Abstract
Heart failure (HF) is a global health burden, with high hospitalization and mortality rates. Timely diagnosis is crucial in the management of HF. While noninvasive imaging techniques generally assess changes in the left atrium (LA) or left ventricle (LV) alone, the application of physiological connections between these chambers as echocardiographic parameters, such as the left atrioventricular coupling index (LACI), may be valuable as an early marker of HF. We conducted a cross-sectional study in which 1145 Vietnamese individuals were selected and 160 subjects met the inclusion criteria. These participants were divided into patient and healthy control groups, with 60 HF patients. LACI levels were significantly greater in HF patients, especially those with preserved ejection fraction (HFpEF), than in those with reduced ejection fraction (HFrEF) and controls (59.16 ± 17.94% vs. 41.28 ± 15.27% and 13.15 ± 3.92%, respectively). The LACI showed strong diagnostic value for HFpEF, with an area under the curve (AUC) of 0.951 and an optimal threshold of 33.07 (sensitivity: 97.1%, specificity: 87.3%). Multivariate analysis confirmed LACI as an independent predictor of HFpEF (OR = 1.144, 95% CI 1.087–1.205). Overall, LACI has emerged as an accessible, promising tool for diagnosing HFpEF.
Similar content being viewed by others
Introduction
Heart failure (HF) is considered the final stage of various cardiovascular diseases, such as hypertension, coronary artery disease, valvular disorders, and structural heart conditions. It presents a major global health challenge, with high rates of hospitalization and mortality1,2,3,4. These factors contribute significantly to the economic burden on both individuals and healthcare systems5. Given these challenges, early diagnosis is crucial, as it can significantly improve both the quality of life and longevity of patients6.
Currently, HF diagnosis largely relies on echocardiography, which is widely regarded as the gold standard for confirming and classifying HF, as recommended by various guidelines worldwide7,8. While diagnosing HF with reduced ejection fraction (HFrEF) is primarily based on the left ventricular ejection fraction (LV EF), diagnosing HF with preserved ejection fraction (HFpEF) is more complex. HFpEF diagnosis requires additional steps to assess left ventricular filling pressures and depends on multiple echocardiographic parameters9. This process demands considerable time and effort from echocardiography specialists.
Several indices, including global longitudinal strain of the left ventricle (LV GLS), the left atrial volume index (LAVI), and left atrial strain, have been investigated for their potential role in diagnosing HFpEF10,11,12,13. However, these indices reflect only individual structural or functional aspects of the left atrium (LA) or left ventricle (LV). In contrast, the pathophysiology of HF indicates a strong physiological relationship between the LA and LV14. This raises the hypothesis that an index assessing both the LA and LV simultaneously could better predict HF than other individual indices of the LA or LV. Recently, the left atrioventricular coupling index (LACI) has emerged as an index capable of simultaneously assessing both the LA and LV. LACI has gained attention in cardiac magnetic resonance imaging, with promising results in its prognostic value for conditions such as atrial fibrillation and HF15,16,17,18,19.
However, the application of LACI in echocardiography remains relatively underexplored, and its diagnostic and classification value in HF remains unclear.
Methods
Study population
This cross-sectional study was approved by the Institutional Review Board of Hue University (No: H2022/044), and it adhered strictly to the principles outlined in the Declaration of Helsinki (2013 version). The participants were adults over 18 years of age who consented to the study before enrollment. One thousand one hundred forty-five selected patients were recruited from a convenience sample from 01/02/2022 to 01/06/2023 at Hue University of Medicine and Pharmacy (the flowchart of participant recruitment in the study is shown in Supplementary Fig S1). Among them, 160 subjects who met the study criteria and were not excluded were divided into patient and control groups. The patient group consisted of 60 adults who were hospitalized and met the selection criteria for the diagnosis of HF according to the 2022 American Heart Association, American College of Cardiology, and Heart Failure Society of America Guideline for the management of HF7. The exclusion criteria included severe comorbidities, pacemaker implantation, atrial fibrillation, severe aortic valve stenosis, severe aortic valve regurgitation, severe mitral valve stenosis, severe mitral valve regurgitation, life-threatening conditions, incomplete clinical data, inadequate echocardiographic data, poor echocardiographic image quality, and incomplete image analysis results. Within the HF patient group, two subgroups were formed: those with an LVEF ≥ 50% (HFpEF) and those with an LVEF < 50% (HFrEF). The control group comprised 100 adults without HF who attended regular check-ups for disease screening without a history of cardiovascular disease. Exclusion criteria included unclear or incomplete echocardiographic images hindering accurate analysis of cardiac chamber images.
Clinical data
All study participants were fully informed about the research’s benefits and risks and provided consent for data collection. Age was recorded on the basis of the participant’s date of birth provided during the initial screening. Gender was self-reported and classified as male or female. Height and weight measurements were obtained with accuracies of 0.5 kg and 1 cm, respectively. Blood pressure was measured according to the American Heart Association’s 2019 recommendations20. The New York Heart Association (NYHA) functional classification was assessed on the basis of patient-reported symptoms and clinical evaluation, with participants categorized into one of four NYHA classes (I, II, III, or IV) according to the severity of their symptoms and limitations in physical activity7. These data were recorded during the initial clinical assessment and were subsequently used in the analysis. Additional parameters, including body mass index, body surface area, and heart rate, were simultaneously recorded during echocardiographic assessment, with heart rate calculated automatically by the echocardiography machine after inputting the required parameters and attaching electrocardiogram electrodes during the procedure. Blood samples for biochemical testing were obtained from venous blood. Quantitative measurement of the N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentration in the blood was performed on a Roche Cobas e 601 automated biochemical analyser.
Transthoracic echocardiography
Our study utilized a Philips Affiniti 70 ultrasound machine, which simultaneously recorded electrocardiographic signals during echocardiography. The measurement protocol followed the comprehensive transthoracic echocardiography guidelines for adults established by the American Society of Echocardiography21.
Two dimensional speckle tracking echocardiography was performed via offline QLAB software version 15. All analyses were performed by a highly echocardiographer blinded to the other patient characteristics. LV GLS was analysed simultaneously via three apical views: the apical four-chamber, the apical two-chamber, and the apical long-axis views. The software automatically tracked the endocardial surface of the LV, creating a region encompassing the entire myocardial thickness throughout the cardiac cycle. If the software failed to automatically detect or incompletely track the endocardial surface, manual adjustments were made to ensure optimal analysis quality and minimize errors. LV GLS was calculated as the change in length divided by the original length of the speckle pattern during the cardiac cycle, expressed as a percentage, with myocardial lengthening represented as positive strain and shortening as negative strain22,23. LV GLS was then averaged across 18 peak systolic strain segments24,25. LA strain was assessed in both the apical two-chamber and apical four-chamber views, with reference points set at the onset of the P wave in the cardiac cycle. LA strain measurements were taken during the reservoir, conduit, and contractile phases of LA function, which were designated left atrium strain reservoir function (LASr), left atrium strain conduit function (LAScd), and left atrium strain contractile function (LASct), respectively25,26,27. The results of the LV strain and LA strains (LAScd and LASct) are conventionally represented as negative values. However, for convenience in analysis and display, we utilized the absolute values of these results.
Two dimensional echocardiographic methods for assessment of left atrioventricular coupling index
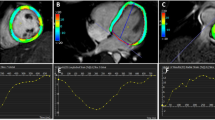
The LACI is the ratio between the left atrial end-diastolic volume (LAEDV) and the left ventricular end-diastolic volume (LVEDV). LA and LV volumes are measured during the end-diastolic phase of the cardiac cycle, identified by the peak of the QRS complex on the electrocardiogram. The measurements were sequentially conducted on four-chamber and two-chamber views. Biplane Simpson’s method was employed to evaluate the LVEDV and LAEDV. The LACI value is expressed as a percentage, with higher LACI values indicating a more significant imbalance between LA and LV volumes during end-diastole, reflecting changes in left atrioventricular coupling16,23. To avoid influencing the research results, the echocardiography procedure for acquiring LACI was conducted by a separate echocardiography specialist independent of the research team. Figure 1 illustrates the procedure for calculating the LACI.
Measurement of the LAEDV and LVEDV via the biplane method to calculate the LACI index via two-dimensional echocardiography. In cases where an electrocardiogram is not simultaneously recorded during echocardiography or when it is difficult to identify the cardiac cycle during LACI measurement, the LAEDV can be considered the smallest LA volume, and the LVEDV can be considered the largest LV volume. Panel A: Measurement of the LV volume in the apical four-chamber view. Panel B: Measurement of the LV volume in the apical two-chamber view. Panel C: Measurement of LA volume in the apical four-chamber view. Panel D: Measurement of LA volume in the apical two-chamber view.
Statistical analysis
All the statistical analyses were performed via IBM SPSS statistical software version 26.0, GraphPad Prism software version 10.1.2.324, and the R Project for Statistical Computing version 4.0.3. The normality of the distribution of variables was assessed via the Kolmogorov–Smirnov test. Categorical variables are presented as frequencies and percentages. Normally distributed continuous variables are expressed as the mean ± standard deviation (X ± SD) and median (interquartile range 25 and 75) if the distribution is nonnormal. The study results are presented in tables and graphs. The χ2 test or Fisher’s exact test was used to compare observed proportions and examine relationships between categorical variables. Independent samples t tests were used to compare the mean values of normally distributed continuous variables between two groups with equivalent variances. One-way ANOVA was used for comparisons involving three or more groups. The Mann‒Whitney U test was used for comparisons between two groups, and the Kruskal‒Wallis test was used for comparisons involving three or more groups when the distribution was nonnormal or when variances were not equivalent. The Pearson correlation coefficient between LACI and other echocardiographic indices was assessed via Pearson correlation (r) to determine whether the distribution was normal or Spearman correlation (rs) to determine whether the distribution was nonnormal. Receiver operating characteristic (ROC) curves were generated to evaluate the diagnostic value of the LACI and other echocardiographic parameters for diagnosing HFpEF, and the area under the curve (AUC), sensitivity, and specificity were assessed. AUC comparison was conducted via the DeLong method to assess the diagnostic value of LACI compared with existing guideline criteria28. Logistic multivariable regression was conducted to assess the risk of HFpEF of the LACI and other echocardiographic values. We randomly selected 30 of the 160 study participants to evaluate the intraclass correlation coefficient (ICC). The intraobserver and interobserver variability of LACI was assessed via the ICC. Additionally, the Bland‒Altman method was used to assess inter- and intraobserver reliability among echocardiography experts. For intraobserver variability, the same operator independently remeasured the data after seven days. For interobserver variability, the data were reanalyzed by a second operator blinded to the initial measurements. All the statistical tests were two-sided, and a p value < 0.05 was considered to indicate statistical significance.
Results
Baseline demographic and clinical features of the study population
In our study, when the HF group and the control group were compared, there were no differences in age, sex, body mass index, or body surface area (p > 0.05). However, there were statistically significant differences in heart rate, systolic blood pressure, and diastolic blood pressure (p < 0.001). The detailed parameters and additional information regarding medical history and treatment are presented in Table 1 and Supplementary Table S1.
In the present study, echocardiographic indices significantly differed between the control and HF groups (p < 0.001). Specifically, the LVMI and LAVI were significantly greater in the HF group than in the control group. Conversely, the LVEF, LV GLS, LASr, LAScd, and LASct were significantly lower in the HF group than in the control group. Details of the echocardiographic parameters are shown in Table 1.
Comparison of LACI and speckle tracking echocardiography parameters of the LA and LV in the HF and control groups
Our study revealed that the LACI in both the HFrEF and HFpEF groups was greater than that in the control group, with values of 41.28 ± 15.27% and 59.16 ± 17.94%, respectively, compared with 13.15 ± 3.92%. The LACI in the HFpEF group was significantly greater than that in the HFrEF group. The LV GLS in the HFrEF and HFpEF groups was lower than that in the control group (11.15 ± 3.29% and 17.46 ± 3.83%, respectively, compared with 20.13 ± 2.34%, respectively; all p < 0.001). Further details are presented in Table 2 and illustrated in Supplementary Fig S2.
Correlation between LACI and other echocardiographic indices in HF patients
The correlation analysis of LA and LV parameters obtained from echocardiography is visualized in Fig. 2 as a heatmap. Significant correlations were observed between LACI and EF, LAVI, LVMI, and LV GLS (EF: r = -0.45, LAVI: rs = 0.59, LVMI: rs = 0.41, LV GLS: r = -0.43, all p < 0.05). In contrast, no correlation was found between LACI and NT-proBNP (rs = -0.15, p > 0.05). Further correlational details are clearly illustrated in the heatmap (Fig. 2).
Heatmap illustrating the relationships between echocardiographic parameters. The color of each cell corresponds to the magnitude and direction of the correlation coefficient, with blue indicating a positive correlation and red indicating a negative correlation. The numerical values within the cells represent the two-way correlation coefficient. NT-proBNP, N-terminal pro–B-type natriuretic peptide; LAVI, left atrial volume index; LVMI, left ventricular mass index; LV EF, left ventricular ejection fraction; E/A, ratio of early to late diastolic filling velocities; e’ lateral, lateral early diastolic mitral annular velocity; e’ septal, septal early diastolic mitral annular velocity; E/e’ ratio, average ratio of early diastolic mitral inflow velocity to early diastolic mitral annular velocity; TRV, maximum tricuspid regurgitant velocity; LV GLS, left ventricular global longitudinal strain; LASr, left atrial reservoir strain; LAScd, left atrial conduit strain; LASct, left atrial contractile strain; LACI, left atrioventricular coupling index.
The value of the LACI and other echocardiographic parameters in diagnosing HFpEF
The sensitivity and specificity of the LACI in diagnosing HFpEF were analysed by constructing ROC curves. The largest area under the ROC curve (AUC) for LACI was 0.951, with an optimal threshold value of 33.07 (sensitivity 97.1%, specificity 87.3%). A statistically significant difference was observed when the AUC of LACI was compared with that of other echocardiographic parameters via the DeLong method (p < 0.05). Additionally, the AUC, cut-off points, sensitivity, and specificity of the other parameters are presented in Table 3; Fig. 3.
Logistic regression analysis for assessing HFpEF risk factors
Logistic regression analysis was conducted to evaluate the risk factors for HFpEF using LACI and other echocardiographic indices as covariates. According to the univariate analysis, higher LACI and lower LASr were identified as risk factors for HFpEF. Furthermore, our multivariate analysis revealed that LACI and LASr are independent factors of HFpEF compared with standard indices for diagnosing HFpEF. Further details are provided in Table 4.
Reliability of LACI measurement
Figure 4 presents the intraobserver and interobserver variability for LACI measurements. The LACI demonstrated good reproducibility, as indicated by high ICC values.
Bland-Altman plot with marginal histograms for interobserver and intraobserver agreement in assessing the reliability of LACI measurement. Panel A: Intraobserver variability of LACI. Panel B: Interobserver variability of LACI. Bias, mean of differences between the two experts, Upper LoA, upper level of limits of agreement = mean of differences + 1.96 standard deviations; Lower LoA, lower level of limits of agreement = mean of differences − 1.96 standard deviations; r, Pearson correlation coefficients; ICC, Intraclass correlation coefficient; SEM, standard error of measurement.
Discussion
Our study aimed to examine LACI among subgroups of HF patients via echocardiography. Our findings were highly promising, as we observed significant differences in LACI among the HFpEF, HFrEF, and control groups. Furthermore, the LACI showed high diagnostic value and accuracy in diagnosing HFpEF compared with commonly used indices (summarized in Supplementary Fig S3). Our study revealed an increase in LACI, particularly in the HFpEF group, followed by a significant decrease in the HFrEF group. The pathophysiology of HF can explain this phenomenon.
In the HFpEF group, there was a characteristic increase in myocardial stiffness due to fibrosis or alterations in the extracellular matrix of the myocardial tissue, leading to impaired ventricular relaxation, particularly in the LV, resulting in diastolic dysfunction29,30. Inadequate ventricular relaxation impedes blood flow from the LA to the LV, causing increased pressure in the LA and pulmonary veins to compensate during the early diastolic phase31. However, prolonged high pressure exerts more pressure on the LA wall, causing dilation of the LA, thereby increasing both the minimal and maximal LA volumes14. This is further supported by the fact that the LA bears direct pressure from the LV during end-diastole, making the LA size an optimal indicator of LV diastolic function18. However, unlike changes in the LA, in HFpEF patients, the predominant change in the LV is concentric hypertrophy, leading to increased LV wall thickness but minimal impact on the LV chamber volume30,32. Epidemiological and clinical imaging studies have consistently shown that in HFpEF patients, the average LV size falls within normal limits32. The LACI, which reflects changes in both the LA and the LV as the ratio of the LAEDV to the LVEDV, increases in HFpEF patients because of a significant increase in LA volume, whereas the LV volume remains relatively unchanged.
Conversely, although the LACI increased in the HFrEF group compared with that in the control group, it tended to be lower than that in the HFpEF group. These findings are consistent because both HFrEF and HFpEF lead to LA dilation33. The key difference, however, lies in the LV changes in HFrEF, where LV dilation leads to an increase in the LVEDV30. Consequently, the increase in LV volume in HFrEF patients results in a decrease in LACI compared with that in HFpEF patients.
Recent studies have highlighted the LACI as an index capable of effectively assessing LV diastolic function and predicting outcomes in conditions such as atrial fibrillation, myocardial infarction, and HF15,16,17,18,19. Our study further supports the role of LACI, suggesting that it could serve as a helpful tool for clinicians in diagnosing HFpEF.
Additionally, when we analysed the correlation between LACI and NT-proBNP, a well-established marker of HF, our study revealed that traditional echocardiographic indices and strain parameters were significantly correlated with NT-proBNP. However, LACI did not demonstrate a strong correlation with NT-proBNP in HF patients. This finding is consistent with the MESA study, which also revealed a weak correlation between LACI and NT-proBNP in individuals free from cardiovascular disease34. NT-proBNP is a widely recognized biomarker indicative of acute hemodynamic stress and myocardial wall stretch and is typically elevated in response to volume or pressure overload. Its levels are highly sensitive to short-term fluctuations caused by changes in preload, afterload, renal function, and therapeutic interventions such as diuretics35,36,37. Conversely, LACI captures the chronic structural remodelling of the LA in relation to the LV, reflecting long-term adaptations to sustained elevated filling pressures, as discussed earlier. Therefore, LACI is less sensitive to short-term hemodynamic changes and may serve as a more stable marker for adverse atrioventricular remodelling. The lack of correlation between LACI and NT-proBNP suggests that LACI may provide independent structural and mechanical information, offering additional insights beyond traditional biochemical markers.
Further evidence supporting LACI’s diagnostic value for HFpEF was found in our study, which revealed that LACI performed well in diagnosing HFpEF. Compared with other modern techniques, such as speckle tracking echocardiography for assessing LV GLS and LA strain, which require sophisticated ultrasound machines and analysis software38,39,40widespread investment and application may be challenging. Moreover, previous studies have focused primarily on assessing LACI via cardiac magnetic resonance imaging17,18,19, which makes the widespread adoption of this index in clinical practice more challenging. In contrast, in our study, LACI is a simple technique that can be performed directly on 2D echocardiographic images available on most current ultrasound devices. Owing to its favourable results and ease of implementation, LACI could be widely applied as a cost-effective technique for diagnosing HFpEF, especially in developing countries lacking resources for other advanced equipment.
Our findings also highlight that LACI demonstrates excellent reproducibility, as indicated by the high ICC values for both intraobserver and interobserver variability. These findings suggest that LACI can be reliably assessed through serial echocardiographic examinations, further supporting its clinical utility. Moreover, the comparable interobserver and intraobserver reproducibility highlights that LACI analysis remains consistent, even when performed by clinicians with varying experience levels. This reinforces its feasibility for widespread application in HF assessment, ensuring reliable monitoring of left atrioventricular coupling over time.
Limitations
Our study provides valuable insights into the LACI among HF patients, especially those with HFpEF. However, certain limitations need to be acknowledged. The small sample size and single-center design limited the study and may have introduced selection bias. Additionally, owing to the limited sample size, the analysis could only compare LACI between HF patients with LVEF < 50% and those with LVEF ≥ 50%. A detailed examination of differences between more specific LVEF levels, such as HFrEF (< 40%), HF with mildly reduced ejection fraction (HFmrEF) (40–50%), and HFpEF (> 50%), was not possible, as the sample size was insufficient for robust comparisons across all three subgroups.
Our study also did not assess patients with specific rhythm disorders, particularly atrial fibrillation, which could influence the progression of HF. As a cross-sectional study, only correlations were established, and causal relationships could not be evaluated. This limitation prevented an assessment of the treatment effects on HF patients in this study. Longitudinal studies are needed to explore these associations further.
Furthermore, the interaction between myocardial deformation and arterial stiffness, or ventricular‒arterial coupling, has been shown to be significantly altered in HFpEF patients compared with those with HFrEF41. However, this comparison was not conducted in our study. Further research is needed to investigate the interactions between the atrium, ventricle, and arteries and their role in HF diagnosis.
Finally, the study relied on a specific ultrasound machine and ultrasound software, which presents a limitation. Future research should aim to compare results from different manufacturers of ultrasound software.
Conclusion
HF patients exhibit increased variability in LACI compared to healthy individuals, with the most significant increase observed in the HFpEF group. Compared with commonly used indices, the LACI demonstrated superior diagnostic performance in identifying HFpEF in current clinical practice. As an easily assessable parameter, the LACI holds significant potential for diagnosing HFpEF. Future research involving larger, more diverse cohorts and longitudinal designs is essential to further validate LACI’s role in HFpEF diagnosis.
Data availability
Research data supporting this publication will be available through direct request to the first author (Hai Nguyen Ngoc Dang). Email: dangnngochai@dtu.edu.vn or ngochai123dc@gmail.com.
References
Virani, S. S. et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation. 141, e139–e596. https://doi.org/10.1161/CIR.0000000000000757 (2020).
Shahim, B., Kapelios, C. J., Savarese, G. & Lund, L. H. Global public health burden of heart failure: an updated review. Card Fail. Rev. 9, e11. https://doi.org/10.15420/cfr.2023.05 (2023).
Davis, R. C. ABC of heart failure: history and epidemiology. BMJ. 320, 39–42.https://doi.org/10.1136/bmj.320.7226.39 (2000).
Dang, H. N. N. et al. Assessing red blood cell distribution width in Vietnamese heart failure patients: A cross-sectional study. PLoS ONE. 19, e0301319. https://doi.org/10.1371/journal.pone.0301319 (2024).
James, S. L. et al. Global, regional, and National incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 392, 1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7 (2018).
Thomson, C., Cuthbert, J. & O’Nwere-Tan, T. A. How can we improve the diagnosis of heart failure? InnovAiT: Educ. Inspiration Gen. Pract. 15, 706–711. https://doi.org/10.1177/17557380221128969 (2022).
Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 145, e895–e1032. https://doi.org/10.1161/CIR.0000000000001063 (2022).
McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. Cardiovasc. Imaging. 42, 3599–3726. https://doi.org/10.1093/eurheartj/ehab368 (2021).
Istratoaie, S. et al. How to diagnose heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging. 25, 1505–1516. https://doi.org/10.1093/ehjci/jeae183 (2024).
Pezel, T. et al. Imaging interstitial fibrosis, left ventricular remodeling, and function in stage A and B heart failure. JACC: Cardiovasc. Imaging. 14, 1038–1052. https://doi.org/10.1016/j.jcmg.2020.05.036 (2021).
Reddy, Y. N. V. et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 21, 891–900. https://doi.org/10.1002/ejhf.1464 (2019).
Dal Canto, E. et al. Diagnostic value of echocardiographic markers for diastolic dysfunction and heart failure with preserved ejection fraction. Heart Fail. Rev. 27, 207–218. https://doi.org/10.1007/s10741-020-09985-1 (2022).
Otani, K., Higa, Y., Kitano, T., Nabeshima, Y. & Takeuchi, M. Prediction of cardiac events using fully automated GLS and BNP titers in patients with known or suspected heart failure. PLoS ONE. 15, e0234294. https://doi.org/10.1371/journal.pone.0234294 (2020).
Sengupta, P. P. & Narula, J. À LA mode atrioventricular mechanical coupling. JACC: Cardiovasc. Imaging. 7, 109–111. https://doi.org/10.1016/j.jcmg.2013.12.001 (2014).
Pezel, T. et al. AI-Based fully automated left atrioventricular coupling index as a prognostic marker in patients undergoing stress CMR. JACC: Cardiovasc. Imaging. 16, 1288–1302. https://doi.org/10.1016/j.jcmg.2023.02.015 (2023).
Leiner, T. Left atrioventricular coupling for early prediction of incident atrial fibrillation. Radiology. 303, 327–328. https://doi.org/10.1148/radiol.213293 (2022).
Kasa, G. et al. Prognostic value of left atrioventricular coupling index in heart failure. Eur. Heart J. Cardiovasc. Imaging. 26, 610–617. https://doi.org/10.1093/ehjci/jeaf010 (2025).
Lange, T. et al. Cardiovascular magnetic resonance-derived left atrioventricular coupling index and major adverse cardiac events in patients following acute myocardial infarction. J. Cardiovasc. Magn. Reson. 25, 24. https://doi.org/10.1186/s12968-023-00929-w (2023).
Backhaus, S. J. et al. Cardiovascular magnetic resonance rest and exercise-stress left atrioventricular coupling index to detect diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 324, H686–H695. https://doi.org/10.1152/ajpheart.00081.2023 (2023).
Casey, D. E. et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: A report of the American college of cardiology/american heart association task force on performance measures. Circ: Cardiovasc. Qual. Outcomes. 12, e000057. https://doi.org/10.1161/HCQ.0000000000000057 (2019).
Mitchell, C. et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J. Am. Soc. Echocardiogr. 32, 1–64. https://doi.org/10.1016/j.echo.2018.06.004 (2019).
Laufer-Perl, M., Gilon, D., Kapusta, L. & Iakobishvili, Z. The role of speckle strain echocardiography in the diagnosis of early subclinical cardiac injury in cancer patients—is there more than just left ventricle global longitudinal strain? J. Clin. Med. 10, 154. https://doi.org/10.3390/jcm10010154 (2021).
Dang, H. N. N., Luong, T. V. & Ho, B. A. Evaluation of the relationship between left atrial stiffness, left ventricular stiffness, and left atrioventricular coupling index in type 2 diabetes patients: a speckle tracking echocardiography study. Front. Cardiovasc. Med. 11, 1372181. https://doi.org/10.3389/fcvm.2024.1372181 (2024).
Yingchoncharoen, T., Agarwal, S., Popović, Z. B. & Marwick, T. H. Normal ranges of left ventricular strain: A Meta-Analysis. J. Am. Soc. Echocardiogr. 26, 185–191. https://doi.org/10.1016/j.echo.2012.10.008 (2013).
Nguyen, N. et al. Assessment of the right ventricular strain, left ventricular strain and left atrial strain using speckle tracking echocardiography in patients with chronic obstructive pulmonary disease. BMJ Open. Resp. Res. 12, e002706. https://doi.org/10.1136/bmjresp-2024-002706 (2025).
Dang, H. N. N., Luong, T. V. & Tran, T. T. Evaluating left atrial function changes by speckle tracking echocardiography in type 2 diabetes patients in central vietnam: a cross-sectional comparative study. Egypt. Heart J. 76, 38. https://doi.org/10.1186/s43044-024-00470-w (2024).
Viet Luong, T., Nguyen Ngoc Dang, H., Nguyen Thi Bich, N., Le Viet Tuan, Q. & Le, M. T. Quantifying left atrial dysfunction in heart failure: A single-center study comparing heart failure with preserved and reduced ejection fractions via speckle tracking echocardiography. J. Diagn. Med. Sonogr. 0 https://doi.org/10.1177/87564793251336381 (2025).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Schwinger, R. H. G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 11, 263–276. https://doi.org/10.21037/cdt-20-302 (2021).
Heinzel, F. R. et al. Left ventricular dysfunction in heart failure with preserved ejection fraction—molecular mechanisms and impact on right ventricular function. Cardiovasc. Diagn. Ther. 10, 1541–1560. https://doi.org/10.21037/cdt-20-477 (2020).
Lester, S. J. et al. Unlocking the mysteries of diastolic function. J. Am. Coll. Cardiol. 51, 679–689. https://doi.org/10.1016/j.jacc.2007.09.061 (2008).
Shah, A. M. Ventricular remodeling in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 10, 341–349.https://doi.org/10.1007/s11897-013-0166-4 (2013).
Melenovsky, V. et al. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ: Heart Fail. 8, 295–303. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001667 (2015).
Pezel, T. et al. Determinants of left atrioventricular coupling index: the Multi-Ethnic study of atherosclerosis (MESA). Arch. Cardiovasc. Dis. 115, 414–425. https://doi.org/10.1016/j.acvd.2022.04.011 (2022).
Knebel, F. et al. NT-ProBNP in acute heart failure: correlation with invasively measured hemodynamic parameters during recompensation. J. Card. Fail. 11, S38–S41. https://doi.org/10.1016/j.cardfail.2005.04.012 (2005).
Täger, T. et al. Hemodynamic determinants of the biologic variation of N-Terminal Pro–B-Type natriuretic peptide in patients with stable systolic chronic heart failure. J. Card. Fail. 23, 835–842. https://doi.org/10.1016/j.cardfail.2017.07.404 (2017).
Kuwahara, K. The natriuretic peptide system in heart failure: diagnostic and therapeutic implications. Pharmacol. Ther. 227, 107863. https://doi.org/10.1016/j.pharmthera.2021.107863 (2021).
Esposito, G. et al. Improving performance of 3D speckle tracking in arterial hypertension and paroxysmal atrial fibrillation by using novel strain parameters. Sci. Rep. 9, 7382. https://doi.org/10.1038/s41598-019-43855-7 (2019).
Piras, P. et al. Left atrial trajectory impairment in hypertrophic cardiomyopathy disclosed by geometric morphometrics and parallel transport. Sci. Rep. 6, 34906. https://doi.org/10.1038/srep34906 (2016).
Piras, P. et al. Homeostatic left heart integration and disintegration links atrio-ventricular covariation’s dyshomeostasis in hypertrophic cardiomyopathy. Sci. Rep. 7, 6257. https://doi.org/10.1038/s41598-017-06189-w(2017).
Pugliese, N. R. et al. Ventricular-Arterial coupling derived from proximal aortic stiffness and aerobic capacity across the heart failure spectrum. JACC: Cardiovasc. Imaging. 15, 1545–1559. https://doi.org/10.1016/j.jcmg.2022.03.024 (2022).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.N.N.D: Conceptualization, methodology, investigation, data curation, writing - original draft, writing - review editing. T.V.L: Conceptualization, methodology, investigation, data curation, writing - original draft, writing - review editing. H.K.T, N.H.T.L, M.H.N.N, T.C.D, H.M.N: Writing - original draft, writing - review editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The research was conducted following the guidelines stipulated in the Declaration of Helsinki 2013. Our research was approved by the Institutional Ethics Committee of Hue University (No approval: H2022/044).
Informed consent
Informed consent was obtained from all participants after providing detailed information about the study, and written consent forms were signed prior to their inclusion.
Disclaimer
The figures presented in this article are exclusively crafted by the author and have been thoroughly checked for any necessary copyright permissions, including those sourced from the internet.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nguyen Ngoc Dang, H., Viet Luong, T., Khanh Tran, H. et al. Left atrioventricular coupling index measured by echocardiography in heart failure with preserved ejection fraction. Sci Rep 15, 20419 (2025). https://doi.org/10.1038/s41598-025-06291-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06291-4