Abstract
Retinal illumination primarily determines pupil size, yet extra-retinal factors like subjective brightness also influence pupillary responses. Previous works reported that in healthy individuals, stimuli whose semantic content evokes brightness cause greater pupillary constriction than control stimuli of similar luminance. This study adapted this approach and tested a passive task to assess consciousness levels of non-communicating patients in intensive care unit. In ten healthy participants and seventeen patients with Disorders of Consciousness (10 in a Minimally Conscious State, 6 in Vegetative State also coined Unresponsive Wakefulness Syndrome), 1 in Emergence from Minimally Conscious State), we measured pupillary responses to photographs of the sun and control stimuli of matched luminance (moon photographs, scrambled sun images, uniform gray squares). At the group level, both healthy participants and Minimally Conscious State patients showed greater pupil constriction for the sun photographs compared to control pictures which elicited a pupillary dilatation. In Vegetative State/ Unresponsive Wakefulness Syndrome patients, this subjective brightness effect on pupillary diameter was not significant. Notably, this effect was observed in only one Vegetative State patient, who regained consciousness a few weeks after the evaluation. The results support that pupillary response to subjective brightness could be a novel method to assess residual cognition at the bedside in non-communicating patients.
Similar content being viewed by others
Introduction
Pupillary light reactivity is a well-described prognostic factor during the acute phase of severe brain damages1,2. However irido-constrictor and dilator centers are not exclusively subject to reflex activity in response to retinal illumination and are also modulated by prefrontal and parieto-frontal cortical (for a review, see3). In healthy participants, pupillary response is modulated by the subjective brightness of a stimulus. While photographs or drawings of the sun elicited a pupillary constriction, moon representations of equal mean luminance were interpreted as darker and elicited a pupillary dilatation4,5. Therefore, pupillary responses to subjective brightness may also be used as a somatic signature of conscious access. In the present study, we assessed whether pupillometry could provide an objective physiological measure of residual cognition in patients with disorders of consciousness.
Previous work in healthy participants reported that pupil diameter increases before perceptual reports of ambiguous stimuli6,7 or along with the recognition stage when presented with ambiguous transforming stimuli8,9. Furthermore, pupillary diameter adapts in the very absence of visual stimulus, for instance in response of passive reading or listening to words associated with bright or dark semantic content10 or during mental imagery of light or dark scenarios (a sunny sky versus a dark room)11. In the absence of semantic interpretation, for example when sun photographs are scrambled4or in the absence of conscious perception, when sun photographs are masked by continuous flash suppression12such subjective brightness effect does not emerge.
In intensive care unit, pupil dilation response has been used by clinicians to monitor nociception13 to communicate14 and to demonstrate command-following14,15,16. Recently, an eye-tracking study in patients with disorders of consciousness17 extended previous results reported in healthy participants18,19 and showed that pupillary responses have been reliably identified as a physiological marker of auditory irregularities discrimination in oddball paradigms.
Patients in a vegetative state, also coined unresponsive wakefulness syndrome (VS/UWS), have to be distinguished from patients in a Minimally Conscious State (MCS) in which non-reflexive cognitively-mediated behaviours demonstrate a richer state associated with richer cortical processing20. In order to improve diagnostic accuracy, rigorous, detailed and repeated behavioural examination is completed with additional measures in multimodal evaluation, using various functional brain-imaging techniques (i.e. functional Magnetic Resonance Imaging (fMRI), electroencephalography (EEG), positron emission tomography (PET), evoked potential, and other physiological measures such as heart rate, olfaction.)20,21,22,23,24,25. Then, pupillometry along a passive visual task, could be a novel and promising approach to assess consciousness level in non-communicating patients.
To test pupillary response to subjective brightness at the bedside of patients, we adapted the experimental protocol developed by Binda and colleagues4. We replicated their results in healthy participants with this adapted protocol and applied it to a cohort of 17 DoC patients − 10 in a Minimally Conscious State, 6 in Vegetative State/Unresponsive Wakefulness Syndrome and 1 in Emergence from Minimally Conscious State (EMCS). We compared pupillary response between four stimulus categories : sun photographs, moon photographs, and two control stimuli of matched luminance (scrambled sun photographs and mean gray squares), regarding patients’ level of consciousness. Our aim was to assess whether the presence of cortical modulation of the pupillary response by visual semantic content could constitute a signature of conscious processing in patients, and therefore by extension, reflect their state of consciousness.
Methods
Participants
Healthy participants
10 healthy participants (median age 28 years, 4 men) were included. All participants had normal or corrected-to-normal vision, and no neurological history. They gave their informed written consent prior to participation and were naïve as to the purpose of the study. The national ethics committee (CPP Ile-de-France VI Pitié-Salpétrière Hospital Group) approved the protocol for the study (C13-41), in accordance with French regulations and the Declaration of Helsinki.
Patients
From December 2020 to July 2023, 73 DoC patients referred to the Neurology Intensive Care Unit of the Pitié-Salpêtrière University Hospital (APHP, Sorbonne University, Paris, France) for an evaluation of consciousness were screened for participation in the study. All behavioural procedures in the present study were performed in accordance with the ethical standards of the Helsinki declaration (1964) as well as its later amendments and were approved by the local ethical committee (Comité de protection des personnes Ile de France I, M-NEURODOC protocol).
Behavioural examination
Clinical examination was performed by an experienced neurologist. It included the Coma Recovery Scale Revised (CRS-R)26 and the assessment of brainstem reflexes. The integrity of the pupillary light reflex of each eye was checked. Comatose patients with an arousal Coma Recovery Scale subscale lower than 1 were excluded, as well as patients with loss of photomotor reflexes. Clinical outcome at 6 months and 1 year after the initial clinical evaluation was assessed using the Glasgow Outcome Scale-Extended (GOSE) and items of the CRS-R through a phone call interview with a close relative, or with patients’ physicians, were extracted from medical records.
Experimental paradigm
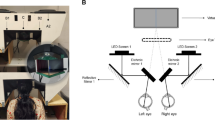
The eye-tracking sessions were carried out in the patients’ rooms at Intensive Care Unit. A CRS-r score was performed just before pupillometric recordings.
Patients were either seated or semi-seated in bed in front of a computer screen positioned between 55 and 85 cm. Stimuli (20 × 20 cm) were presented in the center of the computer screen (537.6 × 21.2 cm) on a gray background.
The healthy participants’ eye-tracking sessions were carried out under conditions similar to those of the patients: participants were tested in a stable noise and luminance environment trying to minimize changes during experimental session, but without a strict control over room brightness or sound volume, in order to match patients’ recording conditions at bedside. Distance from the target presentation screen was approximately 60 to 70 cm.
With their agreement, we used the stimuli developed by Binda and colleagues (Binda, Pereverzeva, et Murray 2013, available at http://faculty.washington.edu/somurray/PupilSun/). There were four stimuli categories: (1) photographs of the sun; (2) uniform gray squares that matched the size and the mean luminance of the sun photographs; (3) phase scrambled images of the sun photographs that preserved mean luminance, and (4) photographs of the moon that matched the mean luminance of the sun photographs. Mean luminance of the stimuli ranged between 22.6 and 60.1 cd/m within each category and was matched across categories. We selected 10 stimuli per category among the 13 stimuli developed by Binda et al.: we excluded photographs of the full moon to avoid confusion with sun photographs and to facilitate semantic content interpretation, and the corresponding stimuli of matched luminance in the 3 other categories.
One experimental run was thus composed of 40 trials, corresponding to the full set of stimuli presented in a random order. Trials started with a 2-s blank pre-stimulus epoch in which subjects fixated the maximum luminance full gray screen. This was followed by a 3-s stimulus epoch in which one of the stimuli was displayed at the screen center.
Experimental run was carried out from 1 to 3 times, depending on the quality of the recording (drowsiness, frequent blinking, participant no longer looking at the screen). For each experimental session, the photomotor reflex was quantified under the experimental conditions that were not matched between patients (no control of the room luminance or light source). The participants were asked to look closely at the images displayed on the screen.
Pupillometry: data acquisition and analysis
Recordings were made using a portable eyeglass eye-tracker model (Pupil Core®, 120 Hz sampling rate). In healthy subject a standard nine-points calibration was performed at the beginning of session. In patients, when achievable, calibration was performed in one point. Trajectories of patient’s fixation point (i.e.: scan path) were analyzed with the Pupil Player® software. Trials in which the patient was no longer looking at the screen were manually rejected. Raw pupil data were first pre-processed with the ET-remove-artifacts toolbox27 (https://github.com/EmotionCognitionLab/ET-remove-artifacts). Dilation speed outliers and eye blinks were detected and removed, and a linear interpolation was used to fill in gaps in the data. A trial was rejected when more than 25% of the data was interpolated. Patients with less than 4 valid trials per category were excluded from the analysis. When the clinical examination revealed an asymmetric pupillary reflex, data from the most reactive eye were analysed. Otherwise, both eyes were analysed, and we kept the best quality dataset (i.e., left or right eye dataset) for further analyses. Then, these pre-processed pupil data were imported into the Brainstorm toolbox (available for download online under the GNU general public license (http://neuroimage.usc.edu/brainstorm)). Data were filtered with a 10 Hz low pass filter, and epochs for each trial were extracted from 500 ms before the stimulus presentation until the end of the stimulus presentation that lasted 3 s.
Statistics
Population characteristics (age, sex, time since brain injury, etiology, medication affecting pupil size) between Vegetative State /Unresponsive Wakefulness Syndrome and Minimally Conscious Sate patients were compared using a Mann–Whitney-U test for continuous data, and the Fisher exact test for categorical variables.
For each trial, we first compared each time point acquired during the 3 s stimulus presentation to the mean baseline computed over a 500 ms time window before stimulus presentation. We used a sample-by-sample Student t-test corrected for multiple comparisons with the false discovery rate (FDR) in time (p ≤ 0.05). This analysis was first performed in the group of healthy participants to define the temporal region of interest for patients: a time window starting 500 ms after stimulus onset and ending 3 s after stimulus onset. We used this temporal window to compute mean pupillary responses.
To compare mean pupil responses between stimulus categories, we first computed a baseline correction. We then computed for each trial the mean pupillary response Then, for each participant or patient, we averaged this mean pupillary response across all the stimuli within each category. We finally tested this average pupil diameter variation response for each category with a Friedman test and then compared it in-pair between in each category by a Wilcoxon test FDR corrected with a significance threshold at p < 0.05. Between-group comparisons of averaged pupil diameter variations were performed by a Kruskal-Wallis test, then compared in pairs by a Mann-Whitney test.
Results
Patients’ characteristics
From January 2021 to July 2023, the 73 patients admitted at the Neurology ICU of the Pitié-Salpêtrière University Hospital were screened for participation in our study. 39 patients were excluded mainly because of vigilance disorders (Fig. 1). The pupillometric data met quality criteria (see Methods section) for 17 patients among the 34 patients who performed our experimental session. Clinical data are presented in Table 1.
The 17 included patients were: 6 patients in a Vegetative State/ Unresponsive Wakefulness Syndrome, 10 in a Minimally Conscious, and 1 in Emergence from Minimally Conscious State. The main aetiologies of consciousness disorders were head injury (35%), stroke (17%) and cardiopulmonary arrest (17%). Sex (9 M/8F), age (median 48 years (min 21, max 60), time between assessment and brain injury (median 70 days (min 12, max 2472) and aetiology were not significantly different between patients in a Minimally Conscious State and those in Vegetative State/ Unresponsive Wakefulness Syndrome (respectively p = 0.2, p = 0.15, p = 0.07, p = 0.6). Analyses were performed on a median number of 9 trials per stimuli category (minimum 4 - maximum 18, Table 2). The number of trials analysed was not significantly different between patients in a Minimally Conscious State and those in Vegetative State/ Unresponsive Wakefulness Syndrome (p = 0.75, Mann-Whitney test).
Replication in healthy participants with the adapted protocol
We adapted the temporal dynamic and number of stimuli of Binda and colleagues’ protocol (see Methods section). We replicated their results4 in healthy participants, without strict control of environment factors but with stable conditions of auditory noise and luminance during testing (see Methods section). We observed a pupillary constriction to sun photographs and a pupillary dilatation for the three other stimulus categories of matched mean luminance: uniform luminance squares, scrambled photographs of the sun and moon photographs.
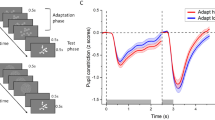
Mean pupil response was significantly different for sun photographs compared to the three control categories (Fig. 2, Friedman test, p = 0.0001, paired Wilcoxon test FDR-corrected for multiple comparisons, sun/moon p = 0.004, sun/scramble p = 0.004, sun/mean p = 0.004). Moon photographs induced a smaller dilatation than mean gray luminance and scrambled sun controls (p = 0.02). Scrambled sun and mean gray luminance control did not induce a significantly different pupillary response. The time-course of pupillary diameter response detailed the dynamics of this effect: sun photographs triggered a pupillary response constriction different from each of the three other categories in a time window spanning from 531 ms post-stimulus onset until 3 s (see Fig. 3). A 500-ms to 3-s post-stimulus presentation window was used for further analyses in both healthy participants and patients.
Mean pupillary responses to pictures of the sun, moon, scrambled sun, and mean luminance images in healthy volunteers, minimally conscious sates patients and vegetative state/unresponsive wakefulness syndrome patients. Baseline-corrected pupil size during the 500 to 3000 ms post-stimulus presentation interval. Lower, upper and median lines of each box indicate respectively the 25th and 75th percentiles and the median of each sample. The lines extending above and below the box represent the sample minimum and maximum values. Individual data are represented by dots. FDR-corrected Wilcoxon paired test results for multiple comparisons are shown: * p < 0.05, ** p < 0.01.
Time course of pupillary diameter variations as a function of stimulus category in healthy volunteers (HV), minimally conscious state patients and vegetative state/unresponsive wakefulness syndrome patients. Mean values and standard deviations are shown from − 500 ms to 3000 ms after stimulus presentation. Intervals during which pupillary variations induced by the presentation of sun photographs differed from those induced by other categories are marked by lines in the corresponding category’s color compared to the sun. Permutation test corrected for multiple comparisons over time using FDR (p < 0.05).
At the individual level, sun photographs induced a pupillary constriction in all healthy volunteers. This pupillary response was significantly different compared to the control stimuli within the expected time window (500 to 3000 ms post-stimulus presentation) in all healthy volunteers. However, a significant difference in the response between the sun and moon stimuli was observed in only 5 healthy volunteers.
Subjective brightness effect on pupillary response in MCS but not vegetative states/unresponsive wakefulness syndrome Patients
At the group level, a pupil response to the sun photographs was found for patients in a Minimally Conscious State: pupillary responses varied according to stimulus category (Fig. 3, Friedman test, p = 0.02). Sun photographs elicited a significantly greater mean pupillary constriction than scrambled sun images (p = 0.02) and mean gray luminance images (p = 0.02). The time course analysis revealed that this effect spanned from 885 ms to 1930 ms post-stimulus presentation, and the effect induced by the sun and mean gray luminance images from 400 to 2000 ms (Fig. 3, permutation test corrected for multiple comparisons over time by FDR, p < 0.05). This time window was in agreement with healthy participants’ results. There was no significant difference between mean pupillary responses induced by sun and moon photographs (p = 0.3), but the time course analysis revealed a significant divergence between the two conditions spanning from 892 ms to 1577 ms post-stimulus presentation. The pupillary response also differed between moon and mean luminance images from 1830 ms to 2500 ms. Crucially, no pupil response to the sun photographs emerged significant at the group level in Vegetative State/ Unresponsive Wakefulness Syndrome patients (Fig. 2, Friedman test, p = 0.8). A linear mixed-effects model revealed a significant interaction between group and image category (p < 0.001), with Minimally Conscious State patients showing markedly stronger pupil responses to sun photographs compared to Vegetative State/ Unresponsive Wakefulness Syndrome patients.
At the individual level, a pupil response to the sun photographs was defined as a significant pupillary constriction compared to the baseline and significantly different from at least one of the control categories within the expected time window (500ms to 3000ms). A pupil response to the sun photographs was found in 7/10patients in a Minimally Conscious Sate (70%) and in the unique Conscious patient of the cohort. In the Vegetative State/ Unresponsive Wakefulness Syndrome group only 1 patient (17%) demonstrated a significant pupillary constriction (see Table 2). The presence of pupil response to the sun photographs did not differ significantly between patients in our study, probably because of the small size of our patient sample. (Fisher’s exact test, p = 0.3; Chi2 = 4.3, sensitivity to discriminate patients in a Minimally Conscious State from those in a Vegetative State / Unresponsive Wakefulness Syndrome 70% (35–93); specificity 83% (36–100); PPV 88% (53–98); NPV 63% (38–82)).
At 6 months, among the 8 patients who showed a pupil response to the sun photographs, 4 patients (50%) were conscious, whereas among the 8 patients who did not show this effect, only one had regained consciousness (13%) (Fischer exact p = 0.3). The only patient classified in a vegetative state/ Unresponsive Wakefulness Syndrome, with a pupil response to the sun photographs regained consciousness shortly after the evaluation.
Discussion
In this study we demonstrated that the presence of cortical modulation of the pupillary response by semantic content could constitute a signature of conscious processing in patients with DoC, and therefore by extension, reflect their state of consciousness.
We first replicated Binda and colleagues’ results4in healthy participants and observed that photographs of the sun prompted pupillary constriction, whereas control images of equivalent luminance did not (moon photographs, scrambled sun, uniform luminance squares)4,5. Previous work by Sperandio et al. suggests that this effect depends on conscious processing, as manipulation of conscious access via continuous flash suppression impeded the emergence of the subjective brightness effect12. Our results provide additional support to this theory. At the group level, patients in a Minimally Conscious State demonstrated more pronounced pupillary constriction in response to sun photographs compared to scrambled sun and uniform luminance squares, while in patients in a Vegetative State/ Unresponsive Wakefulness Syndrome, the pupillary diameter did not vary according to subjective luminance. Contrary to previous findings in healthy participants4,5we did not observe in patients in a Minimally Conscious State significant differences in pupillary responses between photographs of the moon and of the sun. This discrepancy may partly be explained by the difficulty for brain-injured patients to distinguish suns and moons in grayscale photographs. Despite our precaution to exclude full moon pictures which could be confused with sun pictures, some sun pictures could have been interpreted as full moon pictures28. Using stimuli with contextual elements linked to day or night could potentially lead to different outcomes.
At the individual level, cortical modulation of pupillary response was observed in only one of the six patients in a Vegetative State/ Unresponsive Wakefulness Syndrome tested (17%), seven of the ten patients in a Minimally Conscious State. (70%), and the sole patient assessed. Notably, the patient in a Vegetative State/ Unresponsive Wakefulness Syndrome who exhibited this effect regained consciousness a few weeks after the evaluation, indicating a potentially richer level of consciousness than that what was apparent from clinical observations at the time of the study. Six months later, among the eight patients who exhibited sun-induced pupillary constriction, four (50%) had regained consciousness. In contrast, of the eight patients who did not show this effect, only one (13%) had regained consciousness. However, the presence of a subjective brightness effect did not significantly differ between patients in a Vegetative State/ Unresponsive Wakefulness Syndrome and those in a Minimally Conscious Sate in our study, nor was it statistically correlated with prognosis, likely due to the small sample size of our patient cohort.
From a neural perspective, top-down modulation of pupillary constriction requires the contribution of several cortical regions, including the frontal and prefrontal regions29,30and of subcortical structures such as the locus coeruleus and the superior colliculus31,32,33,34.Determining the precise physiological mechanisms underlying these responses will require future studies using functional imaging or neurophysiological methods.
Our results could not allow to conclusively determine whether the preservation of cortical modulation of pupillary diameter in Minimally Conscious State patients reflects the maintenance of conscious semantic processing or simply the sustained functionality of an extensive cortico-subcortical network. However, the preservation of such an expansive cortical network is considered a fundamental prerequisite for a conscious state according to the Global Workspace Neuronal Theory (GNWT) of Consciousness35,36 and by other theories37,38. When access to semantic interpretation is disrupted, such as by scrambling sun images4 or masking them using continuous flash suppression12the pupillary constriction effect is abolished, suggesting that the observed constriction in response to bright images as a top-down modulation of reflex pathways, potentially mediated by higher-order visual or associative cortices. An alternative possibility is that the image of the sun evokes an emotional or homeostatic state—such as calm or safety—rather than being processed purely as a representation of brightness. This highlights the need to consider affective and motivational dimensions in future paradigms, and points to a more complex integration of perceptual, cognitive, and emotional influences on pupillary behaviour39,40. Besides, future studies could explore the use of emotionally salient or personally meaningful stimuli, which may elicit distinct pupillary responses and provide additional insights into residual cognitive processing in patients with disorders of consciousness. Indeed in patients with disorders of consciousness, richer responses have been shown to depend on the motivational context and the personalization of stimuli, particularly when these are tailored to the individual’s personal history (e.g., their own name or a familiar voice)41,42.
In addition to the small size of our patient cohort, a second and major limitation of our research arises from methodological challenges specific to perform pupillometric measurements in brain-injured patients in ICU17,43. Among the 73 patients screened, 39 (53%) could not be assessed using pupillometry, mainly due to disorders of vigilance. Among the remaining 34 patients, only 17 (49%) had pupillometric data with sufficient quality for analyses. The blink rates vary significantly among DoC patients and cannot be controlled44,45. For successful measurements, patients have to remain awake, calm, and focused a sufficient amount of time on the screen. The experimental paradigms for pupillometry in DoC patients face inherent contradictions: they must be brief due to fluctuating attentional capacities, yet require a high number of trials to compensate for the high rate of trial rejection. We adapted the Binda and colleagues’ protocol by reducing both the inter-trial duration and the number of stimuli from 13 to 10 per category4. Despite these changes, the high rate of trial rejection weakened the statistical power required to detect differences between categories. In further studies, a more straightforward approach using only images of the sun and scrambled sun images could have increased the number of trials per category while reducing overall testing time. Additionally, presenting stimuli on a screen has been shown to be suboptimal given the potential distractions in an ICU environment and the low salience of our stimuli (grayscale photographs). While the screen viewing distance may have varied slightly across patients, the distance to the eye tracker remained relatively constant due to the stable positioning provided by the device’s wearable design. Furthermore, Binda et al. demonstrated that pupillary constriction occurred regardless of the image’s position on the screen—whether viewed centrally or peripherally. However, in the absence of precise calibration, we could only exclude trials for which patients did not look at the screen. Using presentation glasses could help to isolate stimuli from external distractions and preclude this issue.
To conclude, this proof-of-concept study showed that cortical modulation of pupillary response by subjective interpretation of brightness may provide a new window into conscious processing that is otherwise inaccessible. Numerous methodological and technical challenges remain to be overcome. Future advances in eye-tracking techniques could help improve data quality and further develop pupillometry in non-communicating patients.
Data availability
The data that support the findings of this study are available from the corresponding author, [AS], upon reasonable request.
References
Peluso, L., Oddo, M., Sandroni, C., Citerio, G. & Taccone, F. S. Early neurological pupil index to predict outcome after cardiac arrest. Intensive Care Med. 48, 496–497 (2022).
Rossetti, A. O., Rabinstein, A. A. & Oddo, M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 15, 597–609 (2016).
Peinkhofer, C., Knudsen, G. M., Moretti, R. & Kondziella, D. Cortical modulation of pupillary function: systematic review. PeerJ 7, (2019).
Binda, P., Pereverzeva, M. & Murray, S. O. Pupil constrictions to photographs of the sun. J. Vis. 13, (2013).
Naber, M. & Nakayama, K. Pupil responses to high-level image content. J. Vis. 13, 7–7 (2013).
Einhäuser, W., Stout, J., Koch, C. & Carter, O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl. Acad. Sci. U S A. 105, 1704–1709 (2008).
Kietzmann, T. C., Geuter, S. & König, P. Overt visual attention as a causal factor of perceptual awareness. PLoS One. 6, e22614 (2011).
Salvi, C., Simoncini, C., Grafman, J. & Beeman, M. Oculometric signature of switch into awareness? Pupil size predicts sudden insight whereas microsaccades predict problem-solving via analysis. Neuroimage 217, 116933 (2020).
Suzuki, Y., Minami, T. & Nakauchi, S. Association between pupil dilation and implicit processing prior to object recognition via insight. Sci. Rep. 8, 6874 (2018).
Mathôt, S., Grainger, J. & Strijkers, K. Pupillary responses to words that convey a sense of brightness or darkness. Psychol. Sci. 28, 1116–1124 (2017).
Laeng, B. & Sulutvedt, U. The eye pupil adjusts to imaginary light. Psychol. Sci. 25, 188–197 (2014).
Sperandio, I., Bond, N. & Binda, P. Pupil size as a gateway into conscious interpretation of brightness. Front. Neurol. 9, (2018).
Paulus, J. et al. Pupillary reflex measurement predicts insufficient analgesia before endotracheal suctioning in critically ill patients. Crit. Care. 17, R161 (2013).
Stoll, J. et al. Pupil responses allow communication in locked-in syndrome patients. Curr. Biol. 23, R647–648 (2013).
Vassilieva, A., Olsen, M. H., Peinkhofer, C., Knudsen, G. M. & Kondziella, D. Automated pupillometry to detect command following in neurological patients: a proof-of-concept study. PeerJ 7, e6929 (2019).
Othman, M. H. et al. Covert consciousness in acute brain injury revealed by automated pupillometry and cognitive paradigms. Neurocrit Care. https://doi.org/10.1007/s12028-024-01983-7 (2024).
Sangare, A. et al. Pupil dilation response elicited by violations of auditory regularities is a promising but challenging approach to probe consciousness at the bedside. Sci. Rep. 13, 20331 (2023).
Friedman, D., Hakerem, G., Sutton, S. & Fleiss, J. L. Effect of stimulus uncertainty on the pupillary dilation response and the vertex evoked potential. Electroencephalogr. Clin. Neurophysiol. 34, 475–484 (1973).
Marois, A., Labonté, K., Parent, M. & Vachon, F. Eyes have ears: indexing the orienting response to sound using pupillometry. Int. J. Psychophysiol. 123, 152–162 (2018).
Naccache, L. Minimally conscious state or cortically mediated state? Brain 141, 949–960 (2018).
Kondziella, D., Friberg, C. K., Frokjaer, V. G., Fabricius, M. & Møller, K. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 87, 485–492 (2016).
Giacino, J. T. et al. Comprehensive systematic review update summary: disorders of consciousness. Neurology 91, 461–470 (2018).
Comanducci, A. et al. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: review of an IFCN-endorsed expert group. Clin. Neurophysiol. https://doi.org/10.1016/j.clinph.2020.07.015 (2020).
Hermann, B. et al. Importance, limits and caveats of the use of disorders of consciousness to theorize consciousness. Neurosci. Conscious 2021, niab048 (2022).
Arzi, A. et al. Olfactory sniffing signals consciousness in unresponsive patients with brain injuries. Nature 581, 428–433 (2020).
Giacino, J. T., Kalmar, K. & Whyte, J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated. Arch. Phys. Med. Rehabil. 85, 2020–2029 (2004).
Mather, M. et al. Isometric exercise facilitates attention to salient events in women via the noradrenergic system. Neuroimage 210, 116560 (2020).
Castellotti, S., Conti, M. & Feitosa-Santana, C. & Del viva, M. M. Pupillary response to representations of light in paintings. J. Vis. 20, (2020).
Ebitz, R. B. & Moore, T. Selective modulation of the pupil light reflex by microstimulation of prefrontal cortex. J. Neurosci. 37, 5008–5018 (2017).
Lehmann, S. J. & Corneil, B. D. Transient pupil dilation after subsaccadic microstimulation of primate frontal eye fields. J. Neurosci. 36, 3765–3776 (2016).
Arnsten, A. F. & Goldman-Rakic, P. S. Selective prefrontal cortical projections to the region of the locus coeruleus and Raphe nuclei in the rhesus monkey. Brain Res. 306, 9–18 (1984).
Gamlin, P. D. R. The pretectum: connections and oculomotor-related roles. In Progress in Brain Research (ed Büttner-Ennever, J. A.) vol. 151, 379–405 (Elsevier, 2006).
Joshi, S. & Gold, J. I. Pupil size as a window on neural substrates of cognition. Trends Cogn. Sci. 24, 466–480 (2020).
Wang, C. A., Boehnke, S. E., White, B. J. & Munoz, D. P. Microstimulation of the monkey superior colliculus induces pupil dilation without evoking saccades. J. Neurosci. 32, 3629–3636 (2012).
Dehaene, S. & Naccache, L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 79, 1–37 (2001).
Dehaene, S. & Changeux, J. P. Experimental and theoretical approaches to conscious processing. Neuron 70, 200–227 (2011).
Lau, H. & Rosenthal, D. Empirical support for higher-order theories of conscious awareness. Trends Cogn. Sci. 15, 365–373 (2011).
Laureys, S. & Schiff, N. D. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 61, 478–491 (2012).
Lang, P. J., Greenwald, M. K., Bradley, M. M. & Hamm, A. O. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 30, 261–273 (1993).
Bradley, M. M., Miccoli, L., Escrig, M. A. & Lang, P. J. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45, 602–607 (2008).
Magliacano, A., De Bellis, F., Galvao-Carmona, A., Estraneo, A. & Trojano, L. Can salient stimuli enhance responses in disorders of consciousness?? A systematic review. Curr. Neurol. Neurosci. Rep. 19, 98 (2019).
Perrin, F., Castro, M., Tillmann, B. & Luauté, J. Promoting the use of personally relevant stimuli for investigating patients with disorders of consciousness. Front. Psychol. 6, (2015).
Johansson, J., Franzon, K., Godbolt, A. K. & Möller, M. C. Methodological aspects of using a wearable eye-tracker to support diagnostic clinical evaluation of prolonged disorders of consciousness. J. Rehabil Med. 53, jrm00213 (2021).
Bonfiglio, L. et al. Spontaneous blinking behaviour in persistent vegetative and minimally conscious states: relationships with evolution and outcome. Brain Res. Bull. 68, 163–170 (2005).
Magliacano, A. et al. Spontaneous eye blinking as a diagnostic marker in prolonged disorders of consciousness. Sci. Rep. 11, 22393 (2021).
Acknowledgements
We would like to express our sincere gratitude to Paola Binda for graciously allowing us to use the stimuli developed in her research. We also acknowledge the support from various funding sources that made this work possible. A.S. was funded by the ‘Bourse poste d’Accueil’ grant from Inserm and by the Foundation “Gueules Cassées” and the Foundation “Matmut Paul Bennetot. L.N. received funding from the ICM, UNIM, and the Dumont family donation.
Author information
Authors and Affiliations
Contributions
A.S, C.E and L.N contributed to the conception and design, the interpretation of data .All authors reviewed the manuscript and contributed to drafting the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sangare, A., Eymond, C., Jodaitis, L. et al. Pupil constrictions to subjective brightness as a gateway to probe consciousness in non communicating patients. Sci Rep 15, 24619 (2025). https://doi.org/10.1038/s41598-025-06941-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06941-7