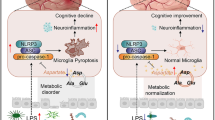
Abstract
The risk of cognitive impairment is markedly elevated in patients with type 2 diabetes mellitus (T2DM). While exercise has been shown to mitigate cognitive deficits associated with diabetes, the underlying mechanisms remain poorly understood. Recent studies suggest that exercise can modulate the composition of the gut microbiota, which, in turn, may influence the central nervous system via the microbiota–gut–brain axis. However, the specific role of gut microbiota in mediating exercise-induced improvements in cognitive function in T2DM remains unclear. In this study, we aimed to investigate whether exercise can alleviate cognitive impairment in T2DM mice by modulating the intestinal microbiota, and to elucidate the mechanisms underlying this effect. This study was conducted using male C57BL/6J mice. Mice fed a normal diet were assigned to the non-diabetic control group (NC), while those fed a high-fat diet were intraperitoneally injected with streptozotocin (STZ) and subsequently divided into the diabetic control group (DM), an exercise group (DM-EXE), and a fecal microbiota transplantation group (DM-FMT). The DM-EXE group underwent treadmill exercise for 8 weeks. During this period, the DM-FMT group received fecal microbiota transplants from the DM-EXE group for 2 consecutive days per week. Following the 8-week intervention, stool samples were collected for 16S rDNA high-throughput sequencing. The fear conditioning test was performed to assess cognitive function. Intestinal mucosa samples were collected to evaluate the expression of intestinal tight junction proteins. Additionally, the expression levels of synaptic proteins, glucose transporters, neurotrophic factors, and inflammatory markers were measured in the hippocampus. Our findings demonstrate that T2DM mice exhibit impaired cognitive function and significant alterations in their gut microbiota compared to non-diabetic controls. Exercise partially reversed these changes in the intestinal microbiota and alleviated cognitive impairment in T2DM mice. Additionally, transplantation of intestinal microbiota from exercised mice improved cognitive function in T2DM mice. Aerobic exercise may mitigate cognitive impairment in T2DM mice by modulating the gut microbiota. The underlying mechanisms appear to involve enhanced neural synaptic plasticity, reduced neuroinflammation, and improved neuronal glucose metabolism.
Similar content being viewed by others
Introduction
The prevalence of diabetes has been rising steadily in recent decades, and the International Diabetes Federation (IDF) predicts that by 2045, 693 million people worldwide will be affected by diabetess1. As a chronic disease, diabetes can lead to various complications. In addition to common conditions such as macrovascular disease, retinopathy, nephropathy, and peripheral neuropathy, the cognitive impairment associated with diabetes has gained increasing attention. Epidemiologically, individuals with type 2 diabetes mellitus (T2DM) are 2.8 times more likely to develop dementia compared to those without T2DM2. Hyperglycemia can impair cognitive function, and individuals with diabetes-related cognitive impairment often experience reduced self-management capabilities and poor treatment adherence, which exacerbates glycemic control and perpetuates a vicious cycle of cognitive decline3.
The pathophysiology of cognitive impairment in diabetes involves impaired hippocampal synaptic plasticity, decreased levels of neurotrophic factors, increased blood–brain barrier permeability, elevated neuroinflammation, and enhanced neuronal apoptosis4,5. Studies have shown that exercise can improve memory and learning in patients with diabetes6, and mechanisms underlying this benefit include enhanced insulin sensitivity, improved synaptic plasticity, reduced neuroinflammation, enhanced energy metabolism, and attenuation of Tau hyperphosphorylation and amyloid deposition6,7,8,9.
With advancing research on the gut microbiota, it has become clear that gut microbiota can influence the central nervous system, giving rise to the concept of the “gut–microbiota–brain axis.” This axis refers to the ability of gut microbiota to affect the central nervous system through the production of metabolic products and modulation of neural signaling, endocrine, and immune pathways10. Current evidence supports the involvement of gut microbiota in neuropsychiatric disorders such as depression, anxiety, cognitive impairment, neurodegenerative diseases, and cerebrovascular conditions11. Gene sequencing studies in diabetic patients have revealed that, compared to healthy individuals, diabetics exhibit reduced abundance of short-chain fatty acid-producing probiotic bacteria in the gut, while opportunistic pathogens are more prevalent12. Dysbiosis of the gut microbiota often leads to increased intestinal endotoxin levels and higher gut permeability, facilitating the entry of toxins into the bloodstream and triggering systemic inflammation13,14. These inflammatory mediators can communicate with the central nervous system via neural and humoral pathways, activating microglial cells and astrocytes. Activated glial cells can induce synaptic loss through phagocytosis and secrete pro-inflammatory cytokines, promoting neuronal apoptosis and ultimately impairing cognitive function15. A study demonstrated that germ-free mice exhibit cognitive decline after receiving fecal microbiota transplants from diabetic mice16, suggesting a direct role of gut microbiota in the onset and progression of diabetes-related cognitive impairment.
Numerous studies have indicated that exercise can modulate gut microbiota17,18. A clinical study reported that athletes show increased gut microbiota diversity and lower levels of systemic inflammation19. Additionally, evidence suggests that the gut microbiome may be a key mediator between exercise and cognitive function18,20. For example, exercise has been shown to improve gut microbiota imbalances induced by surgery and alleviate postoperative cognitive dysfunction in mice. Fecal microbiota transplantation from exercised to non-exercised mice improved the cognitive function of the recipient mice, indicating that exercise enhances cognitive recovery through modulation of the gut microbiota21. Another study found that sedentary obese mice showed improved cognitive function after receiving fecal microbiota transplants from exercise-trained obese mice22.
Given the variable effects of exercise on the gut microbiota across different animal models23, and the limited research on the impact of exercise on the gut microbiota in diabetes, the mechanisms by which exercise improves cognitive function in diabetes remain unclear. This study aims to investigate whether exercise modulates the gut microbiota in diabetic mice and whether this modulation contributes to improvements in cognitive function in T2DM.
Materials and methods
Animals
Five-week-old male C57BL/6J mice (19–22 g) were purchased from Gempharmatech Co., Ltd. (Jiangsu, China). The mice were housed in a controlled environment with a temperature of 22 °C ± 2 °C, relative humidity of 50% ± 5%, and a 12-h light/dark cycle. Mice had ad libitum access to food and water, and they were euthanized via cardiac puncture while under the effects of isoflurane anesthesia. All experimental procedures were approved by the Experimental Animal Management and Use Committee of Nanjing Medical University, and the study was conducted in accordance with the guidelines for laboratory animal use established by the National Institutes of Health.
Induction of T2DM and experimental design
The mice were randomly assigned to either the normal control group or the diabetic group. Both groups were initially fed a standard diet for 1 week. The normal control group continued on the standard diet, while the diabetic group was switched to a high-fat diet for 6 weeks. After 6 weeks of the high-fat diet, mice in the diabetic group were administered an intraperitoneal injection of streptozotocin (STZ; Sigma-Aldrich) at 120 mg/kg in 10 mg/mL citrate buffer (PH = 4.5)24. Mice in the control group were given an equivalent volume of citric acid buffer via the same route. Seven days post-injection, blood glucose levels were measured from tail blood samples. Mice with blood glucose levels ≥ 16.7 mmol/L were classified as diabetic and included in subsequent experiments25. The success rate for establishing the T2DM model was 85.2%, with 23 diabetic mice included in the study. The animals were then randomly assigned to one of the following experimental groups: (1) NC, normal control group (n = 9); (2) DM, diabetic control group (n = 8); (3) DM-EXE, diabetes with aerobic exercise training (n = 8); (4) DM-FMT, diabetes with fecal microbiota transplantation (n = 7).
Exercise protocol
Mice in the DM-EXE group underwent a 3-day acclimation period of treadmill exercise at a zero-degree slope, at a speed of 10 m/min for 15 min daily. Maximum speed (Smax) was determined by progressively increasing the treadmill speed from 8 m/min every 2 min until the mice could no longer maintain the pace for more than 10 s. The average Smax of all mice was calculated and used as the final speed. For formal training, mice initially ran at 40% of Smax for a 5-min warm-up, followed by 60–70% Smax for moderate-intensity exercise. Every 2 weeks, the Smax was retested and the exercise speed was adjusted accordingly. The exercise protocol lasted 8 weeks, with training 5 days per week7.
Fecal microbiota transplantation (FMT)
To deplete the gut microbiota prior to fecal microbiota transplantation, mice in the DM-FMT group were administered an intragastric mixture of antibiotics (vancomycin, 5 mg/mL; metronidazole, 10 mg/mL; cefazolin, 5 mg/mL) for 3 consecutive days26. Fecal samples were then collected from the DM-EXE group under aseptic conditions. Mice were gently restrained by holding the skin at the back of the neck with one hand, while the abdomen was gently massaged with the other hand to induce defecation. Once the mouse defecated, the feces were carefully collected using sterile forceps and placed into a sterile tube. For each sample, 1 small spoon of grinding beads was added to the tube, followed by 1 mL of sterile saline for every 100 mg of feces (approximately 5–6 pieces). The mixture was vortexed to achieve full homogenization, then centrifuged at 800×g for 3 min. Each mouse in the DM-FMT group was gavaged with 100 µL of the supernatant for 2 days per week over an 8-week period27.
Gut microbiota analysis
After the 8-week intervention, feces from all mice were collected as previously described and stored at − 80 °C until use. DNA extraction was performed using the Magnetic Soil and Stool DNA Kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions. DNA concentration and purity were assessed using 1% agarose gels. Based on the concentration, DNA was diluted to 1 ng/μL using sterile water. The V3–V4 hypervariable region of the bacterial 16S rRNA gene was amplified using the primers 341F-806R. All PCR reactions were conducted in 30 μL volumes, containing 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM of forward and reverse primers, and approximately 10 ng of template DNA. PCR products were pooled in equidensity ratios. The pooled PCR products were then purified using the AxyPrep DNA Gel Extraction Kit (AXYGEN). Sequencing libraries were generated using the NEBNext® Ultra™ DNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer’s instructions, and index codes were added. Library quality was assessed using the Qubit® 2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on the Illumina NovaSeq 6000 platform, generating 250-bp paired-end reads. Paired-end reads from the original DNA fragments were merged using FLASH. Sequence analysis was performed using the UPARSE software package with the UPARSE-OTU and UPARSE-OUT ref algorithms. In-house Perl scripts were used to analyze alpha (within-sample) and beta (between-sample) diversity. Sequences with ≥ 97% similarity were grouped into operational taxonomic units (OTUs). Representative sequences for each OTU were selected, and taxonomic classification was performed using the RDP classifier. α-diversity was assessed using the Chao1, Observed species, Simpson, and Shannon indices. Principal coordinate analysis (PCoA) was employed to assess β-diversity. To identify differences in microbial communities between groups, ADONIS tests were performed based on Bray–Curtis dissimilarity distance matrices.
Fear conditioning (FC) test
Fear conditioning (FC) is commonly used to assess associative memory in rodents28. The experiment consists of training and testing phases. During the training phase, mice were placed in a conditioning chamber (FCT-100, Chengdu Techman Software Co., Ltd., China) to freely explore for 3 min. Following this, a sound stimulus (80 dB, 30 s) was introduced, immediately followed by an electric foot shock (0.35 mA, 2 s). This sequence was repeated 10 times. Repeated exposure to both acoustic and electrical stimuli led the mice to associate them with conditioned fear. The testing phase occurred 24 h after training. Mice were reintroduced to the same conditioning chamber, where only the sound stimulus (80 dB, 30 s) was presented. Freezing behavior was recorded for 5 min using a video analysis system.
Western blotting
Colonic mucosa and hippocampal tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer (1X) containing protein phosphatase inhibitors. The homogenates were then centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatant was collected. Protein concentrations were quantified using a BCA protein assay kit (Beyotime, China). Protein samples were separated using SDS–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, USA), which was incubated overnight at 4 °C with the following primary antibodies: β-actin (1:1000; AiFang Biological, China), PSD95 (1:1000; Proteintech, USA), SYN (1:1000; Abcam, UK), BDNF (1:1000; Abcam, UK), GDNF (1:1000; Abcam, UK), GLUT1 (1:1000; Servicebio, China), GLUT4 (1:1000; Cell Signaling, USA), ZO1 (1:1000; Servicebio, China), and Occludin (1:1000; Servicebio, China). After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. Bands were visualized using enhanced chemiluminescence, and the intensity was quantitatively analyzed using ImageJ software.
Enzyme-linked immunosorbent assay (ELISA)
Hippocampal tissue was homogenized, and the total protein concentration was quantified using the BCA assay as described above. The levels of IL-6 and TNF-α were measured according to the manufacturer’s instructions using ELISA kits (MULTI SCIENCES, China). The amounts of these inflammatory cytokines were normalized to the total protein content in each sample and expressed as pg/mg protein.
Hematoxylin and eosin (H&E) staining
Skeletal muscle paraffin sections were dewaxed in xylene twice, each for 10 min. They were then dehydrated in 100% ethanol for two cycles (5 min each), followed by dehydration in 90%, 80%, and 70% ethanol (5 min each). The sections were rinsed in deionized water for 2 min. After removing excess water, hematoxylin solution was applied dropwise to the tissue and stained for 5 min, followed by rinsing with deionized water. The tissue was differentiated using 1% hydrochloric acid–ethanol solution for 2 s, then immediately rinsed with deionized water. The tissue was counterstained in deionized water for 3 min, followed by eosin staining for 40 s. After rinsing with deionized water, the sections were dehydrated in a series of alcohol solutions (70%, 80%, 90%, and 100% ethanol, 2 min each). The sections were then cleared in xylene twice, each for 2 min, and mounted with neutral resin. The skeletal muscle cross-sectional area was quantified using ImageJ software.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26.0 software, and graphical representations were generated using GraphPad Prism 9.4.1. Data are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for comparisons between groups, with p < 0.05 considered statistically significant.
Results
Exercise had no significant effect on random blood glucose and body weight in T2DM mice
Following the successful establishment of the T2DM mouse model, mice were subjected to different groupings and interventions. Weekly measurements of body weight and random blood glucose were performed for all mice. As shown in Fig. 1, compared to the NC group, the DM group exhibited a reduction in body weight and a significant increase in random blood glucose levels. Exercise led to a decrease in weight among T2DM mice but did not significantly alter random blood glucose levels. Fecal microbiota transplantation (FMT) also failed to induce significant changes in body weight or random blood glucose in T2DM mice.
Exercise increased the cross-sectional area of skeletal muscle in T2DM mice
To investigate the impact of exercise on skeletal muscle in T2DM mice, we performed H&E staining to observe muscle morphology and conducted a statistical analysis of the cross-sectional area of skeletal muscle. Compared to the NC group, the DM group showed a trend toward a decreased cross-sectional area of skeletal muscle, although this difference was not statistically significant. However, in the DM-EXE group, the cross-sectional area of skeletal muscle was significantly increased compared to the DM group. The DM-FMT group showed a trend of increased cross-sectional area, though this difference was not statistically significant (Fig. 2). These findings suggest that exercise can enhance skeletal muscle mass in T2DM mice.
Skeletal muscle fibers in T2DM were thickened after exercise. (a) Hematoxylin and eosin (H&E) staining of muscle tissues at the end of the 8-week experimental period. (b) Comparison of average normalized cross-sectional area of gastrocnemius muscle fibers. Normalization was based on the mean cross-sectional area of the NC group. ####P < 0.0001 versus DM group.
Exercise or fecal microbiota transplantation improved cognitive function in T2DM mice
Previous studies have demonstrated that exercise can improve cognitive function in diabetic mice7,29,30. In this study, an exercise protocol was adopted to assess its effect on cognitive function. After the 8-week intervention, cognitive function was evaluated using the fear conditioning test. The greater the number of freezing episodes and the percentage of freezing duration, the stronger the memory capabilities of the mice. Our results indicated that, compared to the NC group, both freezing time and the percentage of freezing duration were significantly reduced in the DM group, suggesting that type 2 diabetes impairs cognitive function. In contrast, the DM-EXE and DM-FMT groups exhibited significantly increased freezing times and freezing percentages compared to the DM group, indicating that either exercise or fecal microbiota transplantation can alleviate cognitive impairment in T2DM mice (Fig. 3).
The effect of exercise on the microbiota of T2DM mice
To further investigate the impact of exercise on the gut microbiota, we performed 16S rDNA sequencing analysis. α-diversity reflects the richness and diversity of microbial species within a single sample. Among the indices used to measure α-diversity, Chao1 and Observed species reflect species richness, while Simpson and Shannon reflect species diversity. Our analysis revealed no significant differences in these indices among the groups (Chao1, p = 0.553; Observed species, p = 0.386; Simpson, p = 0.759; Shannon, p = 0.990) (Fig. 4a–d). This suggests that neither diabetes nor exercise had a significant effect on the overall richness and diversity of the gut microbiota.
Analysis of gut microbiota. α diversity analysis was shown as the comparison of Chao1 index (a), Observed species (b), Simpson (c), and Shannon index (d). E-I PCoA plot established based on the weighted UniFrac distance was used to analyze βdiversity: (e) Comparison among four groups; (f) Comparison between NC group and DM group; (g) Comparison between DM group and DM-EXE group; (h) Comparison between DM group and DM-FMT group; (i) Comparison between DM-EXE group and DM-FMT group. (j–p) Comparison of intestinal flora abundance at different levels: (j–m) Phylum level; (n) Order level; (o) Family level; (p) genus level. *P < 0.05, **P < 0.005, ****P < 0.0001 versus NC group. #P < 0.05, ##P < 0.005 versus DM group.
β-diversity, which reflects the similarities and differences in microbial community structures between samples, was analyzed using principal coordinate analysis (PCoA) based on the weighted UniFrac distance. This analysis revealed significant differences in the microbiota structures among the four groups (Fig. 4e). The DM group and the NC group were clustered in distinct regions, with a significant difference in microbiota composition between the two groups (p = 0.001) (Fig. 4f), indicating that diabetes induces substantial changes in the gut microbiota. Moreover, the microbiota structure of the DM group significantly differed from that of both the DM-EXE group (p = 0.022) (Fig. 4g) and the DM-FMT group (p = 0.008) (Fig. 4h), suggesting that exercise can alter the gut microbiota composition in T2DM mice. Interestingly, the microbiota structure between the DM-EXE and DM-FMT groups was similar (p = 0.401) (Fig. 4i), suggesting that fecal microbiota transplantation was successful in inducing changes similar to those observed with exercise.
Further analysis of the gut microbiota composition at various taxonomic levels revealed significant changes after exercise. At the phylum level, Firmicutes and Bacteroidetes were the dominant phyla. Compared to the NC group, the DM group showed an increased relative abundance of Firmicutes and a decreased relative abundance of Bacteroidetes, resulting in an elevated Firmicutes-to-Bacteroidetes ratio. These changes were partially reversed by exercise. Additionally, the relative abundance of Actinobacteria was reduced in the DM group, with a trend toward increased abundance after exercise (Fig. 4j–m). At the order level, the DM group showed an increased relative abundance of Erysipelotrichales and Clostridiales, while the relative abundance of Bacteroidales was reduced. After exercise, these trends were reversed (Fig. 4n). At the family level, T2DM led to a higher relative abundance of Erysipelotrichaceae, concurrent with a reduction in the abundance of Muribaculaceae (Fig. 4o). At the genus level, the DM group exhibited an increased relative abundance of Dubosiella, Romboutsia, Faecalibaculum, and Lachnospiraceae, whereas the relative abundance of Ruminococcaceae.UCG.014 was decreased. Exercise partially reversed these changes (Fig. 4p).
Exercise improved intestinal barrier in T2DM mice through gut microbiota
Diabetes has been shown to decrease the expression of tight junction proteins between endothelial cells, leading to increased intestinal permeability. The gut microbiota plays a role in modulating intestinal barrier function31. To explore whether exercise could affect intestinal permeability through the gut microbiota, we assessed the expression of tight junction proteins ZO1 and Occludin in the colonic mucosa using Western blotting. Our results demonstrated that compared to the NC group, expression levels of ZO1 and Occludin were significantly reduced in the DM group. However, both tight junction proteins were significantly upregulated in the DM-EXE and DM-FMT groups compared to the DM group (Fig. 5a, b). These findings suggest that diabetes increases intestinal barrier permeability in mice, and that exercise, potentially through alterations in the gut microbiota, can reduce this permeability.
The exploration on the mechanism of exercise alleviating cognitive impairment in T2DM mice through microbiota. (a,b) Western blot analysis for tight junction proteins (Occludin and ZO1) and their quantification in the colonic mucosa. Proinflammatory factors IL-6 (c) and TNF-α (d) in the hippocampus detected by ELISA. (e,f) Western blot analysis for neurotrophic factors (BDNF and GDNF), synaptic proteins (SYN and PSD95), and (g,h) glucose transporter (GLUT1 and GLUT4) in the hippocampus. The membrane images were cropped from different membranes to remove irrelevant parts (Supplementary Fig. S1). *P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001 versus NC group. #P < 0.05, ##P < 0.005, ###P < 0.001, ####P < 0.0001 versus DM group.
Exercise reduces inflammation in the hippocampus of T2DM mice through gut microbiota
Chronic low-grade systemic inflammation is a hallmark pathophysiological feature of diabetes, and neuroinflammation plays a critical role in the onset and progression of cognitive impairment in diabetes32. Increased intestinal permeability allows endotoxins from the gut to enter the bloodstream, which can drive systemic inflammation13. We have previously demonstrated that exercise can reduce intestinal barrier permeability through modulation of the gut microbiota. To investigate whether this reduction in intestinal permeability also alleviates neuroinflammation, we measured the levels of the pro-inflammatory cytokines IL-6 and TNF-α in the hippocampus of T2DM mice using ELISA. Our results revealed that the expression levels of both IL-6 and TNF-α were significantly elevated in the DM group compared to the NC group. Exercise (DM-EXE) and fecal microbiota transplantation (DM-FMT) both significantly reduced the levels of these inflammatory cytokines compared to the DM group (Fig. 5c, d). In summary, hippocampal inflammation was elevated in T2DM mice, and exercise can reduce neuroinflammation in these mice through the modulation of the gut microbiota.
Exercise upregulated the expression of hippocampal neurotrophic factors and synaptic proteins in T2DM mice through gut microbiota
Neurotrophic factors are essential for neuronal proliferation, differentiation, maturation, and synaptic plasticity, which underpins learning and memory processes33. BDNF and GDNF are synthesized and secreted primarily by astrocytes. A decrease in their levels in the hippocampus leads to increased vulnerability of neuronal cells to damage, which constitutes one of the mechanisms underlying diabetic encephalopathy34. Presynaptic proteins such as SYN and postsynaptic proteins like PSD-95 play crucial roles in synaptic plasticity, which is closely linked to cognitive functions35. Zhang Y et al. found that diabetic rats exhibit upregulated expression levels of SYN and PSD-95 in the hippocampus following exercise, with concomitant improvement in cognition8. In this study, we aimed to determine whether exercise improves cognitive functions by upregulating the expression of neurotrophic factors BDNF (Brain-derived neurotrophic factor), GDNF (Glial cell line-derived neurotrophic factor), and synaptic proteins PSD95 and SYN in the hippocampus, potentially through modulation of the gut microbiota. Our results showed that the expression levels of neurotrophic factors and synaptic proteins were significantly reduced in the DM group compared to the NC group. However, both the DM-EXE and DM-FMT groups exhibited increased expression of these factors and proteins relative to the DM group (Fig. 5e, f). These findings suggest that diabetes leads to a decrease in neurotrophic factors and synaptic protein expression in the hippocampus, and exercise can enhance their expression via gut microbiota modulation, thereby improving synaptic plasticity and cognitive function.
Exercise upregulated the expression of glucose transporter proteins in the hippocampus of T2DM mice through gut microbiota
Glucose transporters, GLUT1 and GLUT4, play pivotal roles in glucose uptake and neuronal energy metabolism. GLUT1 is primarily expressed in the endothelial cells of the blood–brain barrier (BBB), and its reduced expression can lead to increased BBB permeability36,37. Chronic hyperglycemia, a hallmark of diabetes, can cause endothelial dysfunction, which increases BBB permeability, promotes neuroinflammation, and contributes to cognitive decline32. Moreover, GLUT1 plays a critical role in cerebral glucose homeostasis, with its dysfunction being mechanistically linked to the pathophysiology of diabetic encephalopathy through impaired blood–brain barrier glucose transport. Si Shi et al. found that T2DM mice exhibited significantly reduced GLUT1 expression in the hippocampus, accompanied by impaired cognitive function34. GLUT4, mainly expressed in neurons, is crucial for neuronal energy metabolism, and its downregulation may impair neuronal plasticity and cognitive function in diabetes38. In this study, we measured the expression levels of GLUT1 and GLUT4 in the hippocampus of T2DM mice using Western blotting. The results showed that GLUT1 expression was significantly decreased in the DM group compared to the NC group. Exercise (DM-EXE) and FMT (DM-FMT) groups showed a significant increase in GLUT1 expression compared to the DM group. However, the expression of GLUT4 did not significantly decrease in the DM group compared to the NC group. Nonetheless, GLUT4 expression was significantly increased in both the DM-EXE and DM-FMT groups (Fig. 5g, h). These findings suggest that exercise may reduce BBB permeability and improve neuronal energy metabolism through modulation of the gut microbiota.
Correlation analysis between microbiota and detected indicators
To explore the relationship between the gut microbiota and the measured biomarkers of cognitive function and inflammation, we performed Pearson correlation analysis and presented the results as a heatmap. PSD95 was negatively correlated with Firmicutes, Clostridiales, Romboutsia, and Faecalibaculum, but positively correlated with Bacteroidetes, Bacteroidales, and Muribaculaceae. Similarly, Occludin, ZO1, and GLUT1 were negatively correlated with Clostridiales, Romboutsia, and Faecalibaculum, but positively correlated with Muribaculaceae. BDNF was positively correlated with Muribaculaceae but negatively correlated with Faecalibaculum. TNF-α showed a positive correlation with the Firmicutes/Bacteroidetes ratio (F/B) and Faecalibaculum, but a negative correlation with Muribaculaceae. Finally, IL-6 was positively correlated with Clostridiales and Romboutsia (Fig. 6).
Discussion
The human gut harbors trillions of microorganisms, with bacterial concentrations reaching up to 1011–1012 per milliliter of intestinal content38. Over the past decade, advances in sequencing technology and data analysis have significantly enhanced our understanding of the gut microbiota, revealing its intricate relationships with various body organs, including the brain39. In particular, the gut microbiota can influence the central nervous system (CNS) through the production of metabolic by-products and the modulation of neural signaling, endocrine pathways, and immune responses10. Based on these insights, we hypothesize that altering the gut microbiota composition may help alleviate cognitive impairments associated with diabetes. Previous studies have demonstrated that exercise can improve cognitive function in diabetes7,8,33, although the underlying mechanisms remain incompletely understood. It has been shown that exercise can modulate the gut microbiota17,18, and we hypothesize that the gut microbiota serves as an intermediary through which exercise improves cognitive function in T2DM.
In our study, no significant differences in α-diversity were observed among the experimental groups, indicating that neither diabetes nor exercise induced alterations in the overall species abundance of the gut microbiota in mice. However, significant disparities in β-diversity were detected, suggesting that diabetes or exercise modified the structural composition of microbial communities and differentially influenced the abundance of specific bacterial taxa. We observed that the relative abundance of Firmicutes was increased, while Bacteroidetes decreased in the intestines of T2DM mice, leading to a higher Firmicutes/Bacteroidetes (F/B) ratio. The F/B ratio has been associated with obesity and metabolic disorders in both animal and human studies40,41. An increased F/B ratio enhances intestinal energy absorption, contributing to body fat accumulation and obesity, a well-established risk factor for T2DM. Additionally, an elevated F/B ratio has been linked to cognitive decline. One study found that the F/B ratio was higher in elderly mice with cognitive impairment42. and a similar trend was observed in Alzheimer’s disease models43. In our study, we also found a negative correlation between the F/B ratio and cognitive function. The mechanism by which an increased F/B ratio may contribute to cognitive decline involves increased permeability of the blood–brain barrier (BBB) and intestinal barrier, along with heightened systemic inflammation. Our findings revealed a positive correlation between the F/B ratio and TNF-α, aligning with clinical observations44. Furthermore, we observed a negative correlation between the F/B ratio and intestinal tight junction proteins and glucose transporter proteins involved in BBB function. Previous studies have also shown that an increased F/B ratio is positively correlated with BBB permeability in aging mice42. The increased BBB permeability in diabetes may allow inflammatory factors to enter the CNS, exacerbating neuroinflammation and contributing to cognitive dysfunction. Interestingly, we found that exercise reduced the F/B ratio in T2DM mice, which is consistent with findings in other animal models21,45. This effect of exercise on the F/B ratio may be mediated by the reduction in bile acid secretion. A study found that physical activity negatively correlates with fecal bile acid content46, and the addition of bile acids to the diet of rats can increase Firmicutes abundance while decreasing Bacteroidetes47.
Additionally, we observed that exercise led to an increase in the abundance of butyrate-producing bacteria such as Ruminococcaceae and Bacteroidales in the intestines of T2DM mice, as well as increased expression of intestinal tight junction proteins. The presence of butyrate, a primary metabolite of these bacteria, plays a crucial role in maintaining intestinal barrier integrity. Butyrate activates AMPK, enhancing the assembly of tight junction proteins between intestinal epithelial cells45,48. Butyrate is the primary metabolic substrate for colonic epithelial cells, providing at least 60–70% of the energy needed for their proliferation and differentiation49. Therefore, exercise may improve cognitive function by increasing the abundance of butyrate-producing bacteria, reducing gut barrier permeability, and decreasing neuroinflammation.
BDNF, the most important neurotrophic factor in the brain, regulates synaptic transmission and plasticity through activation of the Trk receptor. It is considered a key factor in learning, behavior, motor function, memory, and various stress responses50,51. Synaptic proteins are essential for synaptic signal transduction and plasticity52, and BDNF is known to upregulate the expression of synaptic proteins such as PSD95 and SYN53. In this study, exercise increased the expression of BDNF, PSD95, and SYN in T2DM mice. Interestingly, the expression of these proteins also increased in the hippocampus of diabetic mice that did not undergo exercise, following fecal microbiota transplantation. It has been reported that butyrate can promote histone hyperacetylation, leading to increased BDNF expression54,55. As previously mentioned, we found that exercise can increase the abundance of Bacteroidales producing butyrate in the intestines of T2DM mice, and correlation analysis shows that Bacteroidales is positively correlated with BDNF and synaptic proteins. Therefore, we speculate that the reason why exercise causes an increase in the expression of BDNF and synaptic proteins in the hippocampus of diabetic mice may be due to the fact that exercise increases the abundance of butyrate-producing bacteria in the intestine, leading to an increase in butyrate production. This, in turn, enhances synaptic transmission and plasticity, leading to an overall improvement in cognitive function.
Erysipelotrichaceae has been linked to energy metabolism disorders56. In our study, the relative abundance of Erysipelotrichaceae was elevated in the intestines of T2DM mice, but this abundance decreased following exercise. Additionally, we found that Erysipelotrichaceae was negatively correlated with GLUT1. GLUT1 is predominantly expressed in astrocytes, where it influences both the permeability of the blood–brain barrier (BBB) and neuronal energy metabolism. Astrocytes use GLUT1 to transfer glucose from the vascular system to the synapse, thus supporting the energy needs of neurons57. Therefore, we speculate that one of the mechanisms through which exercise improves cognitive function in T2DM mice is by modulating the gut microbiota, which, in turn, enhances hippocampal energy metabolism.
In summary, our results demonstrate that T2DM can alter the intestinal microbiota, impair the intestinal barrier, promote neuroinflammation, reduce the expression of synaptic proteins and glucose transporters in the hippocampus, and lead to cognitive decline. Exercise can improve cognitive function in T2DM mice and partially reverse the intestinal microbiota alterations induced by diabetes. Additionally, fecal microbiota transplantation from diabetic exercise mice to non-exercise diabetic mice also improved cognitive function, suggesting that exercise can enhance cognitive function in T2DM mice through gut microbiota modulation. The underlying mechanisms likely involve reducing gut and BBB permeability, alleviating neuroinflammation, and improving synaptic plasticity and glucose metabolism. While the role of exercise in mitigating diabetic cognitive dysfunction is well-documented, the specific involvement of the gut microbiota has not been explored in depth. This study is the first to confirm the contribution of gut microbiota to exercise-induced improvements in diabetic cognitive dysfunction, identifying altered microbiota profiles that could serve as a foundation for future therapeutic interventions, such as gut microbiota transplantation. Given our findings demonstrating exercise-induced modulations of specific microbial taxa and associated cognitive improvements, these results propose a translational strategy to formulate microbial consortia capsules containing these targeted bacteria. Oral administration of such capsules may ameliorate diabetes-associated cognitive decline. Furthermore, therapeutic supplementation with microbial metabolites (e.g., butyrate) derived from these bacterial communities could be explored as potential interventions for diabetic cognitive dysfunction. However, there are limitations to our study. Although we demonstrated that fecal microbiota transplantation alleviates cognitive impairment in T2DM mice, we have not directly verified the specific role of the differential bacteria. Future research could focus on investigating the role of these specific microbiota in cognitive function through targeted microbiota transplantation. Furthermore, the precise mechanisms by which these bacteria influence cognitive function in T2DM could be explored by knocking out relevant genes.
Data availability
Data are available upon reasonable request from the corresponding author.
Change history
30 January 2026
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-33042-2
Abbreviations
- DM:
-
Diabetic control group
- DM-EXE:
-
Diabetes with aerobic exercise training
- DM-FMT:
-
Diabetes with fecal microbiota transplantation
- IDF:
-
The International Diabetes Federation
- STZ:
-
Streptozotocin
- Smax :
-
The maximum speed
- FMT:
-
Fecal microbiota transplantation
- PCoA:
-
Principal coordinate analysis
- FC:
-
Fear conditioning
- RIPA:
-
Radioimmunoprecipitation
- PVDF:
-
Polyvinylidene fluoride
- ELISA:
-
Enzyme-linked immunosorbent assay
- H&E:
-
Hematoxylin and eosin
- F/B:
-
Ratio of Firmicutes/Bacteroidetes
References
Cho, N. H. et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281 (2018).
Schnaider Beeri, M. et al. Diabetes mellitus in midlife and the risk of dementia 3 decades later. Neurology 63(10), 1902–1907 (2004).
Cai, Y.-H., Wang, Z., Feng, L.-Y. & Ni, G.-X. Effect of exercise on the cognitive function of older patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Hum. Neurosci. 16, 876935 (2022).
Ho, N., Sommers, M. S. & Lucki, I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci. Biobehav. Rev. 37(8), 1346–1362 (2013).
Van Dyken, P. & Lacoste, B. Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Front. Neurosci. 12, 930 (2018).
Wang, Q. et al. Aerobic exercise improves synaptic-related proteins of diabetic rats by inhibiting FOXO1/NF-κB/NLRP3 inflammatory signaling pathway and ameliorating PI3K/Akt insulin signaling pathway. J. Mol. Neurosci. 69(1), 28–38 (2019).
Lang, X. et al. Treadmill exercise mitigates neuroinflammation and increases BDNF via activation of SIRT1 signaling in a mouse model of T2DM. Brain Res. Bull. 165, 30–39 (2020).
Zhang, Y. et al. Treadmill training attenuate STZ-induced cognitive dysfunction in type 2 diabetic rats via modulating Grb10/IGF-R signaling. Brain Res. Bull. 181, 12–20 (2022).
Shima, T. et al. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 60(3), 597–606 (2017).
Cryan, J. F. et al. The microbiota–gut–brain axis. Physiol. Rev. 99(4), 1877–2013 (2019).
Zhu, S. et al. The progress of gut microbiome research related to brain disorders. J. Neuroinflam. 17(1), 25 (2020).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490(7418), 55–60 (2012).
Cani, P. D. et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50(11), 2374–2383 (2007).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56(7), 1761–1772 (2007).
Walker, K. A., Ficek, B. N. & Westbrook, R. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem. Neurosci. 10(8), 3340–3342 (2019).
Yu, F. et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in streptozotocin-induced diabetic mice. Aging (Albany NY) 11(10), 3262–3279 (2019).
Campbell, S. C. et al. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PLoS ONE 11(3), e0150502 (2016).
Kang, S. S. et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 9, 36 (2014).
Clarke, S. F. et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63(12), 1913–1920 (2014).
Gubert, C., Kong, G., Renoir, T. & Hannan, A. J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 134, 104621 (2020).
Lai, Z. et al. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol. Psychiatry 26(12), 7167–7187 (2021).
Li, R. et al. Microbiota from exercise mice counteracts high-fat high-cholesterol diet-induced cognitive impairment in C57BL/6 mice. Oxid. Med. Cell. Longev. 2023, 2766250 (2023).
Cerdá, B. et al. Gut microbiota modification: Another piece in the puzzle of the benefits of physical exercise in health?. Front. Physiol. 7, 51 (2016).
Wang, X. et al. Diabetic cognitive dysfunction is associated with increased bile acids in liver and activation of bile acid signaling in intestine. Toxicol. Lett. 287, 10–22 (2018).
Xu, L. et al. miR-125a-5p ameliorates hepatic glycolipid metabolism disorder in type 2 diabetes mellitus through targeting of STAT3. Theranostics 8(20), 5593–5609 (2018).
Parker, A. et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome 10(1), 68 (2022).
Lu, X.-Y. et al. Pomegranate peel extract ameliorates the severity of experimental autoimmune encephalomyelitis via modulation of gut microbiota. Gut Microb. 12(1), 1857515 (2020).
Zheng, Q. et al. Trisomy 21-induced dysregulation of microglial homeostasis in Alzheimer’s brains is mediated by USP25. Sci. Adv. 7(1), 1eabe340 (2021).
Lin, L. et al. Aerobic exercise improves type 2 diabetes mellitus-related cognitive impairment by inhibiting JAK2/STAT3 and enhancing AMPK/SIRT1 pathways in mice. Dis. Mark. 2022, 6010504 (2022).
de Senna, P. N. et al. Physical exercise reverses spatial memory deficit and induces hippocampal astrocyte plasticity in diabetic rats. Brain Res. 1655, 242–251 (2017).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19(1), 55–71 (2021).
Xu, Y., Zhou, H. & Zhu, Q. The impact of microbiota–gut–brain axis on diabetic cognition impairment. Front. Aging Neurosci. 9, 106 (2017).
Bertram, S., Brixius, K. & Brinkmann, C. Exercise for the diabetic brain: how physical training may help prevent dementia and Alzheimer’s disease in T2DM patients. Endocrine 53(2), 350–363 (2016).
Shi, S. et al. Studies of pathology and pharmacology of diabetic encephalopathy with KK-Ay mouse model. CNS Neurosci. Ther. 26(3), 332–342 (2019).
Liu, Y. et al. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in Mice. J. Neuroinflamm. 15(1), 112 (2018).
Winkler, E. A. et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 18(4), 521–530 (2015).
Koepsell, H. Glucose transporters in brain in health and disease. Pflugers Arch. 472(9), 1299–1343 (2020).
Hu, X., Wang, T. & Jin, F. Alzheimer’s disease and gut microbiota. Science China. Life Sci. 59(10), 1006–1023 (2016).
Sommer, F., Anderson, J. M., Bharti, R., Raes, J. & Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 15(10), 630–638 (2017).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U.S.A. 102(31), 11070–11075 (2005).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: Human gut microbes associated with obesity. Nature 444(7122), 1022–1023 (2006).
Hoffman, J. D. et al. Age drives distortion of brain metabolic, vascular and cognitive functions, and the gut microbiome. Front. Aging Neurosci. 9, 298 (2017).
Brandscheid, C. et al. Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. J. Alzheimers Dis. 56(2), 775–788 (2017).
Verdam, F. J. et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 21(12), E607–E615 (2013).
Evans, C. C. et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 9(3), e92193 (2014).
Wertheim, B. C. et al. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol. Biomark. Prev. 18(5), 1591–1598 (2009).
Islam, K. B. M. S. et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141(5), 1773–1781 (2011).
Mitchell, C. M. et al. Does exercise alter gut microbial composition? A systematic review. Med. Sci. Sports Exerc. 51(1), 160–167 (2019).
Tan, J. et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119 (2014).
Jamali, A., Shahrbanian, S. & Morteza, T. S. The effects of exercise training on the brain-derived neurotrophic factor (BDNF) in the patients with type 2 diabetes: A systematic review of the randomized controlled trials. J. Diabetes Metab. Disord. 19(1), 633–643 (2020).
Huang, E. J. & Reichardt, L. F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003).
Feng, W. & Zhang, M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat. Rev. Neurosci. 10(2), 87–99 (2009).
Leal, G., Comprido, D. & Duarte, C. B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76(Pt C), 639–656 (2014).
Waldecker, M., Kautenburger, T., Daumann, H., Busch, C. & Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 19(9), 587–593 (2008).
Sleiman, S. F. et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 5, e15092 (2016).
Chen, W., Liu, F., Ling, Z., Tong, X. & Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 7(6), e39743 (2012).
Müller, M. S., Fouyssac, M. & Taylor, C. W. Effective glucose uptake by human astrocytes requires its sequestration in the endoplasmic reticulum by glucose-6-phosphatase-β. Curr. Biol. 28(21), 3481–3486 (2018).
Acknowledgements
We are very grateful to all the researchers who participated in this study.
Funding
This study was supported by grants from the Science and Technology Project of Changzhou Health Commission (ZD202317, ZD202434) and the Clinical Research Project of Changzhou Medical Center of Nanjing Medical University (CMCC202310).
Author information
Authors and Affiliations
Contributions
Sp.R performed experiments, gathered and analyzed data, and drafted manuscripts. J.L designed research and conducted experiments. Xq.Y designed research and conducted experiments. Xh.Y secured funding and refined draft. Q.Z designed research, conducted experiments, and refined draft. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethics Committee of The Second People’s Hospital of Changzhou.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in Figure 5 and in the Funding statement. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ruan, S., Liu, J., Yuan, X. et al. Aerobic exercise alleviates cognitive impairment in T2DM mice through gut microbiota. Sci Rep 15, 23917 (2025). https://doi.org/10.1038/s41598-025-07220-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07220-1
Keywords
This article is cited by
-
The effect of different exercise interventions on global cognitive function in patients with type 2 diabetes: a systematic review and network meta-analysis
BMC Public Health (2026)
-
Interactions Between Gut Microbiota and Brain: Possible Effects on Sport Performance
Journal of Science in Sport and Exercise (2025)