Abstract
Strigolactones (SLs) are butenolide-type plant hormones that play several roles in plants, such as suppressing shoot branching and promoting arbuscular mycorrhizal symbiosis. Recently, SLs have been reported to positively regulate disease resistance in plants. In this study, we analyzed the effect of the synthetic SL analog rac-4-bromodebranon (rac-4BD) on systemic acquired resistance (SAR) in rice. First, we demonstrated in vitro that rac-4BD, similar to the common SL analog rac-GR24, promotes the interaction of SL and karrikin receptor, D14 and D14-like (D14L), respectively, with signaling factor D3. Gene expression analysis and inoculation tests indicated that pretreatment with rac-4BD promotes the effect of the SAR inducer BIT. Activation of SAR was also significantly observed in the SL and karrikin signal-deficient rice mutant d3. These results suggest that D3-mediated SL/karrikin signaling by rac-4BD treatment does not directly activate rice immunity but induces a priming state in the plant that enhances SAR induction.
Similar content being viewed by others
Introduction
Rice is an economically important crop because it is the staple food for more than half of the world’s population1. Future population growth will necessitate increased rice production, for which cultivation techniques must be adapted to biotic and abiotic stresses2,3. Previous studies have estimated that up to 40% of rice production is lost annually owing to diseases4. Rice blast disease, caused by Pyricularia oryzae (synonym of Magnaporthe oryzae), is a critical disease in rice cultivation that must be controlled. Numerous fungicides are used in agricultural production to control diseases. However, the emergence of fungicide-resistant strains has become a serious problem, necessitating the development and application of technologies that increase disease resistance in plants. To protect themselves from pathogenic attacks, plants have evolved unique defense mechanisms that are governed by plant hormones, such as salicylic acid (SA) and jasmonic acid (JA)5,6. SA is synthesized at the site of pathogen infection and functions as a signal for inducing systemic acquired resistance (SAR) throughout the plant body7,8,9,10. SAR is effective against various types of pathogens and protects plants from further attacks. Chemicals that activate SAR have been developed and are widely used to control rice blast disease in rice paddy fields. Probenazole and its active metabolite, 1, 2-benzisothiazol-3(2H)-one-1,1-dioxide (BIT), are SAR-inducing agents that activate SA biosynthesis in plants11,12,13. One of the key genes for rice SAR, OsWRKY45, has been identified to act downstream of SA and is used as an SAR marker gene14,15. Overexpression of the transcription factor OsWRKY45 confers strong disease resistance for rice16.
Strigolactones (SLs) and karrikin are butanolide-type compounds (Fig. 1) with diverse effects on plant growth and development17,18. SLs regulate shoot branching and root morphology in plants19,20,21. SLs are involved in biotic interactions, promoting symbiosis with mycorrhizal fungi and the settlement of parasitic weeds22,23. Karrikin, a compound found in smoke emitting from burning wood, affects seed germination rates, seedling photomorphogenesis, and stress tolerance24.
Various SL analogs have been characterized to elucidate SL functions and develop strategies for controlling parasitic weeds25,26. The canonical, natural SL structure includes a tricyclic lactone (ABC ring) and a methylfuranone ring (D ring) connected by an enol ether. The representative synthetic SL analog, rac-GR24, has a four-ring structure similar to canonical SLs (Fig. 1). The smaller compound, rac-4-bromodebranone (rac-4BD), which is an ether linkage of a substituted phenol and D ring, is easier to synthesize than rac-GR2427. Furthermore, rac-4BD was found to have stronger activity for shoot branching inhibition in rice than rac-GR2427.
The mechanism of SL signaling and biosynthesis in rice has been studied through the analysis of dwarf (d) and high-tillering dwarf mutants and SL analog26,28,29. The causative gene for the SL-insensitive mutant d14 encodes the SL receptor, an α/β-fold hydrolase family protein. The D14 protein accepting SL interacts with the F-box protein D330,31 to form the Skp1-Cullin-F-box (SCF) E3 ubiquitin ligase complex with OSK1 (Oryza sativa SKP1-like)32,33. The D14-SCFD3 complex transmits the SL signal by inducing the ubiquitination and degradation of the D53 protein by the proteasome system. Additionally, the D3 protein interacts with the karrikin receptor D14L in a karrikin-dependent manner, whereas the internal ligands for D14L have not been identified34. Thus, D3 is a common pivotal factor in the SL- and karrikin-mediated signaling pathways. In rice, rac-GR24 enhances the interaction of D3 with D14 or D14L31,35, while in Arabidopsis the optical isomers GR245DS and GR24ent-5DS enhance the interaction of D3 homolog MAX2 with AtD14 or KAI2, respectively36,37. While it was previously reported that rac-4BD promotes the interaction between the Arabidopsis SL receptor AtD14, ASK (Arabidopsis SKP1-like), and rice F-box protein D338, the effect of rac-4BD on the interaction of D3 and OSK with D14 or D14L remains to be determined.
The effects of SL on plant-pathogen interactions have been reported in recent years39,40. In rice, SL biosynthesis mutant d17 and SL receptor mutant d14 showed higher susceptibility to P. oryzae than the wild type39. We previously demonstrated that SL signaling enhanced the expression of SAR marker genes in response to bacterial infection and enhanced disease resistance in Arabidopsis using rac-GR24 and an SL-biosynthesis inhibitor40. Therefore, SL signaling has positive effect on disease resistance and treatment with an SL analog is a promising tool for plant disease control. However, further research is necessary to determine whether activation of the SL-mediated signal influences rice immune systems. On the other hand, karrikin has been found to induce disease resistance in Arabidopsis41. Therefore, F-box protein MAX2, Arabidopsis D3 ortholog, is crucial for SL- and karrikin-mediated immune systems in Arabidopsis, which is also presumed to function in rice. Although D3- and MAX2-related signalings by SLs and Karrikins in growth and development have been extensively studied, their function and underlying mechanisms in the immune system are poorly understood. In this study, we analyzed the effects of the SL analog rac-4BD on defense gene expression and disease resistance in rice, with the aim of demonstrating the importance of the D3- and MAX2-related immune systems and providing a chemical tool to elucidate their mechanisms.
Results
Promotion of interaction between D14 or D14L and D3 by rac-4BD
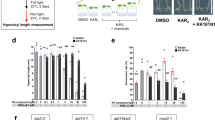
We examined the effects of rac-4BD on interactions between the rice D14, D3, and OSK1 using the yeast three-hybrid (Y3H) system to determine whether rac-4BD acts as an SL-analog in the SL receptor-F-box protein system. The receptor proteins D14L instead of D14 were used to determine whether rac-4BD acts as a karrikin analog. The vector pGADT7-D14, -D14L, or -EV (empty) used as prey expressed the D14 or D14L protein, or nothing, respectively (Fig. 2). The vector pBridge-BD:D3-M:OSK1 used as bait expressed both D3 and OSK1 (Fig. 2). In preliminary experiments without OSK1, the detection of an interaction between the receptor and D3 was not stable (data not shown). The yeast AH109 strain was transformed with pGADT7-D14, -D14L, or -EV and pBridge-BD:D3-M:OSK1 and cultured in media containing rac-GR24 or rac-4BD. All transformants grew in the control medium (SD-Leu, Trp, Met). In the selection medium (SD-Leu, Trp, Met, His, Ade) containing rac-GR24 or rac-4BD, the transformants with pGADT7-D14 or pGADT7-D14L and pBridge-BD:D3-M:OSK1 grew, whereas the transformant with pGADT7-EV and pBridge-BD:D3-M:OSK1 failed to grow (Fig. 2). All transformants did not produce colonies on selection medium without synthetic SL (Mock). These results indicate that D14 or D14L interacts with D3 only when rac-GR24 or rac-4BD exist (Fig. 2). Collectively, rac-4BD acts as both SL analog and karrikin analog in rice and can be used to assess the role of D3-mediated signaling in the rice immune system.
Effect of rac-GR24 and rac-4BD on the interaction between D14 or D14L and D3. Y3H analysis of the interaction between D14 or D14L and D3 in the presence of the third protein OSK1. Yeast (AH109) is transformed with pGADT7-D14, -D14L or -EV and pBridge-BD:D3-M:OSK1. Transformants were spotted on control medium (SD − Leu, Trp, Met) (− LWM) and selection medium (SD − Leu, Trp, Met, His, Ade) (− LWMHA) in the absence or presence of 10 μM rac-GR24 or 10 μM rac-4BD. Mock is the selection medium with acetone added.
rac-4BD has no effect on the growth of rice blast fungus
To properly evaluate the effect of rac-4BD on rice blast disease development, its antimicrobial activity against P. oryzae should be determined. To assess the direct effect of SL analogs on fungal growth, in vitro paper disk assays were performed using rac-GR24 or rac-4BD with P. oryzae. None of the SL analogs at concentrations up to 1 mg/ml had a significant inhibiting effect on the growth of P. oryzae colonies (supplementary Fig. 1). These results suggest that rac-4BD has no direct effect on the development of blast fungus.
Promotion of SAR response in rice by rac-4BD
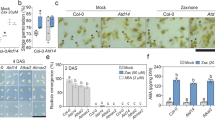
We examined the effects of treatment with rac-4BD on BIT-induced SAR using gene expression analysis and pathogen infection assay. Expression levels of the rice SAR marker gene, OsWRKY45, in rice leaves were analyzed 1 and 3 days after 0.5 mg/pot BIT treatment, following pretreatment with rac-4BD. Whereas rac-4BD did not affect the expression of OsWRKY45, BIT induced the expression of OsWRKY45 in both rac-4BD-pretreated and non-pretreated plants (Fig. 3a). The induction of OsWRKY45 expression 3 days after BIT treatment in the rac-4BD-pretreated plant was significantly higher than that of non-pretreated control plants, which was unclear 1 day after BIT treatments (Fig. 3a). These results indicate that pre-treatment with rac-4BD alone does not affect OsWRKY45 expression but promotes OsWRKY45 induction by BIT, suggesting that rac-4BD enhances the SAR-inducing activity of BIT (Fig. 3a). Subsequently, we analyzed the effects of rac-4BD on SAR induction by BIT using the rice blast inoculation assay. Similar to OsWRKY45 expression levels, the control and rac-4BD treatment did not differ significantly, indicating that rac-4BD treatment alone did not affect disease resistance against rice blast (Fig. 3b, c). Treatment with 0.5 or 0.1 mg/pot BIT reduced the number of lesions of both the rac-4BD-pretreated and non-pretreated plants (Fig. 3b, c). Disease resistance induced by 0.1 mg/pot BIT was significantly higher in rac-4BD-pretreated plants than in non-pretreated plants; however, the results were not significantly different after treatment with 0.5 mg/pot BIT (Fig. 3b). These data indicate that rac-4BD pre-treatment does not affect rice blast resistance but promotes resistance induction by BIT. These results suggest that rac-4BD does not activate SA-mediated defense signaling and resistance but promotes SAR induction by BIT.
Effect of rac-4BD on BIT-induced disease resistance in rice. Rice plants at the 3.5 leaf stage were pretreated with rac-4BD (30 µM in pot) and 1 day later with BIT (0.5 or 0.1 mg in pot) using the soil drenching method prior to RNA analysis and rice blast inoculation. (a) Expression of SAR marker gene. Rice leaves were sampled 1 and 3 days after treatment with the BIT (0.5 mg in pot). The expression levels of OsWRKY45 in the fourth leaves of plants were analyzed using RT-qPCR 1 or 3 days after treatment with compounds. The expression levels were normalized against the expression levels of OsUBQ measured in the same samples. Statistical analysis was performed for each sampling point. (b) Rice blast inoculation assay. Spreading lesions on the fourth leaf were counted five days after inoculation. Statistical analysis was performed for each BIT concentration (0.5 mg or 0.1 mg/pot). (c) Photograph of representative disease symptoms taken 5 days after inoculation. Different letters indicate significant difference. (ANOVA, Tukey’s test, p < 0.05).
Defects of D3-mediated signaling do not affect SAR induction.
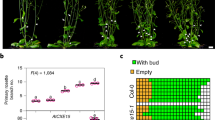
The fact that rac-4BD promoted SAR induction by BIT suggests that SAR induction depends on D3-mediated signaling or is regulated in intensity by D3-mediated signaling. To determine whether SL or karrikin-mediated signaling is required for SAR induction by BIT in rice, we examined the effects of BIT treatment in d3 mutants deficient in those signals. Expression of defense-gene OsWRKY45 and disease resistance against rice blast in d3 mutant and its background wild type ‘Shiokari’ were analyzed 3 days after treatment with 0.5 mg/pot BIT. Treatment with BIT significantly induced the OsWRKY45 expression in leaf tissues of d3 and wild type plants (Fig. 4a). Expression of OsWRKY45 in untreated plants was not significantly different between the d3 mutant and the wild type. However, induction of OsWRKY45 expression in BIT-treated plants was significantly increased by 151% in the wild type compared to the d3 mutant (Fig. 4a). These results indicate that D3-mediated signaling is involved in the induction of SAR by BIT treatment. In addition, in the blast inoculation assay in untreated plants, the number of spreading lesions in the wild type was reduced by 43% compared to the d3 mutant, indicating that D3-mediated signaling is involved in the basal disease resistance of rice plants (Fig. 4b, c). Furthermore, the number of lesions in BIT-treated plants compared to untreated plants was reduced by 65% in the d3 mutant and by 75% in the wild type (Fig. 4b, c), indicating that D3-mediated signaling is involved in the induction of SAR by BIT treatment. Therefore, analyses of defense gene expression and rice blast resistance suggested that D3-mediated signaling is involved in basal disease resistance and SAR induction in rice.
SAR induction in the D3-mediated signal deficient mutant. The d3 and wild type Shiokari plants were treated with BIT (0.5 mg in pot) using the soil-drenching method 3 days prior to gene expression analysis and challenge inoculation with rice blast fungus. (a) Expression of SAR marker gene. The expression levels of OsWRKY45 were normalized against the expression level of OsUBQ measured in the same samples. (b) Rice blast inoculation assay. Spreading lesions on the fourth leaf of rice plants were counted 5 days after inoculation. (c) Photograph of representative disease symptoms taken 5 days after inoculation. Different letters indicate significant differences between samples (ANOVA, Tukey’s test, p < 0.05).
Discussion
Considering future food problems due to population growth, the use of plant immune mechanisms is important. However, SAR, the main technology for this purpose, is affected by environmental factors and is difficult to use for crops other than rice, so a technology to efficiently use plant immunity is needed. Since SL signaling has been shown to enhance SA signaling in Arabidopsis40, we analyzed the effect of SL signaling on SAR and its mechanism in rice. The SL analog rac-4BD was shown by Y3H analysis to be able to act on both SL and karrikin receptors in rice, both of which lead to activation of the D3-mediated signaling pathway, and by gene expression analysis in rice plants and rice blast inoculation tests to enhance induction of SAR. Taken together with the priming effect of D3-mediated signaling on SA-mediated defense signaling, activation of D3-mediated signaling by rac-4BD treatment had the effect of priming the rice immune system, resulting in enhanced activation of SA signaling for SAR induction.
Interactions of D3 and OSK1 with D14 or D14L were promoted by rac-4BD (Fig. 2), however, it is unclear which enantiomer is responsible for the interaction with D14 or D14L. Since rac-4BD has strong inhibitory activity against shoot branching in rice as functional analog of SL27, we can assume that either or both optical isomers of rac-4BD activate signaling through SL in rice. Further analyses using optical isomers will reveal the detailed mechanisms of priming of rice immune system by D3-mediated signaling pathway.
Analysis of SL/karrikin-insensitive rice mutants showed that the number of blast lesions in the rac-4BD-untreated d3 mutant was about twice that of the wild type, suggesting that D3-mediated signaling positively regulates basal disease resistance to rice blast (Fig. 4b). On the other hand, treatment with BIT induced OsWRKY45 expression and rice blast resistance in the d3 mutant as well as in wild type, obviously indicated that SL and karrikin signaling are not essential for SAR induced by SA-mediated signaling. These suggest that D3-mediated signaling activated by rac-4BD plays a role in priming the plant immune system and accelerating the activation of SA-mediated signaling upon BIT treatment. In the case of Arabidopsis primed by rac-GR24 resulted in enhanced disease resistance against a bacterial pathogen40, whereas priming effect of rac-4BD in rice was effective to SAR induction but not to disease resistance against rice blast. The reason for this difference is presumably due to the physiological properties of the plants or the combination of plants and pathogens, rather than to differences in compounds. The high level of endogenous SA in rice is possible reason why rac-4BD alone cannot successfully induce resistance to rice blast42. Another reason is that the priming state of plant immunity may be effective against infection with pathogenic bacteria but ineffective against intracellularly invading filamentous fungal pathogens. Further analyses using pathogenic filamentous fungi in Arabidopsis or pathogenic bacteria in rice would reveal the details of the effects of priming plant immunity by D3-mediated signals.
The positive roles of SL and karrikin in disease resistance have been demonstrated in Arabidopsis40,41. The contribution of SL-mediated signaling to blast resistance in rice was demonstrated by increased susceptibility in the SL biosynthesis mutant d17 and SL receptor mutant d1439. Conversely, it has been reported that suppression of SL signaling with the d14 mutant, the SL biosynthesis mutant d10, or SL biosynthesis inhibitors activates JA signaling and enhances resistance to blast fungus43. These conflicting results about the function of SL signaling in the rice immune system may be caused by regulatory mechanisms of SA and JA signaling. For the semi-biotrophic pathogen P. oryzae, the SA signal is effective during the early biotrophic phase of infection, while the JA signal mainly acts on disease resistance during the late necrotrophic phase44. Furthermore, JA and SA signaling are mutually antagonistic in the pathway of inducing disease resistance in rice45. Unlike the suppression of SL signaling in those reports, this study demonstrated that activation of D3-mediated signaling by rac-4BD had no effect on rice blast infection but promote SAR induction by BIT. However, it is unknown whether SL or karrikin-mediated signaling is more important for priming of rice immune system by rac-4BD. Further investigation using optical isomers of 4BD and mutants defective in D14 or D14L will reveal the roles of SL and karrikin in D3-mediated immunity in rice.
Several SAR inducers, such as probenazole, tiadinil, and isotianil, are widely used to control rice blast disease in rice paddy fields46,47. However, SAR is suppressed by environmental stresses, such as cold and drought, via the antagonistic crosstalk between SA- and abscisic acid-mediated signaling pathways48,49,50. In addition, SAR inducers are difficult to use on other crops, including vegetables, because they affect plant growth. The data presented in this study demonstrate that the activation of D3-mediated signaling is beneficial for increasing the efficacy of SAR inducers, which would contribute to future disease control technologies that exploit plant potentials and reduce the use of SAR inducers as well as antimicrobial agents. Furthermore, the manufacturing cost of rac-4BD is lower than that of SL and its analog rac-GR24 because the synthesis method is less complex. Therefore, the activity of rac-4BD demonstrated in this study will be a useful reference for the development of new crop management technologies in the future and, more importantly from a plant science perspective, will lead to a detailed mechanistic understanding of the priming by D3-mediated signals that regulate the plant immune system.
Materials and methods
Chemicals
Chemicals, (3aR,8bS,E)-3-((((R)-4-methyl-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one (rac-GR24) and 5-(4-bromophenoxy)-3-methylfuran-2(5H)-one (rac-4BD), were synthesized as previously described51,52 and dissolved in acetone; 1,2-Benzisothiazol-3(2H)-one 1,1-Dioxide (BIT) was dissolved in water.
Yeast three-hybrid assay
The yeast three-hybrid assay using the Matchmaker Three-Hybrid System (Takara Bio, Shiga, Japan) was performed as previously described53,54. pGADT7-D14, pGADT7-D14L or pGADT7-EV served as prey and pBridge-BD:D3-M:OSK1 as bait.
Plant materials and chemical treatment
Rice seeds (Oryza sativa cv. Nipponbare, cv. Shiokari and d3 (cv. Shiokari background)) were surface sterilized before sowing by soaking in hot water at 60 °C for 10 min, followed by soaking in 25 °C water for 2 days. Rice was grown in potting soil Honens 1 (Honen Agri, Niigata, Japan) in pots (2.5 cm × 2.5 cm × 4 cm) inside a growth chamber under a 12 h:12 h light: dark regimen at 28 °C with 60% humidity. Seven days after sowing, young seedlings were transferred to 20 mL 0.25% fertilizer solution (Chiyoda Kasei; SunAgro Co., Ltd, Tokyo, Japan) in 50 mL polypropylene tubes. Rice seedlings were treated with rac-4BD (30 µM in tube) and/or BIT (0.5 mg or 0.1 mg in tube) using a soil drenching method 3 days before challenge inoculation.
Pathogen inoculation assay
P. oryzae compatible race hoku-1 (race 007)55,56 was cultured on oatmeal agar medium at 25 °C. Spore formation of P. oryzae was induced by culturing under black light blue fluorescent lamp for 4 days. Rice seedlings were sprayed with a spore suspension (1 × 105 conidia/ml, 0.02% (w/v) Tween 20), maintained under high humidity and dark conditions for 20 h, then grown for 4 days under normal conditions. The number of lesions on the fourth leaf of the rice plants was recorded.
RNA isolation and RT-qPCR
Total RNA isolation from leaf samples, cDNA synthesis, and RT-qPCR were performed as previously described57. The gene-specific primer pairs used are as follows: OsWRKY45 (Os05g0322900), forward 5ʹ-CGGGTAAAACGATCGAAAGA-3ʹ, reverse 5ʹ-TTTCGAAAGCGGAAGAACAG-3ʹ; OsUBQ (Os03g0234200), forward 5ʹ-AACCAGCTGAGGCCCAAGA-3ʹ, reverse 5ʹ-ACGATTGATTTAACCAGTCCATGA-3ʹ. Thermal cycling conditions included 30 s at 95 °C, 40 cycles for 5 s at 95 °C, and 20 s at 60 °C. Transcript levels were normalized to the expression of OsUBQ measured in the same samples.
Statistical analysis
The data are presented as the mean ± standard error. Statistical analysis was performed using GraphPad Prism 9 software (GraphPad, CA, USA). Multiple comparisons were performed using Tukey’s test after analysis of variance (ANOVA). The Student’s t-test was used to analyze the significant differences among groups. All statistical significance was set at p < 0.05.
Data availability
All data presented in this study are included in this article. However, raw data of this manuscript can be provided by corresponding author on reasonable request.
References
Bin Rahman, A. R. & Zhang, J. Trends in rice research: 2030 and beyond. Food Energy Secur. 12, e390 (2023).
He, D., Zhan, J. & Xie, L. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr Agric. 15, 705–715 (2016).
Tyczewska, A., Woźniak, E., Gracz, J., Kuczyński, J. & Twardowski, T. Towards food security: Current state and future prospects of agrobiotechnology. Trends Biotechnol. 36, 1219–1229 (2018).
Oerke, E. & Dehne, H. Safeguarding production—losses in major crops and the role of crop protection. Crop Prot. 23, 275–285 (2004).
De Vleesschauwer, D., Gheysen, G. & Höfte, M. Hormone defense networking in rice: Tales from a different world. Trends Plant Sci. 18, 555–565 (2013).
Gozzo, F. & Faoro, F. Systemic Acquired Resistance (50 Years after Discovery): Moving from the Lab to the Field. J. Agric. Food Chem. 61, 12473–12491 (2013).
Kachroo, A. & Robin, G. P. Systemic signaling during plant defense. Curr. Opin. Plant Biol. 16, 527–533 (2013).
Kachroo, A. & Kachroo, P. Mobile signals in systemic acquired resistance. Curr. Opin. Plant Biol. 58, 41–47 (2020).
Vlot, A. C., Dempsey, D. A. & Klessig, D. F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 47, 177–206 (2009).
Wildermuth, M. C., Dewdney, J., Wu, G. & Ausubel, F. M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565 (2001).
Nakashita, H. et al. Characterization of PBZ1, a probenazole-inducible gene, in suspension-cultured rice cells. Biosci. Biotechnol. Biochem. 65, 205–208 (2001).
Nakashita, H. et al. Probenazole induces systemic acquired resistance in tobacco through salicylic acid accumulation. Physiol. Mol. Plant Pathol. 61, 197–203 (2002).
Yoshioka, K., Nakashita, H., Klessig, D. F. & Yamaguchi, I. Probenazole induces systemic acquired resistance in Arabidopsis with a novel type of action. Plant J. 25, 149–157 (2001).
Nakayama, A. et al. Genome-wide identification of WRKY45-regulated genes that mediate benzothiadiazole-induced defense responses in rice. BMC Plant Biol. 13, 1–11 (2013).
Shimono, M. et al. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 19, 2064–2076 (2007).
Shimono, M. et al. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 13, 83–94 (2012).
Morffy, N., Faure, L. & Nelson, D. C. Smoke and hormone mirrors: action and evolution of karrikin and strigolactone signaling. Trends Genet. 32, 176–188 (2016).
Yang, T., Lian, Y. & Wang, C. Comparing and contrasting the multiple roles of butenolide plant growth regulators: strigolactones and karrikins in plant development and adaptation to abiotic stresses. Int. J. Mol. Sci. 20, 6270 (2019).
Gomez-Roldan, V. et al. Strigolactone inhibition of shoot branching. Nature 455, 189–194 (2008).
Umehara, M. et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200 (2008).
Zwanenburg, B., Pospíšil, T. & Ćavar Zeljković, S. Strigolactones: New plant hormones in action. Planta 243, 1311–1326 (2016).
Cook, C. E., Whichard, L. P., Turner, B., Wall, M. E. & Egley, G. H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 154, 1189–1190 (1966).
Yoneyama, K. et al. Regulation of biosynthesis, perception, and functions of strigolactones for promoting arbuscular mycorrhizal symbiosis and managing root parasitic weeds. Pest Manag. Sci. 75, 2353–2359 (2019).
Nelson, D. C., Flematti, G. R., Ghisalberti, E. L., Dixon, K. W. & Smith, S. M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu. Rev. Plant Biol. 63, 107–130 (2012).
Takahashi, I. & Asami, T. Target-based selectivity of strigolactone agonists and antagonists in plants and their potential use in agriculture. J. Exp. Bot. 69, 2241–2254 (2018).
Zorrilla, J. G., Rial, C., Varela, R. M., Molinillo, J. M. & Macias, F. A. Strategies for the synthesis of canonical, non-canonical and analogues of strigolactones, and evaluation of their parasitic weed germination activity. Phytochem. Rev. 21, 1627–1659 (2022).
Fukui, K., Ito, S. & Asami, T. Selective mimics of strigolactone actions and their potential use for controlling damage caused by root parasitic weeds. Mol. Plant 6, 88–99 (2013).
Mashiguchi, K., Seto, Y. & Yamaguchi, S. Strigolactone biosynthesis, transport and perception. Plant J. 105, 335–350 (2021).
Wang, Y. & Li, J. Branching in rice. Curr. Opin. Plant Biol. 14, 94–99 (2011).
Ishikawa, S. et al. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 46, 79–86 (2005).
Zhou, F. et al. D14–SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature 504, 406–410 (2013).
Marzec, M. Perception and signaling of strigolactones. Front. Plant Sci. 7, 1260 (2016).
Tang, J. & Chu, C. Strigolactone signaling: repressor proteins are transcription factors. Trends Plant Sci. 25, 960–963 (2020).
Kagiyama, M. et al. Structures of D 14 and D 14 L in the strigolactone and karrikin signaling pathways. Genes Cells 18, 147–160 (2013).
Zheng, J. et al. Karrikin signaling acts parallel to and additively with strigolactone signaling to regulate rice mesocotyl elongation in darkness. Plant Cell 32, 2780–2805 (2020).
Scaffidi, A. et al. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol. 165, 1221–1232 (2014).
Flematti, G. R., Scaffidi, A., Waters, M. T. & Smith, S. M. Stereospecificity in strigolactone biosynthesis and perception. Planta 243, 1361–1373 (2016).
Yao, R. et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 536, 469–473 (2016).
Nasir, F. et al. Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa). Plant Physiol. Biochem. 142, 106–116 (2019).
Kusajima, M. et al. Strigolactones modulate salicylic acid-mediated disease resistance in Arabidopsis thaliana. Int. J. Mol. Sci. 23, 5246 (2022).
Zheng, X. et al. The MAX2-KAI2 module promotes salicylic acid-mediated immune responses in Arabidopsis. J. Integr. Plant Biol. 65, 1566–1584 (2023).
Silverman, P. et al. Salicylic acid in rice (biosynthesis, conjugation, and possible role). Plant Physiol. 108, 633–639 (1995).
Lahari, Z. et al. Strigolactone deficiency induces jasmonate, sugar and flavonoid phytoalexin accumulation enhancing rice defense against the blast fungus Pyricularia oryzae. New Phytol. 241, 827–844 (2024).
Val-Torregrosa, B., Bundó, M. & San Segundo, B. Crosstalk between nutrient signalling pathways and immune responses in rice. Agriculture 11, 747 (2021).
Tamaoki, D. et al. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal. Behav. 8, e24260 (2013).
Hirooka, T. & Ishii, H. Chemical control of plant diseases. J. Gen. Plant Pathol. 79, 390–410 (2013).
Yasuda, M., Nakashita, H. & Yoshida, S. Tiadinil, a novel class of activator of systemic acquired resistance, induces defense gene expression and disease resistance in tobacco. J. Pestic. Sci. 29, 46–49 (2004).
Kusajima, M., Okumura, Y., Fujita, M. & Nakashita, H. Abscisic acid modulates salicylic acid biosynthesis for systemic acquired resistance in tomato. Biosci. Biotechnol. Biochem. 81, 1850–1853 (2017).
Kusajima, M. et al. Suppressive effect of abscisic acid on systemic acquired resistance in tobacco plants. J. Gen. Plant Pathol. 76, 161–167 (2010).
Yasuda, M. et al. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 20, 1678–1692 (2008).
Mangnus, E. M., Dommerholt, F. J., De Jong, R. L. & Zwanenburg, B. Improved synthesis of strigol analog GR24 and evaluation of the biological activity of its diastereomers. J. Agric. Food Chem. 40, 1230–1235 (1992).
Fukui, K. et al. New branching inhibitors and their potential as strigolactone mimics in rice. Bioorg. Med. Chem. Lett. 21, 4905–4908 (2011).
Nakamura, H. et al. Triazole ureas covalently bind to strigolactone receptor and antagonize strigolactone responses. Mol. Plant 12, 44–58 (2019).
Takahashi, I., Fukui, K. & Asami, T. Chemical modification of a phenoxyfuranone-type strigolactone mimic for selective effects on rice tillering or Striga hermonthica seed germination. Pest Manag. Sci. 72, 2048–2053 (2016).
Arazoe, T., Kuwata, S., Arie, T. & Ohsato, S. Experimental evidence of a pathogenic change caused by homologous recombination between endogenous and introduced dysfunctional Avr-Pita genes in Pyricularia oryzae. J. Gen. Plant Pathol. 80, 153–157 (2014).
Kunihiro, Y. et al. The new rice variety" Kita-ake". Bulletin of Hokkaido Prefectural Agricultural Experiment Station 59, 67–80 (1989).
Kusajima, M. et al. Characterization of plant immunity-activating mechanism by a pyrazole derivative. Biosci. Biotechnol. Biochem. 84, 1–9 (2020).
Acknowledgements
This work was partially supported by the Ministry of Agriculture, Forestry and Fisheries under Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (27004A) to K.Y., K.A., T.A. and H. Nakashita, by Grant-in-Aid for JSPS Fellow 19J14665 to M.F., by JSPS KAKENHI Grant Numbers 18K05656 to H. Nakashita, and by the Research and implementation promotion program through open innovation grants (JPJ011937) from the Project of the Bio-oriented Technology Research Advancement Institution (BRAIN) to M.K., M.F., T.A., H. Nakashita.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.K., T.A. and H.Nakashita; methodology, M.K., I.T., H.Nakamura, and H.Nakashita; validation, M.K., M.F., K.Y., K.A., K.B., T.A. and H.Nakashita; investigation, M.K., M.F., T.M., I.T., T.Tanaka and T.Thanh; writing, M.K. and H.Nakashita; visualization, M.K.; project administration, T.A. and H.Nakashita; funding acquisition, K.Y., K.A., T.A. and H.Nakashita. All authors have read and agreed to the version of the manuscript submitted for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kusajima, M., Fujita, M., Takahashi, I. et al. Enhancement of systemic acquired resistance in rice by F-box protein D3-mediated strigolactone/karrikin signaling. Sci Rep 15, 23875 (2025). https://doi.org/10.1038/s41598-025-06984-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06984-w