Abstract
Epithelial ovarian cancer (EOC) is characterized by a highly immunosuppressive tumor microenvironment (TME) that enables EOC progression and limits the efficacy of current immunotherapies. In this study, we demonstrated that isogenic BRCA2-mutated PEO1 and BRCA2-wild type PEO4 EOC cells induced immunosuppressive TMEs through distinct mechanisms. PEO1 cells produced IFNγ-induced PD-L1 and expressed CD39 and CD73 for generating adenosine. Treatment with the adenosine antagonist CGS15943 reversed PEO1 cell-mediated suppression of effector T cell activation. In contrast, PEO4 cells secreted IFNγ-induced CXCL10 and promoted up-regulation of FOXP3+ regulatory T cells (Tregs). Treatment with the CXCL10/CXCR3 antagonist AMG487 attenuated PEO4 cell-induced Tregs and decreased IL10 production. In vivo, administration of a monoclonal antibody against CXCR3 effectively hindered the progression of tumor ascites and prolonged survival in the p53(-/-) ID8 EOC syngeneic mouse model. Additionally, AMG487 treatment synergized with the VEGFA inhibitor bevacizumab, significantly reducing tumor ascites and extending mouse survival. Collectively, our results reveal that EOC leverages CXCL10-induced Tregs or adenosine signaling to dampen T cell-mediated anti-cancer immune responses. These findings suggest that targeting CXCL10/CXCR3 and adenosine signaling could effectively counter immunosuppression of EOC, offering a promising therapeutic strategy for improving patient outcomes.
Similar content being viewed by others
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of death among gynecological malignancies in the United States, with an estimated 12,810 women dying from the disease annually and a 5-year relative survival rate of 49% across all stages1. Immune evasion in EOC plays a crucial role in the high recurrence rate and poor survival outcomes, particularly in patients with advanced disease. Cancer cells evade immune surveillance by inducing immune checkpoint molecules, up-regulating regulatory T cells (Tregs), and establishing immunosuppressive networks within the tumor microenvironment (TME)2,3,4. Recent evidence in immune checkpoint blockade therapies has shown promise in achieving durable clinical responses and disease regression across various cancer types5. Immune checkpoints are mechanisms that regulate immune cell activity to prevent tissue damage. However, cancer cells hijack these regulatory mechanisms to evade immune destruction. Therapeutic blockade of immune checkpoints overrides the inhibitory signals, enabling T cells to mount an effective attack on cancer cells5.
Cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and programmed cell death protein 1 (PD1) are two major inhibitory checkpoint molecules of lymphocytes that attenuate T cell activation. Upon engagement with human leukocyte antigen (HLA) and activation of T-cell receptor (TCR), CTLA4 is up-regulated and competes with co-stimulatory receptor CD28 for its ligands CD80 and CD86, thereby dampening TCR signaling6. Blockade of CTLA4 releases this inhibition, promoting T cell activation. Ipilimumab, an FDA-approved monoclonal antibody against CTLA4, has shown efficacy in treating melanoma, small cell lung cancer, and other malignancies. Similarly, PD1, through its interaction with programmed death-ligand 1 (PD-L1) on dendritic and cancer cells, impedes TCR signaling7. PD1 may directly transmit an inhibitory signal or target inhibition of TCR signaling8,9. Pembrolizumab, an FDA-approved monoclonal antibody targeting PD1, has been used for treating endometrial and cervical cancers with mismatch repair deficiency and PD-L1 expression, respectively.
In addition to immune checkpoint molecules, Tregs play a critical role in the immune evasion in cancer. Tregs are a subset of CD4+ T cell populations characterized by expressing the transcriptional regulator forkhead box protein P3 (FOXP3) and exhibiting anti-inflammatory and immunosuppressive properties10. They suppress effector T cell responses for self-tolerance and prevent autoimmunity. Many immunosuppressive mechanisms of Tregs have been proposed: depletion of IL211, secretion of immunosuppressive cytokines (IL10, IL35 and TGFβ) that block the production of pro-inflammatory cytokines and inhibit the effector functions of T cells12,13,14and direct cytolysis of effector T cells via granzymes and perforin15. Tregs constitutively express CTLA-4 interacting with CD80/CD86 to block dendritic cell-mediated priming and activation of effector T cells16,17.
Clinical studies have shown that EOC tumors often contain reduced levels of CD8+ tumor infiltrating lymphocytes (TILs) and high levels of Tregs, both of which correlate with poor response to immune checkpoint blockade and unfavorable survival outcomes18,19. Cancer and dendritic cells produce chemokines C-C motif ligands (CCLs) and C-X-C motif ligands (CXCLs) to recruit TILs and immunosuppressive cells into the TME, including Tregs and myeloid-derived suppressor cells (MDSCs)20,21. These cytokines are key in regulating inflammatory responses in cancer, viral infections, and autoimmune diseases22. Both CXCL10 and CCL5 are considered pro-inflammatory cytokines to initiate and maintain immune responses to eliminate infected and diseased tissues. CXCL10 and CCL5 exert their chemotactic effects by binding to their receptors CXCR3 and CCR5, respectively, on T lymphocytes and other immune cells23,24. CXCL10 is produced by many cell types including epithelial cells, endothelial cells, and fibroblasts, in response to immune cell-produced interferon γ (IFNγ)25,26. CXCL10 serves as a chemoattractant to recruit immunoreactive T cells to the site of inflammation23. Similarly, CCL5 is produced by various cell types and functions to recruit T cells27. Despite their pro-inflammatory and anticancer roles,. CXCL10 and CCL5 have been shown to recruit Treg cells to the TME, promoting immune tolerance and facilitating cancer progression19,24,28,29,30,31.
EOC frequently recurs and develops resistance to conventional therapies including platinum-based chemotherapy and PARP inhibitors. The genetic and phenotypic evolution of EOC also contributes to the development of resistance to immune checkpoint blockade therapies. Although PD1 and CTLA4-targeted therapies have shown efficacy, only a small fraction of EOC patients benefit from these treatments and their effectiveness may diminish over time. Understanding why immune checkpoint blockade therapies fail in most EOC patients and identifying strategies to overcome these limitations are critical. Furthermore, the dynamic interplay between EOC and immune cells must be further investigated to design effective combination therapies. The present study aims to identify and target these interactions between EOC and immune cells to achieve durable treatment response and improve the survival outcomes for all EOC patients.
Results
Isogenic EOC cells exhibit differential profiles of immune checkpoints and cytokines
Differential gene expression between PEO1 and PEO4 cells was analyzed using the Cancer Stem Cell PCR array (Qiagen). Among several up-regulated genes, PEO1 cells exhibited a considerable increase in STAT3 expression compared to PEO4 cells (Table 1). Given that PD-L1 is up-regulated by IFNγ-JAK2-STAT3 and DNA damage signaling pathways32,33PD-L1 expression in response to IFNγ and DNA double strand breaks (DSBs) was determined and compared between both cell lines. Treatment of PEO1 cells with olaparib led to a prominent increase in γH2AX, a marker of DSBs (Fig. 1A and Supplemental Fig. 6). IFNγ treatment induced PD-L1 expression and markedly enhanced olaparib-induced PD-L1 in PEO1 cells. In contrast, PEO4 cells displayed minimal increases in γH2AX and PD-L1 under these conditions (Fig. 1A and B). Furthermore, the PD-L1 reporter assay demonstrated that IFNγ up-regulated PD-L1 transcriptional activity in PEO1 cells, which was sensitive to STAT3 inhibition by stattic (Fig. 1C). Stattic is a small molecule inhibitor of STAT334. Western blot analysis of PD-L1 protein corroborated this observation (Fig. 1D and Supplemental Fig. 7).
Expression of PD-L1 in PEO1 and PEO4 cells. Cells were treated with 10 µM olaparib, 20 ng/mL IFNγ, or both in combination for 24 h. Total protein was analyzed by western blotting for PD-L1, γH2AX, and HSC70. γH2AX was used to confirm olaparib-induced DNA damage. HSC70 was used as a loading control (A). Cells were stained with an anti-PD-L1 antibody and analyzed by a flow cytometer. PD-L1-positive cell populations were gated to determine the percentage of PD-L1-positive cells (B). PEO1 and PEO4 cells were transfected with the PD-L1 luciferase reporter plasmid and the renilla luciferase control plasmid for 24 h and then treated with IFNγ, stattic, or both in combination for 24 h. The firefly luciferase level was normalized to that of the renilla luciferase to determine the fold changes in PD-L1 promoter activity (C). Data are means ± SD (n = 3) from three independent experiments. Cells were treated with olaparib, IFNγ, or both in combination without and with stattic for 24 h. Total protein was analyzed by western blotting for PD-L1 and HSC70 (D).
Cytokine production profiles in PEO1 and PEO4 cells were assessed following IFNγ treatment and STAT3 inhibition. PEO1 cells produced higher basal levels of IL6, IL8, and CXCL1 compared to PEO4 cells. Conversely, PEO4 cells produced elevated basal levels of CCL5 relative to PEO1 cells (Fig. 2A and Supplemental Fig. 1). IFNγ treatment led to pronounced increases in ICAM1 levels in PEO1 cells and CXCL10 levels in PEO4 cells. IFNγ also induced a minor increase in CXCL10 levels in PEO1 cells. IFNγ-induced CXCL10 levels in PEO1 and PEO4 cells were sensitive to STAT3 inhibition by stattic. Further analysis of non-IFNγ-inducible cytokines revealed that PEO1 cells produced higher basal levels of ST2, PTX3, IGFBP3, DKK1, TSP1, and MMP9, while PEO4 cells produced higher basal levels of CHI3L1, IGFBP2, uPAR, and VEGF (Fig. 2B and C, and Supplemental Fig. 2).
Cytokine profiling of conditioned media from PEO1 and PEO4 cells. PEO1 and PEO4 cells were treated with IFNγ, stattic, or both in combination for 24 h. Conditioned media were collected to determine the levels of 36 cytokines using a cytokine immunoarray. The levels of representative cytokines produced by PEO1 and PEO4 cells are shown (A). Conditioned media of PEO1 and PEO4 cells under normal growth conditions after 24 h were collected to determine the levels of 105 cytokines using a cytokine immunoarray. The levels of cytokines over-produced by PEO1 cells (B) or by PEO4 cells (C) were shown. PEO1 and PEO4 cells produced comparable levels of MIF, which are also demonstrated. Data are means ± SD (n = 2) of duplicate measurements from a representative experiment.
PEO1 and PEO4 cells display distinct mechanisms of immunosuppression
The immunosuppressive effects of EOC cells on T cell subsets within peripheral blood mononuclear cells (PBMCs) were evaluated. CD3/CD28 antibodies-stimulated PBMCs were co-cultured with PEO1 or PEO4 cells for 48 h. Both PEO1 and PEO4 cells significantly suppressed the activation of CD8+ T cells, as indicated by the level of the CD8+ CD25+ cell population in PBMCs. Notably, PEO1 cells demonstrated more pronounced suppressive effect on the activation than PEO4 cells (Fig. 3A). In addition, PEO1 and PEO4 cells significantly reduced the activation of SUPT1 cells, as measured by the level of CD8+CD25+ or CD4+CD25+ cell population in SUPT1 cells (Fig. 3B). SUPT1 cells are a precursor T lymphoma/leukemia cell line that expresses both CD4 and CD8, as well as PD135,36. While PEO1 cells had no effects, PEO4 cells significantly increased the population of activated CD4+ T cells as evidenced by an increase in the CD4+CD25+ population in PBMCs (Fig. 3C). Furthermore, PEO4 cells induced a significant increase in the FOXP3+CD4+CD25+ Treg population in PBMCs (Fig. 3D). However, neither PEO1 nor PEO4 cells induced FOXP3+ Treg-like SUPT1 cells (Supplemental Fig. 3).
Immunosuppressive effects of PEO1 and PEO4 cells on PBMCs and SUPT1 cells. PBMCs or SUPT1 cells activated by CD3/CD28 antibodies were co-cultured with PEO1 or PEO4 cells for 48 h. PBMCs were collected, stained, and analyzed by a flow cytometer to determine the percentage of the CD8+CD25+ T cell population in PBMCs (A) and in SUPT1 cells (B), as well as the percentage of the CD4+CD25+ T cell population in PBMCs (C). To determine the level of the Treg population, PBMCs were first gated for CD4+ cells to determine the percentage of the CD4+ T cell population in PBMCs (Top). The CD4+ T cell population was subsequently gated for FXOP3+CD25+ cells to determine the percentage of FOXP3+CD25+ T cells in the CD4+ T cell population (bottom). The bar graph shows the percentage of FOXP3+CD4+CD25+ T cells in PBMCs by calculating FOXP3+CD25+ T cells of CD4+ T cells of total PBMCs (D). Data are means ± SD (n = 3) from three independent experiments.
Given that PEO4 cells expressed high levels of CCL5 and IFNγ-induced CXCL10, the effects of PBMC activation on CCL5 and CXCL10 expression in PEO4 cells were assessed. PEO4 cells were transfected with CCL5- or CXCL10-siRNA and then co-cultured with CD3/CD28 antibodies-stimulated PBMCs. Activation of PBMCs resulted in a 2-fold increase in CCL5 expression and a 25-fold increase in CXCL10 mRNA expression in PEO4 cells (Fig. 4A). Transfection of PEO4 cells with CCL5- or CXCL10-siRNA led to a significant decrease in the corresponding mRNA expression. To investigate the role of CCL5 and CXCL10 expression in the induction of Tregs by PEO4 cells, pharmacological blockade of CCR5 and CXCR3 on T cells was performed. PEO4 cell-induced increase in Tregs was significantly attenuated by the CCL5/CCR5 antagonist TAK77937 and the CXCL10/CXCR3 antagonist AMG48738 (Fig. 4B). These findings suggest that activation of PBMCs signals PEO4 cells to increase the Treg population through the production of CCL5 and/or CXCL10.
Effects of EOC cell-produced CCL5 and CXCL10 on the level of Tregs in PBMCs. PEO4 cells were transfected with non-targeted control (NTC)- and CCL5- or CXCL10-siRNA for 24 h and then co-cultured with CD3/CD28 antibodies-activated PBMCs for 48 h. Total RNA from PEO4 cells was analyzed by quantitative RT-PCR for CCL5 and CXCL10 mRNA expression (A) Effects of CCL5 and CXCL10 blockade on the level of Tregs in PBMCs. PBMCs were activated by anti-CD3/CD28 antibodies and co-cultured with PEO1 or PEO4 cells for 48 h in the presence of 10 µM TAK779 or AMG487. PBMCs were collected, stained, and analyzed by a flow cytometer. CD4+ cells were gated to further determine the percentage of the FOXP3+CD4+CD25+ Treg population. Data are means ± SD (n = 3) from three independent experiments (B). Effects of CXCL10 blockade on IL10 production by PBMCs. PBMCs were activated by anti-CD3/CD28 antibodies and co-cultured with PEO1 or PEO4 cells for 48 h in the absence or presence of 10 and 20 µM AMG487. Conditioned media were collected for analysis of IL10 produced by PBMCs cells using ELISA. Data are means ± SD (n = 3) from a representative experiment (C).
To further elucidate the impact of CXCL10 signaling in Treg activities, the level of IL10 produced by activated PBMCs was determined. IL10 production is a key component of Treg-mediated immunosuppression39. CD3/CD28 antibodies-stimulated PBMCs were co-cultured with PEO1 or PEO4 cells in the presence of AMG487. Activated PBMCs co-cultured with PEO1 or PEO4 cells exhibited a marked increase in IL10 production (Fig. 4C). Treatment with AMG487 had minimal effects on IL10 production in PBMCs co-cultured with PEO1 cells. In contrast, AMG487 significantly reduced IL10 production in PBMCs co-cultured with PEO4 cells. These results suggest that CXCL10-mediated up-regulation of Tregs by PEO4 cells contributes to increased IL10 production. In contrast, PEO1 cells stimulate effector T cells or other immune cell subsets in PBMCs to produce IL10 independently of CXCL10 signaling.
Effects of adenosine signaling blockade on effector T cell activity
To explore additional soluble factors involved in the immunosuppressive effects of EOC, adenosine signaling between EOC and immune cells were examined. CD3/CD28 antibodies-stimulated SUPT1 were co-cultured with PEO1 or PEO4 cells in the presence of the adenosine A2A receptor (A2AR) antagonist CGS1594340, which blocks the effects of adenosine on immune cells. SUPT1 cells express substantial levels of A2AR similar to T cell subsets in PBMCs, as indicated by data from The Human Protein Atlas (Fig. 5A)41,42. Blockade of A2AR by CGS15943 had minimal effects on SUPT1 activation in the absence of co-culture with EOC cells. Notably, CGS15943 partially but significantly reversed PEO1 cell-mediated suppression of SUPT1 cell activation in a dose-dependent manner (Fig. 5B). In contrast, CGS15943 failed to reverse PEO4 cell-mediated suppression of SUPT1 activation. This finding indicates that PEO1 cells suppress effector T cell activation, in part, by adenosine signaling.
Effects of adenosine signaling blockade on effector T cells. Expression of A2AR in T cell subsets in PBMCs and SUPT1 cells. Levels of A2AR RNA expression (nTPM) were compared, using the HTA datasets (n = 5–6) and the leukemia cell line data from the Human Protein Atlas. (A). Effects of adenosine signaling blockade on SUPT1 cell activation. SUPT1 cells were activated by anti-CD3/CD28 antibodies and co-cultured with PEO1 or PEO4 cells for 48 h in the presence of 2 and 10 µM CGS15943. SUPT1 cells were collected, stained, and analyzed by a flow cytometer to determine the percentage of the CD4+/CD8+CD25+ population (B). The levels of CD39 and CD73 expression in PEO1 and PEO4 cells. Total RNA was analyzed by quantitative RT-PCR for the levels of CD39 and CD73 mRNA. Data are means ± SD (n = 3) from three independent experiments. The levels of CD73 protein in PEO1, PEO4, and SUPT1 cells. Total protein was analyzed by western blotting (C).
CD39 and CD73, ectonucleotidases responsible for the conversion of ATP to extracellular adenosine43were assessed to determine the capacity of EOC cells to produce adenosine. Relative expression levels of CD39 and CD73 in PEO1 and PEO4 cells were compared. PEO1 and PEO4 cells expressed comparable levels of CD39 mRNA, with PEO4 cells exhibiting a two-fold higher level than PEO1 cells (Fig. 5C). In contrast, CD79 mRNA levels were significantly lower in PEO4 cells, approximately 20% of those observed in PEO1 cells. Corresponding protein levels of CD73 supported this observation (Fig. 5D and Supplemental Fig. 8). SUPT1 cells exhibited no detectable level of CD73 protein, excluding the possible involvement of SUPT1 cells in adenosine production. These results suggest that adenosine signaling contributes to the immunosuppressive activity mediated by PEO1 cells.
Differential CXCR3 and CCR5 expression among various immune cell types in human PBMCs
Given the crucial roles of CXCR3 and CCR5 in the recruitment of T cells to the TME23,24expression profiles of CXCR3 and CCR5 in CD8 T cells and Tregs were analyzed in human PBMCs using datasets from The Human Protein Atlas. In the Monaco dataset, effector memory CD8 T cells exhibited high CXCR3 expression (181 nTPM) and moderate CCR5 expression (89 nTPM), while Tregs displayed moderate CXCR3 expression (90 nTPM) and low CCR5 expression (8.2 nTPM) (Fig. 6A)44,45. A similar trend was observed in the HPA dataset, where both memory CD8 T cells and Tregs expressed high levels of CXCR3, though CXCR5 expression was comparatively low (Fig. 6B). Further analysis of PBMCs using the Single Cell Portal dataset, with FOXP3 expression indicating Treg populations within CD4+ T cells, revealed that both cytotoxic T cells and CD4+ T cells expressed substantial and comparable levels of CXCR3 (Fig. 6C)46. However, CCR5 expression was high in cytotoxic T cells and relatively lower in CD4+ T cells, consistent with the results from the Human Protein Atlas datasets. Collectively these findings suggest that blockade of CXCR3 would predominantly reduce immunosuppressive CXCR3+ Tregs but preserve CCR5+ CD8 T cells to exert anti-tumor immunity.
CXCR3 and CCR5 expression in T cell subsets in human PBMCs. Levels of CXCR3 and CCR5 RNA expression (nTPM) in MAIT T cells, CD8 T cells, Tregs, and PBMCs were shown, using the Monaco (n = 4–13) (A) and HPA (n = 5–6) (B) datasets from the Human Protein Atlas. MAIT, mucosal-associated invariant T cells. Heatmap of RNA expression levels of T cell markers, CXCR3, and CCR5 in various immune cell types. The color indicates the level of scaled mean RNA expression. The heat map was generated from the dataset of the Single Cell Portal. CD3E is used as a T cell marker to highlight CD8+ cytotoxic T cells and CD4+ (or FOXP3+) T cells (C).
CXCL10/CXCR3 blockade impedes EOC tumor progression in mice
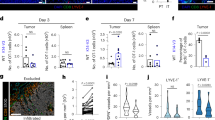
To evaluate the contribution of CXCL10 signaling to EOC tumor growth, pharmacological blockade of CXCR3 was conducted in vivo using a syngeneic EOC tumor mouse model. Immunocompetent C57BL6 mice were implanted intraperitoneally (ip) with syngeneic BRCA-wild type p53(-/-) ID8 EOC cells47,48 and treated with CXCR3 mAb, a monoclonal anti-mouse CXCR3 antibody. The progression of ascitic ID8 tumor, as evidenced by abdominal distension, mirrored the growth of BRCA-wild type PEO4 EOC xenografts in mice49. Tumor progression was assessed by monitoring changes in abdominal circumference (AC) and body condition score (BCS) to determine tumor growth rate and survival endpoints of mice. Treatment with CXCR3 mAb significantly suppressed ascitic tumor growth (Fig. 7A). Kaplan-Meier survival curves based on a 50% increase in AC as the survival endpoint confirmed that CXCR3 mAb treatment suppressed tumor growth by significantly extending median survival time (Fig. 7B). No appreciable toxicity of treatment was observed in mice.
Efficacy of CXCR3 blockade on peritoneal progression of p53(-/-) ID8 EOC and the survival of C57BL6 mice. Mice (Control group, n = 5; CXCR3 mAb group, n = 4) were inoculated ip with ID8 cells and treated ip with vehicle or CXCR3 mAb for 5 weeks. The ACs of mice were measured to determine the percentage increase in AC. The body weight of mice was measured to monitor treatment toxicity (A). The Kaplan-Meier survival curve and median survival time of mice were determined using a 50% increase in AC as the endpoint (B). Mice (n = 5) were inoculated ip with ID8 cells and treated ip with vehicle, AMG487, bevacizumab (Bev), or both in combination for 5 weeks. Bev group, n = 4. The ACs of mice were measured to determine the percentage increase in AC. The body weight of mice was measured to monitor treatment toxicities. Data are means ± SD.*, p < 0.05; **, p < 0.01 (C). The Kaplan-Meier survival curve and median survival time of mice were determined using a 50% increase in AC as the endpoint. p values are shown (D). Mice (n = 5 and n = 3 from two experiments) inoculated ip with ID8 cells were treated with vehicle or AMG487 for 5 weeks. When mice reached the survival endpoint, tumor ascites was obtained and CD3+ T cells were isolated, stained, and analyzed by a flow cytometer to determine the percentages of activated CD8+ T cells and FOXP3+CD4+ Tregs in total CD3+ T cells. Each dot represents the value of an individual mouse. Means and SD of all mice in each treatment group, and p values are shown (E).
The efficacy of pharmacological blockade of CXCR3 or CCR5 using small molecule inhibitors was also explored. C57BL6 mice implanted ip with p53(-/-) ID8 cells were treated with AMG487 or TAK779 in combination with bevacizumab, a monoclonal antibody against VEGFA. VEGFA has been reported to up-regulate Tregs in the TME50. The efficacy of AMG487 and TAK779 in combination with bevacizumab to reduce ascitic tumor growth was compared. Treatment of mice with AMG487 or TAK779 alone appeared to moderately suppress AC increase (Supplemental Fig. 4). Treatment with AMG487, but not TAK779, in combination with bevacizumab caused an evident suppression of AC increase significantly greater than AMG487 or bevacizumab alone.
Thus, the efficacy of the combination of AMG487 and bevacizumab to delay ascitic tumor growth in mice was further investigated. Treatment with bevacizumab or AMG487 alone moderately but significantly suppressed AC increase. Notably, the combination led to a pronounced reduction in AC increase significantly greater than single drugs and the vehicle control (Fig. 7C). These treatments demonstrated no appreciable toxicity in mice. Kaplan-Meier survival curves based on a 50% increase in AC showed that while bevacizumab or AMG487 alone had marginal effects, the combination significantly prolonged the median survival time compared to single-drug treatments or the vehicle control (Fig. 7D). Similar results were obtained using BCS2 as a survival endpoint (Supplemental Fig. 5). These findings suggest that blockade of CXCL10/CXCR3 signaling by AMG487 synergizes with bevacizumab to impede EOC progression in vivo.
To assess the impact of AMG487 on effector T cells and Tregs, tumor ascites was collected from mice treated with vehicle or AMG487, and CD3+ T cells were isolated to evaluate the levels of CD8+ T cells and FOXP3+ Tregs. AMG487 treatment resulted in a reduction in CD8+ T cells (p = 0.0497) and, importantly, a significant decrease in Treg levels (p = 0.0107) in tumor ascites (Fig. 7E and Supplemental Fig. 9). These results support the notion that blockade of CXCL10/CXCR3 signaling by AMG487 reduces the population of immunosuppressive Tregs in tumor ascites, thereby acting synergistically with bevacizumab to impede EOC progression.
Discussion
The mechanisms underlying Immune evasion in cancer are highly dynamic and evolve across different clinical stages of progression. PEO1 and PEO4 cells are isogenic EOC cell lines derived from the same patient at the first and second relapses, respectively51. PEO1 cells harbor a hemizygous mutated BRCA2 gene and display hypersensitivity to platinum drugs and PARP inhibitors. In contrast, PEO4 cells reverse the mutation and regain a wild type BRCA2 gene, thereby exhibiting platinum drug and PARP inhibitor resistance49,52,53. In addition to these distinct characteristics, we observed that PEO4 cells exhibited a cytokine production profile and immune evasion mechanisms that were markedly different from those of PEO1 cells. Given the tumor heterogeneity and genomic instability inherent to EOC, multiple clonal lineages of these tumors may exploit various immunoregulatory pathways to drive disease progression.
Based on our findings, we propose that PEO1 and PEO4 cells utilize distinct immunosuppressive properties (Fig. 8). In response to IFNγ secreted by effector T cells, PEO1 cells up-regulate PD-L1 and produce adenosine, which bind to PD1 and A2AR, respectively, on effector T cells to suppress CD3/CD28-mediated effector T cell activation. In contrast, PEO4 cells produce CXCL10 in response to IFNγ to promote immunosuppression. This is achieved by CXCR3-mediated conversion/polarization of naïve T cells into Tregs54or chemotactic Treg recruitment to attenuate effector T cell activation. Although PEO1 cells produce CXCL10, the level is approximately one-third of that in PEO4 cells (Fig. 2A). This raises the question: why do PEO1 cells not induce Tregs in PBMCs? Multiple lines of evidence indicate that IL6 inhibits the conversion of conventional T cells into Tregs55,56,57. Our cytokine immunoarray analysis shows that PEO1 cells produce high levels of IL6 compared to PEO4 cells (Fig. 2A). We hypothesize that the high IL6 levels in PEO1 cells may prevent the induction of Tregs. Additionally, PEO4 cells lose the ability to up-regulate PD-L1, partly due to the reversion of BRCA2 mutation and restoration of homologous recombination repair capacity58. We propose that PEO4 cells acquire the ability to up-regulate Tregs as a compensatory mechanism to evade immune surveillance. Nonetheless, our proposed models of immunosuppression by PEO1 and PEO4 cells suggest that EOCs not only evolve to survive DNA damaging therapeutic challenges but also adopt different strategies to prevail against immune elimination during the disease progression.
Proposed mechanisms of immunosuppression caused by PEO1 and PEO4 cells. APC(CD3/CD28)-mediated effector T cell activation produces IFNγ that triggers different responses in PEO1 and PEO4 cells. PEO1 cells express PD-L1 in response to IFNγ and generate adenosine via CD39 and CD73 to attenuate effector T cell activation (A). In contrast, PEO4 cells respond to IFNγ by producing CXCL10 to up-regulate and maintain FOXP3+ Tregs. In turn, Tregs exert suppressive effects by producing IL10 and expressing CTLA4 (B). Dashed lines indicate weak or minor signaling events. APC, antigen presenting cells.
CXCL10/CXCR3 signaling is traditionally understood to facilitate immune responses by activating and recruiting effector T cells to the sites of inflammation22. Additionally, CXCL10/CXCR3-mediated recruitment of Tregs is necessary to suppress excessive effector T cell activation. Emerging evidence indicates that CXCL10/CXCR3 signaling plays a role in recruiting Tregs to the TME and promoting tumor growth in many cancers29,30,59. A recent study by Moreno Ayala et al. demonstrates that CXCR3 is required for the accumulation of Tregs in tumors and the attenuation of anti-tumor effector T cell response28. Thus, CXCR3-knockout Tregs enable the priming and activation of effector T cells with dendritic cells, leading to impediment of tumor growth. This finding supports our observation that PEO4 cells up-regulate Tregs via CXCL10 production, thereby facilitating EOC progression. Importantly, we demonstrate that pharmacological blockade of CXCL10/CXCR3 signaling deters EOC growth in mice (Fig. 7). These findings suggest that CXCL10/CXCR3 signaling is a double-edged sword; it has both anti-tumor and pro-tumor properties. We posit that EOC, such as PEO4 cells, exploits the pro-tumor property of CXCL10/CXCR3 signaling to curb anti-tumor immune response in favor of its progression.
Our in vivo analysis of CXCL10/CXCR3 blockade reveals differences in anti-tumor efficacy between the small molecule inhibitor AMG487 and a monoclonal antibody targeting CXCR3. The monoclonal antibody demonstrates greater efficacy, likely due to its extended half-life (6–8 days) compared to the short half-life of AMG487 (a few hours). To reduce the risk of toxicity, we chose to treat mice with AMG487 every 48 h (tiw) at which the dosing interval was likely suboptimal. Additionally, monoclonal antibodies are generally more target-specific than small molecule inhibitors. Future study will optimize the dosing interval of AMG487 to achieve a maximal efficacy and minimal toxicity comparable to CXCR3 mAb.
Besides CXCL10, we observe that PEO4 cells secrete elevated levels of CCL5. Treatment with the CCL5/CCR5 antagonist TAK779 reduced PEO4 cell-induced Tregs in PBMCs (Fig. 4B). These results suggest that CCL5/CCR5 signaling also contributes to the up-regulation of Tregs in the TME. Studies report that CCL5 recruits CCR5+ Tregs to tumors, and blockade of CCL5/CCR5 signaling by TAK779 reduces Treg recruitment and inhibits tumor growth in melanoma and pancreatic cancers24,31. However, in our study, TAK779 did not enhance the efficacy of bevacizumab in delaying EOC progression (Supplemental Fig. 4B). This discrepancy may be attributed to organ- or tumor-specific regulation of Treg recruitment and up-regulation. Moreover, TAK779 has been documented to target both CCR5 and CXCR3 in lung T cell homing pathways60. Our analysis of publicly available datasets indicates that Tregs predominantly express CXCR3, while CD8+ effector T cells express both CXCR3 and CCR5 at considerable levels in PBMCs (Fig. 6). Our findings with mouse tumor ascites demonstrate that blockade of CXCL10/CXCR3 signaling by AMG487 markedly reduced Tregs in mouse tumor ascites (Fig. 7E). Therefore, it is plausible that TAK779 may inhibit the recruitment of both Tregs and CD8+ effector T cells, which may inadvertently compromise anti-tumor immunity in EOC. This hypothesis will be explored using a CCR5 mAb in future studies.
The adenosine signaling pathway also plays a role in EOC-mediated immunosuppression. The adenosine A2A receptor A2AR, expressed by effector T cells, binds adenosine to downregulate T cell activation and effector function61. Our data indicate that PEO1 cells express both CD39 and CD73, enabling adenosine production and contributing to their immunosuppressive activity. Thus, blockade of adenosine binding to A2AR by CGS15943 reverses PEO1 cell-mediated suppression of SUPT1 cell activation (Fig. 5B). However, CGS15943 fails to reverse PEO4 cell-mediated suppression, which could be explained by a relatively low expression of CD73 in PEO4 cells (Fig. 5 C and D), a rate-limiting ectonucleotidase for adenosine production. Thus, the immunosuppressive effects of PEO4 cells may be less adenosine dependent. Furthermore, PEO4 cells may produce other immunosuppressive factors, such as uPAR and VEGFA, as demonstrated in our cytokine immunoarray analysis (Fig. 2 C). Both soluble factors have been implicated in immunosuppressive properties of cancers62,63. These potential mechanisms warrant further investigations.
In conclusion, our study reveals diverse and evolving immune evasion strategies employed by EOC. These findings underscore the need to identify clinical stage-specific immune evasion mechanisms for each EOC. A deeper understanding of these processes will inform the development of innovative strategies to overcome the immunosuppressive TME, ultimately improving patient outcomes and shifting the paradigm in EOC therapy.
METHODS
Cell lines, chemicals, and antibodies
PEO1 and PEO4 are human epithelial ovarian cancer (EOC) cells from the same patient as described previously51. p53(-/-) ID8 cells are a mouse EOC cell line47,48 kindly provided by Dr Iain McNeish (Imperial College London, UK). PEO1, PEO4, and p53(-/-) ID8 cells were grown in DMEM medium supplemented with 10% FBS and penicillin-streptomycin antibiotics. SUPT1 cells (ATCC), a human lymphoblastic leukemia T-cell line, were grown in RPMI medium supplemented with 10% FBS and penicillin-streptomycin antibiotics. Human peripheral blood mononuclear cells (PBMCs) from a single healthy donor were purchased from ATCC (catalog number PCS-800-011). AMG487 was purchased from Cayman Chemical and Selleck Chemicals. TAK779 was purchased from Cayman Chemical and Sigma-Aldrich. InVivoMAb anti-mouse CXCR3 antibody (Clone CXCR3-173) (CXCR3 mAb) was purchased from Bio X Cell. Bevacizumab was purchased from Selleck Chemicals and MedChemExpress.
PCR array analysis
Total RNA was isolated from PEO1 and PEO4 cells and analyzed using RT2 Profiler PCR Array Human Cancer Stem Cells kit (Qiagen), which contained primers of 84 pathway-associated genes, 5 housekeeping genes, and positive and negative controls. Total RNA was first converted to cDNAs, which was mixed with RT2 SYBR Green Master Mix and aliquoted into a 96 well RT2 Profiler PCR array. PCR was performed using the Mx3000p real-time PCR thermal cycler (Strategene). PCR cycle consisted of one cycle of 10 min at 95 °C and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Ct values were obtained to determine relative gene expression levels for 84 genes using the 2−ΔΔCT method.
Cytokine immunoarray assay
PEO1 and PEO4 cells were treated with 5 µM stattic for 30 min and then treated with 50 ng/mL of IFNγ for 3 h. Following medium change, cells were treated with 5 µM stattic for 24 h. The conditioned media from PEO1 and PEO4 cells were analyzed for the levels of cytokines using Proteome Profiler Human (36 cytokines, ARY005B) and Human XL (105 cytokines, ARY022B) Cytokine Array kits (R&D Systems). Cytokines in the medium were captured by antibodies spotted on a nitrocellulose membrane, followed by incubation with biotinylated detection antibodies, streptavidin-HRP and chemiluminescent detection reagents. Signals generated at individual capture spots corresponding to the amount of cytokine bound were detected by the G:BOX gel documentation system and the GeneSnap software (Syngene), and quantified using the ImageJ software (NIH).
Reporter gene assay
PEO1 and PEO4 cells were transfected with the firefly luciferase reporter plasmid, pGL3 3 kb prom.CD274 (ATCC)64 containing the promoter of the PD-L1 gene, and the renilla internal control reporter plasmid, pRL-TK (Promega) at the 10:1 ratio using Lipofectamine 2000 (ThermoFisher). After 24 h, cells were treated with 10 µM stattic for 1 h, and then treated with 20 ng/mL of IFNγ for 24 h. Cells were lysed and assayed for both luciferase activities using the Dual-Luciferase Assay System (Promega) and a TD20/20 Luminometer (Turner Designs). The firefly luciferase activity was normalized to the renilla luciferase activity. The relative PD-L1 promoter activity was shown as a fold increase compared to the non-treated control in each cell line.
Western blot analysis
The methodology was described previously53. The anti-HSC70 antibody was purchased from Santa Cruz. Anti-PD-L1, anti-γ-H2AX (Ser-139), and anti-CD73 (Ab#2) antibodies were purchased from Cell Signaling. Anti-CD73 (Ab#1) antibody was purchased from Biolegend. All images were acquired and processed using the G:BOX gel documentation system and the GeneSnap software (Syngene).
Quantitative RT-PCR
Total RNA was isolated from cells using the RNeasy kit (Qiagen). Target mRNA was quantified using the iTaq Universal One-Step RT-qPCR Kit (Bio-Rad) and the Mx3000p real-time PCR thermal cycler (Strategene). PCR primers used to amplify target genes were: CCL5, 5’-CTTAAGCTTCCGCCGTCTCA-3’ and 5’-CCCCGGGAGGAAATCAAGAG-3’; CXCL10, 5’-GAACCTCCAGTCTCAGCACC-3’ and 5’-TGCAGGTACAGCGTACAGTT-3’; CD39, 5’-CCCCCTGCACCAAGAGATTT-3’ and 5’-CACACTGGGAGTAAGGGCAG-3’; CD73, 5’- GTATCCGGTCGCCCATTGAT-3’ and 5’- AAAGGCCTTCTTCAGGGTGG-3’; GAPDH, 5’-ACAGTCCATGCCATCACTGCC-3’ and 5’-GCCTGCTTCACCACCTTCTTG-3’. PCR was performed consisting of one cycle of 10 min at 50 °C and one cycle of 1 min 95 °C, followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C. Ct values were obtained to determine relative gene expression levels using the 2−ΔΔCT method.
Gene silencing
Cells were plated and transfected with non-targeted control (NTC)-siRNA and CCL5- or CXCL10-siRNA (Millipore-Sigma) using the Lipofectamine 2000 (ThermoFisher) according to the manufacturer’s protocol. After 48 h, cells were lysed for total RNA isolation. Total RNA was analyzed by quantitative RT-PCR to determine the effects of gene silencing.
Co-culture Assay
PEO1 and PEO4 cells were plated into a 6 well plate for 24 h. Inhibitors were added for 30 min and then 1 × 106 PBMCs or 2.5 × 105 SUPT1 cells were added to each well. The ratio of PBMCs to EOC cells was about 2:1. After 30 min, PBMCs were activated by the addition of the Immunocult Human CD3/CD28 T Cell Activator (Stemcell). After 48 h co-culture, the medium containing PBMCs or SUPT1 cells was removed and centrifuged. The medium supernatants were collected and analyzed for cytokines by ELISA. Cell pellets were stained by fluorophore-conjugated antibodies and analyzed for effector T cells and Tregs by flow cytometry.
Immunostaining and flow cytometry
PEO1 and PEO4 cells were stained with the Brilliant Violet 421 anti-PD-L1 (29E.2A3) antibody (Biolegend) to determine the PD-L1-positive cell population. Human PBMCs and SUPT1 cells were stained with Alexa Fluor 488 anti-CD8 (SK1) or Brilliant Violet 421 anti-CD4 (OKT4) and APC anti-CD25 (BC96) antibodies (Biolegend) to determine effector T cell populations using a LSRII flow cytometer (BD). PBMCs were stained with Brilliant Violet 421 anti-CD4, APC anti-CD25, and Alexa Fluor 488 anti-FOXP3 (150D) antibodies (Biolegend) to determine the Treg population. Mouse CD3+ T cells isolated from ascites were stained with FITC anti-mouse CD8a (53−6.7) or Brilliant Violet 421 anti-mouse CD4 (GK1.5) and Alexa Fluor 488 anti-FOXP3 (150D) antibodies (Biolegend) to determine activated (FSC-A high sub-population) CD8+ T cells or FOXP3+CD4+ Tregs, respectively. Cell populations were analyzed using the FlowJo software (BD).
ELISA
The conditioned media of PEO1 and PEO4 cells co-cultured with PBMCs were analyzed for the levels of IL10 using the ELISA MAX Deluxe Set Human IL10 kit (Biolegend). The IL10-specific monoclonal capture antibody was first coated on a 96-well plate for 2 h. Medium samples were added to the wells, followed by the addition of the biotinylated monoclonal anti-IL10 detection antibody. The avidin-HRP reagent was added followed by the addition of the TMB substrate, producing a blue color corresponding to the concentration of IL10 present in the medium. Subsequently the stop solution was added to change the blue color to yellow. The absorbance in wells was read at 450 nm using a Biotek EXL800 microplate reader (Biotek) to calculate the concentrations of IL10 in the samples based on the standard curve determined on the same plate.
Statistical Analysis
Statistical analysis for cell-based assays was performed using unpaired t-test with the Prism software (GraphPad). All tests were two-tailed with an α level at 0.05.
Syngeneic EOC mouse models
The protocol for the in vivo animal studies (IACUC# 2024–20038) was approved by the Yale University Institutional Animal Care and Use Committee in compliance with the US Public Health Policy on Humane Care and Use of Laboratory Animals. Yale University is registered as a research facility with the United States Department of Agriculture, License and Registration number 16-R-0001 The School of Medicine is fully accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). An Animal Welfare Assurance (D16-00146) is on file with OLAW-NIH; Approval Period: May 4, 2023 - May 31, 2027. All methods were performed in accordance with guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. C57BL6 mice were purchased from Charles River Laboratories. Five to 6 weeks old female C57BL6 mice (n = 5) were inoculated intraperitoneally (ip) with 1 × 107 p53(-/-) ID8 EOC cells and treated ip with vehicle or CXCR3 mAb (5 mg/kg, qwk) for 5 weeks. In other experiments, mice were treated ip with vehicle, bevacizumab (5 mg/kg, qwk), AMG487 (5 mg/kg, tiw), or both drugs in combination for 5 weeks. Increases in the abdominal circumference (AC) of mice caused by ascitic ID8 tumor were measured every 2–3 days. The body weight of mice was also measured to monitor the treatment toxicity. The survival endpoint of mice was met when a 50% increase in the AC, or body condition score 2 (BCS2) was observed. Upon reaching the endpoint, mice were removed from the study and euthanized by CO2 inhalation. The percentage of survival was illustrated using the Kaplan-Meier curve and the median survival time was determined by the Prism software (GraphPad). Statistical difference between treatment and vehicle-control groups was evaluated by the Mantel-Cox (log-rank) test using the Prism software.
Immune cell sorting
Upon reaching the survival endpoint, 2–4 mL of tumor ascites was drawn from mice following euthanasia. CD3+ T cells were isolated using the MojoSort™ Mouse CD3 T Cell Isolation kit (Biolegend) based on the principle of negative cell isolation. Tumor ascites was centrifuged to obtain cell pellets, which were washed, resuspended, and incubated with the biotin-antibody cocktail and subsequently with magnetic streptavidin nanobeads. The tube was inserted into magnet to deplete non-CD3+ cells and unlabeled CD3+ T cells were poured out for subsequent immunostaining and flow cytometric analysis.
Data availability
Data are provided within the manuscript or supplementary information files.
Change history
23 July 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-12693-1
References
Siegel, R. L., Miller, K. D., Fuchs, H. E., Jemal, A. & Cancer statistics CA: A Cancer Journal for Clinicians 72, 7–33, (2022). https://doi.org/10.3322/caac.21708 (2022).
Kim, R., Emi, M., Tanabe, K. & Arihiro, K. Tumor-Driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 66, 5527–5536 (2006).
Zhang, Y. & Zheng, J. in Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy (ed Jie Xu) 201–226Springer Singapore, (2020).
Spranger, S. & Gajewski, T. F. Mechanisms of tumor Cell–Intrinsic immune evasion. Annual Rev. Cancer Biology. 2, 213–228. https://doi.org/10.1146/annurev-cancerbio-030617-050606 (2018).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint Blockade therapy. Cancer Discov. 8, 1069–1086. https://doi.org/10.1158/2159-8290.cd-18-0367 (2018).
Lanier, L. L. et al. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. (Baltimore Md. : 1950). 154, 97–105 (1995).
Freeman, G. J. et al. Engagement of the PD-1 Immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Parry, R. V. et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25, 9543–9553. https://doi.org/10.1128/mcb.25.21.9543-9553.2005 (2005).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated Inhibition. Sci. (New York N Y). 355, 1428–1433. https://doi.org/10.1126/science.aaf1292 (2017).
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532. https://doi.org/10.1038/nri2343 (2008).
Pandiyan, P., Zheng, L., Ishihara, S., Reed, J. & Lenardo, M. J. CD4 + CD25 + Foxp3 + regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4 + T cells. Nat. Immunol. 8, 1353–1362. https://doi.org/10.1038/ni1536 (2007).
Lang, R., Patel, D., Morris, J. J., Rutschman, R. L. & Murray, P. J. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 169, 2253–2263. https://doi.org/10.4049/jimmunol.169.5.2253 (2002).
Gorelik, L. & Flavell, R. A. Immune-mediated eradication of tumors through the Blockade of transforming growth factor-β signaling in T cells. Nat. Med. 7, 1118–1122. https://doi.org/10.1038/nm1001-1118 (2001).
Heim, L. et al. Increased expression of the immunosuppressive interleukin-35 in patients with non-small cell lung cancer. Br. J. Cancer. 120, 903–912. https://doi.org/10.1038/s41416-019-0444-3 (2019).
Cao, X. et al. Granzyme B and Perforin are important for regulatory T Cell-Mediated suppression of tumor clearance. Immunity 27, 635–646. https://doi.org/10.1016/j.immuni.2007.08.014 (2007).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725. https://doi.org/10.1084/jem.20082492 (2009).
Walker, L. S. & Treg CTLA-4: two intertwining pathways to immune tolerance. J. Autoimmun. 45, 49–57. https://doi.org/10.1016/j.jaut.2013.06.006 (2013).
Consortium, O. T. T. A. Dose-Response association of CD8 + Tumor-Infiltrating lymphocytes and survival time in High-Grade serous ovarian Cancer. JAMA Oncol. 3, e173290–e173290. https://doi.org/10.1001/jamaoncol.2017.3290 (2017).
Redjimi, N. et al. CXCR3 + T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 72, 4351–4360. https://doi.org/10.1158/0008-5472.can-12-0579 (2012).
Wu, Q., Li, B., Sun, S. & Sun, S. Unraveling adipocytes and Cancer links: is there a role for senescence?? Front. Cell. Dev. Biology. 8, 282. https://doi.org/10.3389/fcell.2020.00282 (2020).
Park, K., Veena, M. S. & Shin, D. S. Key players of the immunosuppressive tumor microenvironment and emerging therapeutic strategies. Front. Cell. Dev. Biology. 10, 830208. https://doi.org/10.3389/fcell.2022.830208 (2022).
Griffith, J. W., Sokol, C. L. & Luster, A. D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 32, 659–702. https://doi.org/10.1146/annurev-immunol-032713-120145 (2014).
Groom, J. R. & Luster, A. D. CXCR3 in T cell function. Exp. Cell Res. 317, 620–631. https://doi.org/10.1016/j.yexcr.2010.12.017 (2011).
Schlecker, E. et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. (Baltimore Md. : 1950). 189, 5602–5611. https://doi.org/10.4049/jimmunol.1201018 (2012).
Luster, A. D. & Ravetch, J. V. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J. Exp. Med. 166, 1084–1097. https://doi.org/10.1084/jem.166.4.1084 (1987).
Liu, M., Guo, S. & Stiles, J. K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2, 583–589. https://doi.org/10.3892/ol.2011.300 (2011).
Marques, R. E., Guabiraba, R., Russo, R. C. & Teixeira, M. M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets. 17, 1439–1460. https://doi.org/10.1517/14728222.2013.837886 (2013).
Moreno Ayala, M. A. et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8(+) T cell antitumor immunity. Immunity 56, 1613–1630e1615. https://doi.org/10.1016/j.immuni.2023.06.003 (2023).
Lunardi, S., Lim, S. Y., Muschel, R. J. & Brunner, T. B. IP-10/CXCL10 attracts regulatory T cells: implication for pancreatic cancer. Oncoimmunology 4, e1027473. https://doi.org/10.1080/2162402x.2015.1027473 (2015).
Kim, M., Choi, H. Y., Woo, J. W., Chung, Y. R. & Park, S. Y. Role of CXCL10 in the progression of in situ to invasive carcinoma of the breast. Sci. Rep. 11, 18007. https://doi.org/10.1038/s41598-021-97390-5 (2021).
Tan, M. C. et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. Journal of immunology (Baltimore, Md.:) 182, 1746–1755, ) 182, 1746–1755, (1950). https://doi.org/10.4049/jimmunol.182.3.1746 (2009).
Garcia-Diaz, A. et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell. Rep. 19, 1189–1201. https://doi.org/10.1016/j.celrep.2017.04.031 (2017).
Kciuk, M. et al. PD-1/PD-L1 and DNA damage response in Cancer. Cells 12 https://doi.org/10.3390/cells12040530 (2023).
Schust, J., Sperl, B., Hollis, A., Mayer, T. U. & Berg, T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 13, 1235–1242. https://doi.org/10.1016/j.chembiol.2006.09.018 (2006).
Kozbor, D., Burioni, R., Ar-Rushdi, A., Zmijewski, C. & Croce, C. M. Expression of members of Immunoglobulin gene family in somatic cell hybrids between human B and T cells. Proc. Natl. Acad. Sci. U S A. 84, 4969–4973. https://doi.org/10.1073/pnas.84.14.4969 (1987).
Islam, S. et al. Co-targeting aurora kinase with PD-L1 and PI3K abrogates immune checkpoint mediated proliferation in peripheral T-cell lymphoma: a novel therapeutic strategy. Oncotarget 8 (2017).
Baba, M. et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. U S A. 96, 5698–5703. https://doi.org/10.1073/pnas.96.10.5698 (1999).
Johnson, M. et al. Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg. Med. Chem. Lett. 17, 3339–3343. https://doi.org/10.1016/j.bmcl.2007.03.106 (2007).
Ng, T. H. S. et al. Regulation of adaptive immunity; the role of Interleukin-10. Front. Immunol. 4 https://doi.org/10.3389/fimmu.2013.00129 (2013).
Ghai, G. et al. Pharmacological characterization of CGS 15943A: a novel nonxanthine adenosine antagonist. J. Pharmacol. Exp. Ther. 242, 784–790 (1987).
The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000128271-ADORA2A/single+cell/t-cells
The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000128271-ADORA2A/cell+line#leukemia
Allard, B., Longhi, M. S., Robson, S. C. & Stagg, J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol. Rev. 276, 121–144. https://doi.org/10.1111/imr.12528 (2017).
The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000186810-CXCR3/single+cell#immune_cell
The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000160791-CCR5/single+cell#immune_cell
The Single Cell Portal. https://singlecell.broadinstitute.org/single_cell/study/SCP424/single-cell-comparison-pbmc-data?genes=FOXP3%2CCD4%2CCD8A%2CIL2RA%2CCD3E%2CCXCR3%2CCCR5&tab=dotplot#study-visualize.
Walton, J. et al. CRISPR/Cas9-Mediated Trp53 and Brca2 knockout to generate improved murine models of ovarian High-Grade serous carcinoma. Cancer Res. 76, 6118–6129. https://doi.org/10.1158/0008-5472.can-16-1272 (2016).
Walton, J. B. et al. CRISPR/Cas9-derived models of ovarian high grade serous carcinoma targeting Brca1, Pten and Nf1, and correlation with platinum sensitivity. Sci. Rep. 7, 16827. https://doi.org/10.1038/s41598-017-17119-1 (2017).
Lin, Z. P. et al. Combination of triapine, olaparib, and cediranib suppresses progression of BRCA-wild type and PARP inhibitor-resistant epithelial ovarian cancer. PloS One. 13, e0207399. https://doi.org/10.1371/journal.pone.0207399 (2018).
Bourhis, M., Palle, J., Galy-Fauroux, I. & Terme, M. Direct and indirect modulation of T cells by VEGF-A counteracted by Anti-Angiogenic treatment. Front. Immunol. 12, 616837. https://doi.org/10.3389/fimmu.2021.616837 (2021).
Langdon, S. P. et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 48, 6166–6172 (1988).
Sakai, W. et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 (2008). Epub 2008 Feb 1110.
Lin, Z. P. et al. In Silico screening identifies a novel small molecule inhibitor that counteracts PARP inhibitor resistance in ovarian cancer. Sci. Rep. 11, 8042. https://doi.org/10.1038/s41598-021-87325-5 (2021).
Karin, N., Wildbaum, G. & Thelen, M. Biased signaling pathways via CXCR3 control the development and function of CD4 + T cell subsets. J. Leukoc. Biol. 99, 857–862. https://doi.org/10.1189/jlb.2MR0915-441R (2016).
Chen, X. et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood 114, 891–900. https://doi.org/10.1182/blood-2009-01-197178 (2009).
Kimura, A. & Kishimoto, T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 40, 1830–1835. https://doi.org/10.1002/eji.201040391 (2010).
Korn, T. et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3 + regulatory T cells. Proc. Natl. Acad. Sci. U S A. 105, 18460–18465. https://doi.org/10.1073/pnas.0809850105 (2008).
Sakai, W. et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 69, 6381–6386. https://doi.org/10.1158/0008-5472.can-09-1178 (2009).
Li, C. X. et al. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J. Hepatol. 65, 944–952. https://doi.org/10.1016/j.jhep.2016.05.032 (2016).
Suzaki, Y. et al. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur. Respir. J. 31, 783–789. https://doi.org/10.1183/09031936.00111507 (2008).
Huang, S., Apasov, S., Koshiba, M. & Sitkovsky, M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated Inhibition of T-cell activation and expansion. Blood 90, 1600–1610 (1997).
Ribatti, D. Immunosuppressive effects of vascular endothelial growth factor. Oncol. Lett. 24, 369. https://doi.org/10.3892/ol.2022.13489 (2022).
Hosen, S. M. Z. et al. Metastatic phenotype and immunosuppressive tumour microenvironment in pancreatic ductal adenocarcinoma: key role of the urokinase plasminogen activator (PLAU). Front. Immunol. 13 https://doi.org/10.3389/fimmu.2022.1060957 (2022).
Coelho, M. A. et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity 47, 1083–1099e1086. https://doi.org/10.1016/j.immuni.2017.11.016 (2017).
Acknowledgements
This work was supported in part by grants from Rivkin Center for Ovarian Cancer Research (Z.P.L) and Peter E. Schwartz Discovery to Cure Program at Yale University (Z.P.L). Yale Flow Cytometry Core was supported in part by an NCI Cancer Center Support Grant # NIH P30 CA016359.
Author information
Authors and Affiliations
Contributions
A.C.L wrote the manuscript. Z.P.L. and E.S.R. conceived the study design, A.C.L., J.M., Y.L.Z., and Z.P.L. conducted the experiments, A.C.L., J.M., Y.L.Z., Z.P.L., and E.S.R. analyzed the results. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the order of the figures. Figure 2 was published as Figure 3, Figure 3 was published as Figure 2, Figure 6 was published as Figure 7, and Figure 7 was published as Figure 6. The Figure legends were correct at the time of publication. Full information regarding the corrections made can be found in the correction for this Article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, A.C., Moscarelli, J., Zhu, YL. et al. CXCL10-induced regulatory T cells and adenosine signaling promote immunosuppression and progression of epithelial ovarian cancer. Sci Rep 15, 20778 (2025). https://doi.org/10.1038/s41598-025-06812-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06812-1