–10951 (2019).
Di Martino, F. et al. FLASH radiotherapy with electrons: issues related to the production, monitoring, and dosimetric characterization of the beam. Front Phys 8, (2020).
Marinelli, M. et al. A diamond detector based dosimetric system for instantaneous dose rate measurements in FLASH electron beams. Phys. Med. Biol. 68, 175011 (2023).
Acknowledgements
The authors would like to thank Francesca Biondi (IN-CNR) for excellent animal care, Elena Novelli (IN-CNR) and Vania Liverani (SNS) for technical assistance and support.
Author information
Authors and Affiliations
Contributions
BDM performed experimental work and wrote the main manuscript text; G Sansevero performed experimental work; BD performed experimental work and contributed to manuscript writing; EDS performed experimental work; GS performed experimental work; AC performed experimental work; LM performed experimental work; MC performed experimental work; FDM conceptualized the study; SC conceptualized the study; DP performed experimental work; FP conceptualized the study; AG performed experimental work; GD performed experimental work; MC conceptualized the study, performed experimental work, wrote the manuscript; ES conceptualized the study, performed experimental work, wrote the manuscript. All the Authors critically read and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Di Marco, B., Sansevero, G., D’Orsi, B. et al. Unraveling the effects of FLASH and conventional irradiation on retinal pigment epithelial cells: in vitro and in vivo studies. Sci Rep 15, 22938 (2025). https://doi.org/10.1038/s41598-025-06101-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06101-x