Abstract
In this paper, a self-healing anticorrosive coating with pH response based on epoxy was prepared. Firstly, triazinyl corrosion inhibitor (Inh) was loaded into mesoporous molecular sieve (MCM-41) by the vacuum impregnation method. Polyethylenimine (PEI) and sodium polystyrene sulfonate (PSS) were coated on the surface through the layer-by-layer (LBL) self-assembly method. Then a pH-responsive self-healing anti-corrosion coating Inh@MS/PEI/PSS-EP was obtained by adding the newly prepared nanocapsules to the organic epoxy coating. Eventually, foutier transform infrared spectroscopy (FT-IR), N2 adsorption/desorption, Zeta potential test, thermogravimetry (TG), testultraviolet-visible absorption spectroscopy (UV–vis), electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), and atomic force microscopy (AFM) were used to investigate the pH response, corrosion resistance, self-healing properties of the prepared coating. The results demonstrate that the coating prepared by this method can actively release Inh in alkaline environment. EIS and surface topography analysis manifest the decay-resistance of the organic epoxy coating was improved. The results of the molecular dynamics (MD) simulation show that Inh can adsorb on the carbon steel surface, which is consistent with the experimental results. The corrosion resistance of the modified epoxy coating was improved, after 30 days of immersion, the |Z|0.01 Hz of the modified epoxy coating is 3 orders of magnitude higher than that of the pure epoxy coating.
Similar content being viewed by others
Introduction
Carbon steel is a kind of important metal material which has good workability, wear resistance, weldability, and is widely used for construction, machinery, automobiles, electric power and other fields 1,2,3,4. However, carbon steel is susceptible to corrosion and rust during use, resulting in environmental pollution and waste of resources that affect social and economic development.
Organic epoxy coatings have received widespread concern in metal corrosion protection field because of their blocking properties and corrosion resistance 5,6. But the traditional epoxy coating is prone to various flaws during the curing process, which promote the penetration of corrosive ions into the coating, resulting in direct contact between the metal substrate and the corrosive medium, thus affecting the corrosion performance of the coating 7,8,9,10. The addition of nanomaterials to epoxy resin can observably reduce the porosity of the coating, thus improving the mechanical properties and thereby improving the corrosion resistance 11,12,13,14,15,16,17. According to reports from the literature, adding corrosion inhibitors directly to the coating can improve its corrosion performance and extend its useful service life 18,19,20. However, compared with the natural release of the inhibitor in the nanocontainer, the inhibition efficiency and service time of the coating can be improved by releasing the inhibitor in response to special stimulus 21. Preparing intelligent nanocapsule by combining corrosion inhibitor and nanocapsule was an effective method of controlling inhibitor release 22,23,24,25,26. When localized corrosion causes environmental factors to change, the intelligent nanocontainer can respond instantly and release the corrosion inhibitor immediately. Then, a protective film is formed on the metal surface through chemisorption or physical adsorption to slow the corrosion rate 27,28,29,30,31,32. Self-healing coating is a self-protective measure to restore the intrinsical properties of the coating without human intervention when the coating is eroded by corrosive material.
Our research group has been devoted to the research of triazine corrosion inhibitors for many years 33,34,35,36,37. Triazine corrosion inhibitors are potential high-efficiency corrosion inhibitors. Triazine derivatives not only contain unpaired N heteroatoms, but also have abundant π electrons, which can interact with the metal surface to produce chelates and form a dense adsorption layer on the metal surface to slow down the corrosion of the metal. Therefore, when these inhibitors were loaded into the nanomaterial, if the coating was damaged, the corrosion inhibitor could be actively released from the nanomaterial and adsorbed to the surface of the metal substrate to form a film, which finally enhanced corrosion inhibition.
At present, the application of inorganic nanocontainers in stimulus-responsive materials is becoming increasingly common. Mesoporous silica nanoparticles (MSNs) are one of the mesoporous materials with excellent properties: (1) The particle size is adjustable, and the specific surface area is large to ensure the high load content and controlled release of the corrosion inhibitor; (2) Good chemical compatibility with many coatings and corrosion inhibitors; (3) The surface is easily chemically modified; (4) High mechanical stability. As one of the most commonly used nanovessels, MCM-41 has a one-dimensional pore structure composed of regular cylindrical mesoporous arrays, and the pore size can be adjusted between 2 and 6.5 nm 38,39,40,41.
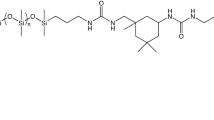
In this paper, in order to improve the prolonged anticorrosion capability of the epoxy coating on Q235 carbon steel, a pH-responsive self-healing anticorrosion coating (Inh@MS/PEI/PSS-EP) was prepared. This paper firstly employed triazinyl corrosion inhibitor to build self-healing anticorrosion coating. The structure of triazinyl corrosion inhibitor is shown in Fig. 1. Foutier transform infrared spectroscopy (FT-IR), N2 adsorption/desorption BET), Zeta potential test, thermogravimetry (TG), testultraviolet-visible absorption spectroscopy (UV–vis), electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), and atomic force microscopy (AFM) were used to investigate the pH response, corrosion resistance, self-healing properties of the prepared coating.
Experimental
Material
Epoxy resin E44, Nantong Xingchen Synthetic Material Co., LTD; Mesoporous molecular sieve MCM-41 (average pore size 20 nm), Tianjin Nanhua Catalyst Co., LTD.; Ethyl acetate, anhydrous ethanol, sodium chloride, analytically pure, Tianjin Damao Chemical Reagent Factory; Ethylenediamine (AR), Tianjin Hengxing Chemical Reagent Manufacturing Co., LTD.; Triazinyl corrosion inhibitor, self-made by experiment; Polyvinyl imide, sodium polystyrene sulfonate, Shanghai Maclin Biochemical Technology Co., LTD.; Carbon steel, Q235, Yangzhou Xiangwei Machinery Co., LTD; KBr (SP), Tianjin Beichen Fangzheng Reagent Factory.
Preparation of Inh@MS/PEI/PSS-EP coating on carban steel
MCM-41 molecular sieve (MS, 1 g) was dispersed in acetone solution (100 mL) containing a certain amount of triazinyl corrosion inhibitor (Inh) 42 (Fig. 1), and the mixture was impregnated vacuum in a vacuum drying oven for 6 h. After filtering, washing, and drying, the mesoporous molecular sieve (Inh@MS) loaded with triazine corrosion inhibitor was obtained. Then Inh@MS was ultrasonically dispersed in 3 mg/mL polyethylenimide (PEI, 40 mL) aqueous solution for 5 min and stirred magnetically for 10 min, the mixture was filtered and washed three times with distilled water until neutral pH is achieved, dried at 60 °C for 3 h to obtain Inh@MS/PEI. Similarly, Inh@MS/PEI was dispersed into 3 mg/mL sodium polystyrene sulfonate (PSS) aqueous solution with 0.5 mol/L NaCl solution. Then, the mixture was filtered and washed three times with distilled water until neutral pH is achieved, dried at 60 °C for 3 h. Finally, microcapsules (Inh@MS/PEI/PSS) loaded with corrosion inhibitors and coated with polyelectrolytes was prepared.
Epoxy resin (EP, 10 g) was dissolved in ethyl acetate (30 mL) to obtain solution A. Inh@MS/PEI/PSS (0.3 g) was dispersed in ethyl acetate (20 mL) to obtain solution B. The two solutions were mixed by high-speed dispersion and ultrasonic dispersion for 10 min. Then, ethylenediamine (1 g) using as the epoxy hardener was added to prepare the coating solution. Before using, Q235 carbon steel plates were polished with sandpapers (240–1000 grit size), degreased with acetone and anhydrous ethanol, washed with distilled water, and dried for use. The coating solution was applied to the prepared steel plates by a coating machine and cured at 70 ℃ for 6 h to obtain a self-healing anticorrosion coating (Inh@MS/PEI/PSS-EP). The thickness of coating is 120 ± 20 μm by the eddy-current coating thickness gauge. The preparation process of self-healing anticorrosion coating is shown in Fig. 2.
Characterization and performance tests
FT-IR measurement
The FT-IR spectra of MS and Inh@MS were recorded with a FT-IR spectrometer (TENSOR 27, Bruker).
TG measurement
Thermo gravimetric (TG) analysis of MS and Inh@MS were performed using a thermal analysis system (DTA7200, HITACHI) with a heating rate of 10 ℃/min from 20 ℃ to 800 ℃ in N2 atmosphere.
Zeta potential measurement
The samples were dispersed in ultra-pure water for 10 min by ultrasound and a small amount of supernatant was taken for the Zeta potential test (Zeta Plus, Brookhaven).
Ultraviolet–visible absorption spectroscopy (UV–vis) measurement
Ultrapure laboratory-made water was used as the reference solution, and the UV–vis spectroscopy scanning wavelength range was 200–500 nm (LAMBDA 365, Perkin Elmer).
N 2 adsorption/desorption test
Liquid N2 was selected as the coolant, and the specific surface area and pore size distribution of the samples were determined by the BET adsorption isotherm equation (ASAP 2020, Micromeritics).
Electrochemical test
Electrochemical impedance spectroscopy (EIS) was performed by electrochemical workstation (CS2350H, CorrTest) to evaluate the anticorrosion ability of the coating. A three-electrode system was used as follows: the coating sample was used as the working electrode, the saturated calomel electrode (SCE) was used as the reference electrode, and the platinum electrode was used as the auxiliary electrode. The corrosive medium was 3.5 wt% NaCl solution, and the contact area between the corrosive solution and the coating was 1 cm2. Before starting the electrochemical impedance test, an open circuit potential (OCP) test was conducted for 30 min to ensure that the surface potential of the sample was stable. The scanning frequency range of the EIS was 10–2–105 Hz, and the AC amplitude was 20 mV. Zsimpwin 3.30 impedance analysis software was used to fit the impedance curve and obtain the relevant parameters.
Surface morphology
The scratches of Inh@MS/PEI/PSS-EP coating were observed by scanning electron microscopy (SEM, Zeiss Sigma 300, Carl Zeiss AG), and the surface elements were analyzed by energy-dispersive X-ray spectrometer (EDS).
Salt spray tests
The salt spray tests were conducted on EP and Inh@MS/PEI/PSS-EP coatings, which were diagonally scratched of 6 cm in length. According to the ASTM B117 standard, the scratched samples were placed into a salt spray chamber containing a 5 wt% NaCl solution. The temperature inside the salt spray chamber was kept at 35 ± 2 °C. The corrosion condition of the coating surface was photographed and documented at regular intervals.
Molecular dynamics simulation
Molecular dynamics (MD) simulation was performed using the Forcite module in Materials Studio 8.0. The simulation process was carried out in a three-dimensional box with periodic boundary condition, and Fe (110) was selected as the adsorption substrate. COMPASS was used as the force field and NVT as the ensemble. The MD simulation was performed at 303 K, time step was 0.1 fs, and the total simulation time was 5000 ps.
Results and discussion
Characterization of Inh@MS and Inh@MS/PEI/PSS nanocontainers
Characterization of Inh@MS
FT-IR spectrum of MS and Inh@MS are shown in Fig. 3a. It can be seen from Fig. 3a, for Inh@MS, –CH3 and –CH2– stretching vibration peaks appeared at 2858 cm-1 and 2927 cm-1, –CH3 and –CH2– flexural vibration peaks appeared at 1357 cm−1 and 1458 cm-1. The peak at 1579 cm-1 belongs to C=N in the triazine. The stretching vibration peak of Si–OH was found at 3449 cm-1. The wide and strong peak at 1093 cm-1 is Si–O–Si antisymmetric stretching vibration peaks, 467 cm-1 and 799 cm-1 are the bending vibration absorption peaks and stretching vibration absorption peaks of Si–O bond, respectively. The analysis of FT-IR spectrum indicates that Inh has been successfully loaded into MS.
The specific surface area and pore volume of MS are 811.08 m2/g and 0.78 cm3/g, respectively, and the average pore size is 3.83 nm. When loaded with inhibitor, the specific surface area and pore volume of Inh@MS particles decreased to 506.35 m2/g and 0.27 cm3/g, respectively, and the average pore size decreased to 2.52 nm, indicating that the Inh molecules were successfully loaded into the mesoporous molecular sieve MS cavity. According to the TG curve (Fig. 3b), the loading amount of Inh is about 19%.
Characterization of Inh@MS/PEI/PSS
Based on Fig. 4, the specific surface area, pore volume and mean pore size of Inh@MS/PEI/PSS particles decrease significantly compared to Inh@MS, which are 242.67 m2/g, 0.21 cm3/g, and 1.83 nm, respectively. The external surface of Inh@MS was coated with polyelectrolyte PEI and PSS by electrostatic force, which reduced the specific surface area, pore volume, and internal pore diameter.
The surface of Inh@MS is electronegative because of the negatively charged surface of the MS. PEI is positively charged in solution and can be automatically coated on the surface of Inh@MS microspheres under electrostatic action, making the Zeta potential of Inh@MS/PEI to 30.33 mV, suggesting the PES was successfully encapsulated. Then, a layer of negatively charged PSS is coated on the surface of Inh@MS/PEI to make Inh@MS/PEI/PSS. Figure 5 shows that the Zeta potential of Inh@MS/PEI/PSS is -23.17 mV, indicating successfully encapsulation of PSS. The positive and negative changes in the surface potential of the microspheres were consistent with the coating process of the two-layer polyelectrolyte. It indicated that the polyelectrolyte PEI and PSS can be well coated on the surface of Inh@MS. Figure 6 is a microscopic diagram of making Inh@MS/PEI/PSS.
pH response of Inh@MS/PEI/PSS microcapsules analysis
To avoid the interaction among the corrosion inhibitor Inh, PEI, and PSS, the three substances were tested by UV–vis absorption spectra (Fig. 7a). As can be seen from the Fig. 7a, polyelectrolyte PEI had no obvious absorption peak in the wavelength range of 200 ~ 500 nm; polyelectrolyte PSS had one strong and one weak absorption peak at 225 nm and 260 nm; the corrosion inhibitor Inh had an absorption peak at 220 and 280 nm, respectively. By comparison of UV–vis absorption spectra, the absorption peak of the corrosion inhibitor Inh at 280 nm was not affected by the absorption peak of polyelectrolyte, therefore, the content of the Inh in the solution can be calculated by the absorption peak at 280 nm.
Due to the different solubility of the Inh at different pH values, different concentrations of Inh solutions were prepared at pH = 3, pH = 7, and pH = 11, respectively. The absorbance of Inh at a wavelength of 280 nm was measured using the standard curve method, as shown in Fig. 7b–d. The results verified that the Inh concentration was linearly related to the absorbance at 280 nm, and that the R2 of the fitted equations had reached above 0.99, which can provide a reliable reference standard for monitoring the Inh release subsequently.
As can be seen from Fig. 8, an almost horizontal release of Inh is observed in a neutral environment (pH = 7), which is may be due to the fact that the positively charged polyelectrolyte PEI and negatively charged polyelectrolyte PSS adsorb each other to form a dense protective film by electrostatic force in a neutral environment 43,44, hindering the release of Inh. Inh releases slowly in an acidic environment (pH = 3 and 5). This is because the protonation of PEI is enhanced in an acidic environment, resulting in a loose chain structure. Inh releases in large quantities within a certain period of time in an alkaline environment (pH = 11 and 9). In the alkaline enhancement, PEI deprotonation destroys the electrostatic force between the polyelectrolyte chain and the chain, while the PSS chain and the chain repel each other, resulting in the release of a large number of corrosion inhibitors 45,46,47.
Anticorrosion and self-healing properties of Inh@MS/PEI/PSS-EP coating analysis
Anticorrosion of Inh@MS/PEI/PSS-EP coating analysis
As can be seen from the Nyquist diagram of EP coating (Fig. 9a), at the initial corrosion stage, the impendance spectra had only one capacitive loop, and the radius of the capacitive loop in the high frequency region decreased after soaking 30 days. It indicated that the corrosion of the carbon steel was controlled by charge transfer and the decay-resistance of the coating gradually decreased. As shown in Fig. 9b, the EIS diagram of the Inh@MS/PEI/PSS-EP coating includes one capacitive loop and diffusion. With the same corrosion time, the capacitive loop radius of the Inh@MS/PEI/PSS-EP coating is much larger than that of the EP coating, indicating long-term anticorrosion property.
Generally, the electrochemical low-frequency impedance modulus |Z|0.01Hz can directly reflect the reactivity of the metal surface coating, and the higher the value |Z|0.01Hz, the better the decay-resistance of the coating 48,49,50. This value reveals the capacity of coating to prevent the transfer of current between the anode and cathode. As can be seen from the Bode diagram, Inh@MS/PEI/PSS-EP coating (Fig. 9b′) has a higher |Z|0.01Hz at each period than that of EP coating (Fig. 9a′), indicating that it has stronger decay-resistance. In addition, after soaking 30 days, the |Z|0.01Hz of the EP coating decreases from 1.70 × 104 Ω cm2 to 2.45 × 103 Ω cm2, while the |Z|0.01Hz of the Inh@MS/PEI/PSS-EP coating only decreases from 8.24 × 106 Ω cm2 to 4.57 × 106 Ω cm2. The results reveals that the Inh@MS/PEI/PSS-EP coating has long-term anticorrosive properties. In addition, concentrated peaks at different frequencies reflect different time constants. When corrosion occurs at the metal/coating interface, the time constant occurs in the low frequency range, while when corrosion occurs at the coating, the time constant occurs in the high frequency range 51,52,53. As shown in the Bode diagram, the time constants of the EP coating move to the low frequency range with prolongation of soaking time, indicating that corrosion occurred at the metal/coating interface. Inh@MS/PEI/PSS-EP coating time constants are concentrated in the high-frequency region, indicating that the coating surface corrosion may occur. This may be due the the fact that the Inh@MS/PEI/PSS microcapsules were closely arranged in EP, which effectively prevented the penetration of corrosive medium and improved the corrosion resistance of the coating.
In order to more directly illustrate the anticorrosive performance of the coating, the EIS curves of the EP coating and Inh@MS/PEI/PSS-EP coating were fitted with equivalent circuit, as shown in Fig. 10. Herein, Fig. 10a was used to fit EIS results for EP coatings. Figure 10b was used to fit EIS results for Inh@MS/PEI/PSS-EP coatings.Where Rs represents the electrolyte solution resistance, Cc is the coating capacitance, Rc is the coating resistance, Rct represents the charge transfer resistance, Cdl represents the double layer capacitance, and Zw represents the Warburg diffusion constant, respectively. It could be attributed to the slower diffusion process in the epoxy/Inh@MS regarding the release of inhibitor in the interface, and the provision of barrier against the diffusion of corrosive species 54.
As can be seen from Table 1, the values of Rct continued to decline indicating that a defect in the coating substrate. Generally, these parameters reflect the decay-resistance of the coating, and the higher the value, the better the corrosion resistance of the coating 55. With prolonging the soaking time, the Rct value of Inh@MS/PEI/PSS-EP coating was much higher than that of EP coating with the same soaking time, indicating that Inh@MS/PEI/PSS-EP coating had better decay-resistance. Cdl is directly related to the area of metal exposed to the electrolyte, which is caused by coating defects and increases as the degree of coating degradation increases 56. As can be seen from Table 1, the Cdl value of EP coating is much higher than that of the Inh@MS/PEI/PSS-EP coating, indicating that Inh@MS/PEI/PSS-EP coating has better decay-resistance.
Self-healing properties of Inh@MS/PEI/PSS-EP coating analysis
Electrochemical test, SEM, and EDS analysis were used to explore the self-healing resistance of the Inh@MS/PEI/PSS-EP coating. Before the experiment, cross scratches were made on the coatings surface with a sharp knife, then the coatings were soaked in 3.5 wt% NaCl solution. After being scratched, the coating/carbon steel system will develop different degrees of corrosion with the change of soaking time.
As far as EP coating is concerned (Fig. 11a), with the immersion time from 2 to 48 h, |Z|0.01Hz decreases from 1.66 × 104 to 4.07 × 103 Ω cm2. It could be attributed to the fact that the electrolyte could quickly reach the damaged surface of the carbon steel when the EP coating scratched samples were immersed in the corrosion solution. Different from EP coating, after being scratched, Inh@MS/PEI/SS-EP coating rapidly releases corrosion inhibitor Inh in response to pH in corrosive medium 30,45. Corrosion inhibitor will be adsorbed on the surface of carbon steel to form a film to prevent further corrosion, which will increase the activation resistance of corrosion reaction. As can be seen from Fig. 11b, when the immersion time of Inh@MS/PEI/PSS-EP coating increases from 2 to 12 h, |Z|0.01Hz increases from 3.16 × 105 to 4.07 × 105 Ω cm2. The high-frequency capacitive loop of Inh@MS/PEI/PSS-EP coating increased within 12 h. This phenomenon may be mainly due to the fact that Inh is released and adsorbed on the surface of carbon steel in the corrosion solution, then a barrier film is formed to prevent the reaction between the corrosive medium and carbon steel. With the increase of soaking time to 48 h, |Z|0.01Hz decreased from 4.07 × 105 to 1.35 × 105 Ω cm2, which was still an order of magnitude higher than the |Z|0.01Hz at the initial EP coating. Comparison of the results, the capacitive loop of EP coating decreased all the time, indicating that the Inh@MS/PEI/PSS-EP coating had good self-healing function and corrosion protection ability.
Figure 12 shows the SEM and EDS graph of scratched EP coating and Inh@MS/PEI/PSS-EP coating after soaking in 3.5 wt% NaCl solution for 12 h. As can be seen from Fig. 13, the EP-coated carbon steel had a rough surface and a serious accumulation of corrosion products on the scratch. As for Inh@MS/PEI/PSS-EP coating, one can see a flat and smooth surface. The scratch line of the matrix was still clearly visible, and no obvious accumulation of corrosion products was found at the scratches. In combination with the EDS analysis diagram and Table 2 of the Inh@MS/PEI/PSS-EP coating, it can be seen that the S and N elements were from the corrosion inhibitor molecules. The SEM and EDS results indicated that the Inh@MS/PEI/PSS-EP coating had self-healing and further anticorrosion function.
Salt spray tests analysis
The corrosion resistance of scratched EP and Inh@MS/PEI/PSS-EP coatings were systematically evaluated using salt spray tests. As showing in Fig. 13, the EP coating is covered with a large amount of rust after 72 h of salt spray corrosion, suggesting that the EP coating lacks sufficient barrier properties to withstand prolonged exposure to corrosive environments. In contrast, the Inh@MS/PEI/PSS-EP coating exhibits only a few insignificant spots after 120 h of salt spray corrosion, suggesting that the corrosion resistance of the Inh@MS/PEI/PSS-EP coating is enhanced compared to that of the Inh@MS-EP coating. This improvement may be attributed to the better dispersion of the microcapsules, the strong physical barrier, and the self-healing ability of the Inh@MS/PEI/PSS-EP coating.
MD simulation
Figure 14 shows the optimal adsorption model of Inh on the Fe (110) surface after the MD simulation. As can be seen from Fig. 14, Inh is adsorbed on the surface of Fe (110) in parallel as a result of the rigid structure of Inh that contains conjugated large π bonds, which can provide electrons for empty d orbitals of Fe atoms to form coordination bonds and adsorb on the surface of carbon steel in the form of chemisorption. The triazine ring and benzothiazole ring in the Inh structure can be adsorbed on the carbon steel surface in parallel, and the long-chain alkane is exposed to the corrosive medium to form a relatively dense hydrophobic layer to protect the metal from corrosion in the medium.
The adsorption and binding energies of Inh on the carbon steel surface were calculated by Eqs. (1) and (2) listed in Table 3.
In the formula, Etotal represents the total energy of the whole system, Esur+water represents the total energy of the Fe surface and water molecules, EInh represents the total energy of the corrosion inhibitor, and the binding energy (Ebingding) value is equal to the opposite value of the adsorption energy (Eads).
In theory, Ebingding reflects the stability of adsorption of corrosion inhibitor molecules on the surface of Fe. The higher the Ebingding, the more stable the adsorption system and the higher the corrosion inhibition efficiency of metal [57]. As can be seen from Table 3, the value of Ebingding is 1058.609 kJ/mol indicating that Inh can stably adsorb on the surface of carbon steel.
Self-healing mechanism
When the coating was damaged, the carbon steel matrix will be exposed in the corrosive medium, causing the surface to be corroded and forming a local corrosion battery. Then the pH of the corrosion site will also increase, thus stimulating Inh@MS/PEI/PSS to release the corrosion inhibitor quickly. The detected Inh on the scratched position from Fig. 12 proved the release of Inh. The inhibition of the corrosion inhibitor was as follows: the pre-adsorbed water molecules at the interface were replaced by corrosion inhibitor molecules, and then the interface formed dense adsorption film. Based on the result of MD (Fig. 14), one can deduces the adsorption of Inh on carbon steel. The N and S atoms in the triazine ring were the main reasons for promoting the adsorption of corrosion inhibitor molecules on the metal surface. On the one hand, Fe2+ was generated on the surface of carbon steel due to corrosion. Thus, the Cl- in the solution was easily adsorbed to the carbon steel surface making the carbon steel negatively charged. The N atom in the corrosion inhibitor was easily protonated in acidic solution, and the protonated molecule will be adsorbed to the surface of carbon steel by electrostatic action (physical adsorption), that is, the triazine ring was adsorbed to the surface of carbon steel. The long-chain alkane extended into the corrosive medium and formed a dense adsorption film that prevented the movement of corrosive ions to the metal surface, thereby corrosion was inhibited. On the other hand, the lone pair electrons of the inhibitor molecule interacted with the d orbital of Fe to form a coordination bond (chemical adsorption), which strengthened the adsorption of the inhibitor molecule on the metal surface, as shown in Fig. 15.
Conclusions
In this paper, Inh@MS/PEI/PSS-EP coating succefully with pH response was prepared, and its anti-corrosion self-healing properties were investigated. The main conclusions are as follows:
-
(1)
The release rate of Inh from Inh@MS/PEI/PSS is much faster under alkaline conditions than in acid or neutral media.
-
(2)
The addition of Inh@MS/PEI/PSS to EP coating could further improved the long-term corrosion decay-resistance of the EP coating.
-
(3)
EDS and electrochemical tests showed that Inh@MS/PEI/PSS-EP coating had self-healing function in 3.5 wt% NaCl solution, and the Inh released from Inh@MS/PEI/PSS can be adsorbed on the carbon steel surface to form a film, thereby inhibiting further corrosion.
Data availability
The authors declare that the data will be available from the corresponding author under reasonable request.
References
Cen, H. Y. et al. Functionalized carbon nanotubes as a novel inhibitor to enhance the anticorrosion performance of carbon steel in CO2-saturated NaCl solution. Corros. Sci. 177, 109011. https://doi.org/10.1016/j.corsci.2020.109011 (2020).
Ye, Y. W. et al. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. 348, 940–951. https://doi.org/10.1016/j.cej.2018.02.053 (2018).
Ana, S. V. et al. Properties of hybrid sol-gel coatings with the incorporation of lanthanum 4-hydroxy cinnamate as corrosion inhibitor on carbon steel with different surface finishes. Appl. Surf. Sci. 561, 149881. https://doi.org/10.1016/j.apsusc.2021.149881 (2021).
Zhang, M. X. et al. Low-cost preparation of durable, transparent, superhydrophobic coatings with excellent environmental stability and self-cleaning function. Surf. Coat. Technol. 438, 128367. https://doi.org/10.1016/j.surfcoat.2022.128367 (2022).
Zhang, Y. et al. Excellent corrosion protection performance of epoxy composite coatings filled with silane functionalized silicon nitride. J. Polym. Res. 25(5), 130. https://doi.org/10.1007/s10965-018-1518-2 (2018).
Dong, Y., Wang, F. & Zhou, Q. Protective behaviors of 2-mercaptobenzothiazole intercalated Zn-Al-layered double hydroxide coating. J. Coat. Technol. Res. 11(5), 793–803. https://doi.org/10.1007/s11998-014-9568-9 (2014).
Pei, W. et al. Research progress of marine anti-corrosion and wear-resistant coating. Tribol. Int. 198, 109864. https://doi.org/10.1016/j.triboint.2024.109864 (2024).
Hou, J. et al. Anticorrosion performance of epoxy coatings containing small amount of inherently conducting PEDOT/PSS on Hull steel in seawater. J. Mater. Sci. Technol. 29(7), 678–684. https://doi.org/10.1016/j.jmst.2013.03.02 (2013).
Zhou, X. et al. Recent advances in synthesis of waterborne polyurethane and their application in water-based ink: a review. J. Mater. Sci. Technol. 31(7), 708–722. https://doi.org/10.1016/j.jmst.2015.03.002 (2015).
Danaee, I. et al. Self-healing and anticorrosive properties of Ce(III)/Ce(IV) in nanoclay-epoxy coatings. Iran. Polym. J. 23(11), 891–898. https://doi.org/10.1007/s13726-014-0288-x (2014).
Chen, Y. et al. Active protection of Mg alloy by composite PEO coating loaded with corrosion inhibitors. Appl. Surf. Sci. 504, 144462. https://doi.org/10.1016/j.apsusc.2019.144462 (2020).
da Silva, R. M. P. et al. Development of an Al3+ ion-selective microelectrode for the potentiometric microelectrochemical monitoring of corrosion sites on 2098–T351 aluminum alloy surfaces. Electrochim. Acta 415, 140260. https://doi.org/10.1016/j.electacta.2022.140260 (2022).
Xu, P. et al. Investigation of the surface modification of magnesium particles with stannate on the corrosion resistance of a Mg-rich epoxy coating on AZ91D magnesium alloy. Prog. Org. Coat. 135, 591–600. https://doi.org/10.1016/j.porgcoat.2019.06.050 (2019).
Sun, D. et al. Robust and impermeable metal shell microcapsules for one-component self-healing coatings. Appl. Surf. Sci. 546, 149114. https://doi.org/10.1016/j.apsusc.2021.149114 (2021).
Ma, Y. N. et al. Enhanced anti-aging and anti-corrosion performance of waterborne epoxy coating layers over the dual effects of g-C3N4 photocatalysis. J. Appl. Polym. Sci. 139(24), 52356. https://doi.org/10.1002/app.52356 (2022).
Cuong, Vu. M. & Quang-Vu, B. Oxidized multiwall carbon nanotubes filled epoxy-based coating: Fabrication, anticorrosive, and mechanical characteristics. Polym. Bull. 78, 2329–2339. https://doi.org/10.1007/s00289-020-03218-z (2021).
Kong, J. Z. et al. Atomic layer-deposited Al2O3 interlayer for improved tribological and anti-corrosion properties of TiN hard coating on 316L stainless steel. JMEPEG 28, 7058–7067. https://doi.org/10.1007/s11665-019-04427-y (2019).
Udoh, I. I. et al. Developments in anticorrosive organic coatings modulated by nano/microcontainers with porous matrices. Adv. Coll. Interface Sci. 330, 103209. https://doi.org/10.1016/j.cis.2024.103209 (2024).
Ismail, N. A., Shakoor, R. A. & Kahraman, R. Corrosion inhibition performance of developed epoxy coatings containing carbon nanocapsules loaded with diethylenetriamine. Prog. Org. Coat. 183, 107716. https://doi.org/10.1016/j.porgcoat.2023.107716 (2023).
Yeganeh, M., Asadi, N., Omidi, M. & Mahdavian, M. An investigation on the corrosion behavior of the epoxy coating embedded with mesoporous silica nanocontainer loaded by sulfamethazine inhibitor. Prog. Org. Coat. 128, 75. https://doi.org/10.1016/j.porgcoat.2018.12.022 (2019).
Manasa, S. et al. Effect of inhibitor loading into nanocontainer additives of self-healing corrosion protection coatings on aluminum alloy A356.0. J. Alloys Compd. 726, 969–977. https://doi.org/10.1016/j.jallcom.2017.08.037 (2017).
Siva, T., Sreejakumari, S. S. & Sathiyanarayanan, S. Dendrimer-like mesoporous silica nanocontainer (DMSN)-based smart self-healing coating for corrosion protection performance. Prog. Org. Coat. 154, 106201. https://doi.org/10.1016/j.porgcoat.2021.106201 (2021).
Sun, X. et al. A novel coating with SiO2 anchored on Mxene loading tannic acid for self-healing anticorrosive performance. J. Alloy. Compd. 928, 167202. https://doi.org/10.1016/j.jallcom.2022.167202 (2022).
Ma, L. et al. Dual0action self-healing protective coatings with photothermal responsive corrosion inhibitor nanocontainers. Chem. Eng. J. 404, 127118. https://doi.org/10.1016/j.cej.2020.127118 (2021).
Hollamby, M. J. et al. Hybrid polyester coating incorporating functionalized mesoporous carriers for the holistic protection of steel surfaces. Adv. Mater. 23(11), 1361–1365. https://doi.org/10.1002/adma.201003035 (2011).
Zmozinski, A. V. et al. Zinc tannate and magnesium tannate as anticorrosion pigments in epoxy paint formulations. Prog. Org. Coat. 121, 23–29. https://doi.org/10.1016/j.porgcoat.2018.04.007 (2018).
Mohammadkhah, S. et al. Construction of a nano-micro nacre-inspired 2D-MoS2-MOF-glutamate carrier toward designing a high-performance smart epoxy composite. J. Ind. Eng. Chem. 121, 358–377. https://doi.org/10.1016/j.jiec.2023.01.039 (2023).
Lashgari, S. M. et al. Application of nanoporous cobalt-based ZIF-67 metal-organic framework (MOF) for construction of an epoxy-composite coating with superior anti-corrosion properties. Corros. Sci. 178, 109099. https://doi.org/10.1016/j.corsci.2020.109099 (2021).
Guo, Y. et al. pH-responsive self-healing anticorrosion coatings based on benzotriazole-containing zeolitic imidazole framework. Colloids Surf. A 561, 1–8. https://doi.org/10.1016/j.colsurfa.2018.10.044 (2019).
Tong, L. et al. Self-healing and corrosion-sensing coatings based on pH-sensitive MOF-capped microcontainers for intelligent corrosion control. Chem. Eng. J. 454, 140335. https://doi.org/10.1016/j.cej.2022.140335 (2023).
Zhang, F. et al. Self-healing mechanismsin smart protective coatings: A review. Corros. Sci. 144, 74–88. https://doi.org/10.1016/j.corsci.2018.08.005 (2018).
Zheng, Z. et al. Self-Healing and antifouling multifunctional coatings based on pHand sulfide ion sensitive nanocontainers. Adv. Funct. Mater. 23(26), 3307–3314. https://doi.org/10.1002/adfm.201203180 (2013).
Zhu, H. L. et al. Intra-/inter-molecular synergistic inhibition effect of sulfonate surfactant and 2-benzothiazolethiol on carbon steel corrosion in 3.5% NaCl solution. Corros. Sci. 182, 109291. https://doi.org/10.1016/j.corsci.2021.109291 (2021).
Jin, X. H. et al. The study of surface activity and anti-corrosion of novel surfactants for carbon steel in 1M HCl. J. Mol. Liq. 353, 118747. https://doi.org/10.1016/j.molliq.2022.118747 (2022).
Hu, Z. Y. et al. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1M HCl. Corros. Sci. 112, 563–575. https://doi.org/10.1016/j.corsci.2016.08.012 (2016).
Zhu, H. L. et al. 2-aminobenzimidazole derivative with surface activity as corrosion inhibitor of carbon steel in HCl: Experimental and theoretical study. J. Mol. Liq. 297, 111720. https://doi.org/10.1016/j.molliq.2019.111720 (2020).
Wang, J. B. et al. Study on corrosion inhibition behavior and adsorption mechanism of novel synthetic surfactants for carbon steel in 1M HCl solution. Sustain. Chem. Pharm. 23, 100500. https://doi.org/10.1016/j.scp.2021.100500 (2021).
Kermannezhad, K. et al. Application of amine-functionalized MCM-41 as pH-sensitive nano container for controlled release of 2-mercaptobenzoxazole corrosion inhibitor. Chem. Eng. J. 306, 849–857. https://doi.org/10.1016/j.cej.2016.08.004 (2016).
Feng, Y. C. & Cheng, Y. F. An intelligent coating doped with inhibitor-encapsulated nanocontainers for corrosion protection of pipeline steel. Chem. Eng. J. 315, 537–551. https://doi.org/10.1016/j.cej.2017.01.064 (2017).
Exbrayat, L. et al. Nanosensors for monitoring early stages of metallic corrosion. ACS Appl. Nano Mater. 2, 812–818. https://doi.org/10.1021/acsanm.8b02045 (2019).
Shchukin, D. G. et al. Active anticorrosion coatings with halloysite nanocontainer. J. Phys. Chem. C 112(4), 958–964. https://doi.org/10.1021/jp076188r (2008).
Zhiyong, Hu. et al. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 112, 563. https://doi.org/10.1016/j.corsci.2016.08.012 (2016).
Elzbieciak, M. et al. Characteristics of model polyelectrolyte multilayer films containing laponite clay nanoparticles. Langmuir 26(1), 277–283. https://doi.org/10.1021/la902077j (2010).
Czerwieniec, B. et al. AFM study of adhesion and interactions between polyelectrolyte bilayers assembly. Colloids Surf. A 555, 465–472. https://doi.org/10.1016/j.colsurfa.2018.07.006 (2018).
He, Y. et al. pH-Responsive nanovalves based on encapsulated halloysite for the controlled release of a corrosion inhibitor in epoxy coating. Roy. Soc. Chem. 5, 90609–90620. https://doi.org/10.1039/c5ra19296j (2015).
Singh, H. K., Yeole, K. V. & Mhaske, S. T. Synthesis and characterization of layer-by-layer assembled magnesium zinc molybdate nanocontainer for anticorrosive application. Chem. Eng. J. 295, 414–426. https://doi.org/10.1016/j.cej.2016.03.055 (2016).
Liu, X. H. et al. Improvement of active corrosion protection of carbon steel by water-based epoxy coating with smart CeO2 nanocontainers. Prog. Org. Coat. 115, 195–204. https://doi.org/10.1016/j.porgcoat.2017.10.015 (2018).
Yeganeh, M. et al. An investigation on the corrosion behavior of the epoxy coating embedded with mesoporous silica nanocontainer loaded by sulfamethazine inhibitor. Prog. Org. Coat. 128, 75–81. https://doi.org/10.1016/j.porgcoat.2018.12.022 (2018).
Huang, Yi. et al. Development of self-healing sol-gel anticorrosion coating with pH-responsive 1H-benzotriazole-inbuilt zeolitic imidazolate framework decorated with silica shell. Surf. Coat. Technol. 466, 129622. https://doi.org/10.1016/j.surfcoat.2023.129622 (2023).
Hu, R. G., Du, R. G. & Lin, C. J. Corrosion behavior of reinforcing steel in concrete subjected to chloride contamination by EIS. Electrochemistry 9(2), 189–195. https://doi.org/10.13208/j.electrochem.2003.02.011 (2003).
Fu, T. et al. Correlation research of phase angle ariation and coating performance by means of Pearson’s correlation coefficient. Prog. Org. Coat. 139, 105459. https://doi.org/10.1016/j.porgcoat.2019.105459 (2020).
Mahdavian, M. & Attar, M. M. Another approach in analysis of paint coatings with EIS easurement: Phase angle at high frequencies. Corros. Sci. 48(12), 4152–4157. https://doi.org/10.1016/j.corsci.2006.03.012 (2006).
Auepattana-Aumrung, K., Phakkeeree, T. & Crespy, D. Polymer-corrosion inhibitor onjugates as additives for anticorrosion application. Prog. Org. Coat. 163, 106639. https://doi.org/10.1016/j.porgcoat.2021.106639 (2022).
Ramezanzadeh, B. et al. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 103, 283–304. https://doi.org/10.1016/j.corsci.2015.11.033 (2016).
Borisova, D. et al. Nanocontainer-based anticorrosive coatings: Effect of the container size on the self-healing performance. Adv. Funct. Mater. 23, 3799–3812. https://doi.org/10.1002/adfm.201203715 (2013).
Zhang, F. et al. Performance and theoretical study on corrosion inhibition of 2-(4-pyridyl-benzimidazole for mild steel in hydrochloric acid. Corros. Sci. 61, 1–9. https://doi.org/10.1016/j.corsci.2012.03.045 (2012).
Acknowledgements
This work was supported by Fundamental research program of Shanxi Province (No.202103021224207)
Author information
Authors and Affiliations
Contributions
Credit authorship contribution statement: Guangqun Cao: Methodolog, Software. Yilei Ruan: Data curation, Writing oridinal draft, Formal Analysis. Jianhua Liu: Investigation. Zhiyong Hu: Project administration. Hailin Zhu: Conceptualization, supervision, Writing–review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, G., Ruan, Y., Liu, J. et al. Preparation and properties study of self-healing anticorrosive coating based on MCM-41 nanocontainer and triazinyl corrosion inhibitor. Sci Rep 15, 22288 (2025). https://doi.org/10.1038/s41598-025-06528-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06528-2