Abstract
Acteoside is the potential bioactive component of Osmanthus fragrans flowers for treating stroke. However, the pharmacological actions, therapeutic targets and underlying mechanisms of acteoside in cerebral ischemia/reperfusion injury remain poorly understood. In this study, we investigated the therapeutic effect, direct target, and the underlying mechanisms of acteoside on cerebral I/R injury in middle cerebral artery occlusion (MCAO) rats. Our study suggested that treatment with OFFE or acteoside significantly reduced cerebral infarct area in MCAO rats. Coagulation function measurement suggested that acteoside treatment increased activated partial thromboplastin time (APTT) and prothrombin time (PT) in vitro. Further molecular docking and enzyme activity assays indicated that acteoside binds to plasma kallikrein (pKal) and markedly inhibits its activity. RNA sequencing, ELISA, and Western blotting revealed that acteoside regulated coagulation to mitigate inflammation by modulating NF-κB signaling and inhibite oxidative stress by suppressing NOX2/NOX4 pathways. These findings reveal that acteoside exerts protective effects against cerebral I/R injury in MCAO rats by attenuating inflammation and oxidative stress as a natural plasma kallikrein inhibitor.
Similar content being viewed by others
Introduction
Stroke is a common disease with high mortality and disability, characterized by neurological damage and long-term disability1,2. About 85% stroke cases belong to ischemic stroke (IS)3. In acute ischemic stroke, cerebral thrombosis blocks brain blood circulation and leads to localized ischemia and tissue hypoxic necrosis. When blood flow is restored after ischemia, reperfusion injury can cause extensive damage to blood vessels and brain tissue, resulting in impaired neurological function4. Thrombolytic therapy remains the cornerstone of treatment for ischemic stroke, aiming to restore cerebral circulation. Nonetheless, the clinical utility of tissue-type plasminogen activator, the sole FDA-approved thrombolytic agent, is constrained by neurotoxicity and adverse effects such as bleeding complications5,6. Thus, there is an urgent need for novel therapeutic strategies to prevent and treat ischemic stroke.
Osmanthus fragrans (Thunb.) Lour., native to East and Southeast Asia, has traditionally been used in Chinese medicine for treating a variety of inflammatory and cardiovascular disorders7. Hitherto, a wide range of compounds including essential oil (e.g. α- and β-linalool), triterpenoid acids (e.g. pomolic acid), esters, lignans and flavonoids have been identified in Osmanthus fragrans flowers extract (OFFE)8. Our study suggested acteoside is one of the most abundant phenylethanol glycosides in osmanthus fragrans flowers9. Previous research suggested acteoside’s efficacy in protecting the brain in mice and improving behavioral and cognitive impairments in D-galactose-induced aging mice10. Additionally, acteoside has been shown to prevented neuro-damage in oxidopamine-induced Parkinson’s disease zebrafish models11. Other study demonstrated that acteoside has antioxidant and neuroprotective effects12. Despite these findings, the direct target and pharmacological mechanisms of acteoside in middle cerebral artery occlusion (MCAO) rats remain unclear.
In this study, we aim to elucidate the pharmacological effects of acteoside, explore its target and therapeutic mechanisms. We carried out this research from the following aspects: (i) evaluating the therapeutic efficacy of OFFE and acteoside in MCAO rats; (ii) identifying acteoside within OFFE using high-performance liquid chromatography-mass spectrometry (HPLC-MS); and (iii) exploring the potential targets and downstream molecular mechanisms of acteoside in the treatment of ischemia-reperfusion (I/R) injury.
Results
OFFE reversed I/R injury in MCAO rats
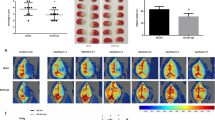
To investigate the anti-I/R injury effects of OFFE in a rat model of cerebral ischemia, we evaluated cerebral infarction using TTC staining and calculated the infarction area percentage in OFFE-treated MCAO rats. Twenty-four-hour post-ischemia/reperfusion, OFFE treatment resulted in a significant reduction in cerebral infarction area in a dose-dependent manner (Fig. 1). These findings suggest that OFFE enhances cerebral blood flow and mitigates ischemic damage in MCAO rats.
OFFE reversed cerebral infarct in middle cerebral artery occlusion (MCAO) rats. After pre-treated with OFFE for 7 days, rats were operated to MCAO and evaluated cerebral infarct, n = 10 in each group. (A) Representative image of brain TTC staining. (B) Infarct area percentage. The values were expressed as Means ± SEM. #P < 0.05 vs. model group. Abbreviations: Sham, sham-operated group; OFFE-Low, Osmanthus fragrans flowers extract-Low dosage group; OFFE-Medium, Osmanthus fragrans flowers extract-Medium dosage group; OFFE-High, Osmanthus fragrans flowers extract-High dosage group; Butyl, Butylphthalide.
Acteoside is a crucial bioactive component of OFFE for its anti-I/R injury effect
HPLC-MS analysis identified acteoside as a major active component of OFFE (Fig. 2). Our pre-experiment comparing the anti- I/R injury effect of OFFE and acteoside, and the result showed that the effect of 5 mg/kg acteoside was comparable to 40 mg/kg OFFE (Supplementary Fig. 1). Acteoside content in Osmanthus fragrans Lour. Flowers is approximately 10% or even higher13. Based on these evidence, we further examined the dose relationship of the anti-ischemia/reperfusion injury effects of acteoside in MCAO rats. Infarction areas were measured 24 h after ischemia/reperfusion. Administration of acteoside at doses of 3 mg/kg and 9 mg/kg significantly reduced cerebral infarction, while a 1 mg/kg dose showed a trend towards reduced infarction (Fig. 3). These results highlight acteoside as a critical bioactive component of OFFE with a dose-dependent efficacy in ameliorating I/R injury.
Identification of acteoside from OFFE through HPLC-MS. (A) Representative LC-MS chromatograms of standard acteoside sample. (B) BPC chromatograms of OFFE. (C) Representative chromatograms of acteoside from OFFE. Abbreviations or term in figure: mrhtg, acteoside; std, standard substance; gutqw or OFFE, Osmanthus fragrans flowers extract; EIC, Extracted Ion Chromatogram; BPC, Base Peak Chromatogram.
Acteoside alleviated cerebral infarct in MCAO rats. MCAO rats were treated with acteoside once and evaluated cerebral infarct 24 h post-reperfusion, n = 10 in each group. (A) Representative image of brain TTC staining. (B) Infarct area percentage. The values were expressed as Means ± SEM. #P < 0.05, ##P < 0.05 vs. model group. Abbreviations: Sham, sham-operated group; Acteoside-Low, Acteoside-Low dosage group; Acteoside-Medium, Acteoside-Medium dosage group; Acteoside-High, Acteoside-High dosage group; Butyl, Butylphthalide.
Plasma Kallikrein is a potential target of acteoside for its anti-I/R injury effect
Coagulation abnormalities are an important pathologic basis for I/R injury. We first assessed the effects of acteoside on plasma coagulation in normal rats in vitro. Acteoside significantly prolonged activated partial thromboplastin time (APTT) at a dose of 1 mg/kg and increased prothrombin time (PT) at 9 mg/kg (Fig. 4A), indicating that acteoside modulates coagulation primarily through the intrinsic pathway.
Plasma kallikrein is a direct target of acteoside. (A) Coagulation function assessment in vitro. (B) Screen the direct target of acteoside by virtual docking. Acteoside docked with candidate proteins or polypeptide by AMDock software, the affinity-score were used to evaluate their binding relationship. (C-E) Enzyme activity assay in cell-free conditions or in vivo. C) Samples were incubated with pure pKal protein and its specific fluorescent substrate in cell-free conditions. The fluorescence intensity was recorded by a fluorescence microplate reader. D-E) After treating MCAO rats with acteoside, plasma or brain samples were collected. The pKal activity assay was performed as C). The values were expressed as Means ± SEM. **P < 0.01, ***P < 0.001 vs. Saline group, or Ctrl group, ##P < 0.01 vs. model group. Abbreviations: APTT, activated partial thromboplastin time; PT, prothrombin time; pKal, plasma kallikrein; FXI, FVIIa, FXa, FXIIA, FIXa, coagulation factor (FXI, FVIIa, FXa, FXIIA, FIXa); OFFE, Osmanthus fragrans flowers extract.
To identify potential targets of acteoside, we performed docking studies using AMDock software with intrinsic pathway-related proteins. Acteoside exhibited favorable binding interactions with plasma kallikrein (pKal) (PDB: 2ANY, Docking_score: −9.0 kcal/mol) (Fig. 4B). Acteoside formed multiple hydrogen bonds with key residues K192, S195, and H57, and additional interactions with residues D189, G219, and S214, suggesting a stable binding configuration that could inhibit pKal.
Enzyme activity assay confirmed the interaction between acteoside and pKal. OFFE and acteoside inhibited pKal activity in a cell-free condition with half maximal inhibitory concentration (IC50) values of 350.6 ± 0.013 µg/mL and 195.2 ± 0.010 µg/mL, respectively (Fig. 4C). In MCAO rats, pKal activity was elevated in plasma but remained low in the brain. Acteoside predominantly inhibited pKal activity in plasma with a tendency to affect brain pKal (Figs. 4D-E). These results indicate that pKal is a relevant target for acteoside’s anti-I/R injury effects.
Acteoside modulates inflammation and oxidative stress via coagulation regulation
We further investigated the molecular mechanism of acteoside for treating I/R injury. We systematically analyzed the I/R injury-related dataset GSE262257 and found that its main functional enrichment focused on coagulation, inflammation and oxidative stress. The pathways were mainly focused on platelet aggregation and nuclear factor kappa-B (NF-κB) pathway (Fig. 5). Ischemia-reperfusion leads to cerebral vascular endothelial damage, following by coagulation activation and thrombosis4,14,15. These evidence indicate that regulating the oxidative stress and inflammation might be the potential molecular mechanism of acteoside.
We measured the oxidative stress- and inflammation-related proteins in MCAO rats. Oxidative stress analysis revealed significant increases in brain NADPH-Oxidase 2 (NOX2) and NADPH-Oxidase 4 (NOX4) protein levels, elevated serum malondialdehyde (MDA), and reduced serum glutamyl-cysteinyl-glycine (GSH) and superoxide dismutase (SOD) in MCAO rats. These oxidative stress markers were notably improved in rats treated with acteoside (Fig. 6). Regarding inflammation, MCAO rats showed significant elevations in brain inhibitor of NF-κB (IκB), p-p65, and serum tumor necrosis factor (TNF-α) and interleukin-1 beta (IL-1β) levels. Acteoside treatment substantially reduced these inflammatory markers (Fig. 7). These findings suggest that acteoside mitigates inflammation and oxidative stress through its regulation of coagulation function.
Acteoside inhibited oxidative stress by NOX2/NOX4 pathway in MCAO rats. MCAO rats were treated with acteoside once and collected blood or brain samples 24 h post-reperfusion, n = 10 in each group. (A) Representative western blot image of NOX2 and NOX4 proteins in infract brain tissue. (B-D) The concentration of pro-oxidant and antioxidant compounds in serum. B) GSH, C) MDA, D) SOD. The values were expressed as Means ± SEM. ***P < 0.001 vs. Ctrl group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model group. Abbreviations: NOX2, NADPH-Oxidase 2; NOX4, NADPH-Oxidase 4; MDA, Malondialdehyde; GSH, Glutamyl-cysteinyl-glycine; SOD, Superoxide dismutase.
Acteoside inhibited inflammation by NF-κB pathway in MCAO rats. MCAO rats were treated with acteoside once and collected blood or brain samples 24 h post-reperfusion, n = 10 in each group. (A) Representative western blot image of IκB, p65, and p-p65 in infract brain tissue. (B-C) The concentration of inflammatory factors in serum. B) TNF-α, C) IL-1β. The values were expressed as Means ± SEM. ***P < 0.001 vs. Ctrl group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. model group. Abbreviations: IκB, inhibitor of NF-κB; TNF-α, Tumor necrosis factor; IL-1β, Interleukin-1 beta.
Discussion
This study investigated the anti-I/R injury effect of acteoside in MCAO rats, explores its direct target and elucidated the downstream molecular mechanism. Acteoside, a natural flavonoid derived from Osmanthus fragrans flowers, has been noted for its therapeutic benefits. Osmanthus fragrans flower can alleviate nerve damage, inhibit neuroinflammation and improve cognitive impairment7. Our study suggested that OFFE alleviated I/R injury and decreased cerebral infraction in MCAO rats. We further investigated the bioactive component of OFFE for its anti-I/R injury effect. Osmanthus fragrans flower is a traditional Chinese flower and is demonstrated to alleviate nerve damage, inhibit neuroinflammation and improve cognitive impairment8. It contains abundant phenylpropanoid glycoside, polyphenols and flavonoids, with acteoside being a major component of phenylpropanoid glycosides. Acteoside can attenuate brain damage, behavioral abnormal and cognitive impairments in various brain diseases10,11,16. In this study, we identified the acteoside from OFFE by HPLC-MS and observed significant alleviation of I/R injury in acteoside-treated MCAO rats. These evidences confirm that acteoside is a critical bioactive component for treating brain damage in MCAO rats.
Hypercoagulability is an independent risk factor for cerebral thrombosis, contributing to cerebral vascular obstruction and I/R injury17. Our study observed that acteoside exerts its anti-coagulation effects mainly through intrinsic thrombosis pathway. We then aimed to explore the direct pharmacological target of acteoside for the anti-coagulant effect. We screened the main proteins involved in the intrinsic thrombosis pathway of APTT and highlighted pKal as a potential target. Subsequent enzyme activity assays in cell-free conditions and in vivo confirmed that acteoside is an inhibitor of protein pKal, a serine protease involved in the kallikrein-kinin system (KKS)18,19. The pKal is transferred from circulating zymogen plasma prekallikrein (PPK) by factor XIIa (FXIIa), and then provide positive feedback amplification of intrinsic thrombosis20. Protein pKal has multiple functions in vascular functions, hemostasis, and thrombosis21,22. Previous studies have indicated that pKal can impair thrombosis by interfering with collagen-mediated platelet activation and its role in vascular thrombosis has been confirmed in animal stroke models23. These evidences suggest that pKal is a potential target for ischemic stroke, with acteoside regulating coagulation and inhibiting thrombosis through pKal inhibition.
To further explore the underlying molecular mechanisms of acteoside targeting pKal to exert its anti-I/R injury effects, we utilized RNA-sequencing transcriptome analysis by online gene expression omnibus (GEO) database. The results revealed that I/R injury predominantly involve in coagulation, inflammation, and oxidative stress, with major pathways including platelet aggregation and the NF-κB pathway. Ischemia-reperfusion induces vascular endothelial injury, activates coagulation and thrombosis, and obstructs blood vessels. It leads to ischemia, hypoxia, and the production of reactive oxygen species (ROS)24,25. ROS following aggravates tissue inflammation and oxidative stress, further damages cerebrovascular structures and promotes thrombosis26. Early studies suggest that NOX2 and NOX4 are ROS-generating enzymes that catalyze the electron transfer from NADPH to molecule O227,28. ROS directly induces oxidative stress to damage neurons and promote I/R injury. This pathological process in turn further up-regulates the expression of NOX2/NOX4, exacerbating oxidative stress damage28,29. Abnormal expression of NOX2 and NOX4 also promote brain damage and neurological disruption in stroke29. Although there is no direct evidence suggests that pKal promotes the expression of NOX2/NOX4, pKal enhances ROS production and decreases GSH in vascular injury, thereby inducing vascular oxidative stress damage30. Our results demonstrated that acteoside significantly inhibited NOX2 and NOX4 expression, decreased pro-oxidant molecule MDA, and increased antioxidant molecules GSH and SOD in brain tissues of MCAO rats, effectively mitigating tissue oxidative stress. The NF-κB pathway is a classical inflammatory pathway. In this pathway, after IκB inhibited, NF-κB-p65 is allowed to enter the nucleus and induce the inflammatory genes transcription31. NF-κB pathway promotes the production, release of inflammatory factors TNF-α and IL-1β, macrophage and neutrophil accumulation, leading to blood-brain barrier disruption and neuronal apoptosis32,33. Current studies report that the KKS can activate NF-κB to promote the expression of inflammatory factors. In additional, pKal can also facilitate the release of TNF and IL34,35. Our results suggested that acteoside inhibited NF-κB pathway activation and reduced inflammatory factors TNF-α and IL-1β release. These evidences i ndicate that acteoside inhibits inflammation and oxidative stress to alleviate I/R injury in MCAO rats through targeting pKal.
In conclusion, this study identifies acteoside, a principal bioactive component of OFFE, as a novel pKal inhibitor that mitigates I/R injury in MCAO rats. Acteoside achieves this by suppressing oxidative stress through NOX2/NOX4 inhibition and reducing inflammation via NF-κB pathway modulation. However, this study has not further verified the relationship between acteoside and pKal through pKal inhibitors or knockout models. Nor has it explored deeply in how acteoside inhibits the downstream NOX2/NOX4 and NF-κB pathways via the inhibition of pKal. Meanwhile, given acteoside’s potent inhibition of pKal activity and anticoagulant properties, the potential for side effects, such as prolonged clotting time, warrants further investigation. Future investigations should prioritize comprehensive pharmacokinetic and toxicological evaluations. And these questions are core aspects for our future research.
Conclusion
Acteoside targets pKal to alleviate I/R injury in MCAO rats by suppressing NOX2/NOX4 oxidative stress and NF-kB associated inflammation.
Method and material
Chemicals and reagents
The OFFE was purchased from Healthy Star Bio-Tech R&D., Ltd (Shanghai, China). Acteoside (purity ≥ 98%) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). The HPLC-grade methanol and acetonitrile were purchased from Thermo Fisher Scientific Inc. (Iowa, United States). Formic acid was purchased from Sigma Chemical Co. (St. Louis, MO, United States). The TNF-α, IL-1β, GSH, MDA, SOD measure kits were purchased from Nanjing Boyan Bio-Technology Co., Ltd. (Nanjing, China). The rabbit primary antibodies (IκB, p65, p-p65, NOX2, NOX4, β-actin) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H + L) were purchased from Abclonal Bio-Technology Co., Ltd. (Wuhan, China). Other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Animal models and treatment
Male Sprague-Dawley (SD) rats (240.0 ± 20 g) were obtained from Charles River Co., Ltd. (Beijing, China). The animals were housed at the Pharmacological Evaluation and Research Animal Center for seven-day acclimatization. They were exposed to 12-h light/12-h dark cycle under a conditions of controlled temperature of 25 ± 2 °C and relative humidity of 45 ± 5%. All rats were provided with standard rat chow and water ad libitum. This study was guided by the Chinese legislation and regulations of Laboratory Animals of the Chinese Animal Welfare Committee. The protocols for this study were approved by the ethics committee of the Center for Pharmacological Evaluation and Research (Shanghai, 200437, China). This study is reported in accordance with ARRIVE guidelines.
The MCAO rats were used to assess the effect of OFFE or acteoside on I/R injury in vivo. After preoperative fasting for 12 h, rats were anesthetized with 1% pentobarbital sodium (40 mg/kg). A midline incision was made to expose the right common carotid artery (CCA) and internal carotid artery (ICA). A 0.26 mm nylon thread was inserted through the CCA and ICA into the middle cerebral artery (MCA). Following 2 h of ischemia, the thread was removed, and the incision was sutured. Successful model induction was confirmed by paraplegia in the contralateral lower extremity.
For pharmacological evaluation of OFFE, normal rats were randomly arranged in five groups, 10 rats in each group. These groups are MCAO group (gavage administration with sterile water), OFFE Low-dosage group (gavage administration with 10 mg/kg OFFE), OFFE Medium-dosage group (gavage administration with 20 mg/kg OFFE), OFFE High-dosage group (gavage administration with 40 mg/kg OFFE), and positive group (intravenous injection with butylphthalide, 2.5 mg/kg). Butylphthalide, an antioxidant commonly used in stroke treatment, can rapidly scavenge oxygen free radicals and alleviate oxidative stress injury in the brain. This study used butylphthalide as a positive drug5. Another sham-operation group was employed for experimental control. After oral administration with 0.9% normal saline or OFFE for 7 days, the MCAO rats were established and sacrificed 24 h post-modeling.
For pharmacological evaluation of acteoside, rats were divided into five groups (10 rats in each group): MCAO group (intravenous injection with 0.9% normal saline), acteoside Low-dose group (1 mg/kg acteoside), acteoside Medium-dosage group (3 mg/kg acteoside), acteoside High-dosage group (9 mg/kg acteoside), and positive group (intravenous injection with butylphthalide, 2.5 mg/kg). MCAO models were established, and rats were administered 0.9% normal saline or drugs by intravenous injection once. The tissue and blood samples were collected 24 h after reperfusion.
Measurement of cerebral infarction area
After euthanizing mice with sodium pentobarbital (150 mg/kg, i.p.), their brain tissues were isolated and stored at −20 °C. After removing the olfactory bulbs, cerebellum, and lower brainstem, the brain was coronally sectioned into five parts as follows: the first incision was made at the midpoint between the anterior pole of the brain and the optic chiasm; the second at the level of the optic chiasm; the third at the infundibular stalk; and the fourth between the infundibular stalk and the caudal pole of the temporal lobe. Coronal brain sections were stained with 2% triphenyltetrazolium chloride (TTC, Sigma, St. Louis, MO, USA) at 37 °C for 30 min. Infarcted areas appeared pale white, while viable tissue appeared dark red. After comparing the color differences between the infarcted and non-infarcted tissues in the same brain, the “Free hand selections” tool in Image J software (Version 1.5.2, https://imagej.net/, National Institutes of Health, Bethesda) was used to manually select the white area of the infarcted side, the left brain side, and right brain sides, calculate the regional areas, and determine the infarct area (%). Infarct volume (%) was calculated as follows: brain infarct area (%) = (Front-infarcted area/Front total area of infarcted hemibrain + Back infarcted area/Back total area of infarcted hemibrain)/2*100%.
Identification of acteoside in OFFE by HPLC-MS
Acteoside was identified using an Agilent 1290 HPLC-MS system. Chromatographic separation was performed on a Waters Xbridge BEH C18 column (2.1 mm × 100 mm, 1.7 μm). The mobile phase comprised 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient was programmed as follows: 0–2 min, 5% B; 2–13 min, 5–95% B; 13–18 min, 95% B; 18–18.1 min, 95 − 2% B; 18.1–20 min, 2% B. Flow rate was set to 0.4 mL/min, column temperature to 40 °C, and injection volume to 2 µL. Mass spectrometric analysis was conducted on a 6545 Quadrupole Time-of-Flight mass spectrometer (Agilent, USA) with an Agilent JetStream Technology electrospray ionization source. Operating parameters were: capillary voltage, 3500 V; gas temperature, 300 °C; drying gas flow, 10 L/min; nebulizer pressure, 35 psi; sheath gas temperature, 350 °C; sheath gas flow, 12 L/min; fragmentor voltage, 100 V. Multi-reaction monitoring mode was used for precursor-to-product ion transitions. Samples and standards were dissolved in acetonitrile at concentrations of 33 mg/mL for OFFE and 3 µg/mL for acteoside, filtered through a 0.22 μm microporous membrane, and analyzed by HPLC-MS.
Coagulation function analysis
Coagulation parameters were assessed in vitro using blood samples from normal rats. Blood samples were collected from the abdominal aorta of rats at study endpoint and anticoagulated with sodium citrate (9:1 ratio of blood to sodium citrate) and mixed with acteoside at concentrations of 1, 3 or 9 mg/mL. Coagulation was evaluated by measuring APTT and PT using a PUN-2048 A blood coagulation analyzer (Beijing, China). Each experimental group consisted of three replicates, and the experiments were performed in triplicate.
Screening potential targets of acteosided
The information of coagulation-related proteins was acquired from CHEMBL database (https://www.ebi.ac.uk/chembl/, downloaded on May 12, 2023). The X-ray crystal structure of proteins was collected from PDB database (https://www.rcsb.org/, downloaded on May 12, 2023). Target screening was performed by AMDock software (Version 1.5.2, github.com/Valdes-Tresa) with comparing Affinity value.
Enzyme activity assays
The effect of acteoside on pKal was investigated in both cell-free and in vivo conditions. Human recombinant plasma kallikrein and protein-specific fluorescent substrates were used according to the manufacturer’s instructions. The kallikrein solution was dissolved in deionized water. Plasma samples were collected from MCAO rats with or without acteoside treatment. Enzyme activity was measured by incubating 50 µL of sample (ranging from 10–2 to 3000 µg/mL OFFE, acteoside, or leupeptin, or plasma samples) with 50 µL of plasma kallikrein substrate solution for 30 min at 37 °C. The fluorescence was measured using a Varioskan LUX microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Autofluorescence was subtracted from the measured values, and relative fluorescence units were normalized to blank samples.
RNA sequencing analysis
RNA sequencing datasets related to stroke were sourced from the GEO database. The dataset GSE262257, which includes brain tissue from MCAO and control mice, was analyzed using R software. Differentially expressed genes (DEGs) were identified with a threshold of |log2 (fold change)| ≥ 1.5 and q < 0.01. Gene Ontology (GO) and KEGG pathway enrichment analyses were conducted for annotation and integrated discovery.
ELISA assay
Rat blood samples were collected via abdominal aorta at the endpoint, centrifuged at 4,000 rpm for 5 min, and the serum was separated. Serum level of GSH, MDA, SOD, TNF-α, IL-1β were quantified using ELISA kits.
Western Blot
Infarct brain tissues were isolated, washed with pre-cold PBS, and homogenized in lysis buffer for 15 min on ice using a tissue homogenizer (Shanghai Jingxin Industrial Development Co., Ltd., Shanghai, China). The homogenates were centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatants were collected. Protein concentrations were quantified using a BSA-protein assay kit (Beyotime, Shanghai, China), then mixed with 1×loading buffer, and heated at 98 °C for 10 min. The mixtures (20 µg protein) were electrophoresed at 100 V for 80 min in a 10% polyacrylamide gel and electrotransferred to polyvinylidene fluoride membranes at 110 V for 70 min. The membranes were blocked with 5% milk-TBST mixture for 60 min at 25 °C, and then treated with primary antibodies against NOX2 (#A19701, 1:1000, Abclonal, Wuhan, China), NOX4 (#A11274, 1:1000, Abclonal), IκB (#A19714, 1:1000, Abclonal), p65 (#A19653, 1:1000, Abclonal), p-p65 (#AP1460, 1:1000, Abclonal), or β-actin (#AC026, 1:10000, Abclonal) at 4 °C overnight, followed by incubating with secondary antibodies (1:5000, Beyotime) at 25 °C for 1 h. The Protein bands were visualized by ECL kit (Beyotime) and analyzed with a Bio-rad chemiluminescence imager.
Statistical analysis
Data was presented as Mean ± SEM. Comparisons between two groups were performed using a two-tailed Student’s t-test. Differences among multiple groups were analyzed using one-way Analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. A P-value < 0.05 was considered statistically significant.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author Li Liu on reasonable request.
Abbreviations
- IS:
-
Ischemic stroke
- OFFE:
-
Osmanthus fragrans flowers extract
- MCAO:
-
Middle cerebral artery occlusion
- HPLC-MS:
-
High performance liquid chromatography-mass spectrometry
- I/R:
-
Ischemia-reperfusion
- APTT:
-
Activated partial thromboplastin time
- PT:
-
Prothrombin time
- pKal:
-
Plasma kallikrein
- IC50:
-
Half maximal inhibitory concentration
- NF-κB:
-
Nuclear factor kappa-B
- NOX2:
-
NADPH-Oxidase 2
- NOX4:
-
NADPH-Oxidase 4
- MDA:
-
Malondialdehyde
- GSH:
-
Glutamyl-cysteinyl-glycine
- SOD:
-
Superoxide dismutase
- IκB:
-
Inhibitor of NF-κB
- TNF-α:
-
Tumor necrosis factor
- IL-1β:
-
Interleukin-1 beta
- KKS:
-
Kallikrein-kinin system
- PPK:
-
Plasma pre-kallikrein
- FXIIa:
-
Factor XIIa
- GEO:
-
Gene expression omnibus
- ROS:
-
Reactive oxygen species
- HRP:
-
Horseradish peroxidase
- SD:
-
Sprague-Dawley
- CCA:
-
Common carotid artery
- ICA:
-
Internal carotid arteries
- MCA:
-
Middle cerebral artery
- TTC:
-
Triphenyltetrazolium chloride
- DEGs:
-
Differentially expressed genes
- GO:
-
Gene ontology
- ANOVA:
-
Analysis of variance
References
Mao, R., Zong, N., Hu, Y., Chen, Y. & Xu, Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci. Bull. 38, 1229–1247 (2022).
Han, J. et al. -)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem Res. 39, 1292–1299 (2014).
Sternberg, Z. & Schaller, B. Central noradrenergic agonists in the treatment of ischemic Stroke-an overview. Transl Stroke Res. 11, 165–184 (2020).
del Zoppo, G. J. & Mabuchi, T. Cerebral microvessel responses to focal ischemia. J. Cereb. Blood Flow. Metab. 23, 879–894 (2003).
Xiong, Y., Wakhloo, A. K. & Fisher, M. Advances in acute ischemic stroke therapy. Circ. Res. 130, 1230–1251 (2022).
Hu, L. et al. Synergistic and efficient thrombolytic nanoplatform: A mechanical method of blasting combined with thrombolytic drugs. Int. J. Nanomed. 17, 5229–5246 (2022).
Pirker, T., Pferschy-Wenzig, E. M., Bampali, E., Bochkov, V. & Bauer, R. Glycolipid-enriched fraction of Osmanthus fragrans inhibits LPS-induced expression of inflammatory genes, COX-2, E-selectin, and Interleukin-8. J. Ethnopharmacol. 309, 116328 (2023).
Wu, L. et al. Exploration of Osmanthus fragrans lour.‘s composition, nutraceutical functions and applications. Food Chem. 377, 131853 (2022).
Liu, S. et al. Osmanthus fragrans flower aqueous extract and its enriched acteoside inhibit melanogenesis and Ultraviolet-induced pigmentation. Nat. Prod. Commun. 13, 575–580 (2018).
Li, M. et al. Acteoside palliates d-galactose induced cognitive impairment by regulating intestinal homeostasis. Food Chem. 421, 135978 (2023).
Li, M., Zhou, F., Xu, T., Song, H. & Lu, B. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food Chem. Toxicol. 119, 6–13 (2018).
Ran, Z. et al. Microbiome-metabolomics analysis reveals the potential effect of verbascoside in alleviating cognitive impairment in db/db mice. Food Funct. 14, 3488–3508 (2023).
Jiang, Y. et al. Phenylethanoid glycoside profiles and antioxidant activities of Osmanthus fragrans lour. Flowers by UPLC/PDA/MS and simulated digestion model. J. Agric. Food Chem. 64, 2459–2466 (2016).
Lehotsky, J. et al. Mechanisms involved in the ischemic tolerance in brain: effect of the homocysteine. Cell. Mol. Neurobiol. 35, 7–15 (2015).
Nong, J., Glassman, P. M. & Muzykantov, V. R. Targeting vascular inflammation through emerging methods and drug carriers. Adv. Drug Deliv Rev. 184, 114180 (2022).
Wu, L. et al. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 40, 2605–2649 (2020).
Gursoy-Ozdemir, Y., Yemisci, M. & Dalkara, T. Microvascular protection is essential for successful neuroprotection in stroke. J. Neurochem. 123 (Suppl 2), 2–11 (2012).
Simão, F., Ustunkaya, T., Clermont, A. C. & Feener, E. P. Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke. Blood 129, 2280–2290 (2017).
Ewald, G. A. & Eisenberg, P. R. Plasmin-mediated activation of contact system in response to pharmacological thrombolysis. Circulation 91, 28–36 (1995).
Renné, T., Schmaier, A. H., Nickel, K. F., Blombäck, M. & Maas, C. Vivo Roles Factor. XII Blood 120, 4296–4303 (2012).
Liu, J. et al. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat. Med. 17, 206–210 (2011).
Kleinschnitz, C. et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J. Exp. Med. 203, 513–518 (2006).
Göb, E. et al. Blocking of plasma kallikrein ameliorates stroke by reducing thromboinflammation. Ann. Neurol. 77, 784–803 (2015).
Busl, K. M. & Greer, D. M. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation 26, 5–13 (2010).
Lahiani, A., Brand-Yavin, A., Yavin, E. & Lazarovici, P. Neuroprotective effects of bioactive compounds and MAPK pathway modulation in Ischemia-Stressed PC12 pheochromocytoma cells. Brain Sci. 8, 32 (2018).
Wang, F. et al. Neuregulin-1 alleviate oxidative stress and mitigate inflammation by suppressing NOX4 and NLRP3/caspase-1 in myocardial ischaemia-reperfusion injury. J. Cell. Mol. Med. 25, 1783–1795 (2021).
Buvelot, H., Jaquet, V., Krause, K. H., Mammalian, N. A. D. P. H. & Oxidases Methods Mol. Biol. 17–36 (2019). (1982).
Magnani, F. & Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 59, 91–97 (2019).
Tarafdar, A. & Pula, G. The role of nadph oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 19, 3824 (2018).
Berger, M. et al. Renal and vascular effects of kallikrein inhibition in a model of lonomia obliqua venom-induced acute kidney injury. PLoS Negl. Trop. Dis. 13, e0007197 (2019).
Oeckinghaus, A., Hayden, M. S. & Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 (2011).
Wang, M. et al. Ethanolic extract of Arctium lappa leaves alleviates cerebral ischemia reperfusion-induced inflammatory injury via HDAC9-mediated NF-κB pathway. Phytomedicine 129, 155599 (2024).
Luo, H. et al. Neutrophil extracellular traps in cerebral ischemia/reperfusion injury: friend and foe. Curr. Neuropharmacol. 21, 2079–2096 (2023).
Yang, C. C., Hsiao, L. D., Shih, Y. F., Yu, Z. Y. & Yang, C. M. Anti-inflammatory effects of rhamnetin on bradykinin-induced matrix metalloproteinase-9 expression and cell migration in rat brain astrocytes. Int. J. Mol. Sci. 23, 609 (2022).
Yang, J. et al. Kallikrein inhibitor derived from immunoglobulin heavy chain junction region possesses anti-thromboinflammation potential. Pharmacol. Res. 209, 107460 (2024).
Funding
This work was sponsored by the Shanghai professional technical service platform for evaluation of the druggability of biological substances (18DZ2290900) and Platform Construction for Lead Compound Druggability Research and Drug Development, 23002400100.
Author information
Authors and Affiliations
Contributions
Z-HW, LL and H-HW conceived and designed the experiments, H-HW, K-RF, and PL performed the experiments and analyzed the data. H-HW, LL and Z-HW wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Hh., Feng, Kr., Li, P. et al. Acteoside ameliorates cerebral ischemia/reperfusion injury in MCAO rats as a natural plasma Kallikrein inhibitor from Osmanthus fragrans flower. Sci Rep 15, 23509 (2025). https://doi.org/10.1038/s41598-025-06553-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06553-1