Abstract
In this study, we synthesized a ZIF-8/graphene (Gr) composite and evaluated its photocatalytic degradation performance toward levofloxacin (LVX) under low-intensity UV light. The composite exhibited improved charge separation and enhanced reactive oxygen species (·OH, ·O2−, h+) generation compared to its individual components. Under optimal conditions (20 mg catalyst, 5 mg/L LVX, neutral pH), a 25.70% degradation was achieved after 120 min of irradiation, following pseudo-first-order (PFO) kinetics. While the degradation efficiency was modest, the catalyst operated effectively under low-energy conditions and demonstrated selective antibacterial activity against E. coli in dark conditions. These findings suggest that ZIF-8/Gr composites hold promise as dual-functional materials for pollutant mitigation and microbial control in niche wastewater applications, particularly where energy availability is limited. Further work is needed to enhance degradation efficiency and assess long-term operational stability.
Similar content being viewed by others
Introduction
Water pollution is a widespread environmental problem caused by human activities, which leads to the contamination of various water bodies such as rivers, lakes, oceans, and groundwater. Contamination can stem from a range of sources, such as industrial discharges, agricultural runoff, sewage disposal, and improper waste management practices. The introduction of harmful substances into water systems has a detrimental impact on water quality and poses serious risks to both human health and ecosystems1,2. It is alarming to note that water pollution-related diseases claim the lives of around 3.4 million people every year, as reported by the World Health Organization (WHO)3. A particularly troubling consequence is the emergence of antibiotic-resistant bacteria, driven by the excessive and improper use of antibiotics in both agriculture and healthcare settings. These resistant strains can persist and proliferate in contaminated water systems, posing serious risks to ecosystems and human health.
Levofloxacin (LVX) is a synthetic antibiotic that falls under the fluoroquinolone class. It is commonly used due to its effectiveness in treating various bacterial infections, such as respiratory tract infections, urinary tract infections, and skin infections. The mechanism of action involves the inhibition of bacterial DNA gyrase and topoisomerase IV, which are crucial for bacterial DNA replication and transcription. LVX being a third-generation fluoroquinolone, is widely recognized for its ability to effectively combat a wide range of bacteria, including both Gram-positive and Gram-negative strains. LVX has a molecular formula of C18H20FN3O4 and a unique structure with a fluorine atom, which boosts its effectiveness against bacteria. The hydrophobic characteristics of the compound are attributed to the presence of the fluorine atom, enabling it to bind effectively to different environmental matrices, such as polystyrene nanoparticles. It has been observed that LVX can withstand degradation during conventional wastewater treatment processes, resulting in its long-lasting presence in aquatic environments. The stability of this compound is due to its unique chemical structure, which enables it to resist microbial and biological oxidation.
Conventional wastewater treatment systems often fall short in effectively removing persistent antibiotics, leading to their accumulation in natural water sources. This inadequacy is primarily due to the limited efficiency of biological treatment processes, which fail to fully degrade antibiotic compounds, resulting in their discharge into the environment4. Among the most studied methods for enhancing antibiotic removal are adsorption5,6,7,8, membrane filtration, advanced oxidation processes (AOPs), and photocatalysis, with the latter showing the greatest potential. Adsorption is one of the most widely researched techniques for removing antibiotics from wastewater. In this method, antibiotic molecules adhere to solid surfaces, reducing their concentration in treated water. Activated carbon and various other adsorbents have been shown to improve the removal efficiency of antibiotics9,10. However, the performance of adsorption is heavily influenced by several factors, such as the type of adsorbent used, the antibiotic concentration, and the presence of competing substances in the wastewater. These factors may limit the scalability and efficiency of adsorption in large-scale applications.
AOPs are characterized by their ability to generate highly reactive radicals that oxidize and degrade organic pollutants, including antibiotics. Methods such as ozonation and UV irradiation have been shown to effectively break down antibiotic compounds in wastewater. Consequently, while AOPs offer a robust method for pollutant degradation, their operational demands and potential side effects limit their widespread application11,12. Among the advanced treatment methods, photocatalysis stands out as the most promising technique for antibiotic removal from wastewater. Photocatalysis utilizes light-activated catalysts to degrade antibiotics and other organic pollutants into less harmful substances. Previous studies have demonstrated that photocatalytic processes using various semiconductor materials can achieve high removal efficiencies, making them a viable option for sustainable wastewater treatment13,14. Key advantages of photocatalysis include its operation under mild conditions, low energy consumption, and the absence of secondary pollutants, which make it highly attractive for large-scale applications. Additionally, advancements in photocatalytic materials, such as polyoxometalates, have further enhanced the performance and efficiency of these systems15,16.
Zeolitic Imidazolate Framework-8 (ZIF-8) is a highly promising semiconductor photocatalyst, especially in solar-driven applications, due to its many advantages. One key benefit is its effectiveness in breaking down various contaminants in water and air via reactive oxygen species (ROS)17,18,19. Although ZIF-8 is widely utilized for the photocatalytic degradation of organic pollutants, its stability in water and low photocatalytic activity remains significant limitations due to poor adsorption efficiency and challenges associated with the migration and separation of electron–hole pairs20,21,22. The stability issue arises from the tendency of ZIF-8 to agglomerate in water, which can hinder their effectiveness in practical applications22. Furthermore, the wide band gap of ZIF-8, approximately 4.9 eV, limits its responsiveness to visible light, necessitating the use of UV light for activation, which is not always feasible in natural settings20. This limitation is compounded by the fact that the photogenerated electron–hole pairs can recombine before participating in the degradation process, thereby reducing the overall efficiency of the photocatalytic reaction21.
Graphene (Gr) is an extremely thin film of carbon atoms that form a distinctive honeycomb pattern. It possesses extraordinary qualities such as outstanding electrical and thermal conductivity, impressive mechanical strength, and remarkable flexibility. The distinctive arrangement of carbon atoms in Gr, with sp2 hybridization, enhances electron mobility, which has led to its potential use in a wide range of applications such as electronics, sensors, and catalysis23. Graphene oxide (GO) and reduced graphene oxide (rGO) a functionalized derivative of Gr are often reported for their tunable surface chemistry, high surface area, and ability to anchor various functional groups or nanoparticles24,25,26. These properties make them highly suitable for applications in environmental remediation, energy storage, catalysis, and biomedical devices. Previous studies have shown that Gr based composites have great potential in the photocatalytic degradation of organic pollutants. For instance, the ZIF-8/GO composite has been shown to improve the degradation of phenol derivatives and pharmaceutical compounds due to the excellent electronic and optical properties of Gr, which facilitate enhanced ROS generation and adsorption capabilities27. Similarly, the ZIF-8-derived carbon modified with GO nanosheets demonstrated a high removal efficiency for malachite green dye, attributed to the hierarchical porosity and conductive network of the composite, which provides abundant active sites for adsorption and degradation28.
While ZIF-8 has shown potential as a photocatalyst, its low stability and limited visible light activity hinder its practical application. In addition, previous studies have examined ZIF-8 composites for various organic pollutants29,30,31, their performance under low-power UV conditions for fluoroquinolones, specifically LVX, remains largely unexplored. Gr and their derivatives offer excellent electron mobility and surface area, yet lacks strong photocatalytic activity alone. By integrating ZIF-8 with Gr via hydrothermal synthesis, this study aims to overcome these limitations. We hypothesize that the resulting ZIF-8/Gr composite will exhibit superior photocatalytic activity and antibacterial properties under low-intensity UV light, specifically targeting the degradation of LVX. Therefore, there is a need to develop more efficient photocatalysts that can degrade antibiotic pollutants. ZIF-8, with its unique visible light harvesting properties and compatibility with Gr, has the potential to address these challenges. However, its application in LVX degradation remains underexplored. This study seeks to fill this gap by fabricating a ZIF-8/Gr and evaluating its photocatalytic performance. The primary goal of this study is to synthesize ZIF-8/Gr composite using the environmentally friendly hydrothermal method. In addition, the study examines the physical and chemical properties of the synthesized photocatalyst and evaluates its effectiveness in the photodegradation of LVX under low intensity UV light source.
Materials and method
Materials
2-methylimidazole (Maclin, China), methanol (HmBG, Malaysia), zinc nitrate hexahydrate (Zn(NO3)2·6H2O (Sigma-Aldrich), and graphene oxide (GO) suspension (Nippon Shokubai, Japan) were purchased for use in this study. Hydrochloric acid (HCl, Sigma-Aldrich), sodium hydroxide (NaOH, Sigma-Aldrich), and levofloxacin (Tokyo Chemical Industry, TCI, Japan) were also obtained. All chemicals were of analytical grade and used without further purification. Solutions were prepared in deionized water (pH 6.5–7) throughout the experiments.
Synthesis of ZIF-8
ZIF-8 is synthesized using the co-precipitation method as shown in Fig. 1a. This method involves the simultaneous precipitation of zinc ions and organic linkers, typically 2-methylimidazole, leading to the formation of ZIF-8 crystals. The co-precipitation technique is favoured for its simplicity and efficiency, enabling the production of ZIF-8 with desirable properties for various applications32. In a typical synthesis method, 1.78 g of Zn (NO3)2·6H2O and 5.26 g of 2-methylimidazole were dispersed in two separate beakers containing 40 ml of methanol each. The suspension was then stirred at room temperature for 6 h. The product was recovered by centrifugation and washed three times with fresh methanol. It was then dried at 80 °C for 12 h, followed by activation in a vacuum oven for an additional 12 h.
Synthesis of ZIF-8/Gr
The synthesis of ZIF-8/Gr was achieved using the hydrothermal method as shown in Fig. 1b. First, 9 g of GO was dispersed in deionized water and sonicated for 3–5 h, ensuring complete dispersion. Following this, 30 mg of as-synthesized ZIF-8 was added to the GO suspension, and the mixture was stirred at 400 rpm for 1 h using a magnetic stirrer. The resulting mixture was then transferred into a 200 mL Teflon-lined autoclave and subjected to hydrothermal treatment at 180 °C for 18 hours. After cooling to room temperature, the ZIF-8/Gr suspension was filtered via vacuum filtration and thoroughly washed with ethanol and distilled water to remove any residual impurities. The filtered product was dried in an oven at 80 °C for 12 h. Finally, the dried composite was carefully collected, ground into a fine powder, and stored for further characterization and analysis. Notably, the resulting carbonaceous phase will be referred to as “Gr”, representing hydrothermally reduced GO.
Characterization
XRD measurements were carried out using a Shimadzu XRD-6000 diffractometer. Scanning was conducted in the 2θ range of 10–80° with a step size of 0.02° and a scanning speed of 1°/min. Crystallite sizes were calculated using the Scherrer equation, focusing on shifts in diffraction patterns to observe any phase changes due to Fe incorporation. FTIR was performed on an Agilent Cary 630 FTIR spectrometer with the KBr pellet method. Spectra were recorded over the 400–4000 cm−1 range with baseline and atmospheric corrections applied, identifying changes in functional groups due to Fe doping. The morphology of the MDW was accessed on a JCM-6000 Versatile Benchtop Scanning Electron Microscope, JEOL Ltd, Japan. Samples were coated with a thin carbon layer (~ 5 nm) to enhance conductivity. UV–Visible absorption spectra were recorded using a Shinco S-3100 spectrophotometer. Absorption edges and bandgap energies (Eg) were determined by constructing Tauc plots.
Photodegradation of LVX
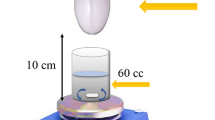
The photocatalytic performance of bare ZIF-8 and ZIF-8/Gr were assessed by examining the degradation of LVX under low intensity UV irradiation. The photocatalytic reactions were carried out in a custom-designed wooden chamber using an Aquarium UV-Sterilizer Lamp (13W) as the light source. Prior to photo-illumination, the system was maintained in the dark for 30 min to evaluate the contribution of the adsorption process. 3.5 mL aliquots were collected at regular intervals to monitor the degradation process. The concentration of LVX in each sample was determined using an IKEMELAB UV1200 UV–Vis spectrophotometer, which operates in the wavelength range of 190–1100 nm. The maximum absorbance wavelength (λmax) for LVX was set at 290 nm. The degradation efficiency was calculated using the following formula:
where C0 represents the initial concentration of LVX, and Ct denotes the concentration of the pollutant after irradiation at time, t minutes.
Antibacterial test
The disk diffusion method was employed to evaluate the antibacterial activity of the prepared ZIF-8/Gr composite. Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli bacteria were grown in Luria Bertania (LB) broth at 37 °C overnight. The bacterial suspensions were inoculated on Mueller–Hinton agar plates at a density of 1.5 × 106 CFU/mL and adjusted to a turbidity of 0.5 McFarland standard. Sterile filter discs (6 mm in diameter) impregnated with 1 mg/mL of ZIF-8/Gr suspensions, dissolved in DMF as the solvent, were prepared separately. Standard susceptibility discs soaked in streptomycin (30 μg) and chloramphenicol (30 μg) were used as positive controls, while discs soaked in sterile deionized water served as the negative control. Four sample holes were made on the agar plates to dispense the suspension of ZIF-8/Gr, streptomycin, chloramphenicol and deionized water. The filter paper discs were placed on top of the Mueller–Hinton agar plates, which were then incubated at 37 °C for 24 h. The antibacterial activity of the ZIF-8/Gr was evaluated by measuring the the zone of inhibition (ZOI) (mm) using a Vernier calliper. All plates. All experiments were carried out in triplicate and data were expressed as mean ± standard deviation (SD).
Antioxidant test
The antioxidant activity of the ZIF-8/Gr was evaluated using the DPPH (2,2-diphenyl 1-picrylhydrazyl) radical scavenging assay. The test is crucial for determining the material’s ability to neutralize harmful free radicals, a key factor in potential environmental applications. The ZIF-8/Gr was dissolved in two different solvents—dimethylformamide (DMF), and methanol to assess how solvent choice influences antioxidant activity. For the DPPH assay, a 1.9 mM solution of DPPH in methanol was prepared, and 0.1 mL of each ZIF-8/Gr solution was added to 2 mL of DPPH solution. The mixtures were incubated in the dark at room temperature for 30 min to avoid light interference. The colour change is determined by measuring absorbance with a spectrophotometer at 517 nm.
Results and discussion
Figure 2a displays the SEM image of reduced Gr, which reveals a characteristic wrinkled and sheet-like structure33,34,35. These morphological features are consistent with the inherent properties of Gr-based materials, providing a high surface area and structural flexibility. The wrinkled surface morphology of Gr facilitates the dispersion of ZIF-8 particles and offers abundant active sites for interaction, making it an ideal substrate for composite formation. Figure 2b shows the SEM image of ZIF-8, which displays irregularly shaped aggregates with a rough and porous surface morphology. Unlike the ideal polyhedral crystals commonly associated with ZIF-8, the observed structure indicates a deviation likely due to synthesis parameters or agglomeration effects36. The rough surface and porosity suggest potential utility in adsorption and catalytic applications, as the increased surface area can provide enhanced interaction sites. The sharp contrast and distinct dispersion confirm the spatial integration of ZIF-8 onto the Gr substrate as shown in Fig. 2c. The Gr network appears as a continuous flexible support, while the ZIF-8 nanocrystals are embedded or partially protruding from the sheet surface, indicating strong interfacial interaction. This structural arrangement promotes interfacial charge transfer, contributing to the enhanced photocatalytic performance discussed later.
The FTIR spectrum of bare ZIF-8 depicted in Fig. 3, reveal distinct vibrational bands characteristic of its structure. Prominent peaks between 900–1500 cm−1 correspond to the C–N stretching and bending vibrations from the imidazole rings that form the ZIF-8 framework. The sharp bands at 995 and 1142 cm−1 correspond to the characteristic stretching vibrations of the C–N bond in the imidazole ring, while the peaks at 1307 and 1145 cm−1 are indicative of the in-plane bending vibrations of the imidazole ring. Similarly, peaks at 1307, 1145, and 993 cm−1 are assigned to the in-plane bending signals of the imidazole ring, reinforcing the notion that these vibrational modes are integral to the structural integrity of ZIF-837. Additionally, C-H stretching vibrations are observed around 2930–2970 cm−1, indicating the presence of alkyl groups within the imidazole linkers. The absence of significant absorption bands beyond 3500 cm−1 suggests minimal surface hydroxylation or moisture adsorption, confirming that the bare ZIF-8 retains its structural integrity without notable modification by environmental exposure. Gr exhibited a broad absorption band between 3000–3500 cm−1, which corresponds to O–H stretching, likely due to the presence of residual hydroxyl groups or adsorbed water, as noted by. The peak at 1700 cm−1 corresponds to the carbonyl group, consistent with previous findings by Zheng and co-workers, who reported similar carbonyl stretching vibrations in their analysis of rGO38,39. Additionally, a prominent peak between 1560 and 1650 cm−1 is attributed to C=C stretching vibrations, confirming the presence of conjugated double bonds in the Gr structure. The weak peaks observed between 1000 and 1500 cm−1 suggest the existence of residual oxygen-containing groups, which could indicate incomplete reduction of GO, showing some retained oxygen functionalities in the graphitic material, a characteristic supported by40. The FTIR spectrum of the ZIF-8/Gr shows a combination of features from both ZIF-8 and Gr, confirming successful integration of the two materials. The characteristic C–N stretching vibrations of ZIF-8 from the imidazole rings appeared to reduce in intensity. This is likely due to the interaction between the functional groups of Gr and the ZIF-8 framework, which can lead to a reduction in the intensity of specific FTIR peaks associated with ZIF-8, such as those related to the imidazole linkers41. “The diminished or less pronounced vibrational bands associated with the imidazole ring (particularly C–N stretching and in-plane bending modes) in the ZIF-8/Gr composite are duly noted. However, such attenuation is consistent with literature reports where strong interfacial interactions between ZIF-8 and Gr may lead to peak suppression or overlap, specifically in the region 900–1500 cm−1 (Jamil et al.41; Zhan et al.37). This is typically attributed to π–π stacking, hydrogen bonding, or physical embedding of ZIF-8 particles into the Gr matrix, which may obscure some functional group vibrations. Despite the reduction in intensity, key vibrational modes around 1145 cm−1 and 1307 cm−1, corresponding to in-plane imidazole ring vibrations, are still discernible (reduced intensity), indicating partial retention of the ZIF-8 structure. Furthermore, the absence of sharp new peaks or strong shifts suggests no decomposition into secondary frameworks.”
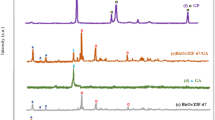
The X-ray diffraction (XRD) patterns of ZIF-8, Gr, and the ZIF-8/Gr composite are presented in Fig. 4. The XRD pattern of bare ZIF-8 displays sharp and intense peaks, particularly in the low-angle region (5°–20°), indicative of its high crystallinity. The prominent diffraction peaks at 7.3°, 10.4°, 12.7°, 14.7°, and 18.0° (2θ) are consistent with the cubic crystal structure of ZIF-8, as reported previously42. These peaks correspond to the (011), (002), (112), (022), and (013) planes, respectively, confirming the successful synthesis of ZIF-8. The crystalline size of ZIF-8 was estimated using the Debye–Scherrer equation, focusing on the most prominent peak at 2θ = 7.3°. The calculation yielded a crystalline size of approximately 15.92 nm. The Gr exhibits a broad peak centered at approximately 26.3° (2θ), corresponding to the (002) plane of graphitic carbon. The broad nature of this peak is indicative of disordered stacking of Gr layers and partial reduction of oxygen-containing groups. Additionally, a secondary peak at 44° is observed, attributed to the (101) plane, suggesting some retained graphitic domains43. This pattern confirms the presence of a partially reduced Gr structure with a mixture of amorphous and crystalline phases. The XRD pattern of the ZIF-8/Gr shows diminished low-angle ZIF-8 peaks and the emergence of new peaks > 40°, consistent with partial transformation into ZnO during hydrothermal treatment. This confirms that the final material is a ZnO/Gr hybrid derived from ZIF-8. The disappearance or attenuation of ZIF-8’s characteristic low-angle peaks (e.g., 2θ = 7.3°, 10.4°, 12.7°) and the appearance of new peaks at higher angles (> 40°) suggest that partial thermal decomposition of ZIF-8 may have occurred during hydrothermal treatment at 180 °C. This transformation is likely facilitated by the presence of Gr sheets which may promote the formation of ZnO or other zinc-rich phases, as also reported in studies where ZIF-8 was treated hydrothermally in oxidative or acidic environments44,45. The peaks appearing above 40° are consistent with the (101), (102), and (110) planes of wurtzite ZnO, suggesting partial conversion of ZIF-8 to ZnO during the composite synthesis. This phase evolution does not negate composite formation but rather indicates that the final product is a ZIF-8-derived ZnO/Gr hybrid, as observed in other metal-organic framework (MOF)-derived systems.
The UV–Vis absorption spectra and the corresponding Tauc plots of ZIF-8, Gr and the ZIF-8/Gr composite are shown in Fig. 5a,b, respectively. The absorption spectrum of ZIF-8 displays a distinct absorption band in the ultraviolet region below 300 nm, characteristic of its electronic transitions46. This is attributed to ligand-to-metal charge transfer (LMCT) within the ZIF-8 framework. The relatively sharp absorbance peak reflects the intrinsic properties of the ZIF-8 crystal structure. The reduced Gr exhibits a broader absorption profile, with a peak centered around 270 nm, corresponding to the π–π ∗ transitions of the conjugated sp2 bonded carbon framework39,47. The broad nature of the spectrum across a wide range of wavelengths indicates its semiconducting properties with some optical absorption in the visible region. For the ZIF-8/Gr composite, the spectrum shows features from both ZIF-8 and Gr. The enhanced absorption across the UV and visible regions suggests strong interactions between ZIF-8 and the Gr, which enhance light absorption and broaden the composite’s spectral response. This synergistic effect can be attributed to the electronic coupling and interfacial charge transfer between ZIF-8 and Gr. The optical band gaps (Eg) of the materials were calculated using the Tauc plot method, which involves plotting (αhν)2 versus photon energy (hν) and extrapolating the linear portion of the curve to the x-axis. The calculated Eg values for ZIF-8, Gr and ZIF-8/Gr is 3.89, 3.23 and 3.49 eV, respectively. The observed optical band gap of 3.49 eV arises due to quantum confinement effects, residual oxygen-containing functional groups, and lattice defects present in the rGO structure. This is consistent with previous literature, where rGO samples with varying degrees of reduction show band gaps typically in the range of 2.5–3.5 eV depending on synthesis conditions48. The intermediate band gap of ZIF-8/Gr reflects the synergistic interaction between ZIF-8 and Gr, likely resulting from charge transfer at the interface, which alters the electronic structure of the composite. The UV–Vis and Tauc plot analysis confirm the successful integration of ZIF-8 with Gr. The reduced band gap of the composite, compared to bare ZIF-8, implies improved light-harvesting properties and enhanced electronic interactions. It is noted that the UV–Vis absorbance of the ZIF-8/Gr composite appears to approach the upper detection limit of the instrument, particularly in the UV region below 300 nm. The strong absorbance is likely due to the combined effect of ZIF-8’s ligand-to-metal charge transfer (LMCT) and rGO’s broad π–π* transitions. While this may introduce slight deviations in absorbance intensity, the bandgap estimation derived from the Tauc plot remains consistent with values reported for similar ZIF-derived Gr hybrids.
Photodegradation test
The photocatalytic degradation performance of Gr, ZIF-8, and ZIF-8/Gr was evaluated over a 120-min period under low-intensity UV light irradiation, preceded by a 30-min dark phase to establish adsorption equilibrium. As shown in Fig. 6a, during the dark phase, adsorption of LVX onto the catalyst surfaces was observed, with ZIF-8/Gr exhibiting the highest adsorption capacity due to its enhanced surface area and abundant active sites provided by the synergistic interaction of ZIF-8 and Gr. This adsorption phase is crucial as it ensures that any initial reduction in LVX concentration arises from adsorption alone, thereby providing a baseline for distinguishing between adsorption and photocatalytic mechanisms49,50. ZIF-8/Gr demonstrated the highest degradation efficiency, achieving a C/C₀ value of 0.80, followed by Gr and ZIF-8 with values of 0.81 and 0.84, respectively. Gr, despite its remarkable electrical conductivity and high surface area, has limited intrinsic light absorption, which restricts its direct photocatalytic performance. Furthermore, its thin optical thickness and short-lived photogenerated carriers complicate photocurrent formation, further reducing its light absorption efficiency. Bare ZIF-8 recorded the lowest degradation rate and kinetic constant, a result of its poor stability in aqueous conditions51. The dissolution of ZIF-8 in water leads to the formation of zinc and imidazolate ions, which negatively impact its performance. Figure 6b depicts the corresponding rate constants (k) calculated using pseudo-first-order (PFO) kinetics which follow the trend k (ZIF-8/Gr) > k (Gr) > k (ZIF-8). The superior performance of ZIF-8/Gr is attributed to the synergistic interaction between ZIF-8 and Gr, which improves charge separation, reduces electron–hole recombination, and facilitates the generation of ROS. While Gr’s photocatalytic performance is inherently limited by its lack of a bandgap, its high surface area and adsorption capacity enhance its overall activity. ZIF-8, by contrast, suffers from poor aqueous stability, which limits its standalone utility. Integrating Gr with ZIF-8 addresses these shortcomings by improving charge transport and providing additional active sites for pollutant adsorption and degradation.
The amount of catalyst used in photocatalytic degradation plays a critical role in determining the efficiency and rate of the reaction. In this study, three different catalyst loadings of ZIF-8/Gr—5 mg, 10 mg, and 20 mg—were investigated for the photocatalytic degradation of LVX. The results presented in Fig. 7a indicate a direct correlation between the amount of catalyst used and the efficiency of the photocatalytic degradation process. Increasing the catalyst loading from 5 to 20 mg led to a significant improvement in degradation efficiency. The findings aligned with existing literature, which suggests that insufficient catalyst quantities can result in suboptimal photocatalytic performance52. At 5 mg, the degradation efficiency was minimal, with rate constant (k) of 5.64 × 10−4 min−1 after 120 min. Increasing the catalyst loading to 10 mg resulted in k value of 0.00152 min−1, reflecting a notable enhancement in degradation efficiency. The highest loading of 20 mg achieved the maximum performance, with k value of 0.00187 min−1. This enhanced performance is attributed to the increased availability of active sites, which facilitates greater generation of ROS and accelerates the photocatalytic degradation process53. Higher catalyst concentrations generally promote faster reactions due to more efficient utilization of light and reactive species. However, beyond a certain threshold, further increases in catalyst loading may lead to diminishing returns due to excessive turbidity in the reaction mixture, which scatters light and diminishes its effectiveness. High turbidity reduces light penetration, thereby decreasing the effective photon absorption and limiting the overall photocatalytic efficiency54. The degradation kinetics is depicted in Fig. 7b. The results clearly indicate that increasing the catalyst loading from 5 to 20 mg resulted in a 3.3-fold increase in the rate constant.
In this study, the influence of initial LVX concentrations on the photocatalytic degradation efficiency was evaluated using three concentrations: 5 mg/L, 10 mg/L, and 20 mg/L. The results presented in Fig. 8a demonstrate an inverse correlation between the initial pollutant concentration and the degradation efficiency. At a lower concentration of 5 mg/L, the degradation efficiency was the highest, with LVX concentration reducing to 3.686 mg/L after 120 min. For 10 mg/L, the degradation was moderately efficient, with the concentration dropping to 8.126 mg/L, while for 20 mg/L, the degradation was minimal, with the concentration stabilizing at 18.08 mg/L. This behavior is attributed to the saturation of active sites on the catalyst surface at higher pollutant concentrations, which limits the degradation rate due to competitive adsorption and insufficient active sites. The degradation kinetics were also analyzed using the PFO model, as shown in Fig. 8b. The rate constant (k) values decreased with increasing LVX concentration, reflecting reduced degradation efficiency at higher concentrations. Specifically, the k value was 0.00219 min−1 for 5 mg/L, 0.00187 min−1 for 10 mg/L, and 5.87 × 10−4 min−1 for 20 mg/L. These results indicate a significant 3.7-fold decrease in the rate constant when the initial LVX concentration increased from 5 mg/L to 20 mg/L. This trend highlights the critical role of pollutant concentration in determining the efficiency and kinetics of the photocatalytic process. The reduced efficiency at higher concentrations may also result from the absorption of incident light by LVX molecules, which reduces the light available for photocatalytic activation of the catalyst55.
The effect of solution pH on the photocatalytic degradation LVX was systematically investigated, as shown in Fig. 9a. Three pH levels were studied: acidic (pH 4.01), neutral (pH 7.01), and basic (pH 9.15). The results indicate that the neutral pH condition provided the highest degradation efficiency, with the C/C₀ value decreasing to 0.74 after 120 min. In contrast, the degradation efficiency was significantly lower under acidic (pH 4.01) and basic (pH 9.15) conditions, with C/C₀ values of 0.93 and 0.97, respectively, over the same duration. The reduced efficiency at acidic pH can be attributed to protonation of the catalyst’s active sites, which hinders interactions between the catalyst and LVX molecules56. At basic pH, the generation of hydroxyl ions competes with ROS formation, further limiting the degradation process57.
The degradation kinetics were analyzed as depicted in Fig. 9b. The rate constant (k) was highest under neutral conditions, with a value of 0.00283 min−1, compared to 5.63 × 10−4 min−1 at pH 4.01 and 3.12 × 10−4 min−1 at pH 9.15. These findings highlight a strong linear correlation between − ln(C/C₀) and time at neutral pH, indicative of efficient degradation kinetics. The lower rate constants at acidic and basic pH levels reflect the suboptimal interactions between the catalyst and LVX under these conditions. Overall, the results emphasize that maintaining a neutral pH is critical for maximizing photocatalytic degradation efficiency and optimizing reaction kinetics.
The role of ROS in the photocatalytic degradation of LVX by ZIF-8/Gr was investigated using radical scavengers, as shown in Fig. 10. Various scavengers were introduced to assess the involvement of superoxide radicals (·O2)−, hydroxyl radicals (·OH), and photogenerated holes (h+) in the degradation process, following similar approaches used in previous studies on radical trapping mechanisms58. The baseline degradation efficiency without any scavenger was 26%, serving as a control for comparison. The addition of ascorbic acid, a known scavenger of ·O2,− led to a notable decrease in the degradation rate to around 10%, indicating that ·O2 − play a significant role in the degradation of LVX, consistent with findings from related work on the photocatalytic degradation of similar pollutants. EDTA, which effectively scavenges h+, caused the most dramatic reduction in degradation, down to about 5%, suggesting that h+ are a major contributor to the reaction mechanism. These findings aligned with prior studies emphasizing the importance of h+ in catalytic degradation processes59. On the other hand, the introduction of methanol and tert-butyl alcohol, which are scavengers for ·OH, resulted in more moderate reductions in degradation rates, approximately 10% and 15%, respectively. This suggests that ·OH are involved in the degradation process but are not the primary reactive species compared to ·O2− and h+. The results of the radical trapping experiments indicated that the degradation of LVX is primarily mediated by the following reactive species: h+ > ·O2− > ·OH. These findings provide a basis for optimizing photocatalyst designs to enhance ROS production and improve overall degradation efficiency.
The reusability and stability of the ZIF-8/Gr catalyst were evaluated through three successive photocatalytic degradation cycles of LVX, as shown in Fig. 11. The initial LVX concentration was controlled at 5 mg/L, and the reaction conditions were kept constant to assess the consistency of the photocatalytic degradation performance. After each trial, the catalyst was centrifuged at 4000 rpm to separate it from the water, washed with ethanol three times, and dried for 12 h at 80 °C to ensure reusability in subsequent trials. The initial degradation efficiency was 23.92% in the first cycle but decreased to 10.04% and 5.71% in the second and third cycles, respectively. This decline in performance can be attributed to potential catalyst deactivation caused by several factors, including the accumulation of residual by-products on the catalyst surface, incomplete regeneration during washing and drying, and structural degradation due to repeated use60. Ethanol washing, while effective for initial cleaning, may have altered the surface structure of the ZIF-8/Gr composite, reducing its active sites and photocatalytic activity61. Additionally, photo-corrosion or leaching of active components, commonly observed in some MOFs, could contribute to the observed reduction in catalytic performance over multiple cycles62. Despite the decrease in degradation efficiency, the ZIF-8/Gr composite demonstrated adequate reusability for practical applications, as the catalyst retained a portion of its activity after three cycles. Further optimization of catalyst regeneration methods, such as alternative washing techniques or surface reactivation treatments, may improve its long-term stability. These findings highlight the importance of addressing catalyst durability to ensure sustained performance in large-scale photocatalytic applications.
Possible photodegradation mechanism of LVX
A potential photocatalytic degradation mechanism for the ZIF-8/Gr composite under low-power intensity UV light irradiation can be proposed as shown in Fig. 12. The conduction band (CB) of ZIF-8 lies at approximately – 0.9 V vs NHE, while its valence band (VB) lies above + 3.0 V. Upon UV irradiation, electrons are excited from the VB to the CB. These electrons are transferred to Gr, whose Fermi level (~ – 0.2 V) makes it an ideal sink, facilitating charge migration and inhibiting recombination. The presence of Gr plays a crucial role in this process, acting as an electron percolation network63. The remaining holes in the VB participate in the generation of hydroxyl radicals (·OH), while electrons on Gr reduce O2 to superoxide radicals (·O2−). This directional charge transfer underpins the observed synergistic enhancement in photocatalytic degradation.
This interfacial electron transfer reduces the likelihood of electron–hole recombination within ZIF-8, thereby enhancing the availability of charge carriers for ROS generation. The transferred electrons reduce dissolved oxygen to superoxide radicals (·O2−), while the remaining holes in ZIF-8 oxidize water or OH− to generate ·OH. These reactions are in agreement with the scavenger experiments, which identified h+ and ·O2− as the dominant species. Thus, the ZIF-8-to-Gr charge transfer pathway is central to the composite’s improved photocatalytic performance under energy-constrained UV conditions. The degradation of LVX via this mechanism can be expressed through the following sequence of reactions:
-
1.
Photoexcitation:
ZIF-8/Gr + hν (UV light) → ZIF-8/Gr (e− + h+)
-
2.
Charge separation enhancement by Gr:
Gr reduces electron–hole recombination, improving the efficiency of charge carriers.
-
3.
Reactive species formation:
h+ + H2O → ·OH
e− + O2 → ·O2−
-
4.
Degradation of LVX:
LVX + h+/·OH /·O2− → Oxidized intermediates → CO2 + H2O
The possible degradation pathway of LVX during the photocatalytic reaction is also proposed based on literature and theoretical understanding64,65. The degradation process begins with the excitation of ZIF-8/Gr under UV light, generating ROS that interact with the LVX, initiating oxidative breakdown at specific functional groups. The degradation starts with the decarboxylation of the methylmorpholine group in LVX resulting in a fragment with a molecular ion peak close to m/z = 336, indicating the loss of a carboxyl group. The degradation is followed by oxidation of the piperazine ring in which the N-methyl piperazine ring undergoes oxidation, forming a stable intermediate. Subsequently, de-alkylation reactions, defluorination and hydroxylation were predicted. The intermediates will undergo further oxidative cleavage, breaking down into small organic acids and eventually mineralizing into carbon dioxide and water. This hypothesized pathway highlights the stepwise transformation of LVX into smaller intermediates, driven by the synergistic effects of ZIF-8/Gr. One previous study using visible-light-driven peroxymonosulfate activation over CuInS2/g-C3N4 proposed four distinct LVX pathways (e.g., hydroxylation, fluorine removal, piperazine cleavage, and C-N bond cleavage)66. The proposed mechanism provides insights into how LVX undergoes oxidative degradation, emphasizing the role of ROS in breaking down complex structures into simpler, environmentally benign products. Although no LC–MS or GC–MS analyses were conducted in this study, a potential sequence of intermediate transformations is hypothesized to elucidate the degradation mechanism.
Antibacterial test
The antibacterial activity of ZIF-8/Gr dissolved in DMF was evaluated against two bacterial strains: E. coli and S. aureus. The results, as depicted in Fig. 13 and summarized in Table 1, indicate that ZIF-8/Gr exhibited a moderate antibacterial effect, particularly when compared to standard antibiotics such as chloramphenicol and streptomycin. The mechanical damage is a primary mechanism by which Gr exerts its bactericidal effects, as demonstrated by the ability of Gr nanospikes to physically rupture bacterial membranes and induce oxidative stress, which together enhance their antibacterial efficacy67. Previous study posited that the excellent antibacterial activity of GO based Co-MOF is linked to synergistic effect of sharp edges of the GO sheets and the toxic effect of cobalt ions Co2+ which are released from their surfaces68.
For E. coli, the ZIF-8/Gr composite exhibited notable antibacterial activity, with a ZOI measuring 1.4 ± 0.85 cm. This result indicates the material’s potential to inhibit the growth of this Gram-negative bacterium. While the ZOI is smaller compared to conventional antibiotics like chloramphenicol and streptomycin, the results indicate that ZIF-8/Gr is still effective to some degree against E. coli. In contrast, for S. aureus, the ZIF-8/Gr results are considered negative in terms of antibacterial activity, with a ZOI of 0.9 ± 0.85 cm, which is quite low. This suggests that the material has little to no significant antibacterial effect against this Gram-positive bacterium69. The ZOI is much smaller compared to those of the control antibiotics, indicating that ZIF-8/Gr is not effective against S. aureus. The differential antibacterial activity of ZIF-8/Gr against E. coli and S. aureus can be attributed to several factors, including the structural differences between Gram-negative and Gram-positive bacteria, the specific properties of the ZIF-8/Gr, and the mechanisms of action involved. E. coli has an outer membrane that can act as a barrier to certain antibacterial agents, whereas S. aureus, lacks this outer membrane but has a thicker peptidoglycan layer69. It is important to note that DMF employed as a solvent control, exhibited minimal to no antibacterial activity, with ZOIs of 1.5 ± 0.80 cm for E. coli and 0.90 ± 0.85 cm for S. aureus. These findings reinforce the hypothesis that the observed antibacterial effects are attributable to the ZIF-8/Gr material itself, rather than the solvent70. Overall, while ZIF-8/Gr shows potential as an antibacterial agent, further optimization may be required to enhance its effectiveness, particularly in comparison to established antibiotics.
Antioxidant test
The DPPH assay was further used to evaluate the antioxidant activity of the ZIF-8/Gr composite, specifically its ability to scavenge free radicals, which are reactive species capable of causing cellular and tissue damage. As illustrated in Fig. 14a,b, no observable color change was detected in the DPPH assay, indicating that ZIF-8/Gr does not effectively donate electrons to neutralize the DPPH radical. This result suggests that the material lacks significant antioxidant activity. In the context of photocatalytic applications, particularly in wastewater treatment, the absence of antioxidant properties in nanomaterials can be advantageous. A lack of electron-donating activity implies that the material will not neutralize ROS, which are essential for the degradation of organic pollutants. Therefore, the limited antioxidant activity of ZIF-8/Gr may enhance its photocatalytic efficiency by allowing the material to fully participate in ROS-driven reactions, which are critical for the breakdown of pollutants.
Although Gr has previously been reported to exhibit antioxidant behaviour, this property is highly dependent on its surface chemistry. The antioxidant capabilities of Gr are closely tied to the presence of functional groups, such as hydroxyl or carboxyl groups, which facilitate interactions with free radicals71. However, these functional groups were likely absent or reduced in the ZIF-8/Gr composite due to the hydrothermal treatment employed during synthesis, which may have significantly reduced oxygenated functional groups on the surface. These functional groups play a key role in the physical adsorption of superoxide anion radicals, thereby enhancing Gr’s antioxidant capacity, ZIF-8, on the other hand, is primarily recognized for its catalytic and adsorption properties rather than its antioxidant potential. The Zn ions in ZIF-8, along with its imidazolate linkers, are not structurally equipped to engage in electron donation or free radical scavenging, which are typical mechanisms involved in antioxidant processes. Moreover, the structural stability of MOFs under oxidative conditions can be a limiting factor. For instance, Mn-based MOFs have demonstrated oxidative degradation under such conditions, which could compromise their functionality72. Therefore, the absence of antioxidant properties in ZIF-8/Gr aligns with its design and purpose in photocatalysis rather than radical scavenging.
Cost analysis
The cost analysis for the synthesis of ZIF-8/Gr composite was conducted to evaluate its economic viability for potential large-scale applications. The synthesis of ZIF-8/Gr was evaluated for cost efficiency, yielding approximately 4.5 g of the catalyst at a total production cost of RM 242.95, which corresponds to RM 53.99 per gram as shown in Table 2. This analysis considered the cost of all raw materials, including Zn(NO₃)2·6H2O, 2-Methylimidazole and GO. The calculations were based on current market prices and the quantities of each material used in the optimized synthesis procedure. The total raw material cost is at RM 217.18. A key observation from this analysis is the significant contribution of Gr to the overall cost. While ZIF-8 synthesis itself is relatively inexpensive due to the readily available and affordable precursors, the incorporation of Gr substantially increases the final product cost. This cost increase is attributed to the more complex production processes and higher market value associated with Gr materials. This finding highlights a critical trade-off in the development of ZIF-8/Gr composites. While rGO imparts desirable properties such as improved stability, and increased surface area, its inclusion comes at a considerable economic premium. It is also important to note that economies of scale could potentially reduce the cost per gram in larger production runs. The hydrothermal method employed for synthesizing ZIF-8/Gr is energy-intensive (RM 6.48) but offers high yields and superior material properties, such as controlled crystallite size and phase purity. The operational costs associated with maintaining a reaction temperature of 180 °C for 15 h are a significant factor. The operational energy requirement for photocatalytic applications is notably low due to the catalyst’s ability to function under low-intensity UV light. Using a low-power (13 W) UV lamp significantly reduces electricity costs, making this catalyst ideal for low-energy, environmentally friendly applications.
Conclusion
This study synthesized a ZIF-8/Gr composite via a hydrothermal method and evaluated its performance for the photocatalytic degradation of LVX under low-intensity UV irradiation. The ZIF-8/Gr composite exhibited improved charge separation, enhanced ROS (·OH, ·O2−, h+) generation, and moderate degradation efficiency compared to its individual components. The optimal condition of 20 mg catalyst in 5 mg/L LVX solution at neutral pH; achieved a 25.70% degradation after 120 min. While this efficiency is relatively low for practical wastewater treatment applications, the ability to function under low-energy UV light presents potential for energy-constrained or pre-treatment settings. Additionally, the composite demonstrated antibacterial activity against E. coli in dark conditions, highlighting its dual functionality. However, the reusability test revealed a marked decline in performance over three cycles, indicating a need for improved regeneration strategies or catalyst stabilization. Overall, this work contributes to the ongoing development of MOF-based photocatalysts and provides foundational insights into the synergistic interactions between ZIF-8 and Gr. Future studies should aim to enhance degradation efficiency, investigate pollutant mineralization pathways through LC–MS validation, and improve long-term catalyst stability for real-world applications.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Bashir, I. et al. Concerns and threats of contamination on aquatic ecosystems. In Bioremediation and Biotechnology 1–26 (Springer, 2020). https://doi.org/10.1007/978-3-030-35691-0_1
Lin, L., Yang, H. & Xu, X. Effects of water pollution on human health and disease heterogeneity: A review. Front. Environ. Sci. 10, 880246 (2022).
Tiwari, A., Kapoor, R., Jain, J. & Sankhla, J. Water purifications methods in Ayurveda and contemporary methods. 10 (2022).
Huang, A. et al. A review of processes for removing antibiotics from breeding wastewater. Int. J. Environ. Res. Public Health 18, 4909 (2021).
Anuar, N. F. et al. The removal of antibiotics in water by chemically modified carbonaceous adsorbents from biomass: A systematic review. J. Clean. Prod. 401, 136725 (2023).
Mangla, D., Annu Sharma, A. & Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard Mater. 425, 127946 (2022).
Dutta, J. & Mala, A. A. Removal of antibiotic from the water environment by the adsorption technologies: A review. Water Sci. Technol. https://doi.org/10.2166/wst.2020.335 (2020).
Peng, B. et al. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π–π interactions. Sci. Rep. 6, 31920 (2016).
Grandclément, C. et al. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 111, 297–317 (2017).
Alvarino, T., Suarez, S., Lema, J. & Omil, F. Understanding the sorption and biotransformation of organic micropollutants in innovative biological wastewater treatment technologies. Sci. Total Environ. 615, 297–306 (2018).
Cheng, D. L. et al. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 621, 1664–1682 (2018).
Liu, P., Zhang, H., Feng, Y., Yang, F. & Zhang, J. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 240, 211–220 (2014).
Iyyappan, J. et al. Critical review on wastewater treatment using photo catalytic advanced oxidation process: Role of photocatalytic materials, reactor design and kinetics. Case Stud. Chem. Environ. Eng. 9, 100599 (2024).
Ahtasham Iqbal, M. et al. Advanced photocatalysis as a viable and sustainable wastewater treatment process: A comprehensive review. Environ. Res. 253, 118947 (2024).
Lan, J., Wang, Y., Huang, B., Xiao, Z. & Wu, P. Application of polyoxometalates in photocatalytic degradation of organic pollutants. Nanoscale Adv. 3, 4646–4658 (2021).
Wu, J., Wu, D., Peng, W., Ji, Y. & Tong, H. Research progress of polyoxometalates photocatalyst for degradation of organic wastewater. Appl. Chem. Eng. 5, 92–106 (2022).
Malik, A., Nath, M., Mohiyuddin, S. & Packirisamy, G. Multifunctional CdSNPs@ZIF-8: Potential antibacterial agent against GFP-expressing Escherichia coli and Staphylococcus aureus and efficient photocatalyst for degradation of methylene blue. ACS Omega 3, 8288–8308 (2018).
Yang, R.-G. et al. ZIF-8/covalent organic framework for enhanced CO2 photocatalytic reduction in gas-solid system. Chem. Eng. J. 450, 138040 (2022).
Wang, T. et al. Thermally treated zeolitic imidazolate framework-8 (ZIF-8) for visible light photocatalytic degradation of gaseous formaldehyde. Chem. Sci. 11, 6670–6681 (2020).
Senapati, D., Swain, J. & Priyadarshini, A. Photocatalytic dye degradation by ZIF-8@BiVO4 composite. https://doi.org/10.21203/RS.3.RS-4599818/V1 (2024)
Guo, R. et al. Facile synthesis of BIVO4@ZIF-8 composite with heterojunction structure for photocatalytic wastewater treatment. Materials 14, 7424 (2021).
Han, X. et al. A bifunctional BiOBr/ZIF-8/ZnO photocatalyst with rich oxygen vacancy for enhanced wastewater treatment and H2O2 generation. Molecules 28, 2422 (2023).
Novoselov, K. S. et al. A roadmap for graphene. Nature 490, 192–200 (2012).
Zheng, A. L. T. & Andou, Y. Hybrid three-dimensional (3D) graphene architectures for photocatalysis of noxious pollutants. In Green Nanoarchitectonics 47–72 (Jenny Stanford Publishing, 2022). https://doi.org/10.1201/9781003318606-3
Zheng, A. L. T., Ohno, T. & Andou, Y. Recent progress in photocatalytic efficiency of hybrid three-dimensional (3D) graphene architectures for pollution remediation. Top. Catal. 65, 1634–1647 (2022).
Zheng, A. L. T., Sabidi, S., Ohno, T., Maeda, T. & Andou, Y. Cu2O/TiO2 decorated on cellulose nanofiber/reduced graphene hydrogel for enhanced photocatalytic activity and its antibacterial applications. Chemosphere 286, 131731 (2022).
Min, H. S. et al. Graphene oxide nanocomposites for the photodegradation of phenol derivatives and pharmaceutical active compounds. In Reference Module in Materials Science and Materials Engineering (Elsevier, 2024). https://doi.org/10.1016/B978-0-323-95486-0.00002-8
Qiu, M. et al. A novel adsorptive and photocatalytic system for dye degradation using ZIF-8 derived carbon (ZIF-C)-modified graphene oxide nanosheets. J. Taiwan Inst. Chem. Eng. 143, 104674 (2023).
Ahmad, U. et al. ZIF-8 composites for the removal of wastewater pollutants. ChemistrySelect 9, 64 (2024).
Li, Y., Zhang, X., Huang, C. & Lyu, H. A g-C3N4/Mn3O4/ZIF-8 composite for efficient degradation of dyes by activating peroxymonosulfate. Opt. Mater. (Amst) 149, 114994 (2024).
Sarwar, B. et al. Comparative study of ZIF-8-materials for removal of hazardous compounds using physio-chemical remediation techniques. Environ. Res. 220, 115168 (2023).
Chin, M. et al. Rhodamine B degradation by nanosized zeolitic imidazolate framework-8 (ZIF-8). RSC Adv. 8, 26987–26997 (2018).
Sethumadhavan, S. C., Pottail, L., Sharma, S. C., Chithambharan, A. & Ballal, S. Structural and morphological characterization of bio-templated reduced graphene oxide and their antibacterial efficacy. J. Clust. Sci. 33, 1997–2008 (2022).
Viprya, P., Kumar, D. & Kowshik, S. Study of different properties of graphene oxide (GO) and reduced graphene oxide (rGO). In RAiSE-2023 84 (MDPI, Basel Switzerland. https://doi.org/10.3390/engproc2023059084 (2023).
Zheng, A. L. T., Boonyuen, S., Ohno, T. & Andou, Y. Accessing effects of aliphatic dicarboxylic acid towards the physical and chemical changes in low temperature hydrothermally reduced graphene hydrogel. J. Porous Mater. 28, 1291–1300 (2021).
Xing, X. et al. ZIF-8 micro-polyhedron MOF-transformed ZnO/ZnFe2O4 nanosheets for highly selective detection of ppb-level isoprene. Sens. Actuat. B Chem. 372, 132669 (2022).
Zhan, Y., Wang, Y., Wang, M., Ding, X. & Wang, X. Improving the curing and mechanical properties of short carbon fibers/epoxy composites by grafting nano ZIF-8 on fibers. Adv. Mater. Interfaces 7, 1901490 (2020).
Zheng, A. L. T., Boonyuen, S., Ohno, T. & Andou, Y. Hydrothermally reduced graphene hydrogel intercalated with divalent ions for dye adsorption studies. Processes 9, 169 (2021).
Zheng, A. L. T., Boonyuen, S., Li, G. Y., Ngee, L. H. & Andou, Y. Design of reduced graphene hydrogel with alkylamine surface functionalization through immersion/agitation method and its adsorption mechanism. J. Mol. Struct. 1245, 131008 (2021).
Fu, H., Qu, X., Chen, W. & Zhu, D. Transformation and destabilization of graphene oxide in reducing aqueous solutions containing sulfide. Environ. Toxicol. Chem. 33, 2647–2653 (2014).
Jamil, N. et al. Green one-pot synthesis and characterisation of hybrid reduced graphene oxide/zeolitic imidazole framework-8 (rGO/ZIF-8). J. Iran. Chem. Soc. 18, 363–373 (2021).
Wang, S. & Zhang, S. Study on the structure activity relationship of ZIF-8 synthesis and thermal stability. J. Inorg. Organomet. Polym. Mater. 27, 1317–1322 (2017).
Chadha, N., Sharma, R. & Saini, P. A new insight into the structural modulation of graphene oxide upon chemical reduction probed by Raman spectroscopy and X-ray diffraction. Carbon Lett. 31, 1125–1131 (2021).
Mani, G., Kumar, A. V. & Mathew, S. ZIF-8 derived ZnO: A facile catalyst for ammonium perchlorate thermal decomposition. RSC Sustain. 1, 2081–2091 (2023).
Ikram, M., Lv, H., Liu, Z., Shi, K. & Gao, Y. Hydrothermally derived p–n MoS2–ZnO from p–p MoS2-ZIF-8 for an efficient detection of NO2 at room temperature. J. Mater. Chem. A Mater. 9, 14722–14730 (2021).
Xiao, J. et al. Zinc-metal–organic frameworks with tunable UV diffuse-reflectance as sunscreens. J. Nanobiotechnol. 20, 87 (2022).
Emiru, T. F. & Ayele, D. W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 4, 74–79 (2017).
Lian, K.-Y. et al. Big bandgap in highly reduced graphene oxides. J. Phys. Chem. C 117, 6049–6054 (2013).
Samriti, M., Chen, Z., Sun, S. & Prakash, J. Design and engineering of graphene nanostructures as independent solar-driven photocatalysts for emerging applications in the field of energy and environment. Mol. Syst. Des. Eng. 7, 213–238 (2022).
Li, X., Yu, J., Wageh, S., Al-Ghamdi, A. A. & Xie, J. Graphene in photocatalysis: A review. Small 12, 6640–6696 (2016).
Jafari, S., Pourmortazavi, S. M., Ehsani, A. & Mirsadeghi, S. CuO-ZIF-8 modified electrode surface as a new electrochemical sensing platform for detection of free chlorine in aqueous solution. Sci. Rep. 14, 18961 (2024).
Kaur, A. et al. Visible light driven photocatalytic degradation of fluoroquinolone levofloxacin drug using Ag2O/TiO2 quantum dots: A mechanistic study and degradation pathway. New J. Chem. 41, 12079–12090 (2017).
Iqbal, R. M. A. et al. Development of Ag0.04ZrO2/rGO heterojunction, as an efficient visible light photocatalyst for degradation of methyl orange. Sci. Rep. 12, 1–12 (2022).
Zhang, D., Lv, S. & Luo, Z. A study on the photocatalytic degradation performance of a [KNbO3]0.9-[BaNi0.5Nb0.5O3−δ]0.1 perovskite. RSC Adv. 10, 1275–1280 (2020).
Hadi, A., Niaei, A., Seifi, A. & Rasoulzadeh, Y. The impact of operational factors on degradation of formaldehyde as a human carcinogen using Ag3 PO4/TiO2 photocatalyst. Health Promot. Perspect. 13, 47–53 (2023).
Rincón, A. G. & Pulgarin, C. Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2: Implications in solar water disinfection. Appl. Catal. B 51, 283–302 (2004).
Rodríguez, E. M., Fernández, G., Álvarez, P. M., Hernández, R. & Beltrán, F. J. Photocatalytic degradation of organics in water in the presence of iron oxides: Effects of pH and light source. Appl. Catal. B 102, 572–583 (2011).
Luo, J., Zhou, X., Zhang, J. & Du, Z. Fabrication and characterization of Ag2CO3/SnS2 composites with enhanced visible-light photocatalytic activity for the degradation of organic pollutants. RSC Adv. 5, 86705–86712 (2015).
Yu, Z. et al. Photoatalytic degradation of organic pollutants in water under visible light by NH2-MIL-125(Ti-Zr)@BiOClxI1-x composite photocatalyst. ChemistrySelect 7, e202201958 (2022).
Piera, E., Ayllón, J., Doménech, X. & Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 76, 259–270 (2002).
Ataee Khorrami, M., Sohrabnezhad, S. & Asadollahi, A. Enhanced visible-light-active MOF-based PbO photocatalyst for binary dye decolorization: A comprehensive study. J. Mol. Liq. 402, 124722 (2024).
Muelas-Ramos, V., Belver, C., Rodriguez, J. J. & Bedia, J. Synthesis of noble metal-decorated NH2-MIL-125 titanium MOF for the photocatalytic degradation of acetaminophen under solar irradiation. Sep. Purif. Technol. 272, 118896 (2021).
Tiong, P., Lintang, H. O., Endud, S. & Yuliati, L. Improved interfacial charge transfer and visible light activity of reduced graphene oxide–graphitic carbon nitride photocatalysts. RSC Adv. 5, 94029–94039 (2015).
Prabavathi, S. L., Saravanakumar, K., Park, C. M. & Muthuraj, V. Photocatalytic degradation of levofloxacin by a novel Sm6WO12/g-C3N4 heterojunction: Performance, mechanism and degradation pathways. Sep. Purif. Technol. 257, 117985 (2021).
Rameel, M. I., Wali, M., Al-Humaidi, J. Y., Liaqat, F. & Khan, M. A. Enhanced photocatalytic degradation of levofloxacin over heterostructured C3N4/Nb2O5 system under visible light. Heliyon 9, e20479 (2023).
Zhong, X. et al. Enhanced degradation of levofloxacin through visible-light-driven peroxymonosulfate activation over CuInS2/g-C3N4 heterojunctions. Nanomaterials 14, 74 (2023).
Zheng, A. L. T. et al. Accessing the anti-microbial activity of cyclic peptide immobilized on reduced graphene oxide. Mater. Lett. 304, 130621 (2021).
Hatamie, S. et al. Antibacterial properties of nanoporous graphene oxide/cobalt metal organic framework. Mater. Sci. Eng. C 104, 109862 (2019).
Li, P. et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 10, 1–10 (2019).
Chauhan, H. P. S., Carpenter, J. & Joshi, S. Mixed bis(morpholine-4-dithiocarbamato-S,S′)antimony(III) complexes: Synthesis, characterization and biological studies. Appl. Organomet. Chem. 28, 605–613 (2014).
Demianenko, E., Sencha-Hlevatska, K., Sementsov, Y. & Kartel, M. Quantum-chemical investigation of the superoxide radical scavenging by graphene oxide surface. Low Temp. Phys. 49, 1088–1092 (2023).
Khramenkova, E. V., Polynski, M. V., Vinogradov, A. V. & Pidko, E. A. Degradation paths of manganese-based MOF materials in a model oxidative environment: A computational study. Phys. Chem. Chem. Phys. 20, 20785–20795 (2018).
Acknowledgements
This research was funded by Universiti Putra Malaysia under grant GP-IPM/9750400. No other funding sources were utilized. The funder played no part in the study’s design, data acquisition, analysis, publication decision, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
C.M.M.: Conceptualization, Methodology, Investigation, Data curation, Writing—Original Draft. A.L.T.Z.: Supervision, Funding acquisition, Writing – Review & Editing, Project administration. R.K.: Formal analysis, Validation, Resources, Writing—Review & Editing. S.S.: Experimental design, Visualization, Software, Data analysis. T.Y.Y.H.: Interpretation of analytical results, Resources. S.B.: Antibacterial testing, Data interpretation, Writing—Review & Editing. J.L.: Literature review, Manuscript proofreading, Reference management. Y.A.: Conceptual advice, Graphene-related methodology, Critical revision of the manuscript. T.K.B.: Formal analysis, Validation. All authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors reviewed and agreed to the final manuscript for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Manivel, C.M., Zheng, A.L.T., Kannan, R. et al. ZIF-8/graphene composite for photocatalytic degradation under low intensity UV irradiation and antibacterial applications . Sci Rep 15, 33875 (2025). https://doi.org/10.1038/s41598-025-07444-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07444-1
Keywords
This article is cited by
-
Duckweed-derived magnetic adsorbent for efficient removal of methylene blue
Biomass Conversion and Biorefinery (2026)