Abstract
Estimated pulse wave velocity (ePWV) has been proposed as a potential predictor of mortality in patients with acute ischemic stroke (AIS). This study aimed to evaluate the relationship between ePWV and all-cause mortality in AIS patients. Among 2,416 AIS patients initially screened, 2176 met the inclusion criteria and were included in the final analysis. ePWV was calculated using a validated formula based on age and mean blood pressure. Patients were stratified into high and low ePWV groups using a cut-off value of 12.33 m/s derived from the ROC curve for 360-day mortality. Cox proportional hazards models, adjusted for clinical and laboratory variables, were used to assess the association between ePWV and mortality at 30, 90, 180, and 360 days. Restricted cubic spline (RCS) analysis was performed to explore potential non-linear associations. Predictive performance was evaluated using area under the ROC curve (AUC), and subgroup analyses were conducted across demographic and clinical strata. Higher ePWV was independently associated with increased mortality at all time points, with adjusted hazard ratios of 1.54 (95% CI 1.26–1.90) at 30 days, 1.58 (1.32–1.89) at 90 days, 1.58 (1.33–1.88) at 180 days, and 1.60 (1.36–1.89) at 360 days (all P < 0.001). Kaplan–Meier analysis showed significantly lower survival in the high ePWV group (P < 0.001). RCS analysis suggested a positive linear relationship between ePWV and mortality. ePWV showed modest discriminative power (AUC 0.62–0.63), outperforming MBP but slightly inferior to age. However, the combination of ePWV with the SOFA score improved prognostic accuracy (AUC up to 0.72), outperforming the age + SOFA model at all time points. Elevated ePWV is independently associated with increased risk of mortality in patients with AIS. As a simple, non-invasive indicator of arterial stiffness, ePWV may serve as a valuable tool for risk stratification and early identification of high-risk patients who may benefit from intensified monitoring and management.
Similar content being viewed by others
Introduction
Acute ischemic stroke (AIS) is a leading cause of death and disability globally, contributing to significant morbidity and placing a substantial burden on healthcare systems1,2. AIS results in over 5 million deaths annually and remains a major cause of long-term disability, particularly among aging populations3. Despite advances in early therapeutic interventions such as thrombolysis and thrombectomy, AIS patients continue to face high risks of poor outcomes, including mortality4,5. Therefore, the early identification of high-risk patients and the accurate assessment of prognosis are essential to improving clinical management and outcomes.
Pulse wave velocity (PWV) serves as a reliable indicator of arterial stiffness and vascular elasticity, reflecting the cumulative effect of cardiovascular risk factors on the arterial walls6. Increased PWV has been consistently linked to a higher risk of cardiovascular events and mortality in diverse populations, including those with hypertension7, diabetes8, and coronary artery disease9. In the context of stroke, higher PWV has been linked to the development of delayed cerebral infarction and poorer short-term outcomes10,11. However, the clinical utility of PWV is limited by the requirement for specialized equipment and technical expertise, restricting its widespread application. To overcome these limitations, estimated PWV (ePWV) has been developed as a surrogate marker derived from routinely available clinical parameters such as age and blood pressure12. ePWV offers a practical and non-invasive alternative for assessing arterial stiffness, making it accessible for use in diverse clinical settings. Recent research has shown that ePWV acts as an independent predictor of mortality in individuals with cardiovascular diseases13, suggesting its potential applicability in broader patient populations.
Despite the promising evidence supporting ePWV, the relationship between ePWV and mortality risk in AIS patients remains poorly understood. Existing research has predominantly focused on traditional PWV measurements, leaving a significant gap in our knowledge regarding the prognostic value of ePWV within the AIS population. Understanding this association is crucial for integrating ePWV into clinical practice as a reliable prognostic tool, which could facilitate the early identification of high-risk patients and enable timely therapeutic interventions.
This study aims to evaluate the association between ePWV and risk of mortality in AIS patients. By elucidating the clinical utility of ePWV in AIS, this research seeks to advance the development of more accurate prognostic tools for AIS patients.
Material and methods
Data source and study population
This study analyzed data from the Medical Information Mart for Intensive Care version 2.2 (MIMIC-IV 2.2) database, which comprises de-identified health records of patients admitted to the intensive care units (ICUs) at Beth Israel Deaconess Medical Center in Boston, Massachusetts14. The MIMIC-IV database provides extensive information on patient demographics, clinical characteristics, diagnoses, treatments, laboratory results, and outcomes. Access to the database was granted to Guangdong Wang (Record ID: 60106105) upon completion of the CITI program, ensuring adherence to ethical standards in data utilization.
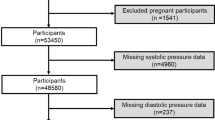
The study cohort was identified from 50,920 first-time ICU admissions recorded in the MIMIC-IV database. Among these, 2416 patients were diagnosed with AIS based on ICD codes. After applying specific inclusion and exclusion criteria, a total of 2176 patients were selected for the final analysis. Exclusion criteria encompassed ICU stays < 24 h (n = 240), patients < 18 years (n = 0), and those lacking data on ePWV (n = 0). The final cohort was stratified into two groups according to mortality outcomes: the survival group (n = 1408) and the mortality group (n = 768). The patient selection process is detailed in Fig. 1.
Data collection ePWV measurement
The data for this study were obtained from the MIMIC-IV 2.2 database through structured query language (SQL) queries. Demographic data included age, gender, and race. Vital signs collected were heart rate, mean blood pressure (MBP), respiratory rate, oxygen saturation (SpO2), and body temperature. Medical history variables included the presence of chronic heart failure (CHF), chronic pulmonary disease (CPD), hypertension, diabetes, and renal disease. Laboratory data, including white blood cell (WBC) count, platelet count, hemoglobin levels, blood urea nitrogen (BUN), creatinine, glucose, potassium, sodium, and prothrombin time (PT), were collected to assess patient condition. Finally, treatment information such as the use of mechanical ventilation, continuous renal replacement therapy (CRRT), vasopressors, anticoagulants, antiplatelet agents, recombinant tissue plasminogen activator (rtPA), and thrombectomy procedures were gathered.
ePWV was calculated using the formula15: ePWV = 9.587 − 0.402 × age + 4.560 × 10–3 × age2 − 2.621 × 10–5 × age2 × MBP + 3.176 × 10–3 × age × MBP − 1.832 × 10–2 × MBP. Age and MBP values used in this formula were derived from the first 24 h following ICU admission, aiming to capture the initial hemodynamic status of patients after acute ischemic stroke onset. Based on the optimal cut-off value of 12.33 m/s derived from the receiver operating characteristic (ROC) curve for 360-day mortality, patients were classified into low (< 12.33 m/s) and high (≥ 12.33 m/s) ePWV groups for subsequent analysis.
Clinical scores
Several established clinical scoring systems were included in this study to assess illness severity and comorbidity burden. The Acute Physiology Score III (APS III), Sequential Organ Failure Assessment (SOFA), and Simplified Acute Physiology Score II (SAPS II) are commonly used ICU severity scores, with higher values indicating more severe physiological derangement or organ dysfunction. The Charlson Comorbidity Index (CCI) quantifies baseline comorbid conditions, where a higher score reflects a greater comorbidity burden. The Glasgow Coma Scale (GCS) evaluates neurological function, with lower scores indicating more severe impairment (Table S1). The availability of these scores in the patient dataset is summarized in Table S2. All scores were used as continuous variables in the analyses.
Endpoints of interest
The primary outcomes of this study were all-cause mortality at 30, 90, 180, and 360 days following ICU admission in patients with AIS. Mortality was defined as death from any cause during the respective follow-up intervals.
Statistical analysis
Continuous variables with a normal distribution were expressed as means ± standard deviation (SD), and comparisons between groups were performed using the Student’s t-test. Continuous variables with skewed distributions were presented as median [interquartile range (IQR)], with comparisons conducted using the Mann–Whitney U test. Categorical variables were reported as counts and percentages [n (%)], with intergroup differences assessed using the chi-squared (χ2) test.
We first compared the baseline characteristics of the 360-day survival and mortality groups. Differences in severity scores and mortality between these ePWV groups were compared. For variables showing significant differences between the survival and mortality groups at 360 days, we applied Least Absolute Shrinkage and Selection Operator (LASSO) regression to reduce multicollinearity and identify candidate predictors. These 24 selected variables were then analyzed using univariate Cox regression. Variables with P values < 0.05 were further assessed for multicollinearity using variance inflation factor (VIF) analysis, and those with VIF > 5 were excluded. The remaining variables were included in the final multivariate Cox regression model to adjust for potential confounders.
To assess the nonlinear relationship between ePWV and mortality risk, we used restricted cubic splines (RCS), which offer greater flexibility in modeling continuous exposures and outcomes. The predictive value of ePWV, SOFA score, and the combination of both for 30-, 90-, 180-, and 360-day mortality was compared using ROC curves.
Subgroup and interaction analyses were conducted based on age, gender, and the presence of CHD, CPD, hypertension, diabetes, renal disease, and SOFA score. Data missing from less than 15% of cases were handled by multiple imputation using the random forest algorithm, as described in Table S2. Data analysis was performed using R software version 4.4.1 and Free Statistics software version 2.0. A P value < 0.05 was considered statistically significant.
Results
Baseline characteristics of included patients
The baseline characteristics of the 2,176 patients with AIS are summarized in Table 1. The mean age of the total cohort was 69.38 ± 15.75 years, with the mortality group being significantly older than the survival group (P < 0.001). Female patients and those categorized as non-White (including Black, Asian, and Other) were more common in the mortality group compared with the survival group (P = 0.011 and P = 0.006, respectively). Vital signs also differed between the groups, with the mortality group exhibiting a higher heart rate (P < 0.001), lower MBP (P = 0.006), higher ePWV (P < 0.001), and increased respiratory rate (P < 0.001). Regarding medical history, the mortality group had significantly higher rates of CHF, CPD, hypertension, diabetes, and renal disease (all P < 0.001).
The severity scores, including APSIII, SOFA, SAPSII, and CCI, were significantly higher in the mortality group, indicating greater disease severity and comorbidity burden (all P < 0.001). Laboratory findings revealed higher WBC, BUN, creatinine, glucose, and potassium levels in the mortality group, while hemoglobin was lower (all P < 0.001). The mortality group also had significantly higher rates of ventilation, CRRT, and vasopressor use (all P < 0.001), but there were no significant differences in the use of anticoagulants, rtPA, or thrombectomy.
Severity scores and mortality between ePWV group
Severity scores and mortality rates between the low and high ePWV groups are compared (Table 2). The high ePWV group had significantly higher APS III (P = 0.002), SAPSII (P < 0.001), and CCI (P < 0.001) scores, while the GCS score was lower (P < 0.001), indicating more severe neurological impairment. There was no significant difference in SOFA scores (P = 0.174). Mortality rates at 30, 90, 180, and 360 days were significantly higher in the high ePWV group (all P < 0.001), with mortality rates of 31.16%, 39.8%, 44.31%, and 48.69%, respectively, compared to 17.65%, 22.44%, 24.76%, and 27.52% in the low ePWV group.
Table S3 shows positive correlations between ePWV and APSIII (r = 0.048, P = 0.025), SAPS II (r = 0.305, P < 0.001), and CCI (r = 0.528, P < 0.001), and negative correlations with SOFA (r = − 0.073, P = 0.001) and GCS (r = − 0.188, P < 0.001).
Association between ePWV and all-cause mortality
LASSO regression was performed to address multicollinearity and identify key predictors, as shown in Fig. S1. Table S4 presents the results of univariate Cox regression analysis for the 24 selected variables. Variables with P values < 0.05 were subsequently evaluated for multicollinearity using VIF analysis, as detailed in Table S5. Variables with VIF values > 5 were excluded from further modeling, including age, which exhibited high collinearity with ePWV. The remaining variables were included in the final multivariate Cox regression analysis to adjust for potential confounders. After adjustment for gender, race, vital signs, medical history, comorbidities, severity scores, laboratory parameters, and treatments, higher ePWV remained independently associated with increased risk of mortality at all time points (Table 3). The adjusted hazard ratio (HR) for high ePWV was 1.54 (95% CI 1.26–1.90; P < 0.001) at 30 days, 1.58 (1.32–1.89; P < 0.001) at 90 days, 1.58 (1.33–1.88; P < 0.001) at 180 days, and 1.60 (1.36–1.89; P < 0.001) at 360 days.
Kaplan–Meier survival curve
Kaplan–Meier survival curves in Fig. 2 showed that, compared to the low ePWV group, the 30, 90, 180, and 360-day cumulative survival rates of patients with high ePWV were significantly lower (all P < 0.001). These results were consistent across all time points, indicating that high ePWV is associated with worse survival.
Non-linear association between ePWV and mortality
Figure 3 illustrates the non-linear relationship between ePWV and all-cause mortality at 30, 90, 180, and 360 days. No significant non-linearity was observed for 30-day, 90-day, or 180-day mortality (P = 0.168, 0.298, and 0.142, respectively), while a marginally significant non-linear trend was noted for 360-day mortality (P = 0.052). Overall, the mortality risk tended to increase with higher ePWV values, suggesting a positive association between arterial stiffness and mortality.
Restricted cubic spline analysis depicting the relationship between ePWV and the risk of all-cause mortality. (A) 30-day, (B) 90-day, (C) 180-day, and (D) 360-day mortality. The solid line represents the adjusted hazard ratio across the continuous range of ePWV, with the median value set as the reference (HR = 1). Shaded areas indicate 95% confidence intervals. The overlaid blue bars at the bottom of each panel show the distribution of ePWV among all patients in the study cohort.
The predictive performance of ePWV on mortality
Figure 4 and Table S6 present the predictive performance of ePWV for all-cause mortality. For 30-day mortality, ePWV (cut-off 10.93) yielded an AUC of 0.62. For 90-, 180-, and 360-day mortality, ePWV cut-offs of 12.34 and 12.33 yielded AUCs of 0.62, 0.62, and 0.63, respectively. The combination of ePWV with the SOFA score significantly improved predictive accuracy, achieving the highest AUC of 0.72 at 90, 180, and 360 days. To further contextualize the predictive value of ePWV, we compared its performance with that of age and MBP (Fig. S2). Across all time points, ePWV consistently outperformed MBP (AUCs: 0.62–0.63), but remained slightly inferior to age alone (AUCs: 0.64–0.66). Notably, when integrated into a combined model, ePWV + SOFA demonstrated superior predictive performance compared to age + SOFA at all four time points (Fig. S3), with AUCs reaching 0.71–0.72 and all comparisons yielding P < 0.001.
Subgroup analysis
Subgroup analyses were performed based on age, gender, and the presence of comorbidities (CHF, CPD, hypertension, diabetes, renal disease) as well as SOFA score (Fig. 5). No significant interactions were observed between ePWV and these subgroups, indicating that the results was consistent across these variables.
Discussion
In this study, we investigated the association between ePWV and mortality in patients with AIS. Our findings indicate that higher ePWV is associated with an increased risk of mortality at 30, 90, 180, and 360 days, suggesting that ePWV could serve as a valuable prognostic biomarker for both short-term and long-term outcomes in AIS patients. Notably, when combined with the SOFA score, ePWV significantly enhanced predictive performance, indicating its complementary value to traditional severity assessment tools and reinforcing its potential role in clinical risk stratification.
Arterial stiffness, characterized by the reduced elasticity of the arteries, is a key contributor to cardiovascular diseases, including stroke16,17. It results from factors such as aging, hypertension, and atherosclerosis, which increase vascular resistance and strain the cardiovascular system. PWV is a widely used measure of arterial stiffness, reflecting the speed at which pressure waves travel through the arteries18,19. Traditionally, PWV is measured invasively, but ePWV provides a non-invasive alternative by calculating arterial stiffness using simple clinical parameters such as age and blood pressure12. ePWV has been shown to correlate strongly with traditional PWV, making it a reliable marker for assessing arterial stiffness20. Studies have demonstrated that ePWV is a predictor of mortality in patients with subarachnoid hemorrhage (SAH) and coronary heart disease (CHD). Chen et al. found that ePWV ≥ 12.10 was associated with increased risks of both 30-day and 1-year mortality in SAH patients, with the combined use of ePWV and SOFA score improving predictive performance21. Similarly, Li et al. confirmed that high ePWV levels were associated with higher inpatient mortality among critically ill patients with non-traumatic subarachnoid hemorrhage22. Gu et al. further expanded the role of ePWV, showing that it is a strong independent predictor of both short- and long-term mortality in critically ill patients with CHD23. Their study also found that integrating ePWV into SOFA score, significantly improved predictive accuracy in this population.
Several recent studies have highlighted the prognostic relevance of ePWV in cerebrovascular disease. Jae et al. demonstrated that elevated ePWV was independently associated with increased risk of both ischemic and hemorrhagic stroke in a long-term cohort of middle-aged men, even after adjusting for traditional risk factors including age and pulse pressure24. Similarly, Gu et al. confirmed the positive association between higher ePWV and incident stroke in three large, nationally representative aging cohorts from the United States, the United Kingdom, and China25. Most recently, Li et al. extended these findings by showing that ePWV independently predicted all-cause and cardiovascular mortality in a nationally representative stroke population, and even outperformed the Framingham Risk Score in mortality prediction26. While these studies collectively support the role of ePWV as a vascular biomarker for stroke incidence and prognosis, they predominantly focus on general or ambulatory populations, rather than critically ill patients. In contrast, our study specifically investigates the predictive value of ePWV for short- and long-term mortality in patients with AIS admitted to the ICU, a population characterized by greater clinical complexity and higher baseline risk. Moreover, our study comprehensively evaluate the additive prognostic benefit of combining ePWV with the SOFA score, a widely used ICU severity index, demonstrating that the integration significantly enhances mortality prediction across multiple time points. Our results not only reinforce ePWV’s utility as an independent prognostic marker but also provide evidence for its potential integration into critical care risk stratification strategies for stroke.
The association between elevated ePWV and increased mortality risk in AIS patients is likely mediated by several interrelated mechanisms, particularly the effect of arterial stiffness on vascular health, cerebrovascular function, and systemic inflammation. Increased arterial stiffness impairs large arteries’ ability to buffer pressure fluctuations during the cardiac cycle, leading to elevated systolic blood pressure and pulse pressure27,28. This dysfunction compromises cerebral hemodynamics by reducing the efficiency of collateral circulation and impairing autoregulatory capacity, which makes the brain more vulnerable to ischemic injury. Furthermore, arterial stiffness is closely linked to endothelial dysfunction and vascular remodeling, processes that are exacerbated by chronic conditions such as hypertension and diabetes29,30. These changes, including thickened arterial walls and increased collagen deposition, increase the risk of ischemic damage. Elevated arterial stiffness is also associated with systemic inflammation and oxidative stress, both of which play crucial roles in the pathogenesis of atherosclerosis and neuronal injury during stroke31. The release of pro-inflammatory cytokines and reactive oxygen species may result in blood–brain barrier disruption and subsequent secondary brain injury. Additionally, renal dysfunction, often observed in patients with high ePWV, further exacerbates cardiovascular instability and contributes to worse stroke outcomes by increasing blood pressure and promoting fluid retention.
Interestingly, a modest yet statistically significant inverse correlation was observed between ePWV and the SOFA score (r = − 0.073, P = 0.001), indicating that patients with greater arterial stiffness tended to exhibit lower degrees of acute organ dysfunction at ICU admission. This paradoxical relationship likely reflects the distinct physiological constructs captured by these two indices. Specifically, ePWV serves as a surrogate marker of chronic vascular burden, primarily shaped by long-standing exposure to cardiovascular risk factors such as aging, hypertension, and diabetes. In contrast, the SOFA score evaluates the severity of acute organ failure, which is often driven by transient critical illnesses, including sepsis and multiorgan dysfunction. It is plausible that patients with elevated ePWV may retain relatively preserved organ function upon ICU admission, particularly if their clinical deterioration is primarily driven by chronic comorbidities rather than an acute systemic insult. Conversely, individuals presenting with high SOFA scores may be acutely ill due to severe systemic derangements that mask or supersede the contributions of underlying vascular pathology. As such, the observed inverse association may reflect a temporal and pathophysiological disconnect between chronic vascular stiffness and acute organ dysfunction. This reinforces the notion that ePWV and SOFA capture different but complementary aspects of mortality risk, chronic versus acute, and supports the value of using both in combination for prognostication in AIS patients.
While age demonstrated slightly better predictive performance than ePWV when assessed individually, ePWV captured vascular pathology that chronological age alone may not reflect. Notably, the integration of ePWV with the SOFA score substantially enhanced mortality prediction, with AUCs reaching 0.72 across multiple time points. This synergistic improvement suggests that ePWV provides complementary information regarding chronic vascular burden, which traditional severity scores may overlook.
In clinical practice, elevated ePWV in AIS patients should alert clinicians to the presence of underlying vascular pathology, which may complicate recovery. Elevated ePWV suggests an increased risk of cardiovascular events and warrants a comprehensive evaluation for co-morbidities like hypertension, diabetes, and dyslipidemia. Management of these patients should prioritize controlling modifiable risk factors, such as blood pressure, lipids, and smoking, while considering therapies that target vascular health, such as ACE inhibitors or statins. Regular cardiovascular monitoring and rehabilitation should be integrated into the care plan to mitigate long-term risks. Furthermore, incorporating ePWV into clinical decision-making, alongside established severity scores like the SOFA score, can enhance risk stratification and assist clinicians in making more informed decisions regarding patient management. Ultimately, addressing vascular health in AIS patients with elevated ePWV may improve long-term outcomes and reduce mortality.
The strengths of this study include the use of ePWV as a non-invasive biomarker, providing valuable prognostic insights, and the comprehensive dataset with longitudinal follow-up, enabling robust analysis of long-term outcomes. However, several limitations should be noted. First, as an observational design, it precludes any causal inference between ePWV and mortality. Second, the estimation of ePWV may be subject to measurement bias. Although we used a validated formula based on age and MBP, this surrogate marker can still be influenced by factors such as patient positioning, hemodynamic instability, and measurement conditions in the ICU. Third, ePWV was calculated only once, using MBP recorded during the first 24 h after ICU admission. While this time window reflects the early hemodynamic status following acute ischemic stroke, it may not account for temporal fluctuations in blood pressure. Consequently, the single-timepoint measurement may not fully capture the dynamic nature of arterial stiffness. Future studies should consider repeated measurements to improve the reliability and temporal resolution of ePWV assessment. Lastly, the study did not include the NIHSS score, a key clinical indicator of stroke severity. Incorporating NIHSS could have enhanced the granularity of risk adjustment.
Conclusion
In conclusion, our study demonstrates that increased ePWV is independently associated with higher short-term and long-term mortality in patients with AIS. The findings suggest that ePWV could serve as an important prognostic marker, aiding in the identification of high-risk patients who may benefit from intensified management.
Data availability
The data used in this study were obtained from the publicly available MIMIC-IV 2.2 database. This database can be accessed through the PhysioNet platform at the following URL: https://physionet.org/content/mimiciv/2.2/.
References
Feigin, V. L. & Owolabi, M. O. Pragmatic solutions to reduce the global burden of stroke: A World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 22, 1160–1206. https://doi.org/10.1016/s1474-4422(23)00277-6 (2023).
Feigin, V. L., Norrving, B. & Mensah, G. A. Global burden of stroke. Circ. Res. 120, 439–448. https://doi.org/10.1161/circresaha.116.308413 (2017).
Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20, 795–820. https://doi.org/10.1016/s1474-4422(21)00252-0 (2021).
Owolabi, M. O. et al. The state of stroke services across the globe: Report of World Stroke Organization-World Health Organization surveys. Int. J. Stroke 16, 889–901. https://doi.org/10.1177/17474930211019568 (2021).
Review of response to the thunderstorm asthma event of 21–22 November 2016, Authorised and published by the Victorian Government, 1 Treasury Place, Melbourne.
Xu, L. et al. A flexible ultrasound array for local pulse wave velocity monitoring. Biosensors (Basel) https://doi.org/10.3390/bios12070479 (2022).
Palombo, C. & Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul. Pharmacol. 77, 1–7. https://doi.org/10.1016/j.vph.2015.11.083 (2016).
Cheong, S. S. et al. Prognostic value of pulse wave velocity for cardiovascular disease risk stratification in diabetic patients: A systematic review and meta-analysis. J. Diabetes Complicat. 38, 108894. https://doi.org/10.1016/j.jdiacomp.2024.108894 (2024).
Lyu, Y. et al. Classification of coronary artery disease using radial artery pulse wave analysis via machine learning. BMC Med. Inform. Decis. Mak. 24, 256. https://doi.org/10.1186/s12911-024-02666-1 (2024).
Gąsecki, D. et al. Pulse wave velocity is associated with early clinical outcome after ischemic stroke. Atherosclerosis 225, 348–352. https://doi.org/10.1016/j.atherosclerosis.2012.09.024 (2012).
Kim, J. et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension 64, 240–246. https://doi.org/10.1161/hypertensionaha.114.03304 (2014).
Greve, S. V., Laurent, S. & Olsen, M. H. Estimated pulse wave velocity calculated from age and mean arterial blood pressure. Pulse (Basel) 4, 175–179. https://doi.org/10.1159/000453073 (2017).
Prelević, V. et al. Estimated pulse wave velocity and all-cause and cardiovascular mortality in the general population. J. Clin. Med. https://doi.org/10.3390/jcm13123377 (2024).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Cheng, W. et al. Superior predictive value of estimated pulse wave velocity for all-cause and cardiovascular disease mortality risk in U.S. general adults. BMC Public Health 24, 600. https://doi.org/10.1186/s12889-024-18071-2 (2024).
Mitchell, G. F. et al. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 121, 505–511. https://doi.org/10.1161/circulationaha.109.886655 (2010).
Lever-Megina, C. G. et al. Association between pulse wave velocity and cerebral microbleeds: A systematic review and meta-analysis. Hypertens Res. https://doi.org/10.1038/s41440-024-01963-6 (2024).
Laurent, S. et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 27, 2588–2605. https://doi.org/10.1093/eurheartj/ehl254 (2006).
O’Rourke, M. F. & Franklin, S. S. Arterial stiffness: Reflections on the arterial pulse. Eur. Heart J. 27, 2497–2498. https://doi.org/10.1093/eurheartj/ehl312 (2006).
Greve, S. V. et al. Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J. Hypertens. 34, 1279–1289. https://doi.org/10.1097/hjh.0000000000000935 (2016).
Chen, M., Fan, H., Xie, L., Zhou, L. & Chen, Y. Association between estimated pulse wave velocity and the risk of mortality in patients with subarachnoid hemorrhage: A retrospective cohort study based on the MIMIC database. BMC Neurol. 24, 408. https://doi.org/10.1186/s12883-024-03897-5 (2024).
Li, J., Zhang, M., Ye, B., Lu, M. & Liao, G. Association between estimation of pulse wave velocity and all-cause mortality in critically ill patients with non-traumatic subarachnoid hemorrhage: An analysis based on the MIMIC-IV database. Front Neurol. 15, 1451116. https://doi.org/10.3389/fneur.2024.1451116 (2024).
Gu, Y. et al. Prognostic significance of the estimated pulse wave velocity in critically ill patients with coronary heart disease: analysis from the MIMIC-IV database. Eur. Heart J. Qual. Care Clin. Outcomes https://doi.org/10.1093/ehjqcco/qcae076 (2024).
Jae, S. Y., Heffernan, K. S., Kurl, S., Kunutsor, S. K. & Laukkanen, J. A. Association between estimated pulse wave velocity and the risk of stroke in middle-aged men. Int. J. Stroke 16, 551–555. https://doi.org/10.1177/1747493020963762 (2021).
Gu, Y. et al. Estimated pulse wave velocity and stroke among middle-aged and older population: Insights from 3 prospective cohorts. J. Am. Heart Assoc. 14, e038376. https://doi.org/10.1161/jaha.124.038376 (2025).
Li, J. et al. Estimated pulse wave velocity is associated with all-cause and cardiovascular mortality in individuals with stroke: A national-based prospective cohort study. Medicine (Baltimore) 104, e41608. https://doi.org/10.1097/md.0000000000041608 (2025).
Alsomali, A. et al. Associations between central and brachial blood pressure in patients with hypertension and aortovascular disease: Implications for clinical practice. Curr. Probl. Cardiol. 50, 102874. https://doi.org/10.1016/j.cpcardiol.2024.102874 (2025).
Wang, X., Keith, J. C. Jr., Struthers, A. D. & Feuerstein, G. Z. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc. Ther. 26, 214–223. https://doi.org/10.1111/j.1755-5922.2008.00051.x (2008).
Della Corte, V. et al. Inflammation, endothelial dysfunction and arterial stiffness as therapeutic targets in cardiovascular medicine. Curr. Pharm. Des. 22, 4658–4668. https://doi.org/10.2174/1381612822666160510124801 (2016).
Jekell, A., Malmqvist, K., Wallén, N. H., Mörtsell, D. & Kahan, T. Markers of inflammation, endothelial activation, and arterial stiffness in hypertensive heart disease and the effects of treatment: Results from the SILVHIA study. J. Cardiovasc. Pharmacol. 62, 559–566. https://doi.org/10.1097/fjc.0000000000000017 (2013).
Sugiura, T. et al. Oxidative stress is closely associated with increased arterial stiffness, especially in aged male smokers without previous cardiovascular events: A cross-sectional study. J. Atheroscler. Thromb. 24, 1186–1198. https://doi.org/10.5551/jat.39289 (2017).
Funding
No specific fund supports the current analysis.
Author information
Authors and Affiliations
Contributions
YZ: Methodology, Writing—original draft. PC, YW: Formal analysis, Visualization. YY, ZC: Validation, Investigation. GW: Data curation. MZ and WZ: Supervision, Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the relevant ethical review boards. Since the data used in this study were obtained from the publicly available MIMIC-IV 2.2 database, which is anonymized and does not contain personally identifiable information, the study was exempt from the requirement for individual patient consent. The MIMIC-IV database is provided under strict ethical guidelines, ensuring that the data is used responsibly and in compliance with all applicable regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Cai, P., Wu, Y. et al. Association between estimated pulse wave velocity and mortality risk in patients with acute ischemic stroke. Sci Rep 15, 22237 (2025). https://doi.org/10.1038/s41598-025-07082-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07082-7