Abstract
Vitiligo, a depigmentation disorder, significantly impacts the well-being of affected individuals. The induction of vitiligo by pharmacological agents is a critical concern, with prior research establishing a link between antineoplastic medications and the onset of vitiligo. This study aims to assess the reported association between vitiligo and antineoplastic drugs using the FAERS. The study encompassed FAERS reports spanning the years 2004 to 2024. Medical Dictionary for Regulatory Activities (MedDRA) was used to identify cases of vitiligo. The Reporting Odds Ratio, Proportional Reporting Ratio, Bayesian Confidence Propagation Neural Network, and Empirical Bayes Geometric Mean were calculated to assess the reported associations between available drugs and vitiligo. A significant statistical association was considered when a drug signal met the criteria of all four algorithms. Our analysis of the FAERS database revealed 533 adverse event (AE) reports implicating antineoplastic drugs in the development of vitiligo, with a higher prevalence among females compared to males. The 18–65 age group accounted for the majority of cases, with the United States contributing the most reports. Malignant melanoma was the most frequently reported underlying condition. Nivolumab and Pembrolizumab were the most commonly implicated drugs, with 147 and 126 reports, respectively. Disproportionality analysis identified 14 antineoplastic drugs with a significant association with vitiligo-related AEs, including the monoclonal antibody Mogamulizumab, immune checkpoint inhibitor Ipilimumab, and oncolytic virus Talimogene Laherparepvec, with Mogamulizumab exhibiting the highest correlation. These findings underscore the necessity for heightened clinical vigilance regarding the safety profiles of specific medications. This study represents the inaugural investigation into the real-world incidence of antineoplastic drug-induced vitiligo utilizing the FAERS database. Our findings reveal a strong association between vitiligo and immunomodulatory therapies, including immune checkpoint inhibitors and monoclonal antibodies. There is an imperative need for vigilant patient monitoring during the clinical administration of these agents to promptly identify and address potential AEs such as vitiligo.
Similar content being viewed by others
Introduction
Vitiligo, a common pigmentary disorder, arises from the dysfunction or loss of melanocytes, resulting in localized or generalized depigmentation of the skin1. The prevalence of vitiligo is estimated to be between 0.5–2% worldwide, with no significant variation in incidence across gender, ethnicity, or geographical regions2,3,4,5,6. This condition exerts a profound impact on the psychological well-being and quality of life of affected individuals7. Previous studies have identified various risk factors for vitiligo, encompassing immune, genetic, and environmental factors7. Genetic predisposition plays a crucial role in the susceptibility to vitiligo, with genome-wide association studies having identified approximately 50 genetic loci associated with disease risk, predominantly involving the regulation of immune responses and highlighting the centrality of the immune system in the pathogenesis of vitiligo8,9. Environmental factors, notably exposure to phenolic compounds, also contribute to the development of vitiligo10,11,12,13. These compounds, by structurally resembling tyrosine, interfere with melanin biosynthesis and may precipitate autoimmune responses targeting melanocytes.
Antineoplastic drugs are also one of the primary causes of vitiligo. Among these agents, immune checkpoint inhibitors (ICIs) have been associated with the development of vitiligo14. Recent reports have indicated that cyclin-dependent kinase (CDK) 4 and 6 inhibitors can induce vitiligo15. To our knowledge, no studies to date have specifically utilized the FAERS database to explore vitiligo induced by antineoplastic drugs. This study aims to assess the reported association between antineoplastic drugs and vitiligo using the FAERS database.
Methods
Data source
This retrospective pharmacovigilance study utilizes the FAERS database, a global resource for post-marketing drug safety monitoring and signal detection. The database comprises reports submitted voluntarily by healthcare providers, as well as mandatory submissions from pharmaceutical companies. Information on drugs, including their names, active ingredients, routes of administration, and roles in adverse events (AEs), along with codes for different drug interactions such as primary suspect (PS), secondary suspect, interacting, and concomitant medications, is accessible within the FAERS. Each report identifies a primary suspect drug, enumerates one or more adverse drug reactions (ADRs), and may detail additional medications ingested by the patient.
Study design
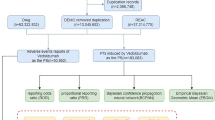
This retrospective pharmacovigilance study encompassed FAERS data from January 2004 to June 2024. To account for multiple submissions with updated information, duplicate reports were identified and excluded based on case numbers, with only the most recent version retained for analysis. A case–control analysis was performed using FAERS to investigate the association between drug exposure and vitiligo reports. In this analysis, ‘cases’ corresponded to reports of AEs of interest, while ‘controls’ comprised all other AE reports not related to the AE under scrutiny. Classification of cases and controls was based on exposure or non-exposure to the drug in question. The reporting odds ratio (ROR) and its 95% confidence interval (CI) were calculated as a measure of association. The ROR specifically indicates whether an AE is disproportionately reported in relation to all other AEs associated with a particular exposure, thus reflecting the reporting odds of the AE of interest between those exposed and those not exposed to the drug. Additionally, the proportional reporting ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayes Geometric Mean (EBGM) were employed to detect drug signals.
Data exposure and adverse drug reaction definition
This study used the preferred term ‘vitiligo’ from the Medical Dictionary for Regulatory Activities (MedDRA) to identify AEs of vitiligo in the REAC files. Given that reporters can assign various roles to the drugs in question, the assessment of drug exposure focused solely on those designated with the ‘PS’ role code. DrugBank was used to standardize different drug names in the ‘drugs’ table, such as brand names, generic names, synonyms, or abbreviations. All drugs ultimately appeared in the standardized generic name format.
Statistical analysis
Disproportionate analysis is extensively applied for identifying signals of ADRs16. In this study, we employed four analytical approaches, conducting statistical analyses based on the construction of a 2 × 2 contingency table17. The methodologies encompassed both frequency-based metrics, namely the ROR and the PRR, and Bayesian methodologies, including the BCPNN and the EBGM. The Bayesian methods, while computationally more intensive, offer a significant advantage over the frequency-based methods by mitigating the risk of false positives associated with sparse AE reporting18. The synergistic application of these four algorithms enhances the robustness and reliability of the findings. All data processing and statistical analyses were performed utilizing R software, version 4.4.1.
Results
Case characteristics
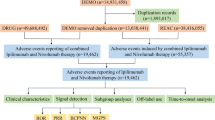
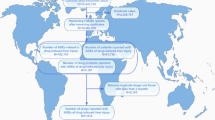
From the first quarter of 2004 to the second quarter of 2024, this study extracted a total of 21,433,114 adverse event reports from the FAERS database, which, after deduplication, resulted in 18,182,912 independent AE reports. Ultimately, 1,726 reports related to vitiligo were identified, involving 553 adverse reaction reports associated with antineoplastic drugs and vitiligo. Figure 1 presents the number of AEs reports filed each year. Between 2018 and 2024, immune checkpoint inhibitors (ICIs) saw a dramatic expansion in clinical use, with approvals and indications extended for several major cancer types, including non-small-cell lung cancer, urothelial cancer, renal cancer, and hepatocellular carcinoma19,20. The approval of mogamulizumab in 2018 also expanded its clinical application, creating a new patient population exposed to this specific drug21,22. Although the novel coronavirus first appeared at the end of 2019, the major public health impact and the global response escalated in early 2020. The first Emergency Use Authorizations for COVID-19 vaccines in the US were granted by the FDA in December 2020 for the Pfizer-BioNTech and Moderna mRNA vaccines23. Widespread public exposure to COVID-19 vaccines did not begin until the end of 2020 and early 2021, which was later than the initial increase in trends reported in 2019. Therefore, while COVID-19 vaccines may have contributed to the further increase in reports since 202124,25,26,27,28, the primary initiating factor for the observed trend is more likely related to the aforementioned drug factors. Among the reports included in the analysis, there were more reports for females (44.5%) than for males (40.7%). In terms of body weight, patients weighing 50–100 kg accounted for 15.6%. Regarding age, the majority of patients were between 18 and 65 years old (42.0%), followed by those aged 66–85 (24.2%). The majority of reports were provided by Physicians (38.3%) and Health-professionals (22.4%). Geographically, the United States had the highest proportion of reports (36.9%), followed by France (12.7%), Germany (6.1%), Spain (3.4%), and Japan (3.3%). Malignant melanoma (25.9%) was the most frequently reported indication, followed by metastatic malignant melanoma (23.5%), breast cancer (3.4%), breast cancer metastatic (3.3%), and non-small cell lung cancer (2.7%). Table 1 presents detailed clinical characteristics of the reports related to antineoplastic drugs and vitiligo.
Antineoplastic drugs used for vitiligo
In the 1,726 AE reports related to vitiligo identified in this study, a total of 491 drug names were listed as “PS”. After consolidating different names, including brand names and generic names, 29 antineoplastic drug classes with more than three reports were ultimately identified. This study conducted a disproportionality analysis on the 29 drugs with more than three reports and initially identified 18 drugs that met the ROR algorithm criteria (Table 2). The drug with the highest number of reports associated with AEs of vitiligo was Nivolumab (n = 147), followed by Pembrolizumab (n = 126), Ipilimumab (n = 67), Ribociclib (n = 37), and Atezolizumab (n = 30). To more accurately reveal the relationship between antineoplastic drugs and vitiligo AEs, this study further analyzed drugs that met four disproportionality analysis methods (Table 3). Among the 14 significantly associated drugs identified, Mogamulizumab, Ipilimumab, Talimogene Laherparepvec, Pembrolizumab, and Nivolumab were included. Mogamulizumab had the highest signal (ROR 85.15, 95% CI 47.03–154.16), which suggests a strong association between this drug and AEs. Ipilimumab and Pembrolizumab showed higher ROR values of 46.32 and 29.97, respectively, which may reflect the strong link to AEs of vitiligo in the context of their use in oncology. Although Talimogene Laherparepvec had only four reports, its ROR value reached 45.03, indicating a need for particular attention to its safety monitoring in clinical use. Concurrently, we noted that Imatinib, Cyclophosphamide, Vemurafenib, and Fludarabine were not classified as significantly associated with AEs of vitiligo due to not meeting four disproportionality analyses. Although the four disproportionality analyses reveal a more stable and reliable association, drugs with a higher number of adverse event reports in the real world, even if they only meet one statistical method, still warrant our further clinical attention and vigilance.
Discussion
Vitiligo is a dermatological condition characterized by the decline of melanocyte function, leading to localized or widespread depigmentation that significantly impacts the physical and mental health of affected individuals. The disease primarily affects melanocytes located in the epidermal hair follicles, with its progression predominantly driven by autoimmune mechanisms. The progressive autoimmune destruction of dermal melanocytes by CD8+ T cells is the main pathogenic mechanism29. Patients exhibit a high presence of cytotoxic T cells targeting melanocytes in peripheral blood, accompanied by the infiltration of T cell, resulting in the loss of melanocytes. Gamma-interferon (IFN-γ) secreted by T cells exacerbates this process by stimulating keratinocytes to produce CXCL9 and CXCL10, thereby attracting more T cells30. The IFN-γ signaling pathway involves binding to cell surface receptors, activating JAK1 and JAK2 kinases, leading to STAT phosphorylation and nuclear translocation, and activating downstream genes31,32. Recurrences of vitiligo may be associated with the reactivation of functional CD8 tissue-resident memory T cells (TRM). TRM can detect self-antigens in the skin during periods of disease stability, producing cytokines such as IFN-γ, CXCL9, CXCL10, and TNF-α, which are responsible for recruiting circulating effector cells33. Oxidative stress is also a significant contributor to vitiligo. Under stress, melanocytes release reactive oxygen species (ROS), leading to an imbalance between pro-oxidants and antioxidants, accelerating cellular senescence34,35. The generation and accumulation of ROS can also cause DNA damage, protein oxidation, and lipid peroxidation, thereby impairing cellular function36.
The clinical characteristics data associated with reports of antineoplastic drugs and vitiligo reveal several key insights. Firstly, the proportion of reports for females exceeds that for males, which may be related to a higher sensitivity to certain drugs in women. The significant absence of weight data limits our analysis of the potential association between obesity and vitiligo. The age distribution indicates that vitiligo can affect patients across various age groups, but is predominantly concentrated in the adult population, with the highest proportion in the 18 to 65 age group and a substantial proportion in the 66 to 85 age group, suggesting a need for caution when treating elderly patients. The diversity of report sources reflects a global awareness and willingness to report vitiligo, with the highest number of cases reported by physicians, highlighting the pivotal role of physicians in identifying and reporting adverse drug reactions. Geographically, the United States, France, Germany, Spain, and Japan report a higher number of cases, which may be related to the pharmaceutical market distribution and drug regulatory capabilities in these countries. Lastly, we observed that the indications are primarily focused on malignant melanoma, breast cancer, and non-small cell lung cancer.
The six most frequently implicated antineoplastic drugs in FAERS reports for vitiligo are Nivolumab (n = 147), followed by Pembrolizumab (n = 126), Ipilimumab (n = 67), Ribociclib (n = 37), and Atezolizumab (n = 30). The high ROR and PRR values for these drugs indicate a statistically significant association with vitiligo. For instance, the ROR values for Ipilimumab, Pembrolizumab, and Nivolumab are 46.32, 29.97, and 28.52, respectively, suggesting that these drugs increase the risk of vitiligo in specific patient populations. Furthermore, the EBGM and IC (Information Component) values substantiate the consistency and biological plausibility of these associations. Notably, some drugs with relatively few reports still exhibit high ROR and PRR values, particularly Mogamulizumab, which, despite only 11 reports in the FAERS database, has an ROR of 85.15 and a PRR of 84.92; similarly, Talimogene Laherparepvec has only four reports but an ROR of 45.03, indicating that the association between certain drugs and vitiligo remains noteworthy even with a relatively low number of reports. It is important to note that, while our FAERS analysis identified mogamulizumab as having the highest ROR value, only six cases have been reported in which patients with Sézary Syndrome developed vitiligo following the treatment of mogamulizumab37,38,39,40. The onset of vitiligo occurred 4 to 8 months after the initiation of the therapy. Among these patients, five achieved complete remission of both blood and skin at the time of vitiligo AE, and maintained the remission regardless of treatment discontinuation during the time of follow-up37,38,39,40. Thus, while the occurrence of vitiligo may serve as a favorable predictive marker for treatment response, there is no direct evidence linking it to improved overall survival or median survival time. Talimogene laherparepvec also exhibits a high ROR value, and the vitiligo may be associated with favorable outcomes. Some cases have correlated the development of vitiligo following talimogene laherparepvec treatment with long-term complete remission and other positive therapeutic responses41. The differences in ROR values between nivolumab and pembrolizumab, both PD-1 monoclonal antibodies, are likely driven by a combination of factors. Despite targeting the same molecule, the agents exhibit differences in binding sites, affinity, and specificity for PD-142. Whether these molecular distinctions influence the risk of vitiligo remains uncertain. Moreover, clinical studies indicate that nivolumab and pembrolizumab are largely comparable in efficacy and safety profiles43. However, the heterogeneity of indications, particularly the high incidence of melanoma44, and differential reporting behaviors driven by regulatory, market, and incentive factors likely contribute to numerical disparities and reporting biases in AE counts45. These factors are probably the primary drivers of the differences in ROR. The limitations of the FAERS preclude a straightforward interpretation of ROR differences as true risk differences. Thus, the ROR variations between the two should be interpreted with caution. They may reflect a signal influenced by database characteristics and reporting dynamics, rather than a direct reflection of pharmacological differences. Further validation is required to elucidate the underlying causes.
Our findings underscore the importance of identifying and monitoring potential risk factors for vitiligo, especially when using antineoplastic drugs known to be associated with an increased risk of vitiligo. Additionally, this highlights the need for increased vigilance in signal detection and assessment of AEs of drugs in pharmacovigilance and clinical practice. These data may also provide crucial information for drug development and drug safety regulation. For instance, further research may be required to explore the mechanisms of antineoplastic drugs associated with an increased risk of vitiligo and to develop strategies for prevention or mitigation of the condition. Lastly, our study suggests that surveillance of AEs of drugs should not be limited to the post-marketing phase but should also occur during drug development and clinical trials. This facilitates early identification of potential risks and the implementation of measures to reduce the incidence of AEs.
Patients with melanoma treated with ICIs such as Pembrolizumab often exhibit vitiligo, suggesting that its onset may serve as a marker of successful immunotherapy46. Studies indicate that drug-induced vitiligo correlates with prognosis in some cases; for instance, in patients with metastatic melanoma treated with Pembrolizumab or Nivolumab, the appearance of drug-induced vitiligo is often associated with favorable outcomes, such as longer overall survival or better therapeutic responses47,48. Conversely, the resolution of drug-induced vitiligo may signal disease progression49. Monoclonal antibodies targeting the programmed death receptor-1 (PD-1), including Nivolumab, Pembrolizumab, and Atezolizumab, as well as Ipilimumab targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), are ICIs effective against malignancies like melanoma and non-small cell lung cancer. Ipilimumab appears to have a higher incidence of skin adverse events, including vitiligo, compared to anti-PD-1 or anti-PD-L1 drugs50, aligning with our research findings. Among patients treated with ICIs, a subset may develop vitiligo-like lesions, a phenomenon thought to be related to immune activation. PD-1 inhibitors may enhance the immune response against tumor cells, indirectly promoting the destruction of normal melanocytes. Melanocytes may be targeted during immunotherapy due to the expression of antigens similar to those on tumor cells, leading to skin depigmentation and vitiligo-like areas51,52. Immunohistochemical studies have revealed T-cell infiltration in vitiligo lesions, where T-cells recognize and respond to melanocyte antigens, triggering melanocyte destruction and subsequent skin depigmentation53,54. Further research indicates that PD-1, primarily expressed in activated T-cells, inhibits T-cell function by binding to its ligand PD-L1. PD-1 inhibitors enhance T-cell activity by blocking the PD-1/PD-L1 signaling pathway, potentially targeting shared antigens such as gp100, MelanA/MART-1, and tyrosinase, leading to melanocyte destruction55,56. Additionally, the PD-1/PD-L1 pathway may play a role in maintaining peripheral tolerance of melanocyte proteins, and disrupting PD-1 signaling could induce autoimmune vitiligo57. Studies also suggest that the transcription factor microphthalmia-associated transcription factor (MITF), crucial in regulating genes essential for melanocyte survival, may play a key role in drug-induced vitiligo. MITF is a master regulator of melanocyte development and melanin protein transcription, including tumor-specific neoantigens and melanocyte lineage-specific antigens. The immune system activated by anti-PD-1 treatment may target MITF-associated epitopes, leading to the destruction of both melanoma and normal melanocytes58. CTLA-4 is an immune modulatory molecule expressed on T-cells, primarily involved in maintaining immune tolerance to self-antigens by inhibiting excessive activation of T-cell to prevent autoimmune diseases59. CTLA-4 inhibitors, such as Ipilimumab, enhance the attack of immune system on tumors by blocking CTLA-4 interactions with its ligands CD80 and CD86, thereby releasing the inhibition of T-cell activation60,61. However, this intervention may lead to erroneous attacks on normal self-tissues, triggering autoimmune responses59. CTLA-4 is not only expressed on T-cells but also in melanoma cells, suggesting a role in tumor immune evasion. Specifically, the IFN-γ signaling pathway activates CTLA-4 gene expression specifically in melanocytes and melanoma cells, involving JAK1/2-dependent STAT1 phosphorylation. In melanoma cell lines, high baseline CTLA-4 expression is dependent on constitutive activation of the MAPK pathway, indicating that melanoma cells may maintain high CTLA-4 expression by activating the MAPK pathway to facilitate immune evasion of tumor62. Therefore, CTLA-4 inhibitors may cause skin pigment loss and vitiligo by enhancing T-cell activation, leading to overactivation of the immune system and erroneous attacks on normal melanocytes. Additionally, CTLA-4 inhibitors may directly impair melanocyte function and affect melanin production by disrupting the IFN-γ signaling pathway, altering the composition and function of immune cells in the tumor microenvironment. CDK4/6 inhibitors, such as Ribociclib and Abemaciclib, are used to treat hormone receptor-positive and human epidermal growth factor receptor 2—negative metastatic breast cancer63. The pathogenesis of vitiligo induced by CDK4/6 inhibitors remains speculative. These inhibitors block cancer cell proliferation by disrupting key regulatory points in the cell cycle, potentially affecting the proliferation of keratinocyte precursors and inducing melanocyte apoptosis, leading to skin pigment loss64. Furthermore, CDK4/6 inhibitors may activate the immune system against normal melanocytes expressing breast cancer-related antigens, with this cross-reactivity potentially leading to an immune response against melanocytes, making vitiligo-like toxicity irreversible and possibly contributing to the control of metastatic disease65.
In this study, we leveraged the FAERS database to retrospectively assess the reported associations between antineoplastic drugs and vitiligo using pharmacovigilance methods. To our knowledge, this is the first real-world study utilizing the FAERS database to investigate the induction of vitiligo by antineoplastic drugs. Our findings reveal a significant correlation between the occurrence of vitiligo and immune checkpoint inhibitors, monoclonal antibodies, and several other medications, which primarily affect signaling pathways such as PD-1/PD-L1, CTLA-4, and CDK4/6 (Table 4), potentially increasing the risk of vitiligo. The results underscore the need for close monitoring of patients in clinical settings when these antineoplastic drugs are used, to promptly identify and manage adverse events such as vitiligo. This study also provides direction for future research, including further exploration of the specific mechanisms by which antineoplastic drugs induce vitiligo and how to better prevent and manage these adverse reactions. It is important to note that this study has certain limitations. Firstly, there is a potential for reporting bias in the data, as AEs are reported voluntarily, which may lead to underreporting or overreporting, significantly influencing the ROR analysis. For instance, clinicians and researchers may be more vigilant in detecting and reporting vitiligo in patients with melanoma receiving PD-1/PD-L1 inhibitors, potentially leading to overreporting of vitiligo cases in this subgroup. This selection bias could impact conclusions about the true relationship between antineoplastic drugs and vitiligo in a broader patient population. Secondly, the present study is retrospective in nature, thereby precluding the direct inference of a causal relationship between the medication and vitiligo from the outcomes. For example, if the increase in reports is confounded by the impact of COVID-19, this could lead to an overestimation of the association between antineoplastic drugs and vitiligo. Thirdly, the FAERS database lacks comprehensive patient-level data, which limits the ability to assess confounding factors and conduct reliable statistical analyses. For example, the lack of height data prevents the analysis of body mass index. Lastly, the FAERS database does not provide detailed information on the timing of drug exposure, thus precluding the calculation of incidence rates and the quantification of individual effects of multiple drug exposures. Therefore, future studies should consider prospective designs to more comprehensively collect and analyze data on the relationship between antineoplastic drug exposure and the occurrence of vitiligo, in order to further validate and expand the findings of this study. Secondly, the necessity of standardizing patient population reporting should be emphasized to reduce bias and enhance the generalizability of the results. Lastly, for special conditions such as COVID-19, they can be included as covariates in the analysis to adjust for their potential confounding effects, and through sensitivity analysis to assess the robustness of the results in the presence of potential bias. In summary, this study provides important insights into the relationship between antineoplastic drugs and vitiligo and offers valuable references for drug use and patient management in clinical practice.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. The data supporting the results of this study are available in the FDA Adverse Event Reporting System (FAERS) Public Dashboard, which can be accessed at https://www.fda.gov/drugs/fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard.
References
Bergqvist, C. & Ezzedine, K. Vitiligo: A review. Dermatology (Basel, Switzerland) 236(6), 571–592. https://doi.org/10.1159/000506103 (2020).
Alikhan, A., Felsten, L. M., Daly, M. & Petronic-Rosic, V. Vitiligo: A comprehensive overview Part I Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J. Am. Acad. Dermatol. 65(3), 473–491. https://doi.org/10.1016/j.jaad.2010.11.061 (2011).
Boisseau-Garsaud, A. M. et al. Epidemiology of vitiligo in the French West Indies (Isle of Martinique). Int. J. Dermatol. 39(1), 18–20. https://doi.org/10.1046/j.1365-4362.2000.00880.x (2000).
Howitz, J., Brodthagen, H., Schwartz, M. & Thomsen, K. Prevalence of vitiligo Epidemiological survey on the Isle of Bornholm, Denmark. Arch. Dermatol. 113(1), 47–52. https://doi.org/10.1001/archderm.113.1.47 (1977).
Krüger, C. & Schallreuter, K. U. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int. J. Dermatol. 51(10), 1206–1212. https://doi.org/10.1111/j.1365-4632.2011.05377.x (2012).
Zhang, Y. et al. The prevalence of vitiligo: A meta-analysis. PLoS ONE 11(9), e0163806. https://doi.org/10.1371/journal.pone.0163806 (2016).
Rodrigues, M., Ezzedine, K., Hamzavi, I., Pandya, A. G. & Harris, J. E. New discoveries in the pathogenesis and classification of vitiligo. J. Am. Acad. Dermatol. 77(1), 1–13. https://doi.org/10.1016/j.jaad.2016.10.048 (2017).
Jin, Y. et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat. Genet. 48(11), 1418–1424. https://doi.org/10.1038/ng.3680 (2016).
Spritz, R. A. & Andersen, G. H. Genetics of vitiligo. Dermatol. Clin. 35(2), 245–255. https://doi.org/10.1016/j.det.2016.11.013 (2017).
Harris, J. E. Chemical-induced vitiligo. Dermatol. Clin. 35(2), 151–161. https://doi.org/10.1016/j.det.2016.11.006 (2017).
Romaguera, C. & Grimalt, F. Occupational leukoderma and contact dermatitis from paratertiary-butylphenol. Contact Dermatitis 7(3), 159–160. https://doi.org/10.1111/j.1600-0536.1981.tb04598.x (1981).
Sasaki, M. et al. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 27(5), 754–763. https://doi.org/10.1111/pcmr.12269 (2014).
Tokura, Y. et al. Biochemical, cytological, and immunological mechanisms of rhododendrol-induced leukoderma. J. Dermatol. Sci. 77(3), 146–149. https://doi.org/10.1016/j.jdermsci.2015.02.001 (2015).
Anthony, N. et al. Drug-induced vitiligo: A case/non-case study in Vigibase(®), the WHO pharmacovigilance database. Fundam. Clin. Pharmacol. 34(6), 736–742. https://doi.org/10.1111/fcp.12558 (2020).
Algethami, A. K. et al. Palbociclib-induced vitiligo-like lesions: A report of a challenging case. Cureus 16(6), e62293. https://doi.org/10.7759/cureus.62293 (2024).
Huang, J., Su, A., Yang, J., Zhuang, W. & Li, Z. Post-marketing safety concerns of teprotumumab: A real-world pharmacovigilance assessment. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgae417 (2024).
Mao, X. et al. A pharmacovigilance study of FDA adverse events for sugammadex. J. Clin. Anesth. 97, 111509. https://doi.org/10.1016/j.jclinane.2024.111509 (2024).
Glasby, T. M. Surface composition and orientation interact to affect subtidal epibiota. J. Exp. Mar. Biol. Ecol. 248(2), 177–190. https://doi.org/10.1016/s0022-0981(00)00169-6 (2000).
Miller, S. R. et al. Pan-cancer survival impact of immune checkpoint inhibitors in a national healthcare system. Cancer Med. 13(21), e70379. https://doi.org/10.1002/cam4.70379 (2024).
Younis, A. & Gribben, J. Immune checkpoint inhibitors: Fundamental mechanisms. Curr. Status Future Dir. 4(3), 186–210 (2024).
Blackmon, A. L. & Pinter-Brown, L. Spotlight on Mogamulizumab-Kpkc for use in adults with relapsed or refractory mycosis fungoides or sézary syndrome: Efficacy safety, and patient selection. Drug Des. Dev. Therapy 14, 3747–3754. https://doi.org/10.2147/dddt.S185896 (2020).
Fernández-Guarino, M., Ortiz, P., Gallardo, F. & Llamas-Velasco, M. Clinical and real-world effectiveness of mogamulizumab: A narrative review. Int. J. Mol. Sci. https://doi.org/10.3390/ijms25042203 (2024).
Teo, S. P. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 35(6), 947–951. https://doi.org/10.1177/08971900211009650 (2022).
Herzum, A., Micalizzi, C., Molle, M. F. & Parodi, A. New-onset vitiligo following COVID-19 disease. Skin Health Dis. 2(1), e86. https://doi.org/10.1002/ski2.86 (2022).
Lim, S. H. et al. Autoimmune and autoinflammatory connective tissue disorders following COVID-19. JAMA Netw. Open 6(10), e2336120. https://doi.org/10.1001/jamanetworkopen.2023.36120 (2023).
Macca, L. et al. Vitiligo-like lesions and COVID-19: Case report and review of vaccination- and infection-associated vitiligo. Vaccines https://doi.org/10.3390/vaccines10101647 (2022).
Mormile, R. De novo vitiligo following covid-19 infection and vaccination: A door open to future events?. Arch. Med. Res. 55(2), 102961. https://doi.org/10.1016/j.arcmed.2024.102961 (2024).
Singh, R., Cohen, J. L., Astudillo, M., Harris, J. E. & Freeman, E. E. Vitiligo of the arm after COVID-19 vaccination. JAAD Case Rep. 28, 142–144. https://doi.org/10.1016/j.jdcr.2022.06.003 (2022).
Wańkowicz-Kalińska, A. et al. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab. Investig. 83(5), 683–695. https://doi.org/10.1097/01.lab.0000069521.42488.1b (2003).
Rashighi, M. et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med. 6(223), 223. https://doi.org/10.1126/scitranslmed.3007811 (2014).
Damsky, W. & King, B. A. JAK inhibitors in dermatology: The promise of a new drug class. J. Am. Acad. Dermatol. 76(4), 736–744. https://doi.org/10.1016/j.jaad.2016.12.005 (2017).
Jacquemin, C. et al. Heat shock protein 70 potentiates interferon alpha production by plasmacytoid dendritic cells: relevance for cutaneous lupus and vitiligo pathogenesis. Br. J. Dermatol. 177(5), 1367–1375. https://doi.org/10.1111/bjd.15550 (2017).
Richmond, J. M. et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J. Investig. Dermatol. 139(4), 769–778. https://doi.org/10.1016/j.jid.2018.10.032 (2019).
Bergqvist, C. & Ezzedine, K. Vitiligo: A focus on pathogenesis and its therapeutic implications. J. Dermatol. 48(3), 252–270. https://doi.org/10.1111/1346-8138.15743 (2021).
Maresca, V. et al. Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J. Investig. Dermatol. 109(3), 310–313. https://doi.org/10.1111/1523-1747.ep12335801 (1997).
Bickers, D. R. & Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 126(12), 2565–2575. https://doi.org/10.1038/sj.jid.5700340 (2006).
Algarni, A. S., Ram-Wolff, C., Bagot, M. & De Masson, A. Mogamulizumab-induced vitiligo in patients with Sézary syndrome: Three cases. Eur. J. Dermatol. EJD 31(2), 213–216. https://doi.org/10.1684/ejd.2021.4002 (2021).
Bagot, M., Ram-Wolff, C., Battustella, M. & De Masson, A. Long-term complete remission induced by mogamulizumab in a severe Sezary patient, together with five different possible auto-immune manifestations. Eur. J. Cancer 101, S22. https://doi.org/10.1016/j.ejca.2018.07.263 (2018).
Bonnet, P. et al. Association of autoimmunity and long-term complete remission in patients with Sézary syndrome treated with mogamulizumab. Br. J. Dermatol. 180(2), 419–420. https://doi.org/10.1111/bjd.17320 (2019).
Serrano, L., Wanat, K. A. & Chaney, K. 34539 Vitiligo associated with mogamulizumab. J. Am. Acad. Dermatol. https://doi.org/10.1016/j.jaad.2022.06.906 (2022).
Iglesias, P. et al. Induced vitiligo due to talimogene laherparepvec injection for metastatic melanoma associated with long-term complete response. Acta Derm. Venereol. 99(2), 232–233. https://doi.org/10.2340/00015555-3061 (2019).
Jeong, T. J. et al. The high-resolution structure reveals remarkable similarity in PD-1 binding of cemiplimab and dostarlimab, the FDA-approved antibodies for cancer immunotherapy. Biomedicines https://doi.org/10.3390/biomedicines10123154 (2022).
Fessas, P., Lee, H., Ikemizu, S. & Janowitz, T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin. Oncol. 44(2), 136–140. https://doi.org/10.1053/j.seminoncol.2017.06.002 (2017).
Lo, J. A., Fisher, D. E. & Flaherty, K. T. Prognostic significance of cutaneous adverse events associated with pembrolizumab therapy. JAMA Oncol. 1(9), 1340–1341. https://doi.org/10.1001/jamaoncol.2015.2274 (2015).
Hammad, T. A., Naylor, M., Ely, D. M. & Davies, S. Exploring the complexities of disproportionality analysis in pharmacovigilance: reflections on the READUS-PV guideline and a call to action. Front. Pharmacol. 16, 1573353. https://doi.org/10.3389/fphar.2025.1573353 (2025).
Hua, C. et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 152(1), 45–51. https://doi.org/10.1001/jamadermatol.2015.2707 (2016).
Nakamura, Y. et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J. Dermatol. 44(2), 117–122. https://doi.org/10.1111/1346-8138.13520 (2017).
Ramondetta, A. et al. Clinical and pathological relevance of drug-induced vitiligo in patients treated for metastatic melanoma with anti-PD1 or BRAF/MEK inhibitors. Acta Derm. Venereol. 100(1), 00001. https://doi.org/10.2340/00015555-3319 (2020).
Babai, S., Voisin, A. L., Bertin, C., Gouverneur, A. & Le-Louet, H. Occurrences and outcomes of immune checkpoint inhibitors-induced vitiligo in cancer patients: A retrospective cohort study. Drug Saf. 43(2), 111–117. https://doi.org/10.1007/s40264-019-00875-6 (2020).
Sibaud, V. Dermatologic reactions to immune checkpoint inhibitors : skin toxicities and immunotherapy. Am. J. Clin. Dermatol. 19(3), 345–361. https://doi.org/10.1007/s40257-017-0336-3 (2018).
Boniface, K., Dutriaux, C., Prey, S., Taieb, A. & Seneschal, J. Vitiligo-like lesions in patients receiving anti-programmed cell death-1 therapies are distinct from spontaneously occurring active vitiligo. J. Am. Acad. Dermatol. 78(1), e17–e18. https://doi.org/10.1016/j.jaad.2017.08.028 (2018).
Larsabal, M. et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J. Am. Acad. Dermatol. 76(5), 863–870. https://doi.org/10.1016/j.jaad.2016.10.044 (2017).
Pulkkinen, L. et al. Predominance of the recurrent mutation R635X in the LAMB3 gene in European patients with Herlitz junctional epidermolysis bullosa has implications for mutation detection strategy. J. Investig. Dermatol. 109(2), 232–237. https://doi.org/10.1111/1523-1747.ep12319752 (1997).
van Geel, N. A. et al. First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res. 23(3), 375–384. https://doi.org/10.1111/j.1755-148X.2010.00703.x (2010).
Mandelcorn-Monson, R. L. et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J. Investig. Dermatol. 121(3), 550–556. https://doi.org/10.1046/j.1523-1747.2003.12413.x (2003).
Yee, C. et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: Direct evidence of t cell-mediated vitiligo. J. Exp. Med. 192(11), 1637–1644. https://doi.org/10.1084/jem.192.11.1637 (2000).
Tewalt, E. F. et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120(24), 4772–4782. https://doi.org/10.1182/blood-2012-04-427013 (2012).
Byrne, E. H. & Fisher, D. E. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 123(S11), 2143–2153. https://doi.org/10.1002/cncr.30444 (2017).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3(5), 541–547. https://doi.org/10.1016/1074-7613(95)90125-6 (1995).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271(5256), 1734–1736. https://doi.org/10.1126/science.271.5256.1734 (1996).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348(6230), 56–61. https://doi.org/10.1126/science.aaa8172 (2015).
Mo, X. et al. Interferon-γ signaling in melanocytes and melanoma cells regulates expression of CTLA-4. Can. Res. 78(2), 436–450. https://doi.org/10.1158/0008-5472.Can-17-1615 (2018).
Spring, L. M. et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet (London, England) 395(10226), 817–827. https://doi.org/10.1016/s0140-6736(20)30165-3 (2020).
Sollena, P. et al. Vitiligo-like lesions in patients with advanced breast cancer treated with cycline-dependent kinases 4 and 6 inhibitors. Breast Cancer Res. Treat. 185(1), 247–253. https://doi.org/10.1007/s10549-020-05914-w (2021).
Pasqualoni, M. et al. Case report: Vitiligo-like toxicity due to ribociclib during first-line treatment of metastatic breast cancer: two cases of premature interruption of therapy and exceptional response. Front. Oncol. 13, 1067264. https://doi.org/10.3389/fonc.2023.1067264 (2023).
Funding
The study was supported by a grant from the High Level Chinese Medical Hospital Promotion Project (No. HLCMHPP2023027 and No. HLCMHPP2023088), the Escort Project of Guang’anmen Hospital, China Academy of Chinese Medicine Science-Backbone Talent Cultivation Project (No. GAMHH9324021) and the Scientific Research Foundation for New recruits, China Academy of Chinese Medical Sciences (No. ZZ16-XRZ-045). Funders had no role in the study design, data collection, analysis and interpretation, writing of the report, or decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
YYX and LDT: study design, analysing data and writing the article. XHZ and JY: references research and supplementary materials. CBN and ZJH: supervision of research and editing and review of the article. XZS: funding acquisition, supervision of research, study design, and editing and review of the article. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y., Xie, H., Li, D. et al. Real world pharmacovigilance study of antineoplastic drug related vitiligo risks. Sci Rep 15, 22733 (2025). https://doi.org/10.1038/s41598-025-06314-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06314-0
Keywords
This article is cited by
-
The association between colorectal cancer drugs and heart failure: a real-world pharmacovigilance study of the FAERS database
Naunyn-Schmiedeberg's Archives of Pharmacology (2026)