Abstract
This study aims to evaluate lamina cribrosa (LC) parameters, macular ganglion cell layer thickness (GCLT) and peripapillary retina nerve fiber layer thickness (RNFLT) in Graves’ Ophthalmopathy (GO) patients using spectral domain optical coherence tomography (SD-OCT). Seventy GO patients and 70 healthy subjects were included in the study. LC thickness (LCT), LC depth (LCD), LC curvature depth (LCCD), LC curvature index (LCCI) measurements, peripapillary RNFLT and macular GCLT were evaluated with SD-OCT, and proptosis was evaluated with Hertel exophthalmometer. Clinical activity was assessed using the clinical activity score (CAS). Disease severity was evaluated according to the European Group on Graves Orbitopathy (EUGOGO) stage. SD-OCT revealed significant differences in the LC morphology between the affected and control groups. LCT was significantly decreased in the GO group (236.7 ± 37.1 μm) compared to the control group (276.1 ± 36.3 μm) (p < 0.001). Median LCCD was significantly higher in the patient group [95.5 (80.5–127.5) µm] than in the control group [78 (66–90.25) µm] (p < 0.001). LCCI was 6.08 (4.7–7.5) in the GO group and 4.9 (4.3–5.6) in the control group. The difference between the two groups regarding LCCI was statistically significant (p < 0.001). The mean LCD, central foveal GCLT, peripapillary global RNFLT and central foveal thickness were not significantly different between the two groups (p = 0.124, p = 0.536, p = 0.226 and p = 0.958, respectively).LC morphology may change in patients with GO. LCT, LCCI and LCCD showed a good ability to differentiate GO from healthy controls. Therefore, they can be used as supporting parameters for diagnosis.
Similar content being viewed by others
Introduction
Graves’ ophthalmopathy (GO) is the most common extrathyroidal involvement of Graves’ disease1. GO, also known by different names such as thyroid eye disease, infiltrative ophthalmopathy, thyroid orbitopathy, is an inflammatory autoimmune disease of the eye that is often accompanied by hyperthyroidism. GO is the most common cause of proptosis and orbital inflammation in adults2.
The most common signs and symptoms seen in patients are eyelid retraction, proptosis, extraocular muscle dysfunction, pain and tearing. Additionally, severe GO, which occurs in approximately 5%, progresses with compressive optic neuropathy and corneal ulceration3.
Autoantibodies develop against thyroid stimulating hormone (TSH) receptors, a common antigen in the thyroid gland and orbital tissue, and target fibroblasts in intraorbital and extraocular muscles. Activated fibroblasts due to increased inflammation enhance glycosaminoglycan (GAG) synthesis. Excessive accumulation of GAGs in intraorbital tissues and extraocular muscles constitutes the clinical presentation of this disease. Additionally, the expansion of muscles at the orbit apex leads to compressive optic neuropathy. In addition, the stimulated fibroblasts transform into adipocytes and begin the synthesis of fatty tissue. As a result, an increase in fat volume is observed in orbit4.
In some patients, optic nerve compression can develop even if there is no change in visual acuity. Optic disc edema is mostly seen in these patients but can sometimes appear normal, hyperemic, or pale depending on optic atrophy5. In clinical practice, signs and symptoms indicating optic nerve dysfunction such as decreased visual acuity, color vision disturbances, visual field defects, and afferent pupillary defects are observed. Optic nerve damage can be demonstrated with optical coherence tomography (OCT). Changes in macular ganglion cell layer thickness (GCLT), peripapillary retina nerve fiber layer thickness (RNFLT), and lamina cribrosa thickness (LCT) can be used in the diagnosis as supportive measures in the early stages of optic nerve compression6.
OCT is a non-invasive method used to evaluate retinal layers such as the macular ganglion cell layer (GCL), peripapillary retinal nerve fiber layer (RNFL), and optic disc7,8. Moreover, the enhanced deep imaging (EDI) mode of OCT allows for clearer imaging of these structures.
The lamina cribrosa (LC), the primary supporting element of the optic nerve, is an important marker for early diagnosis of certain diseases. LC is a functional barrier zone located between the intraocular and subarachnoid spaces. The pressure difference between these two spaces is referred to as the translaminar pressure difference. Increased intracranial pressure or decreased cerebrospinal fluid (CSF) pressure leads to high translaminar pressure. Increased translaminar pressure in glaucoma patients causes structural changes in LC and results in damage to the nerve fibers9,10. We thought that there could be morphological changes in the LC due to increased intraorbital pressure leading to elevated translaminar pressure in patients with GO.
In this study, we investigated whether OCT could be useful as an auxiliary imaging method in early diagnosis and early treatment initiation by looking at the patient’s LC-related measurements in mostly mild and inactive patients and compared them to healthy controls. In other words, the aim is to evaluate clinically invisible LC changes with OCT imaging.
Results
The clinical and demographic characteristics of the groups are shown in Table 1. In the study, there were 15 (21.4%) males and 55 (78.6%) females in the patient group. The control group consisted of 26 (37.1%) males and 44 (62.9%) females (p = 0.063).
The median age was 45 (34.0–52.0) years in the patient group and 43 (30.0–58.0) years in the control group (p = 0.502). Both in the patient and control groups, best-corrected visual acuity (BCVA) was 20/20 according to the Snellen chart and there was no statistically significant difference in intraocular pressure (IOP) (p = 0.652).
The mean clinical activity score (CAS) in the patient group was 0.7 ± 1.2. According to EUGOGO, severity was mild in 53 patients (75.7%), moderate in 15 patients (21.4%), and 2 patients (2.9%) had sight-threatening ophthalmopathy. Both patients in the sight-threatening ophthalmopathy group had corneal involvement.
The measurement of the lid aperture was significantly higher in the patient group [13.50 (12.0–15.0) mm] compared with the control group [11 (10.0–12.0) mm] (p < 0.001). Margin-reflex distance-1 (MRD-1) [5.4 (5.0–6.0) mm vs. 4.6 (4.0–5.0) mm] and margin-reflex distance-2 (MRD-2) [8 (7.0–10.0) mm vs. 6 (5.0–7.0) mm] measurements were significantly higher in the patient group than the control group (p < 0.001). The amount of exophthalmos was found to be higher in the patient group [18 (16.75–21.25) mm] than in the control group [15 (14–16) mm] (p < 0.001) (Table 1).
In the comparison between the GO group (447.5 ± 129.8 μm) and the control group (418.7 ± 85.7 μm), no statistically significant difference was found in LC depth (LCD) (p = 0.124). Median LC curvature depth (LCCD) was significantly higher in the patient group [95.5 (80.5–127.5) µm] than in the control group [78 (66–90.25) µm] (p < 0.001). In the patient group, mean LCT was found to be 236.7 ± 37.19 μm, while in the control group, it was 276.1 ± 36.3 μm. Mean LCT in the patient group was significantly lower compared to the control group (p < 0.001). Median LCCI was significantly higher in the patient group [6.08 (4.7–7.5)] than in the control group [4.9 (4.3–5.6)] (p < 0.001) (Table 2).
In the patient group, the mean central foveal thickness was 265.7 ± 20.1 μm, while in the control group, it was 265.8 ± 21.4 μm, and the difference between them was not statistically significant (p = 0.958) (Table 3). The median central foveal GCLT was 13 (11–15) µm in the patient group and 13 (11–15) µm in the control group. No statistically significant difference was found between the two groups (p = 0.536). Furthermore, GCLT in all sectors did not differ significantly between GO and control groups (Table 3). Mean peripapillary RNFLT was 102.8 ± 8.5 μm in the patient group and 100.7 ± 11.8 μm in the control group. No statistically significant difference was found between the two groups (p = 0.226) (Table 3).
The relation between disease severity and LC parameters in GO patients is shown in Table 4. No significant relation was observed between disease severity and LC parameters (Moderate to severe and sight-threatening ophthalmopathy groups were considered as one group due to the small number of patients).
A receiver operating characteristic (ROC) analysis was conducted to investigate the parameters differentiating GO from healthy controls and to determine cut-off values for them. ROC analysis revealed that LCT, LCD and LCCI showed a good ability to differentiate GO from healthy controls (p < 0.001, for all) (Table 5; Fig. 1).
Discussion
The LC is located behind the optic nerve head and is primarily composed of collagen fibers. It is a lattice-like structure through which axons of ganglion cells pass. Additionally, this structure, shaped like a sieve, allows the passage of the central retinal artery and vein. It supports and protects the retinal nerve fibers. It is believed that LC is the main area affected by glaucomatous damage in patients with glaucoma11.
It is assumed that morphological changes in the sclera are due to many different underlying mechanisms in different diseases. In GO patients, compression of the LC is caused by enlarged extraocular muscles at the orbital apex, which impairs axoplasmic flow. The optic nerve is also stretched due to severe proptosis with impaired axonal function and optic nerve blood flow. This occurs much less frequently than the compressive mechanism12. High intraorbital pressure causes compression of the optic nerve and increases translaminar pressure gradient. High translaminar pressure causes structural changes in the sclera and leads to nerve fiber damage9,10. In addition, deformation and compression of the sclera can contribute to optic nerve ischemia, as the tension within the sclera can potentially cause blockage of laminar capillaries13. While the normal range of IOP varies between 10 and 21 mmHg, the normal range of cerebrospinal fluid pressure varies between 5 and 15 mmHg14. Therefore, generally having a positive translaminar pressure gradient is possible, which results in the optic nerve bending backward. According to previous studies, it is known that an increase in intraorbital pressure causes compression around the optic nerve in the subarachnoid space and decreases the CSF pressure in that area15. In another study conducted on patients with GO, a decrease in subarachnoid space volume and morphological changes in the LC were observed16. Therefore, a decrease in CSF pressure around the optic nerve leads to an increase in translaminar pressure. Morgan et al. have conducted studies examining the effect of altering the pressure in the intraocular and subarachnoid spaces on the LC. They have reported that the variability of CSF pressure has a similar effect on the LC as changing the IOP17. Lee et al. found a positive correlation between the difference in translaminar pressure and the backward displacement of lamina cribrosa curvature in a study conducted on 24 healthy individuals10. In another study, it was reported that when IOP decreased in glaucoma patients, LCCI decreased as well18. The lack of difference in IOP between the groups in our study suggests that other factors contribute to LCCI changes. Based on this information, we propose that a reduction in CSF pressure around the optic nerve in GO patients leading to an increase in translaminar pressure gradient causes an increase in LCCI and a decrease in LCT in our patient group. In previous studies, the most commonly used parameter to characterize LC morphology is LCD which is the depth of LC measured from the level of Bruch’s membrane opening19. High translaminar pressure increases LCD. However, in our study, no statistically significant difference was found regarding LCD between GO patients and the control group. Since LC measurements are made from the Bruch’s membrane opening, which also includes choroidal thickness, these measurements can provide misleading results. Additionally, studies have shown that there are differences in choroidal thickness among individuals20. Therefore, measurements directly targeting the interior of the LC seem more appropriate. Lee et al. suggested that the evaluation of LC curvature using LCCI may be superior to LCD as a parameter related to optic nerve head biomechanics21. Considering this information, the insignificant statistical difference in LCD between the patient and control groups in our study may be due to the difference in choroidal thickness between the groups. In a study evaluating LCD after intravenous methylprednisolone treatment in GO patients, no significant difference was found between the patient and control groups22. Our study appears consistent with the research conducted by Ozer et al. in this regard.
The number of studies on LCT in GO patients is very low. However, LCT has been described as a more useful diagnostic tool for normal tension glaucoma (NTG), particularly for detecting early glaucomatous changes, compared to RNFL measurement23. In one study, it was reported that LCT was significantly thinner in eyes with NTG and primary open-angle glaucoma compared to the control group24. Additionally, in pathological myopia, it has been reported that LC is thinner compared to ametropic eyes, thus making it more sensitive to the increased translaminar pressure gradient25. This finding may explain the increased risk of optic nerve damage in highly myopic eyes. In vivo studies of LC revealed that the LCT is thinner in eyes with glaucoma compared to normal controls, and LCD is significantly deeper compared to normal controls24,26. Romano et al. observed that the LCT is thinner in GO patients6. In our study, LCT was found to be lower in the patient group in line with Romano et al.’s study.
Romano et al. have found a significant decrease in the RNFLT, GCLT and macular thickness of patients with GO compared to the control group in their study. They have suggested that this could be used as an early indicator for determining optic nerve damage in GO. In our study, there was no statistically significant difference between the patient and control groups regarding RNFLT, GCLT, and macular thickness. Therefore, we speculate that morphological changes in the LC may have begun prior to changes in RNFLT, GCLT and macular thickness. Indeed, in a study evaluating NTG patients, thinning in LC was found to have higher sensitivity and specificity in detecting early glaucomatous changes compared to RNFL23.
Contradictory results have been observed in previous studies regarding the thickness of macula and peripapillary RNFLT in patients with GO. In a study conducted by Sayın et al. comparing macular thickness and peripapillary RNLFT between GO patients and healthy controls, both macular thickness and peripapillary RNLFT were found to be significantly thinner in GO patients compared to the control group. They think that the reason for this might be that the IOP and proptosis values are higher in the GO group compared to the control group, and optic nerve damage could occur as a result of the expansion of retrobulbar connective tissue while maintaining a constant orbit volume, particularly with thinning, initially in the GCL, which could result in macular thinning27. However, Meirovitch et al. reported a significant increase in peripapillary RNFLT when comparing GO patients with the control group, suggesting that inflammatory involvement of orbital tissues could lead to additional damage to the optic nerve28. Considering the background of this study, it is natural that our study did not find a significant increase in peripapillary RNFLT in our patient group compared to the control group, as inflammatory activity is minimal. Danesh-Meyer et al. have argued in their studies conducted at different times that the decrease in peripapillary RNFLT may be associated with the compression zone in compressive optic neuropathy29,30. Since no signs of compressive optic neuropathy were found in any of the patients included in our study, we concluded that it was not a prevalent condition in our sample.
In a study, it was found that, compared to the healthy control group, the macular thickness and the GCLT were significantly lower in patients with GO31. In our study, no statistically significant difference was found in macular thickness measurements and GCLT measurements between patient and control groups in all segments using OCT. We hypothesize that the primary target region of damage associated with GO is ganglion cell axons at the LC level. Therefore, the absence of thinning in the ganglion cell layer explains the absence of thinning in macular thickness as well.
Our study has limitations in several aspects. First, the study was conducted cross-sectionally. If future prospective studies are carried out with a larger number of patients, it will support the progression of LC measurement. Additionally, due to both the low number of clinically active patients and advanced-stage patients, correlation analysis with LC parameters could not be determined; therefore, its reliability could be low. Furthermore, axial lengths which may affect LC parameters, were not assessed between the groups. However, since there are no current studies specifically evaluating LCCI and LCCD in GO patients, this study may be instructive. Lastly, LCCI and LCCD measurements were done manually; however, implementing a standardized program could lead to more consistent measurements.
To our knowledge, Our study is the first to evaluate the LCCI and LCCD in GO patients. In our patient group, LCCI and LCCD were found to be significantly higher compared to the control group. Furthermore, ROC analysis showed that LCT, LCCD and LCCI had a good ability to differentiate GO from healthy controls. We concluded that LCT may decrease in GO patients, and this decrease could be related to increased intraorbital pressure. Since LCD is affected by choroidal thickness, it is not a reliable parameter to demonstrate LC morphology. If supported by more comprehensive and prospective studies to confirm their diagnostic potential, LCT, LCCD and LCCI could be used as supporting parameters for diagnosing GO patients. It would be valuable to support the morphologic changes in LC, which we have shown to start before the changes in RNFLT, GCLT and macular thickness, with prospective studies that will evaluate whether they will guide the course and treatment of the disease.
Methods
In this study, the principles of the Helsinki Declaration were followed. Ethics committee approval was obtained from Kocaeli University Research and Application Hospital (GOKAEK-2023/13.31). A study was conducted comparing follow-up patients diagnosed with GO in the Oculoplastics Clinic and healthy control volunteers referred to our clinic for refractive glasses prescriptions. Seventy GO patients and 70 healthy individuals, totaling 140 eyes, were included in the study.
In this study, the electronic medical records of patients who were followed up at Kocaeli University from January 2018 to May 2024 were reviewed. Informed consent was obtained from all participants included in the study. Measurements were taken from patients in both groups using Heidelberg SD-OCT. LC parameter measurements, peripapillary RNFLT, GCLT, and macular thickness were evaluated using SD-OCT. The amount of proptosis was measured using a Hertel exophthalmometer (Bernell Corp, Mishawaka, Ind.)
Baseline examination
All patients underwent a thorough ophthalmic evaluation. Demographic data (age and sex), blood thyroid hormones levels, measurement of IOP (Canon TX-20P tonometer), lid aperture, MRD-1 and MRD-2 measurements, amount of proptosis, and CAS32 were recorded. MRD-1 is the measurement in millimeters from the light reflex on the patient’s cornea to the upper eyelid margin, with the patient gazing in the primary position. MRD-2 measures from the corneal light reflex to the lower eyelid margin, with the patient’s eyes in the primary gaze. For the evaluation of the CAS, spontaneous retrobulbar pain, pain with eye movements, eyelid redness, conjunctival redness, chemosis, eyelid swelling and caruncle and/or plica inflammation were noted. The patients were divided into two groups based on CAS (active and inactive). BCVA was determined according to the Snellen chart. OCT was performed on all patients and control subjects by an experienced technician.
Exclusion criteria were as follows: eye disease other than ocular involvement associated with Graves’ disease for GO group, diabetes mellitus, cardiovascular disease, cancer, or another systemic disease, a history of ocular surgery, opacity in the optical media, a history of ocular trauma, BCVA of < 20/20, high myopia (> −6D) and high hyperopia ( > + 5D) for both groups.
OCT scan protocol
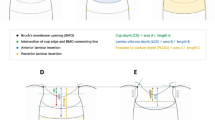
B-scans of the optic nerve head (ONH) were obtained using a Spectralis OCT (Heidelberg Engineering GmbH, Heidelberg, Germany). EDI mode was preferred in OCT for better visualization. LC is accepted as a region with high reflectivity in OCT. LCT and LCD were measured. Retinal vessel shadows can prevent hyperreflectivity of the LC, and these shadows make measurement difficult. Therefore, the ONH center was preferred as the measurement site (without including the main vessels). The LCD was determined as the distance between the horizontal line connecting Bruch’s membrane limit points (Bruch’s membrane opening (BMO)) and the anterior surface of the LC. The first horizontal line where the hyperreflectivity of the LC begins was accepted as the anterior lamina cribrosa surface. The vertically lowest hyperreflective horizontal line of the LC was accepted as the lower limit. Thickness was measured as the distance between these two horizontal lines. LCCI was determined by measuring the width of the LC curve reference line (LCCW) and then measuring the LCCD. The LCCW was defined as the width of the line connecting the two points on the anterior LC surface meeting the lines drawn from each BMO termination point perpendicular to the BMO reference line. The maximum depth from the reference line to the anterior LC surface was defined as the LCCD. The LCCI was calculated as (LCCD/LCCW) × 100 (Fig. 2).
Lamina cribrosa (LC) measurements in the enhanced-deep-imaging optical coherence tomography image through the optic nerve head. The LC depth (yellow line) is measured as the distance between the horizontal line (red line) connecting Bruch’s membrane limit points (Bruch’s membrane opening (BMO)) and the anterior surface of the LC. LC thickness (black line) is measured as the vertical height between the anterior and posterior surfaces of the LC. The LC surface reference line (LCCW) (green line) was defined as the width of the line connecting the two points on the anterior LC surface that met the lines drawn from each BMO termination point perpendicular to the BMO reference line. The maximum depth from the reference line to the anterior LC surface was defined as the LC curve depth (LCCD) (blue line). The lamina cribrosa curvature index (LCCI) was calculated as (LCCD/LCCW) X 100.
The radial scanning protocol, consisting of 6 linear scans equidistantly centered at the optic nerve head, was performed with a scanning angle of 30°. The parameters of LC were obtained by averaging the measurements taken in these 6 radial sections (Fig. 3).
Measurements were performed for the peripapillary RNFL and macular GCL of the patients. Total macular thickness and macular GCLT measurements were separately calculated within circular areas of 1-3-6 mm in a total of 9 sectors using OCT. Peripapillary RNFL was evaluated in 6 different sectors (Fig. 4).
Statistical analysis
All statistical analyses were performed using IBM SPSS for Windows version 20.0 (IBM Corp., Armonk, NY, USA) and MedCalc 14.0. Kolmogorov-Smirnov test was used to assess the normality assumption. Continuous variables were presented with mean ± standard deviation or median (IQR: Interquartile range). Categorical variables were summarized as counts and percentages. Comparisons between groups were carried out using independent samples t-test for normally distributed variables and Mann-Whitney U test for non-normally distributed variables. Associations between continuous variables were determined by Spearman correlation analysis and associations between categorical variables were examined by Chi-square test. ROC analysis was used to determine the AUC, sensitivity, specificity, and cut-off values. p-value < 0.05 was considered statistically significant.
Data availability
Data available on request from the first author “Abdullah Ergen”.
References
Burch, H. B. & Wartofsky, L. Life-threatening thyrotoxicosis: Thyroid storm. Endocrinol. Metab. Clin. North Am. 22.2, 263–277 (1993).
El-Kaissi, S., Frauman, A. G. & Wall, J. R. Thyroid‐associated ophthalmopathy: A practical guide to classification, natural history and management. Intern. Med. J. 34.8, 482–491 (2004).
Wiersinga, W. M. & Bartalena, L. Epidemiology and prevention of graves’ ophthalmopathy. Thyroid 12.10, 855–860 (2002).
Prabhakar, B. S., Bahn, R. S. & Smith, T. J. Current perspective on the pathogenesis of graves’ disease and ophthalmopathy. Endocr. Rev. 24.6, 802–835 (2003).
Mckeag, D. et al. Clinical features of dysthyroid optic neuropathy: A European group on graves’ orbitopathy (EUGOGO) survey. Br. J. Ophthalmol. 91.4, 455–458 (2007).
Romano, M. R. et al. Optic nerve compression: The role of the lamina cribrosa and translaminar pressure. Int. J. Ophthalmol. 10.12, 1883 (2017).
Spaide, R. F., Koizumi, H. & Pozonni, M. C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 146.4, 496–500 (2008).
Vizzeri, G. et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br. J. Ophthalmol. 93.6, 775–781 (2009).
Jonas, J. B. et al. Estimated trans-lamina cribrosa pressure difference versus intraocular pressure as biomarker for open‐angle glaucoma. The B Eijing eye study 2011. Acta Ophthalmol. 93 (1), e7–e13 (2015).
Lee, D. S. et al. Influence of translaminar pressure dynamics on the position of the anterior lamina cribrosa surface. Investig. Ophthalmol. Vis. Sci. 56.5, 2833–2841 (2015).
Nadler, Z. et al. Automated lamina cribrosa microstructural segmentation in optical coherence tomography scans of healthy and glaucomatous eyes. Biomed. Opt. Express. 4.11, 2596–2608 (2013).
Rose, G. E. & Vahdani, K. Optic nerve stretch is unlikely to be a significant causative factor in dysthyroid optic neuropathy. Ophthalmic Plast. Reconstr. Surg. 36, 157–163 (2020).
Burgoyne, C. F. et al. The optic nerve head as a Biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 24.1, 39–73 (2005).
Berdahl, L. P., Allingham, R. R. & Johnson, D. H. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 115.5, 763–768 (2008).
Volpe, N. J. et al. Occult thyroid eye disease in patients with unexplained ocular misalignment identified by standardized orbital echography. Am. J. Ophthalmol. 142.1, 75–81e1 (2006).
Ossoining, K. C. The role of standardized ophthalmic echography in the management of graves’ ophthalmopathy. Dev. Ophthalmol. 20, 28–37 (1989).
Morgan, W. H. et al. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Investig. Ophthalmol. Vis. Sci. 39.8, 1419–1428 (1998).
Lee, S. H. et al. Reduction of the lamina cribrosa curvature after trabeculectomy in glaucoma. Investig. Ophthalmol. Vis. Sci. 57.11, 5006–5014 (2016).
Lee, E. J., Kim, T. W., Weinreb, R. N. & Kim, H. Reversal of lamina cribrosa displacement after intraocular pressure reduction in open-angle glaucoma. Ophthalmology 120.3, 553–559 (2013).
Rhodes, L. A. et al. Peripapillary choroidal thickness variation with age and race in normal eyes. Investig. Ophthalmol. Vis. Sci. 56.3, 1872–1879 (2015).
Lee, S. H. et al. Diagnostic power of lamina cribrosa depth and curvature in glaucoma. Investig. Ophthalmol. Vis. Sci. 58.2, 755–762 (2017).
Ozer, F. et al. Evaluation of lamina cribrosa and peripapillary vascular density in thyroid orbitopathy and effect of intravenous methylprednisolone therapy. Can. J. Ophthalmol. 59, e489–e495 (2024).
Park, H. Y. L. Diagnostic capability of lamina cribrosa thickness by enhanced depth imaging and factors affecting thickness in patients with glaucoma. Ophthalmology 120.4, 745–752 (2013).
Park, H. Y. L., Jeon, S. H. & Park, C. K. Enhanced depth imaging detects lamina cribrosa thickness differences in normal tension glaucoma and primary open-angle glaucoma. Ophthalmology 119.1, 10–20 (2012).
Miki, A. et al. Defects of the lamina cribrosa in high myopia and glaucoma. PLoS ONE. 10.9, e0137909 (2015).
Park, S. C. et al. Lamina cribrosa depth in different stages of glaucoma. Investig. Ophthalmol. Vis. Sci. 56.3, 2059–2064 (2015).
Sayın, O., Yeter, V. & Arıtürk, N. Optic disc, macula, and retinal nerve fiber layer measurements obtained by OCT in thyroid-associated ophthalmopathy. J. Ophthalmol. 2016.1, 9452687 (2016).
Meirovitch, S. B. et al. Retina and nerve fiber layer thickness in eyes with thyroid-associated ophthalmopathy. Isr. Med. Assoc. J. 19.5, 277–281 (2017).
Danesh-Meyer, H. V. et al. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Investig. Ophthalmol. Vis. Sci. 49.5, 1879–1885 (2008).
Danesh-Meyer, H. V. et al. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in Chiasmal compression. Investig. Ophthalmol. Vis. Sci. 47.11, 4827–4835 (2006).
Casini, G. et al. Retinal, choroidal and optic disc analysis in patients with graves’ disease with or without orbitopathy. Int. Ophthalmol. 40, 2129–2137 (2020).
Bartalena, L. et al. Consensus statement of the European group on graves’ orbitopathy (EUGOGO) on management of GO. Eur. J. Endocrinol. 158.3, 273–285 (2008).
Acknowledgements
Thanks to Assoc. Prof. Sibel Balci, Kocaeli University, Faculty of Medicine, Department of Biostatistics and Medical Informatics.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.E, B.Y.T; Methodology: B.Y.T; Formal analysis and investigation: A.E, B.Y.T; Writing - original draft preparation: A.E, B.Y.T; Writing - review and editing: B.Y.T, A.E; Funding acquisition: none; Resources: none; Supervision: B.Y.T. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Local ethic committee registration number of this study is KÜ GOKAEK-2023/13.31.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ergen, A., Yılmaz Tuğan, B. Lamina cribrosa curvature depth and index as novel parameters in Graves’ ophthalmopathy. Sci Rep 15, 22981 (2025). https://doi.org/10.1038/s41598-025-06584-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06584-8