Abstract
Stroke poses a significant mortality threat and frequently results in survivors experiencing varying degrees of residual disability. The objective of this meta-analysis was to elucidate the association between C-reactive protein-to-albumin ratio (CAR) and prognosis in patients with stroke. We searched the databases of PubMed, Embase, the Cochrane Library, and CNKI for relevant studies up to 26th January 2024. The quality of the included studies was evaluated using the Newcastle Ottawa Quality Assessment Scale (NOS). Pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated for the association between CAR and poor functional outcomes and mortality. A total of nine studies with 2954 patients were included. The pooled results demonstrated that elevated CAR was associated with poor functional outcomes in patients with stroke (OR: 2.24; 95% CI: 1.81–2.78; P < 0.00001). Similarly, elevated CAR was associated with mortality in patients with stroke (OR: 2.11; 95% CI: 1.51–2.94; P < 0.0001). CAR has prognostic value for poor functional outcomes and mortality in patients with stroke.
Similar content being viewed by others
Introduction
-
Stroke has a significant mortality rate and is one of the primary causes of disability and death globally1. Intravenous recombinant tissue plasminogen activator and cardiovascular therapy are effective treatments for acute stroke2. Nevertheless, patient selection criteria are important because of their limited therapeutic windows. Moreover, no approved drug possesses overprotective qualities as of yet. This led us to search for a biomarker that would facilitate prompt diagnosis and prognosis prediction for patient selection.
-
Research indicates a connection between inflammation and stroke, playing a pivotal role in their mechanisms3,4. C-reactive protein (CRP), used as an inflammatory marker in clinical practice, is elevated during inflammation and infection4. Meanwhile, albumin, a liver-produced negative acute phase reactant, decreases during inflammatory processes. Both CRP and albumin levels are associated with disease severity and mortality5.
-
The C-reactive protein-to-albumin ratio (CAR) serves as an inflammation-based prognostic marker, reflecting the interplay between CRP and albumin levels in critical diseases and stroke prognosis6,7.The relationship between CAR and outcomes in patients with stroke is still controversial. Some studies found that elevated CAR levels were correlated with outcomes in patients with stroke8,9. Nevertheless, some studies failed to find correlation between CAR and outcomes in patients with stroke10. Therefore, we conducted this meta-analysis to assess the relationship between CAR and outcomes in patients with stroke.
Results
Study selection and characteristics
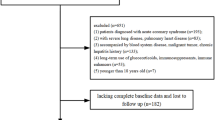
A total of 2564 studies were identified based on an online database search and manual search, of which only 9 eligible articles11,12,13,14,15,16,17,18,19 were included in the meta-analysis. Specifically, six studies reported functional outcomes12,13,16,17,18,19, four reported mortality11,12,14,15, and one study reported both12. The search flow chart is shown in Fig. 1, and the complete search strategy is shown in Supplementary Table 1. The basic characteristics of the included studies are shown in Table 1. In terms of the methodological quality of the studies, the overall NOS scores ranged from 5 to 7. Notably, no evidence of publication bias was observed, as confirmed by the funnel plot displayed in Supplementary Fig. 1.
Poor functional outcomes
Six studies12,13,16,17,18,19 reported data on the association between CAR and poor functional outcomes. The pooled results demonstrated that CAR was associated with poor functional outcomes in patients with stroke (OR: 1.96; 95% CI: 1.48–2.60; P < 0.00001), although there was considerable heterogeneity (I2 = 70%). So a leave-one-out sensitivity analysis was conducted to identify potential sources of heterogeneity in the quantitative analysis (Supplementary Table 2). Notably, the observed heterogeneity primarily stemmed from methodological differences in study design, specifically the timing of outcome assessment. While five studies12,13,16,18,19 evaluated functional outcomes within 3 months post-stroke or at discharge, Zhang et al.17 extended follow-up to 6 months post-discharge. This discrepancy in assessment windows may introduce variability due to delayed neurological recovery and potential recurrent events. After excluding Zhang et al.‘s study17, the results showed that CAR was closely related to poor functional outcomes in patients with stroke (OR: 2.24; 95% CI: 1.81–2.78; P < 0.00001; I2 = 0%) (Fig. 2).
Mortality
Four studies11,12,14,15 reported the relationship between CAR and mortality in patients with stroke. The pooled results showed that elevated CAR was strongly associated with mortality in patients with stroke (OR: 2.24; 95% CI: 1.43–3.52; P = 0.0004), without significant heterogeneity (I2 = 39%) (Fig. 3).
Subgroup analysis
To detect potential heterogeneity, subgroup analyses were performed for poor functional outcomes and mortality (Table 2) (Supplementary Table 3). For each subgroup, P1 indicates the significance of the pooled OR (Z-test), P2 reflects heterogeneity within the subgroup (Q-test), and P3 tests for subgroup differences (chi-square interaction test). A P3 < 0.05 suggests significant variation in CAR’ s prognostic effect across subgroups. Notably, neither CAR’ s association with poor functional outcomes nor mortality exhibited significant variation across subgroups. For poor functional outcomes, mechanism of pathology (acute primary injury vs. secondary complication-driven, P for interaction = 0.74), country of publication (China vs. Other countries, P = 0.45), NOS score (NOS < 7 vs. NOS ≥ 7, P = 0.74), average age (≤ 60 vs. >60 years, P = 0.62), sample size (≤ 300 vs. >300, P = 0.99), and CAR cut-off (≤ 0.8 vs. >0.8, P = 0.44) (Supplementary Fig. 3–8). For mortality, country of publication (China vs. Other countries, P = 0.82), NOS score (NOS < 7 vs. NOS ≥ 7, P = 0.63), average age (≤ 60 vs. >60 years, P = 0.39), sample size (≤ 300 vs. >300, P = 0.06), and CAR cut-off (≤ 0.8 vs. >0.8, P = 0.82) (Supplementary Fig. 9).
Discussion
In this meta-analysis of nine randomized controlled trials of CAR in patients with stroke, we found that CAR in stroke patients was strongly associated with poor functional outcomes and mortality. As far as we know, no meta-analysis has examined the relationship between CAR and stroke patients’ outcomes. This study is the first meta-analysis that explores the prognostic value of CAR in patients with stroke. Notably, much of the existing research has concentrated on CRP or serum albumin for predicting outcomes in patients with stroke. For instance, higher circulating CRP levels are linked to an increased risk of all-cause mortality in patients with acute ischemic stroke, according to a meta-analysis by Yu et al.20 Furthermore, Zhou et al.21 discovered that in individuals suffering from acute ischemic stroke or transient ischemic attack low serum albumin levels are prognostic of poor functional outcome and mortality.
The exact processes behind the complex still unclear relationship between high CAR and poor outcomes in stroke patients. A few theories can account for them. To begin with, inflammation can both precede and contribute to stroke22,23. It affects all phases of the atherosclerotic process, as well as brain damage and brain healing23,24,25. In stroke patients, inflammatory cytokines may be linked to a decline in their neurological function25. Additionally, it has been shown that the systemic immune-inflammation index has predictive significance in some malignant solid tumors26,27,28,29,30. Second, inflammatory circumstances have an independent effect on both CRP and albumin; as inflammation rises, albumin metabolism rises and synthesis falls30. Third, the CAR value is obtained through the ratio of CRP and albumin. Thus, CAR reflects both the pro-inflammatory condition and the nutritional status.
The results of our study highlight CAR’ s potential as an affordable, accessible prognostic biomarker in stroke. Unlike CRP or albumin alone, CAR integrates complementary inflammatory and nutritional pathways, offering a more comprehensive reflection of systemic inflammation31,32,33. While CAR requires the calculation of the CRP-to-albumin ratio, this process utilizes routinely measured parameters without additional costs, preserving its clinical feasibility. However, CAR’ s dependence on two variables introduces variability risks, particularly in settings with inconsistent assay standardization. Notably, our systematic evaluation of heterogeneity through subgroup analyses (encompassing country, NOS score, age, sample size, and CAR cutoffs) revealed no significant interaction effects with minimal between-study heterogeneity (I2 = 0% in most subgroups), reinforcing the robustness of CAR-outcome associations across diverse settings.
Limitations
As for the limitation of our study, the first thing is that the most studies we included were retrospective in nature with selection bias, potentially impacting the reliability of our findings. Second, variations in the mechanisms of pathology underlying poor functional outcomes across studies may have introduced heterogeneity, though subgroup analyses stratified by mechanism (acute primary injury vs. secondary complication-driven) demonstrated negligible within-group heterogeneity (I² = 0%) and consistent prognostic effects (P for interaction = 0.74), reinforcing the robustness of CAR’ s association with adverse outcomes. A key limitation is that the country-specific sensitivity analysis included only Turkey, potentially restricting the generalizability of findings across diverse geographic or healthcare contexts. However, subgroup analyses demonstrated no significant heterogeneity (P for interaction > 0.05) in the relationship between CAR and adverse functional outcomes/mortality among countries, implying that the observed associations may remain consistent despite limited regional representation. Future multi-country studies are warranted to confirm these results and enhance external validity.
Implications of these findings in practice
The findings underscore the clinical utility of the CAR as a readily accessible prognostic tool in stroke management. Elevated CAR levels were robustly associated with poor functional outcomes and mortality, highlighting its potential to identify high-risk patients early in the clinical course. Since CAR integrates inflammatory (CRP) and nutritional (albumin) biomarkers that both routinely measured in acute care, its adoption does not incur additional costs or require specialized assays, enhancing its feasibility in diverse healthcare settings. Clinicians could leverage CAR to stratify patients, enabling tailored monitoring or intensified rehabilitation for those at elevated risk.
Implications of these findings in future research
We suggest that future multi-center studies prioritize comparative validation of CAR against emerging composite biomarkers, like low-density lipoprotein cholesterol to lymphocyte ratio34, monocyte to high-density lipoprotein ratio35 or neutrophil to lymphocyte ratio36, to clarify its unique prognostic value in stroke. Meta-regression analysis could theoretically enhance our understanding of heterogeneity by elucidating how covariates modulate CAR’ s prognostic performance. However, our systematic review identified nine eligible studies that below the recommended threshold of at least 10 studies for stable regression modeling37. Future research should prioritize meta-regression analyses in larger, multi-center cohorts to explore interactions between CAR and clinical variables.
Conclusion
In this study, CAR demonstrates potential as a prognostic marker for poor functional outcomes and mortality in patients with stroke. However, its clinical utility requires validation through large-scale prospective studies.
Methods
Search strategy
We conducted the systematic review and meta-analysis following the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (PRISMA). We searched the PubMed, Embase, the Cochrane Library, and CNKI databases for relevant studies up to January 26, 2024. There were no language restrictions applied. We employed the following keywords and their synonyms: “C-reactive protein,” “CRP,” “Albumin,” and “Stroke”. Two investigators (JY and YC) independently evaluated the titles and abstracts of the records, excluding articles that did not meet the eligibility criteria. The reviewers evaluated the full-text articles. Additionally, we examined the reference lists and recent reviews to identify potentially relevant studies.
Study selection
The eligibility criteria employed aimed to minimize clinical heterogeneity: (a) patients diagnosed with acute ischemic stroke; (b) albumin counts and CRP were assessed on or after admission or could be calculated; (c) survival outcomes or functional outcomes included odds ratio (OR) or risk ratio (HR) with a 95% confidence interval (CI); and (d) eligible studies were prospective or retrospective cohort studies.
Exclusion criteria included: (a) conference abstracts, letters, case reports, reviews, and unrelated articles; (b) studies where the endpoint event was not death, disability, or major disability; (c) studies lacking sufficient data (referring to the absence of OR or related data for odds ratio estimation, as well as a lack of CAR or functional outcomes after discharge). Abstracts, title and abstract screening, and full-text screening were conducted by at least two reviewers. The senior author was tasked with resolving conflicts between the two reviewers.
Data extraction
We conducted a preliminary extraction from a subset of included studies to construct a data extraction sheet. Two reviewers extracted the required data from each of the included papers. The extraction sheet encompassed author, year of publication, country, age, sample size, number of males, study design, outcome measure, time points for CRP measurements, optimal cut-off value and NOS score. Any disagreements were resolved through consensus between the two investigators.
Outcomes
During clinical follow-up, the Modified Rankin Scale (mRS) was used to evaluate functional status, mRS = 3–5 was poor functional status, and mRS = 6 mortality was used to measure survival outcome. CAR was calculated as the ratio of serum CRP (mg/L) to serum albumin (g/dL), measured at admission or within 24 h using automated analyzers. This method is consistent with prior studies6,32,33, and all included articles directly reported CAR values derived from this standardized approach.
Quality assessment
Two authors independently evaluated the quality of the included studies, employing the Newcastle-Ottawa scale designed for cohort studies. The scale comprises three parts: selection (4 items), comparability (2 items), and outcome (3 items), yielding a cumulative score ranging from 0 to 9. Any discrepancies were ultimately resolved by a leading expert through arbitration. Studies scoring higher than 7 were categorized as high quality, those scoring between 5 and 7 were considered moderate quality, and those scoring lower than 7 were deemed low quality.
Statistical analyses
We applied Review Manager (version 5.4.1) and Stata (version 15.0) software for the analysis. We estimated the prognostic value of CAR in stroke patients using forest and funnel plots. Given the large sample size, we employed the OR as the effect size for this meta-analysis. To address heterogeneity in CAR operationalization across studies, we harmonized all effect sizes by converting raw ORs to logORs. This transformation linearizes the multiplicative scale of ORs into an additive scale, aligning both continuous and categorical definitions under a unified metric37. The log (OR) and its standard error were calculated using the OR and 95% CI, and these values were utilized for aggregation. We combined the OR and 95% CI to analyze the implications of CAR with death and poor endpoints. Additionally, heterogeneity was comprehensively assessed through subgroup analysis. Heterogeneity among the studies was evaluated using Cochran’s Q test and the I2 statistic. A sensitivity analysis using the “leave-one-out” method was conducted to evaluate the impact of individual studies on the overall outcome. We employed a random-effects model to calculate pooled OR and 95% confidence intervals (CI) in the presence of significant heterogeneity among the enrolled studies (I2 > 50%, or P < 0.10). Otherwise, the fixed-effects model was utilized (I2 < 50% or P > 0.10).
Data availability
All relevant data are within the manuscript and its Supporting Information files.
References
Nagre, A. S. Perioperative stroke - Prediction, prevention, and protection. Indian J. Anaesth. 62, 738–742 (2018).
Cornelius, J. F., Sandu, N., Perez-Pinzon, M. A. & Schaller, B. Treatment of acute ischemic stroke: role of ischemic tolerance in intravenous and endovascular therapies. Expert Rev. Cardiovasc. Ther. 7, 331–332 (2009).
Jiang, T. et al. Soluble TREM1 concentrations are increased and positively correlated with total Tau levels in the plasma of patients with alzheimer’s disease. Aging Clin. Exp. Res. 31, 1801–1805 (2019).
Mészáros, Á. et al. Neurovascular inflammaging health disease. Cells 9, 1614 (2020).
Sproston, N. R. & Ashworth, J. J. Role of C-Reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754 (2018).
Kario, K., Okura, A., Hoshide, S. & Mogi, M. The WHO global report 2023 on hypertension warning the emerging hypertension burden in Globe and its treatment strategy. Hypertens. Res. 47, 1099–1102 (2024).
Lu, Z., Fu, S., Li, W., Gao, X. & Wang, J. Prognostic role of C-reactive protein to albumin ratio in lung cancer: an updated systematic review and meta-analysis. Chronic Dis. Transl Med. 10, 31–39 (2023).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/american stroke association. Stroke 50, e344–e418 (2019).
Luan, Y. Y. & Yao, Y. M. The clinical significance and potential role of C-Reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 9, 1302 (2018).
Oh, J. et al. High-sensitivity C-reactive protein/albumin ratio as a predictor of in-hospital mortality in older adults admitted to the emergency department. Clin. Exp. Emerg. Med. 4, 19–24 (2017).
Li, M. et al. Dose-Response relationship between C-Reactive protein/albumin ratio and In-Hospital mortality in elderly patients with acute ischemic stroke. Gerontology 70, 125–133 (2024).
Huang, L. et al. Hypersensitive C-reactive protein-albumin ratio is associated with stroke-associated pneumonia and early clinical outcomes in patients with acute ischemic stroke. Brain Behav. 12, e2675 (2022).
Kanal, Y. et al. CRP albumin ratio May predict no reflow in patients undergoing percutaneous coronary intervention for saphenous vein graft stenosis. Angiology 74, 55–61 (2023).
Kocatürk, M. & Kocatürk, Ö. Assessment of relationship between C-reactive protein to albumin ratio and 90-day mortality in patients with acute ischaemic stroke. Neurol. Neurochir. Pol. 53, 205–211 (2019).
Sonsöz, M. R. et al. İ. C-Reactive protein to albumin ratio predicts In-hospital mortality in patients with acute heart failure. Turk. Kardiyol Dern Ars. 51, 174–181 (2023).
Xu, T. et al. High ratio of C-reactive protein to albumin is associated with hemorrhagic transformation and poor functional outcomes in acute ischemic stroke patients after thrombolysis. Front. Aging Neurosci. 15, 1109144 (2023).
Zhang, Y., Li, H. & Chang, H. Relationship between serum NSE, PLR, CRP/ALB and severity and prognosis of patients with ischemic stroke. Chin. J. Hygiene Eng. 22, 400–402 (2023).
Tang, Q. & Zhang, W. Predictive effect of crp/albumin ratio on the prognosis of thrombolytic patients with ischemic stroke. Chin. J. Experimental Diagnostics. 24, 1985–1986 (2020).
Chen, J., Tang, K. & Chen, S. The relationship between crp/albumin ratio and prognosis of ischemic stroke. J. Immunoass. Clin. Immunol. 27, 665–669 (2020).
Yu, B., Yang, P., Xu, X. & Shao, L. C-reactive protein for predicting all-cause mortality in patients with acute ischemic stroke: a meta-analysis. Biosci. Rep. 39, BSR20181135 (2019).
Zhou, H. et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. 6, 458–466 (2021).
Chiba, T., Itoh, T., Tabuchi, M., Nakazawa, T. & Satou, T. Interleukin-1β accelerates the onset of stroke in stroke-prone spontaneously hypertensive rats. Mediat. Inflamm. 2012, 701976 (2012).
Finnie, J. W. Neuroinflammation: beneficial and detrimental effects after traumatic brain injury. Inflammopharmacology 21, 309–320 (2013).
Das, M., Mohapatra, S. & Mohapatra, S. S. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J. Neuroinflammation. 9, 236 (2012).
Diedler, J. et al. C-reactive-protein levels associated with infection predict short- and long-term outcome after supratentorial intracerebral hemorrhage. Cerebrovasc. Dis. 27, 272–279 (2009).
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20, 6212–6222 (2014).
Geng, Y. et al. Systemic Immune-Inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: A propensity Score-matched analysis. Sci. Rep. 6, 39482 (2016).
Passardi, A. et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 7, 33210–33219 (2016).
Xie, Q. K. et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J. Transl Med. 16, 273 (2018).
Huang, H. et al. Prognostic value of preoperative systemic Immune-Inflammation index in patients with cervical Cancer. Sci. Rep. 9, 3284 (2019).
Idicula, T. T., Waje-Andreassen, U., Brogger, J., Naess, H. & Thomassen, L. Serum albumin in ischemic stroke patients: the higher the better. The Bergen stroke study. Cerebrovasc. Dis. 28, 13–17 (2009).
Kinoshita, A. et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 22, 803–810 (2015).
Zhang, D. et al. C-Reactive protein/albumin ratio correlates with disease severity and predicts outcome in patients with aneurysmal subarachnoid hemorrhage. Front. Neurol. 10, 1186 (2019).
Güneş, M. & Büyükgöl, H. A novel predictive marker for In-Hospital mortality in acute cerebral infarction: Low-Density lipoprotein cholesterol to lymphocyte ratio. Cureus 12(8), e9986. https://doi.org/10.7759/cureus.9986 (2020).
Bolayir, A. et al. Monocyte/high-density lipoprotein ratio predicts the mortality in ischemic stroke patients. Neurol. Neurochir. Pol. 52 (2), 150–155. https://doi.org/10.1016/j.pjnns.2017.08.011 (2018).
Paudel, S. S., Thapa, B. & Luitel, R. Neutrophil lymphocyte ratio as a prognostic marker in acute ischemic stroke: a systematic review and Meta-analysis. J. Nepal. Health Res. Counc. 18(4), 573–579. https://doi.org/10.33314/jnhrc.v18i4.3143 (2021).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 1 (2), 97–111. https://doi.org/10.1002/jrsm.12 (2010).
Acknowledgements
We would like to express our heartfelt gratitude to all authors who provided published data for our systematic review.
Funding
This work is supported by National Natural Science Foundation of China (82271364), the innovation team project of Affiliated Hospital of Clinical Medicine College of Chengdu University.
Author information
Authors and Affiliations
Contributions
JY and ZY originated and designed the study. JY, YC, JW, FL, XY conducted the literature search, data extraction, data analysis and interpretation and drafting of the manuscript. BS and NL conducted the data interpretation and methodology. JY and ZY conducted critical revision of article and final approval. All authors offered comments and suggestions, and gave final approval for its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethics approval was not obtained because this study did not involve human or animal subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, J., Chen, Y., Wan, J. et al. Prognostic value of the C-reactive protein to albumin ratio in patients with stroke: a meta-analysis. Sci Rep 15, 21150 (2025). https://doi.org/10.1038/s41598-025-07327-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07327-5
Keywords
This article is cited by
-
Association of the C-reactive protein–to–albumin ratio (CAR) with clinical outcomes in chronic kidney disease patients undergoing surgery: a retrospective cohort analysis of the INSPIRE database
BMC Nephrology (2026)
-
The Ratio Paradox in Stroke Prognosis: Abundant Evidence, Absent Utility
Translational Stroke Research (2026)