Abstract
Emerging tick-borne infections pose public health challenges and may complicate treatment decisions. The EMBio study, a multicenter observational study, aims to describe erythema migrans (EM), an early localized manifestation of Borrelia burgdorferi sensu lato (s.l.) infection, and investigate the occurrence of tick-borne co-infections among patients presenting with this skin lesion. Additionally, the study seeks to determine relations between EM morphology, other clinical manifestations, specific pathogens, and disease prognosis. Clinical characteristics, skin biopsies, and blood samples were analyzed from 26 patients to assess co-infections, quantity, Borrelia species, and spirochete load. Borrelia DNA was detected in 88% of EM skin lesions, with Borrelia afzelii as the predominant species. Two cases of co-infections were identified, one involving two Borrelia species and one involving Borrelia afzelii and the intracellular bacterium Neoehrlichia mikurensis. Notably, homogeneous EM lesions harbored significantly higher spirochete quantities in the central zone compared to annular lesions, suggesting that lesion morphology reflects local bacterial density. This supports the value of molecular diagnostics in detecting mixed infections and supports morphology-guided biopsy strategies in the clinical assessment of cutaneous infections. This study contributes to a better understanding of co-infection dynamics and may improve diagnostic accuracy and patient management in endemic settings.
Similar content being viewed by others
Introduction
Tick-borne human pathogens are highly prevalent in ticks from Sweden1. Ticks can carry multiple pathogens, which may be simultaneously transmitted to humans through tick bites. The EMBio study, a multicenter observational study, aims to describe erythema migrans (EM), an early localized manifestation of Borrelia burgdorferi sensu lato (s.l.) infection, and investigate the occurrence of tick-borne co-infections among patients presenting with this skin lesion. Additionally, the study seeks to determine relations between EM morphology, other clinical manifestations, specific pathogens, and disease prognosis.
Two out of 26 EM patients had co-infections (8%, Table 1) detected by PCR. One patient had co-infection with B. afzelii and B. garinii, both detectable by PCR in the obtained skin biopsies. The patient had a homogeneous EM on one leg and reported fatigue and vertigo upon inclusion in the study. The co-infection was suspected due to multiple signals in the electropherogram and confirmed through RipSeq Mixed sequence analysis. Only B. garinii was detected in the central zone of the EM, whereas both B. afzelii and B. garinii were detected in the border zone. Another patient, who had an EM on the abdomen and tested positive for B. afzelii in the skin biopsy, was also positive for Neoehrlichia mikurensis in the blood at the time of study inclusion. This patient did not report any clinical symptoms except the EM. PCR positivity for N. mikurensis did not persist at the one-month follow-up.
Twenty-four patients completed the one-month follow-up. No patients reported fever. Other symptoms and characteristics are presented in Table 1. Eleven patients were aware of a tick bite preceding the appearance of EM, and eight of them estimated that they removed the tick within 24 h. The EM were mostly (14 of 26) located to the lower extremities. The EM were of mixed morphology, 11 were annular (with central clearing) and 11 homogeneous (without central clearing). Morphology could not be assessed in four patients due to missing photography or allergic reaction to the local anesthetic. Twelve patients reported that they had previously been diagnosed with LB, and one reported previous Human Granulocytic Anaplasmosis. Thirteen patients reported no previous tick-borne infection.
Twelve patients were seropositive for B. burgdorferi s. l. IgG (VlsE) antibodies at the time of inclusion in the study, and six patients were seropositive for IgM (VlsE, OspC) antibodies. Interestingly, only one patient seroconverted in IgM at the one-month follow-up, and only two patients seroconverted in IgG. Borrelia DNA was detected in skin biopsies taken from the EM in 23 out of 26 patients. B. afzelii was identified in fifteen patients, and B. garinii in two patients. Borrelia species could not be determined for the remaining seven patients with detected Borrelia DNA as nested PCR did not produce a product of expected length or readable sequence. The patients with the highest number of spirochetes were also IgG seropositive by inclusion in the study, confirming lack of protective effect from VlsE-specific IgG. This was further confirmed by correlation analyses, where levels of neither IgM nor IgG were negatively correlated to the number of spirochetes in the biopsies (IgM: rs = 0.25, 95% CI -0.15 to 0.58; IgG: rs = 0.023, 95% CI -0.37 to 0.41).
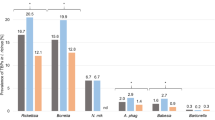
Homogeneous EM had higher numbers of Borrelia spirochetes in the central zone compared to annular EM (Fig. 1, two-sided Wilcoxon rank sum test, p = 0.014, 95% CI 12.7 to 6134). This information may assist clinicians in determining the most appropriate biopsy location for detecting Borrelia DNA by PCR, particularly in cases of diagnostic uncertainty. We suggest sampling from the border zone of annular EM, and both central and border zones of homogeneous EM. Results from previous studies2,3,4in which biopsies were only from the border zone of EM, have concluded that annular EM, compared to homogeneous EM, have a higher burden of Borrelia spirochetes, but this could not be confirmed in the present study.
The correlation coefficient between diameter and patient’s delay was rs 0.59 (p = 0.0058, 95% CI 0.17 to 0.83) (Fig. S1). The velocity of spirochetal migration in vivo is uncertain, yet crucial to understand concerning the course of the infection ranging from tick bite to disseminated infection. Expansion of EM was estimated by two different linear models based on time elapsed from discovery of the EM until inclusion in the study. The first linear regression model, excluding samples with negative Borrelia PCR, resulted in a co-efficient of 0.085, which suggests that EM expands by around 0.85 mm per day after discovery of the EM. The second model also excluded outliers with a longer patient’s delay than four weeks due to the risk of recall bias, this resulted in an estimate of around 3.8 mm migration velocity per day. Estimates from these clinical cases are uncertain due to unreliability of self-reported estimates and the low sample size, and therefore require further confirmation in larger studies. Previous in vitro work by Moriarty5 estimated that the rate of spirochetal extravasation is 3.4 μm/min, which can be extrapolated to 4.9 mm per 24 h. If we assume that spirochetal extravasation is relevant for transmission or movement in skin, this may be compared to our estimates. The rate of extravasation is higher than the estimates in this study, however estimates may vary due to differences between Borrelia species, as well as various in vivo factors and uncertainty due to our small sample size.
Our data (Fig. S2) does not show a clear correlation between patient sex and EM morphology. Unfortunately, we have too few confirmed cases of B. garinii infection to assess the impact of species. Previous studies in the Nordic countries6,7 on EM have suggested that women and B. garinii infections are more likely to be associated with homogeneous EM. Since there is a clear difference in number of spirochetes based on EM morphology and location of the biopsy within the EM – we hypothesize that the morphology is a consequence of the distribution of occurring number of spirochetes rather than the host’s sex.
In conclusion, EM following a tick bite in Sweden indicates an on-going Borrelia infection, which may involve multiple simultaneous species of Borrelia. There is also the possibility of infection by other tick-borne pathogens such as N. mikurensis. The significance of N. mikurensis and other tick-borne pathogens such as Anaplasma phagocytophilum or Rickettsia helvetica in EM patients remains uncertain, and their implications for treatment and clinical outcome require further study. In a clinical setting where confirmation of Borrelia diagnosis is necessary, our results indicate that a biopsy for analysis of Borrelia DNA should be taken from the border zone of annular EM and either the central area or both central and border zones of homogeneous EM to successfully detect Borrelia DNA. VlsE-specific immunoglobulins do not appear to reduce the number of spirochetes in the skin.
Methods
Study design
The diagnostic criterium for EM in Sweden is an expanding red or blue-red skin lesion (≥ 5 cm in diameter) with or without central clearing. The study design is summarized in Fig. S3. Adult patients capable of informed consent with clinical diagnosis of EM from participating outpatient clinics were considered eligible. Patients in whom a biopsy was contraindicated (such as known allergies to local anesthetics, or inappropriate localization of the EM for a biopsy) by the physician were excluded. All patients were treated according to standard care. Geographical bias was addressed through a multicenter design, and eligible patients were included after written informed consent at nine outpatient clinics (Fig. S4) in South-Eastern Sweden during 2018–2022. At the time of inclusion, physicians and patients completed questionnaires (S5) containing clinical data, self-reported symptoms and, if applicable, date and location of the tick bite. The EM was photographed. One to three biopsies were taken. Patients were included in the study even if they only consented to a single biopsy. The three punch biopsies were, in order of priority: Outer border of EM (4 mm), visually unaffected skin outside the EM (2 mm), and center of EM or visible tick-bite reaction (2 mm). Biopsies were immediately submerged in RNALater (Thermo-Fisher Scientific, USA), and sent by mail to the National Reference Laboratory (NRL) for Borrelia and other tick-borne bacteria (NRL) at the Department of Clinical Microbiology, County Hospital Ryhov, Jönköping, Sweden, and upon arrival frozen at − 20 °C. Blood and serum samples were also acquired from the patient, and frozen at − 70 °C. After one month, patients received another questionnaire by mail and complementary blood samples were acquired.
Ethical statement
The study was approved by the Swedish Ethical Review Authority (Linköping: 2017/486 − 31, 2018/310 − 32) and conducted in accordance with the Declaration of Helsinki and local guidelines and regulations. All patients were included in the study after written informed consent. Human biological samples are stored at the Biobank facility at County Hospital Ryhov, Jönköping, Sweden according to the Swedish Biobank Act (2023:38).
PCR detection methods
Detection of Borrelia spp., Rickettsia spp., Babesia spp., Neoehrlichia mikurensis, Anaplasma phagocytophilum, and relapsing fever Borrelia miyamotoi by real-time PCR was performed on biopsies and blood samples. Total nucleic acids were extracted using EZ1 RNA Tissue Mini Kit according to supplementary protocol “Isolation of total nucleic acids from animal and human tissues using the EZ1 RNA Tissue Mini Kit” (Qiagen, Kista, Sweden). Complementary DNA synthesis was performed using Illustra™ Ready-to-Go RT-PCR Beads kit (GE Healthcare, Chicago, USA) according to the manufacturer’s instructions. Borrelia afzelii was extracted from culture at a known concentration to enable real-time PCR-based quantification ex vivo. Number of spirochetes in biopsies were estimated from ct-values from the real-time PCR and normalized by area of the punch biopsy. Mixed electropherogram with suspected co-infection infection were analyzed using RipSeq Mixed web application (http://www.ripseq.com/) (Pathogenomix, Santa Cruz, California, USA). Probe-based assays utilized Maxima Probe qPCR Master Mix (Thermo-Fisher Scientific), and SYBR Green assays utilized Maxima SYBR Green Master Mix (Thermo-Fisher Scientific).
Detection of Borrelia spp. was analyzed by real-time PCR targeting a 116 bp amplicon of 16 S rRNA, as previously described by Gyllemark et al.8. The primers used were: forward - GCT GAG TCA CGA AAG CGT AG, reverse - CAC TTA ACA CGT TAG CTT CGG TA, and the probe FAM-CGC TGT AAA CGA TGC ACA CTT GGT-MGB. The thermal cycling protocol consisted of an initial denaturation at 95° C for 5 min, followed by 50 cycles of 95° C for 10 s and 60° C for 60 s.
Genospecies identification in PCR-positive samples was carried out by nucleotide sequencing (Macrogen Inc., Amsterdam, the Netherlands) of amplicons generated by nested PCR. This assay targeted the intergenic spacer region (IGS) between 5 S and 23 S rRNA genes, following the method described by Wilhelmsson et al.9.
Samples that did not produce an amplicon of expected length or failed to yield a readable sequence from the IGS region were further analyzed by nested PCR of MLST housekeeping genes as previously described by Margos et al.10.
Samples in which the species could not be determined through sequencing of either IGS region or MLST genes were classified as Borrelia spp., without species-level identification.
Detection of B. miyamotoi was analyzed by a species-specific real-time PCR targeting a 156 bp amplicon of flaB gene, previously described by Hovius et al.11. The primers used were: forward - AGA AGG TGC TCA AGC AG, reverse - TCG ATC TTT GAA AGT GAC ATA T, and the probe FAM-AGC ACA GGA GGG AGT TCA AGC-BHQ1. The thermal cycling protocol consisted of an initial denaturation at 95° C for 10 min followed by 45 cycles of 95° C for 5 s and 60° C for 35 s.
Detection of A. phagocytophilum was analyzed by a species-specific real-time PCR targeting a 64 bp amplicon of gltA gene, as previously described by Henningsson et al.12. Primers used were: forward - TTT TGG GCG CTG AAT ACG AT, reverse - TCT CGA GGG AAT GAT CTA ATA ACG T, and the probe FAM-TGC CTG AAC AAG TTA TG-BHQ1. The thermal cycling protocol consisted of an initial denaturation at 95° C for 2 min, followed by 45 cycles of 95° C for 30 s and 60° C for 1 min.
Detection of Rickettsia spp. was analyzed by real-time PCR targeting a 75 bp amplicon of gltA gene, as previously described by Stenos et al.13. Primers used were: forward - TCG CAA ATG TTC ACG GTA CTT T, reverse - TCG TGC ATT TCT TTC CAT TGT G, and the probe FAM-TGC AAT AGC AAG AAC CGT AGG CTG GAT G-BHQ1. The thermal cycling protocol consisted of 3 min of 50° C, 5 min of 95° C, followed by 60 cycles of 95° C for 20 s and 60° C for 40 s.
Detection of N. mikurensis was analyzed by real-time SYBR green PCR targeting a 106 bp amplicon of 16 S rRNA gene, as previously described by Labbé Sandelin et al.14. The primers used were: forward - GTA AAG GGC ATG TAG GCG GTT TAA, and reverse - TCC ACT ATC CTC TCT CGA TCT CTA GTT TAA. The thermal cycling protocol consisted of an initial denaturation at 95° C for 3 min, followed by 60 cycles of 95° C for 15 s, 60° C for 30 s, and 72° C for 30 s, followed by melting curve analysis.
Detection of Babesia spp. was analyzed by real-time SYBR green PCR targeting a 411 to 452 bp region of 18 S rRNA gene, as previously described by Wilhelmsson et al.15. The primers used were: forward - GTC TTG TAA TTG GAA TGA TGG, and reverse - TAG TTT ATG GTT AGG ACT ACG. The thermal cycling protocol consisted of an initial denaturation at 94° C for 10 min, followed by 35 cycles of 94° C for 1 min, 55° C for 1 min, and 72° C for 2 min. The protocol concluded with a final extension at 72° C for 5 min, followed by melting curve analysis.
Serological assays
Anti-Borrelia IgM was assessed with recombinant VlsE and OspC antigen (VirClia, Vircell, Granada, Spain), and positive results in IgM were confirmed by VlsE and OspC antigens immunoblot (Anti-Borrelia EUROLINE-RN-AT IgM, EUROIMMUN, Lübeck, Germany) from B. afzelii, B. garinii and B. burgdorferi sensu stricto (s.s.). Anti-Borrelia IgG was assessed with recombinant VlsE antigens (Liaison, DiaSorin, Saluggia, Italy). Serological analyses were performed at the NRL in Jönköping using assays that were part of the clinical routine and conducted according to manufacturer’s instructions.
Boxplot of spirochete counts by biopsy location and morphology. Box limits are interquartile range, and center line is median. Whiskers represent 1.5x IQR. Homogeneous EM lesions contain a higher number of spirochetes in the central zone compared to annular EM lesions (Two-sided Wilcoxon rank sum test: p = 0.014, 95% CI 12.7 to 6134). Dots colorized red if there was a visible tick bite reaction within the EM.
Assessment of photographs and clinical data
Photographs were assessed by SC according to a predetermined protocol (S6) developed by dermatologist and Professor CA. Clinical data was extracted from questionnaires. Patient’s delay was defined as the time elapsed, in days, between when the patient discovered the rash, until inclusion in the study.
Statistics
Data normality was estimated using the Shapiro-Wilk test. Correlations were estimated using Spearman rank correlation coefficient. Estimation of EM’s expansion was performed with two different linear regression models (Fig. S1), one excluding Borrelia PCR negative patients, and one also excluding study participants with extended patient’s delay (> 28 days). The two-sided Wilcoxon rank sum test was used to assess the difference between groups. Participants with missing data were excluded from analyses where applicable (Table 1). Statistical analyses were performed in the R statistical language16and plots were created using Tidyverse17. A p-value < 0.05 was considered significant.
Data availability
Data is available online by DOI 10.6084/m9.figshare.28614677.
References
Cialini, C. et al. Prevalence of tick-borne pathogens in feeding and questing Ixodes ricinus ticks from Southern Sweden. Ticks Tick. Borne Dis. 16, 102453 (2025).
O’Rourke, M. et al. Quantitative detection of borrelia burgdorferi sensu Lato in erythema Migrans skin lesions using internally controlled duplex real time PCR. PLoS ONE. 8, e63968 (2013).
Liveris, D. et al. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema Migrans or Lyme arthritis. J. Clin. Microbiol. 40, 1249–1253 (2002).
Li, X. et al. Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. Arthr. Rhuem. 63, 2238–2247 (2011).
Moriarty, T. J. et al. Effect of gender on clinical and epidemiologic features of Lyme borreliosis. PLoS Pathog. 4, e1000090 (2008).
Bennet, L. S. & Berglund, L. Effect of gender on clinical and epidemiologic features of Lyme borreliosis. Vector-Borne Zoonotic Dis. 7, 34–41 (2007).
Carlsson, S. A., Granlund, H., Jansson, C., Nyman, D. & Wahlberg, P. Characteristics of erythema Migrans in Borrelia afzelii and Borrelia garinii infections. Scand. J. Infect. Dis. 35, 31–33 (2003).
Gyllemark, P. et al. Are other tick-borne infections overlooked in patients investigated for Lyme neuroborreliosis? A large retrospective study from South-eastern Sweden. Ticks Tick-borne Dis. 12, 101759 (2021).
Wilhelmsson, P. et al. Prevalence and diversity of Borrelia species in ticks that have bitten humans in Sweden. J. Clin. Microbiol. 48, 4169–4176 (2010).
Margos, G. et al. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc. Natl. Acad. Sci. 105, 8730–8735 (2008).
Hovius, J. W. R. et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382, 658 (2013).
Henningsson, A. J. et al. Detection of Anaplasma phagocytophilum in Ixodes ricinus ticks from Norway using a realtime PCR assay targeting the Anaplasma citrate synthase gene GltA. BMC Microbiol. 15 (2015).
Stenos, J., Graves, S. R. & Unsworth, N. B. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am. J. Trop. Med. Hyg. 73, 1083–1085 (2005).
Labbé Sandelin, L. et al. Candidatus Neoehrlichia mikurensis in ticks from migrating birds in Sweden. PLOS ONE. 10, e0133250 (2015).
Wilhelmsson, P. et al. Clinical/serological outcome in humans bitten by Babesia species positive Ixodes ricinus ticks in Sweden and on the Åland Islands. Ticks Tick-borne Dis. 11, 101455 (2020).
R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2024).
Wickham, H. et al. Welcome to the tidyverse. J. Open. Source Softw. 4, 1686 (2019).
Acknowledgements
We would like to greatly thank all participating patients for their valuable contribution to this study. The participating outpatient clinics and their staff, whose collaboration has been crucial to this study, are greatly thanked.Therese Sandberg and Anna Angel are acknowledged for their excellent assistance with study coordination and laboratory analyses. Dr. Malin Lager is acknowledged for kindly providing primers for the Borrelia MLST assay. A special thank you goes to the Biobank Facility, County Hospital Ryhov, Jönköping, Sweden, for their support.
Funding
Open access funding provided by Linköping University. The Medical Research Council of Southeast Sweden under Grant FORSS-855141 and FORSS-940983 and FORSS-931010 and FORSS-1012268; Region Jönköpings County under Grant FUTURUM-983017, FUTURUM-995198; Region Östergötland under Grant RÖ-990411, RÖ-979010, RÖ-937675 and the EU-Interreg NSR programme NorthTick.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. I.T., M.W., and A.H. facilitated contacts with outpatient clinics across various regions of Sweden. C.A. developed the protocol for photograph assessment. S.C. assessed the photographs, conducted laboratory and statistical analyses, prepared figures, and wrote the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
PEL is a senior external scientific advisor of Pfizer Inc., US, and Novo-Nordisk A/S, Denmark.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cronhjort, S., Wilhelmsson, P., Tjernberg, I. et al. Investigation of erythema migrans patients identifies Borrelia species and Neoehrlichia mikurensis with implications for clinical assessment. Sci Rep 15, 20293 (2025). https://doi.org/10.1038/s41598-025-07291-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07291-0