Abstract
We investigated the association of cystatin C with incident chronic kidney disease (CKD), defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2. We enrolled patients with an eGFR ≥ 60 mL/min/1.73 m2 who underwent an oral glucose tolerance test (OGTT) between 2011 and 2013. Patients with diabetes were excluded from the analysis. We measured their cystatin C levels, and all patients’ renal function and survival status were followed until March 2023. Cox-proportional hazard models were conducted to examine the association of cystatin C with incident CKD and all-cause mortality. A total of 146 patients were analyzed. After a median follow-up period of 10.7 years, patients with a higher cystatin C (≥ median vs. < median) were independently associated with a higher risk of incident CKD and all-cause mortality (adjusted hazard ratio [HR] 2.96, 95% CI 1.09 to 8.09, p = 0.034). The findings were consistent when cystatin C was analyzed as a continuous variable. The association was mainly observed in patients with prediabetes (adjusted HR 5.75, 95% CI 1.34 to 24.69, p = 0.019). In summary, a higher cystatin C was independently associated with risk of incident CKD and all-cause mortality in non-diabetes patients with an eGFR ≥ 60 mL/min/1.73 m2.
Similar content being viewed by others
Introduction
The prevalence of CKD has increased substantially all over the world1. It was estimated that in 2017, CKD had a global prevalence of 9.7%, a 29.3% increase from 1990. According to clinical practice guidelines, the definition of CKD is structural or functional abnormalities of the kidneys for more than 3 months2. A urine albumin-to-creatinine ratio (UACR) ≥ 30 mg/g or an eGFR < 60 mL/min/1.73 m2 are usually used to define CKD in epidemiologic studies and clinical research. CKD patients are at a high risk of all-cause and cardiovascular mortality, and treatment for these patients is increasing the burden on healthcare systems3,4,5,6. Hence, identifying people who are at risk of CKD development is important.
Cystatin C is a low-molecular-weight (13-kD) basic protein, and has been implicated as a marker for renal dysfunction. Nucleated cells produce cystatin C at a constant rate, and it is reabsorbed and catabolized by proximal tubule cells7. Therefore, any abnormality of reabsorption in the proximal tubules can cause significant increase in urinary levels of cystatin C. Serum levels of cystatin C have been used to estimate GFR, and cystatin C-based eGFR has been associated with adverse renal outcomes8,9,10. In clinical practice guidelines, the use of cystatin C-based eGFR is suggested in adults who have a creatinine-based eGFR between 45 and 59 mL/min/1.73 m2 without markers of kidney damage, to confirm the diagnosis of CKD11.
Despite the findings described above, usage of serum cystatin C remains limited in daily practice. Diabetes is a well-established risk factor of CKD, and patients with type 2 diabetes have a higher circulating cystatin C level than healthy controls12. Nevertheless, there was a high variability in terms of the performance of cystatin C in the estimation of renal function in patients with diabetes13. In this study, we investigated the association of serum cystatin C levels with incident CKD (defined as an eGFR < 60 mL/min/1.73 m2) in patients with normal glucose tolerance and prediabetes.
Patient and methods
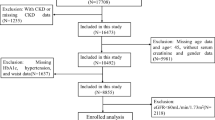
This study was a prospective cohort study. The study protocol was approved (approval number C08215B) by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan. We conducted this study in accordance with the Declaration of Helsinki. Written informed consent from all study participants was obtained. Patients with no history of diabetes who underwent an OGTT to screen for diabetes at outpatient clinics between 2011 and 2013 were enrolled14. We excluded patients who had an eGFR < 60 mL/min/1.73 m2, liver failure, malignancy, pregnancy or lactation, abnormal thyroid function, or those receiving glucocorticoid treatment. Laboratory data assessment at enrollment included total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, cystatin C, and UACR. Information on demographics, past history, and use of medications were collected from electronic medical records.
All patients’ glucose regulation state (normal glucose tolerance, prediabetes, and newly diagnosed diabetes) was determined according to the results of OGTT15. Patients with newly diagnosed diabetes were excluded from the analyses. We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation16. Circulating cystatin C levels were measured using an ELISA kit (Cystatin C Quantikine ELISA kit, Cat. No. DSCTC0; R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions. The mean minimum detectable dose of serum cystatin C was 0.102 ng/ml, and the intra- and inter-assay coefficients of variation were both < 7.0%.
After the baseline assessment, all study participants were treated and followed up at our outpatient clinics. The frequency of laboratory data follow-up was at the discretion of their providers (usually 1 to 3 times per year). We determined our patients’ eGFR during the follow-up period according to their serum creatinine using the CKD-EPI equation. The outcomes of interest were incident CKD and all-cause mortality. Incident CKD was defined as an eGFR < 60 ml/min/1.73 m2 of at least 3 months’ duration. We obtained data on all-cause mortality (updated to March 2023) from the Ministry of Health and Welfare, R.O.C. De-identified data were used for analyses.
Statistical analysis
All statistical analyses were conducted using the Statistical Package for the Social Sciences (IBM SPSS version 22.0; International Business Machines Corp., NY, USA). We stratified our patients according to their glucose regulation status (normal glucose tolerance vs. prediabetes). We determined between-group differences in clinical variables for statistical significance using the Student’s t-test for continuous variables, and the Chi-square test for categorical variables. Linear regression analyses were used to examine the associations of clinical variables with cystatin C levels. Kaplan-Meier survival curves were plotted for CKD-free survival according to baseline cystatin C levels (≥ median vs. < median). We investigated the association of cystatin C levels (≥ median vs. < median, and, as a continuous variable) with incident CKD and all-cause mortality using Cox-proportional hazard models with multivariate adjustment. Cubic spline of cystatin C levels versus risk of incident CKD and all-cause mortality by Cox proportional hazards model was performed. Cubic spline was plotted by R software (Version 2.15.3, cubic spline package: smooth HR). In all of these statistical analyses, a two-sided p value < 0.05 was considered statistically significant.
Results
Table 1 shows the baseline characteristics of the study participants according to their glucose regulation status and cystatin C levels. Patients with prediabetes were older (60.6 ± 10.6 vs. 57.0 ± 9.9 years, p = 0.035), had higher systolic (128.4 ± 14.2 vs. 123.0 ± 17.4 years, p = 0.042) and diastolic blood pressure (76.2 ± 10.0 vs. 72.8 ± 11.1 years, p = 0.047), and more hypertension (95.9% vs. 83.3%, p = 0.012), compared to those who had normal glucose tolerance. There were no significant between-group differences in body mass index, eGFR, UACR, albuminuria (≥ 30 mg/g), and the rates of using ACEI or ARB and statins. Interestingly, patients with prediabetes had higher cystatin C levels (627 ± 316 vs. 500 ± 321 ng/mL, p = 0.017) than those with normal glucose tolerance.
We analyzed cystatin C as a dependent variable, as shown in Table 2. In univariate analysis, prediabetes (vs. normal glucose tolerance) was positively associated with cystatin C (β coefficient 127.09, 95% CI 22.81 to 231.37, p = 0.017). After multivariate adjustment, the association remained statistically significant (β coefficient 120.31, 95% CI 10.64 to 229.97, p = 0.032).
After a median follow-up of 10.7 years, there were 17 incident CKD and 13 all-cause deaths. There was no patients progressed to end-stage kidney disease requiring renal replacement therapy. Figure 1 illustrates the cumulative CKD-free survival rates according to cystatin C levels. Patients with cystatin C ≥ median (677 ng/mL) had lower CKD-free survival than those with cystatin C < median (log-rank p = 0.024). Table 3 shows the association of cystatin C with incident CKD and all-cause mortality. In the unadjusted model, cystatin C ≥ median was associated with a higher risk of incident CKD and all-cause mortality (HR 2.85, 95% CI 1.11 to 7.33, p = 0.030), compared with cystatin C < median. The risk remained significant after multivariate adjustment (HR 2.96, 95% CI 1.09 to 8.09, p = 0.034). The findings were comparable when cystatin C was analyzed as a continuous variable. Cystatin C (as a continuous variable) was positively associated with incident CKD and all-cause mortality after multivariate adjustment (HR 1.002, 95% CI 1.001 to 1.004, p = 0.006, Table 3). Figure 2 shows the cubic spline of cystatin C versus risk of incident CKD and all-cause mortality (p = 0.002). The cystatin C level above which the risk of incident CKD and all-cause mortality increased was approximately 600 ng/mL.
Table 4 shows the associations of cystatin C with incident CKD and all-cause mortality in various subgroups. The associations were similar among the subgroups of age (< 65 vs. ≥65 years), sex (male vs. female), body mass index (< 25 vs. ≥ 25 kg/m2), systolic BP (< 130 vs. ≥ 130 mm Hg), and use of ACEI or ARB (no vs. yes). It is interesting to note that the association between cystatin C and incident CKD and all-cause mortality was more prominent in patients with prediabetes (adjusted HR 5.75, 95% CI 1.34 to 24.69, p = 0.019), compared to those with normal glucose tolerance (adjusted HR 1.52, 95% CI 0.26 to 9.04, p = 0.643).
Discussion
In this study, we demonstrated that prediabetes (vs. normal glucose tolerance) was positively associated with serum cystatin C level (Table 2). Moreover, a higher cystatin C (≥ median vs. < median) was independently associated with risk of incident CKD and all-cause mortality (Table 3). The findings were comparable when cystatin C was analyzed as a continuous variable. Moreover, the association between cystatin C and risk of incident CKD and all-cause mortality was more prominent in patients with prediabetes (Table 4). Our findings suggest that cystatin C might be a biomarker to predict risk of adverse renal outcomes in non-diabetes patients with an eGFR ≥ 60 mL/min/1.73 m2.
Our findings are in line with previous studies. In a large cohort study that included 4637 patients aged ≥ 65 years, cystatin C was a stronger predictor than creatinine for the risk of all-cause death and cardiovascular events during a median follow-up of 7.4 years17. Post-hoc analysis of the study revealed that a higher cystatin C (≥ 1.0 mg/L vs. < 1.0 mg/L) was associated with a 4-fold risk of progression to CKD after 4 years of follow-up in patients with a baseline eGFR ≥ 60 mL/min/1.73 m218. Similar findings were noted in patients with a baseline eGFR < 60 mL/min/1.73 m2. In a multicenter cohort study that included nondiabetic patients with stage 3–4 CKD, a 1-SD decrease in 1/cystatin C was associated with a significantly higher risk of kidney failure (adjusted HR 2.36, 95% CI 2.10 to 2.66) during a median follow-up period of 10 years19.
However, in the aforementioned studies, the proportion of patients with diabetes was only 20% or less. Diabetes is a well-known risk factor of incident CKD, but data on cystatin C in patients with diabetic kidney disease were limited in a previous review20. Cystatin C-based estimation of GFR appeared to improve risk stratification of end-stage kidney disease (ESKD), compared with creatinine-based estimation of GFR, in patients with type 1 diabetes and type 2 diabetes who had stages 1–3 CKD at baseline after an average of 8–10 years’ follow-up10. In this study, data on glucose-lowering medications were not reported for patients who had type 2 diabetes. This issue might be a confounding factor, since some glucose-lowering drugs may have independent reno-protective effects in patients who had type 2 diabetes21,22. In a systematic review and meta-analysis, there was a high variability among 24 reported cystatin C-based equations of eGFR for diabetes patients13. Hence, the use of cystatin C as a biomarker for CKD in diabetes patients merits further investigation23, especially in Asian populations24,25. In our study, we investigated the association between cystatin C and incident CKD in patients with OGTT-determined prediabetes and normal glucose tolerance. Our results showed that prediabetes was independently associated with cystatin C (Table 2). Furthermore, a higher cystatin C was associated with a significantly greater risk of decline in eGFR to < 60 mL/min/1.73 m2 (Table 3), especially in those with prediabetes (Table 4). These findings suggest that prediabetes (vs. normal glucose tolerance) was associated with cystatin C, which in turn was related to a higher risk of incident CKD.
Type 2 diabetes patients were found to have significantly higher levels of serum cystatin C compared to healthy controls (standard mean difference 1.39, 95% CI 0.92 to 1.86, p < 0.001) in a meta-analysis12. Moreover, patients with type 2 diabetes had a higher velocity of increase in serum cystatin C26. Our patients with prediabetes had non-significantly lower eGFR (89.6 ± 13.3 vs. 92.5 ± 15.3, p = 0.225) and higher UACR (17.7 ± 82.7 vs. 10.4 ± 21.7, p = 0.470) than those with normal glucose tolerance (Table 1). Hence, cystatin C may have value as a biomarker of early renal damage in patients with prediabetes. In a previous cohort study, researchers determined CKD (eGFR < 60 mL/min/1.73 m2) using creatinine and cystatin C, and assessed risks of adverse outcomes (death, cardiovascular events, heart failure, and ESKD) in two racially diverse cohorts9. Among patients with decreased eGFR (< 60 mL/min/1.73 m2) according to creatinine but not cystatin C, the proportion of diabetes patients was around 12–13%. In contrast, the proportion was 18–19% among patients with decreased eGFR according to cystatin C but not creatinine. Moreover, adverse outcomes in patients with creatinine-determined CKD were limited to the subset who also had a low eGFR according to cystatin C. Taken together, these findings indicate that cystatin C could be a valuable biomarker for predicting adverse renal outcomes, especially in patients at high risk, such as those with prediabetes.
Our study had several limitations. First of all, the sample size was relatively small. Second, we used an arbitrary cutoff (≥ median vs. < median) to investigate the association of cystatin C with incident CKD. When cystatin C was treated as a continuous variable, its association with incident CKD remained significant (HR 1.002, 95% CI 1.001 to 1.004, p = 0.006 after multivariate adjustment, Table 3). Nevertheless, the optimal cutoff for its clinical use to determine risk of adverse renal outcomes deserves further investigation. We conducted cubic spline analysis, and observed that an increase in cystatin C was associated with a higher risk of incident CKD and all-cause mortality (p = 0.002, Fig. 2). In a post hoc analysis of pooled data from phase 3 randomized controlled trials, the correlation between creatinine-determined and cystatin C-determined eGFR was poor in patients with known diabetes27. Estimated GFR assessed by creatinine may be underestimated, and some patients may be misdiagnosed with CKD. We suggest that cystatin C is a clinically relevant indicator for assessing renal function and risk of adverse renal outcomes. More data are needed to justify its use in various populations at high risk of CKD.
In conclusion, we demonstrated that cystatin C was independently associated with incident CKD and all-cause mortality. Cystatin C may be used as a biomarker to predict risk of incident CKD in non-diabetes patients with an eGFR ≥ 60 mL/min/1.73 m2.
Data availability
Data is provided within the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Global National burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395, 709–733 (2020).
Levey, A. S. et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from kidney disease improving global outcomes. Kidney Int. 72, 247–259 (2007).
Matsushita, K. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375, 2073–2081 (2010).
Wen, C. P. et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371, 2173–2182 (2008).
Vanholder, R. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13, 393–409 (2017).
Bello, A. K. et al. Assess. Global Kidney Health Care Status JAMA 317, 1864–1881 (2017).
Coll, E. et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am. J. Kidney Dis. 36, 29–34 (2000).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl. J. Med. 367, 20–29 (2012).
Peralta, C. A. et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J. Am. Soc. Nephrol. 22, 147–155 (2011).
Krolewski, A. S. et al. Serum concentration of cystatin C and risk of end-stage renal disease in diabetes. Diabetes Care. 35, 2311–2316 (2012).
Stevens, P. E. & Levin, A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158, 825–830 (2013).
Ma, C. C., Duan, C. C., Huang, R. C. & Tang, H. Q. Association of circulating cystatin C levels with type 2 diabetes mellitus: a systematic review and meta-analysis. Arch. Med. Sci. 16, 648–656 (2019).
Cheuiche, A. V., Queiroz, M., Azeredo-da-Silva, A. L. F. & Silveiro, S. P. Performance of cystatin C-based equations for estimation of glomerular filtration rate in diabetes patients: A Prisma-Compliant systematic review and meta-analysis. Sci. Rep. 9, 1418 (2019).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 45, S17–S38 (2022).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Shlipak, M. G. et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl. J. Med. 352, 2049–2060 (2005).
Shlipak, M. G. et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann. Intern. Med. 145, 237–246 (2006).
Menon, V. et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann. Intern. Med. 147, 19–27 (2007).
Colhoun, H. M. & Marcovecchio, M. L. Biomarkers of diabetic kidney disease. Diabetologia 61, 996–1011 (2018).
DeFronzo, R. A., Reeves, W. B. & Awad, A. S. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 17, 319–334 (2021).
Nelson, A. J. et al. Incorporating SGLT2i and GLP-1RA for cardiovascular and kidney disease risk reduction: call for action to the cardiology community. Circulation 144, 74–84 (2021).
Machado, J. D. et al. Combined creatinine-cystatin C CKD-EPI equation significantly underestimates measured glomerular filtration rate in people with type 2 diabetes mellitus. Clin. Biochem. 53, 43–48 (2018).
Tsuda, A. et al. Poor glycemic control is a major factor in the overestimation of glomerular filtration rate in diabetic patients. Diabetes Care. 37, 596–603 (2014).
Li, H. X., Xu, G. B., Wang, X. J., Zhang, X. C. & Yang, J. M. Diagnostic accuracy of various glomerular filtration rates estimating equations in patients with chronic kidney disease and diabetes. Chin. Med. J. (Engl). 123, 745–751 (2010).
Wang, N. et al. Serum cystatin C trajectory is a marker associated with diabetic kidney disease. Front. Endocrinol. (Lausanne). 13, 824279 (2022).
Mende, C., Katz, A. & Cystatin, C. Creatinine-based estimates of glomerular filtration rate in Dapagliflozin phase 3 clinical trials. Diabetes Ther. 7, 139–151 (2016).
Funding
Our study was funded by Taichung Veterans General Hospital, Taichung, Taiwan [grant numbers TCVGH-1123502C and TCVGH-1133503C]. The funder was not involved in the study design, data collection, analysis, interpretation of the results, preparation of the article, or the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
GHL contributed to conceptualization, data curation, and investigation, and draft the original version of the manuscript. CHL contributed to data curation, investigation, and reviewed and edited the manuscript. JSW contributed to conceptualization, data curation, formal analysis, methodology, investigation, and draft the original version of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, GH., Lin, CH. & Wang, JS. Associations of cystatin C with incident chronic kidney disease and all-cause mortality in patients with normal glucose tolerance and prediabetes. Sci Rep 15, 23092 (2025). https://doi.org/10.1038/s41598-025-07159-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07159-3