Abstract
Recent studies suggest that high-dose hemodiafiltration (HDF) may reduce mortality more effectively than high-flux hemodialysis (HD), though the mechanisms remain unclear. Traditional metrics such as Kt/V and convective volume do not fully capture overall dialysis efficiency. This study proposes a novel approach using circulating beta-2-microglobulin (ß2M) levels to estimate an equivalent Continuous Dialytic Clearance (eCDCß2M), reflecting an equivalent glomerular filtration rate. Using data from the FRENCHIE study, we calculated eCDCß2M and assessed its association with patient outcomes, including all-cause and cardiovascular mortality, in comparison with traditional dialysis dose metrics. Our analysis showed that HDF achieved higher treatment efficiency than high-flux HD, with a mean increase of + 1.5 ml/min in eCDCß2M. Moreover, eCDCß2M demonstrated superior predictive value for mortality risk compared to Kt/V. These findings support eCDCß2M as a meaningful and physiologically relevant measure of dialysis efficiency and adequacy. By better reflecting the continuous function of the native kidney, this approach may improve patient stratification and outcome prediction across all forms of kidney replacement treatment schedule. Further validation in independent patient cohorts is warranted.
Similar content being viewed by others
Introduction
Several recent studies, including the CONVINCE trial and the updated individual patient data meta-analysis, have demonstrated that high-dose hemodiafiltration (HDF) is superior to high-flux hemodialysis (HD) in reducing all-cause mortality risk for end-stage kidney disease (ESKD) patients treated by dialysis1,2,3,4,5. However, the exact mechanisms and biomarkers supporting these benefits remain debated or hypothetical6. While the removal of larger uremic toxins is a potential explanation, quantifying the true efficiency of these kidney replacement therapies is challenging in clinical practice7,8,9,10.
Current approaches to quantifying the efficiency of kidney replacement treatment rely on surrogate markers. In hemodialysis, a diffusive-based modality, Kt/V, a dimensionless ratio, estimates the sessional fractional urea clearance11,12,13. In HDF, a convective-based modality, the convective volume delivered per session serves as a proxy of middle and large sized uremic solute clearances14,15,16,17,18,19,20. These methods have limitations, as highlighted by the CONVINCE study, which did not report any specific biomarker to support the beneficial effects of HDF except for these two surrogate markers of small and middle-sized solute clearance3. Combining clearances of small and middle to large molecular weight uremic markers will provide a more comprehensive approach to quantify overall efficiency across a larger spectrum of uremic compounds and all dialysis modalities21,22. Therefore, a more objective measure reflecting overall kidney replacement therapy efficiency and more closely resembling the continuous function of native kidney function is needed.
In this context, beta-2 Microglobulin (ß2M) is potentially a highly valuable biomarker for this purpose23,24. Established in renal physiology, circulating ß2M levels or ß2M clearances can be used to estimate glomerular filtration rate (GFR)23,25. This characteristic makes it a promising biomarker to quantify overall kidney replacement therapy efficiency, including residual kidney function23,24,25,26. Furthermore, circulating levels of ß2M go beyond kidney function or dialysis efficiency, as ß2M may also serve as a cardiovascular risk marker27, relevant not only for chronic kidney disease patients but also for those with other acute or chronic disease conditions28,29,30,31.
In this study, our aim was to explore an innovative approach to estimating the equivalent of a continuous dialytic clearance based on circulating levels of ß2M (eCDCß2M). To achieve this, we utilized the dataset from the French HDF study (FRENCHIE trial), which collected plasma/serum samples at various time points during the study and conducted centralized laboratory testing on various biomarkers, including ß2M, as part of the two-year follow-up32. Subsequently, we calculated an equivalent of glomerular filtration rate namely equivalent Continuous Dialytic Clearance (eCDCß2M) based on ß2M concentrations established by the CKD-EPI consortium23,33,34. Additionally, we assessed the effect of this eCDCβ2M equivalent of glomerular filtration rate on patient survival and compared this to conventional urea derived clearances by evaluating the predictive effect on patient survival in ESKD patients treated by both hemodialysis and hemodiafiltration.
Material and methods
Study design: the FRENCHIE trial
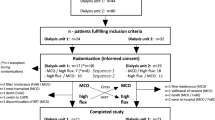
This study is a post hoc analysis of the FRENCHIE trial, which was a multicenter prospective randomized trial with open labeling32. The original study enrolled 381 elderly (over 65 years old) prevalent chronic hemodialysis (HD) patients, out of which 286 were studied as they had blood samples stored for post hoc analysis. These patients were divided equally (1:1 ratio) into two groups: one receiving high-flux HD and the other receiving postdilution HDF. The primary endpoint was to assess patient outcome (intradialytic morbidity) according to the dialysis modality from day 30 to day 120. Secondary outcomes included biological markers associated with cardiovascular disease, hospital admissions for any cause and those specifically due to cardiovascular conditions, death from any cause and cardiovascular-related deaths over a two-year follow-up period.
Detailed steps for calculating eCDC from time averaged β2M (ß2MTAC) concentrations and kidney replacement therapy efficiency indicators are provided in the appendix. Postdialysis β2M was adjusted to align with a two-compartment model by accounting for extracellular volume contraction and incorporating a 60-min postdialysis equilibration phase, as described by Ward et al.35. The midweek time-averaged concentration of β2M was calculated using the pre and post hemodialysis concentrations from the mid-week session.
Calculation of kidney replacement therapy efficiency indicators
Percent reduction (PR) of urea was calculated individually as:
where ureapre and ureapost refer to pre and post dialysis urea concentrations in mmol/L.
Single pool Kt/V urea was calculated individually using the Daugirdas formula:
where Ureapre and Ureapost refer to pre and post dialysis urea concentrations in mmol/L, BWpre and BWpost for pre and post body weight in kg and tHD for treatment duration in hours. Postdialysis blood samples were taken after reducing blood pump for about 3 min.
Calculation of equivalent Continuous Dialytic Clearance from ß2M (\({eCDC}_{\ss 2M}\)) circulating concentrations
To estimate the equivalent continuous dialytic clearance (\({eCDC}_{\ss 2M}\)), we used two midweek ß2M plasma concentration measurements (pre and post dialysis) collected during the FRENCHIE trial, applying an emulation of a two-compartment kinetic model of ß2M. To correct for extracellular volume contraction due to ultrafiltration, the post-dialysis ß2M concentrations were adjusted using Bergström’s formula36. Additionally, to account for the compartmentalization effect and 60-min rebound, the Tattersall equation was applied37,38. Pre- and post-dialysis ß2M concentrations (ß2Mpre and ß2Mpost) were measured using the COBAS Roche platform, with the Tina-quant β2-Microglobulin assay performed on an automated analyzer (Cobas 8000, Roche Diagnostics, Meylan, France).
Corrected postdialysis ß2M concentrations for hemoconcentration:
The correction accounts for extracellular volume changes due to net ultrafiltration and is calculated as:
where, WL represents the weight loss during dialysis (in kg) used as proxy for ultrafiltration volume; \(O.2 . {BW}_{post}\) estimates the extracellular volume (ECV) in liters, based on post-dialysis body weight; the ratio \(\left(WL/0.2 x {BW}_{post}\right)\) serves as a proxy for hemoconcentration (ECV contraction) resulting from fluid removal.
Postdialysis ß2M concentration corrected for the compartmentalization effect (two pool model):
This used Tattersall’s equation, which estimates the equilibrated postdialysis ß2M concentration by accounting for a 60-min rebound period, thereby reflecting the compartmentalization effect as reported in Ward’s two-pool ß2M kinetic model39.
The percent reduction of ß2M (\({PR}_{\ss 2M}\)), in%, was calculated individually as:
The Time Averaged Concentration of ß2M \({(\ss 2M}_{TAC}\) ), in mg/l was calculated as follows:
This formula uses the mid-week logarithmic mean value of ß2M as the best estimate of its concentration over the dialysis cycle. This approach has been recently validated in a direct dialysate quantification study40:
Effective ß2M clearance (\({K}_{\ss 2M}\)) in ml/min, was calculated as:
where ECV refers to extracellular volume as \({O.2 x BW}_{post}\) in liters, \({\ss 2M}_{pre}\) and \({\ss 2M}_{post.eq}\) are the predialysis and postdialysis ß2M concentrations respectively, in mg/l and \({t}_{HD}\) is the dialysis treatment duration in minutes.
ß2M mass removal (\({\ss 2M}_{MR}\)), expressed in mg per session was calculated as:
with \({\ss 2M}_{TAC}\) in mg/l, \({K}_{\ss 2M}\) in ml/min and \({t}_{HD}\) in minutes.
Calculation of estimated Continuous Dialysis Clearance of ß2M (\({eCDC}_{\ss 2M}\)), expressed in ml/min, from the time-averaged concentration ( \({\ss 2M}_{TAC}\))
\({\ss 2M}_{TAC}\) and \({eCDC}_{\ss 2M}\) were calculated individually for the entire population (including both HDF and HD patients) and averaged over the two year-follow-up period. Please note that two to three values per patient were used for these calculations.
Endpoints
Patient all-cause and cardiovascular mortality were defined as the time from study inclusion to death from all-cause and the time from study inclusion to death from cardiovascular cause, respectively. Patient deaths were classified as either having occurred or not from all causes at the completion of the study.
Ethics
After approval by the ethics committee at Montpellier University Hospital, the FRENCHIE trial was registered with the French Ministry of Health and Solidarity ("Ministère de la Santé et des Solidarités"). Funding was exclusively provided by the Ministry of Health through a program called " Hospital Clinical Research Project" (PHRC) and registered as Clinical Trial ID# NCT01327391. All procedures were conducted in strict accordance with relevant guidelines and regulations. Written informed consent was obtained from all participating patients to ensure compliance with ethical standards.
Statistical analysis
Descriptive statistics of patient characteristics, dialysis prescription and dialysis efficiency parameters were obtained for all groups (HDF or high-flux HD) and analyzed using R software. Quantitative variables were described using medians and interquartile ranges (25th and 75th percentiles) and compared between groups using the Wilcoxon rank-sum test. Qualitative variables were described using frequency counts and percentages. Comparison between groups was performed using either Fisher exact test or the Chi-square test for qualitative variables, depending on the expected counts. Dialysis parameters were collected at baseline, 12 and 24 months. The average over these time points was computed for each parameter and used for descriptive analysis. eCDCß2M was compared between treatment modalities (HDF vs. HD) using the Wilcoxon rank-sum test.
The survival curves for all-cause mortality comparing eCDCβ2M values categorized into three groups based on tertiles from the total cohort were analyzed using Kaplan–Meier plots. The Peto and Peto log-rank test for crossing curves was used to compare survival between groups. Cardiovascular mortality for eCDCβ2M groups was estimated based on a competing risk analysis considering other types of death as competing events. Cumulative incidence functions for cardiovascular mortality were calculated and the Gray test used to compare curves.
The effect of eCDCß2M and Kt/V on patient death for the total cohort was analyzed using a multivariate Cox proportional hazards model. In order to explore the potential effect of other factors affecting the risk of death; age, sex and comorbidities (diabetes, hypertension, heart failure, ischemic cardiac disease and arrhythmia) were included in the model, alongside eCDCß2M or Kt/V. A multivariate model selection based on a step backward approach was performed and the final model only included statistically significant variables.
Results
A total of 286 patients from the original FRENCHIE cohort (n = 386) were included in the present analysis, 144 were treated by HD and 142 by HDF. The 286 patients were selected on the availability of a consistent number of blood samples, all of which were collected and processed centrally. Standardized ß2M measurements were performed in a uniform and centralized manner. The main findings of the study are presented and organized into four sections:
This section presents the baseline characteristics of the study population, followed by an analysis of treatment prescription and efficiency of the two dialysis modalities.
Table 1 details the baseline characteristics of the elderly stage 5 CKD dialysis (CKD5D) population (median age 76.2 years, 60.5% male, median post-dialysis body weight 68.7 kg) evenly divided between high-flux HD and hemodiafiltration (HDF). The two treatment groups were well balanced and did not show statistically significant difference in terms of comorbidities; 39.0% had diabetes mellitus, 76.6% hypertension, 14.9% congestive heart failure, 35.8% ischemic cardiac disease, 12.1% arrhythmia, 2.8% heart valve disease, and 2.5%-6.8% had peripheral vascular disease, along with similar sex distribution and anthropometric characteristics including body weight. Pre- to post-dialysis body weights were averaged over multiple time points to assess session weight loss as a surrogate of net ultrafiltration volume. Inflammatory status assessed using high-sensitivity CRP showed no significant differences (p 0.311) between groups (median [Q1; Q3] CRP level: 5.1 [2.6;12.6] mg/L in HD vs. 7.0 [3.0;15.7] mg/L in HDF). Importantly, all patients were prevalent dialysis patients, with a mean dialysis vintage of 4.6 years in the HD group and 5.0 years in the HDF group. Residual kidney function was considered negligible across the entire cohort, as patients had no significant residual urine output at baseline.
Table 2 outlines the dialysis prescription and effective treatment delivered over the two-year follow-up period. Across the entire cohort, the median session time was 240 min based on thrice-weekly treatments. The median blood flow was 336.7 ml/min with a dialysate flow of 500 ml/min, and average weight loss of 2.1 kg per session, corresponding to a net ultrafiltration rate (NET QUF) of 8.7 ml/min. All patients were treated using single-use high-flux synthetic dialyzers with a median surface area of 1.8 m2. The choice of dialyzer was left to the discretion of the prescribing physician, and brand names were not collected for confidentiality reasons. Compared to HD, the HDF group had significantly higher blood flow (345.0 ml/min vs. 325.8 ml/min; p = 0.038) and achieved substitution and total ultrafiltration flows [Refer to Appendix for Complementary Information] of 89.0 and 97.7 ml/min, respectively, resulting in a median total ultrafiltration volume of 23.2 L per HDF session.
Table 3 presents key metrics of dialysis efficiency and adequacy. HDF demonstrated superior solute clearance compared to high-flux HD. The reduction ratio for small solute urea was higher in the HDF group (78.5% vs. 75.9%; p = 0.008), along with greater single-pool Kt/V (1.79 vs. 1.66; p = 0.009) and total Kt (64.8 vs. 62.4 l/session; p = 0.013). Middle molecule clearance using ß2M-related parameters also favored HDF. The ß2M reduction ratio was significantly higher in the HDF group (69.6% vs.58.7%; p < 0.001) as was the effective ß2M clearance (59.3 vs. 44.1 ml/min; p < 0.001) and ß2M mass removal (208.5 vs 183.2 mg/session; p = 0.002), resulting in a lower ß2M time averaged concentration (β2M TAC: 17.3 vs. 15.1; p < 0.001). Nutritional parameters, including normalized protein catabolic rate (nPCR, g/kg/24h) and serum albumin, were comparable between groups. In terms of middle molecule clearance, HDF significantly outperformed HD, achieving a ß2M reduction ratio of 69.6% versus 58.7%, along with superior clearance and mass removal, resulting in lower ß2M plasma concentrations.
This section reports treatment efficiency as assessed by the estimated continuous dialytic clearance of ß2M (eCDCß2M) and explores its association with the convection volume delivered during dialysis.
Figure 1 shows eCDCß2M values stratified by treatment modality presented as medians with interquartile ranges over a two-year period. Patients treated with HDF exhibited significantly higher eCDCβ2M values compared to those on HD, with a median of 13.2 ml/min versus 11.7 ml/min (p < 0.001), suggesting that HDF provided an additional 1.5 ml/min of effective ß2M clearance under comparable clinical conditions.
Figure 2 shows box plots of log-transformed eCDCβ2M values plotted against convective volume (CV) per session, grouped into four predefined CV groups across the entire cohort. The relationship between CV and eCDCβ2M can be estimated using the equation log (eCDCβ2M) = 2.42 + 0.0007 CV (p < 0.0001), indicating a statistically significant linear increase in log(eCDCβ2M) with increasing CV. The first CV group (0–15 L/session) corresponds to patients whose convective volume reflects net ultrafiltration aimed solely at fluid removal. Median log(eCDCβ2M) values for each group, using eCDCβ2M values averaged over two years, demonstrated a consistent upward trend with increasing CV, highlighting the positive impact of higher convection volumes on middle molecule clearance efficiency.
This section examines the prognostic value of the estimated continuous dialytic clearance of ß2M (eCDCß2M) on both all-cause and cardiovascular mortality.
Figure 3 illustrates all-cause survival probability stratified by tertiles of eCDCβ2M for the combined cohort of HD and HDF patients. Patients in the highest tertile (> 13.52 ml/min) demonstrated significantly greater 2-year survival compared to those in the middle (11.23–13.52 ml/min) and lowest (≤ 11.23 ml/min) tertiles (p = 0.021). These findings suggest that a higher delivered dialysis dose, as reflected by eCDCβ2M above 13.52 ml/min, is associated with a meaningful survival benefit.
Figure 4 presents the cumulative incidence of cardio-vascular mortality for the same eCDCβ2M tertiles. In contrast to all-cause mortality, no statistically significant difference was observed between tertiles (p = 0.83), indicating that eCDCß2M may not independently predict cardiovascular mortality in this population.
This section reports the comparative predictive effect of eCDCß2M and urea-based Kt/V on the risk of all-cause mortality in the overall patient cohort.
Table 4 shows the results of Cox proportional hazard (PH) models evaluating the association of eCDCß2M and urea Kt/V with all-cause mortality in both HD and HDF patients. Higher eCDCβ2M values were significantly associated with a reduced mortality risk (HR 0.81; 95% CI [0.72; 0.92]), indicating a protective effect of greater middle molecule clearance. In contrast, urea-based Kt/V was not significantly associated with mortality (HR 0.81; 95% CI [0.28; 2.34]). Among clinical covariates, older age, female sex, diabetes, hypertension, and heart failure did not show a significant impact on mortality risk. However, the presence of ischemic heart disease was associated with an increased risk of death (HR 1.9; 95% CI [1.10; 3.40]). Notably, CRP levels did not significantly influence mortality when modeled alongside eCDCß2M.
Table 5 shows a multivariate Cox PH model after covariable selection, focusing on the predictive value of eCDCß2M for all-cause mortality. Consistent with the previous model, eCDCβ2M remained a strong and independent predictor of survival (HR 0.82; 95%CI [0.74; 0.92)] with higher values associated with a greater protective effect. In this refined model, ischemic cardiac disease continued to be significantly associated with increased mortality (HR 2.04; 95% CI [1.20; 3.60]).
Table 6 presents a breakdown of mortality and hospitalization by cause, expressed as events per 100 patient-years, extrapolated from the original study report. As shown, there is a trend toward lower mortality with HDF compared to HD 11.51 vs. 13.82 for all-cause mortality and 21.74 vs. 26.35 for cardiac-related deaths. However, these differences, including those related to hospitalization causes, did not reach statistical significance.
Discussion
The original aim and findings of this study were to quantify and compare the efficiency of kidney replacement therapies (HD vs. HDF) using a biomarker reported to reflect glomerular filtration rate and providing a proxy for a continuous dialysis clearance, allowing for a comparison with native kidney function. For this purpose, we utilized plasma ß2M levels measured in patients enrolled in our previous FRENCHIE study32. All ß2M samples were measured in a central laboratory, so reducing or mitigating inter-laboratory variations.
As established by the CKD-EPI consortium and subsequently validated by Argyropoulos and colleagues, using a large dataset of patients collected through their meta-analyses spanning various stages of chronic kidney disease and utilizing advanced simulation modeling, plasma ß2M concentrations may be accurately correlated with measured glomerular filtration rates (GFR)23,24,26, with plasma ß2M concentrations displaying an exponential correlation with glomerular filtration rate. To address fluctuations in plasma ß2M levels from varying HD and HDF treatments, we calculated the time-averaged concentration of ß2M (ß2MTAC) over the mid-week dialysis session40,41 for each patient. This midweek ß2MTAC was used to estimate the equivalent continuous dialytic clearance (eCDCß2M) achieved by the two treatment modalities.
In brief, postdilution HDF with a medium convection volume averaging 23.2 L per session provides significantly higher efficiency across all molecular weight spectrums for small and middle molecules as represented by ß2M. As reported in our study, HDF yields a 12.7% lower β2MTAC (15.1 mg/l) compared to HD (17.3 mg/l), and HDF yields a 12.8% higher eCDCß2M (13.2 ml/min) than high-flux HD (11.7 ml/min). Other indicators of dialysis efficiency for urea Kt/V, percent reduction of urea and β2M, as well as effective ß2M clearances (59.3 vs 44.1 ml/min) and β2M mass removal (209.5 vs 183.2 mg per session) confirmed the superiority of HDF compared to high-flux HD. Furthermore, the superior efficiency of HDF may be summarized in a + 1.5 ml/min eCDCß2M for the same treatment time and conditions compared to a standard 4-h HD-treatment. In addition, the data show that eCDCß2M increases as the total convection volume rises, reinforcing the survival benefits of delivering higher convective volumes to patients. However, the number of sessions achieving high convective volumes (> 25 L) was limited, so preventing further exploration of the impact of these higher volumes on eCDCß2M.
Interestingly, survival expectancy, as calculated by Kaplan Meyer analysis across the entire population and stratified by tertiles of eCDCß2M, confirmed the survival advantage of higher eCDCß2M values (> 13.52 ml/min) compared to the lower tertiles. The higher predictive and protective value of eCDCß2M, as compared to Kt/V, was further supported by Cox regression multivariate analysis, even after adjusting for confounding factors including advanced age, female sex, hypertension, cardiac ischemic disease, and inflammation.
Notably, the use of eCDCß2M offers a practical marker for assessing the overall kidney replacement therapy efficiency, including when applicable, the contribution of residual kidney function42,43,44,45. Several studies have consistently highlighted the significant impact of residual kidney function on lowering ß2M circulating levels and improving patient outcomes in a dose-dependent manner46,47. Furthermore, ß2M levels have been proposed as a useful tool to guide and tailor incremental dialysis regimens, whether peritoneal dialysis or hemodialysis48,49,50,51,52,53,54,55. By utilizing eCDCβ2M, dialysis patient management can be simplified, as it eliminates the need for routine urine collection and the calculation of residual kidney function. This streamlines the process for clinicians and healthcare providers while reducing the burden on dialysis patients.
This proposed concept of eCDCβ2M should be integrated into the ongoing search for more suitable markers of dialysis efficiency, alongside traditional urea fractional clearance (Kt/V), particularly when considering convective-based therapies43. In this context, the metabolic, pharmacokinetic, and toxic properties of ß2M make it an ideal biomarker for this purpose. ß2M reflects kidney function, inflammation, cell activation, and the risk of poor outcomes associated with uremic toxin exposure24,26,56,57. Our approach and findings suggest that eCDCß2M may offer a novel metric for assessing dialysis efficiency, one that is more closely aligned with the native glomerular filtration rate (GFR). Additionally, it provides a means to compare the efficiency of various treatment regimens, from incremental to intensive dialysis, as well as conventional thrice-weekly sessions. This concept is in line with a previous proposal by Casino and co-workers, which proposed the use of a more appropriate metric than urea Kt/V to assess effective dialysis efficiency, advocating an equivalent of urea clearance as a proxy marker of a weekly continuous clearance58,59. Despite the interest in this original concept, the Casino formula never really gained popularity as it was solely based on urea, a poor marker of uremic toxicity, and an inadequate indicator of glomerular filtration rate58,59.
Using ß2M kinetic modeling analysis or percent reduction, it has been demonstrated that HDF provides a removal rate capacity up to 30% higher than high flux HD39,60,61,62. Additionally, the percent reduction or effective clearance of ß2M is positively correlated with convection volume63,64. The superior efficiency of HDF has been confirmed using a global removal score approach that uses markers across a broad molecular weight spectrum, ranging from 60 Daltons to 41 kDa, and adjusted for albumin loss65,66. Such superior removal rate translates into higher mass removal that tends to reduce ß2M circulating levels over time. In this context, a post-hoc analysis of the HEMO study showed that predialysis ß2M concentrations represented a biomarker for an increased risk of mortality for HD patients, with a threshold value of 27 mg/l67,68. Interestingly, this relative risk increased up to 60% when ß2M levels reached 50 mg/l. The membrane permeability outcome (MPO) study subsequently showed in its primary outcome that high-flux HD improved survival expectancy of diabetic and hypoalbuminemic HD patients, suggesting a benefit of clearing middle molecular weight uremic compounds69. Unfortunately, ß2M concentrations were not reported in the MPO study. Based on these findings, the European Best Practices Guidelines pointed out the need for clearing and assessing middle molecule uremic toxins with a particular focus on ß2M. In addition, the Japanese Society for Dialysis Therapy included ß2M concentrations as a marker of HDF efficiency in their own best practice guidelines, setting pre-dialysis target levels of ß2M of 25 mg/l for HDF and 27 mg/l for high flux HD70.
Recent update reviews have confirmed the increasing interest in monitoring ß2M in chronic kidney disease patients for assessing both kidney function and mortality risk. This interest in ß2M is not only limited to dialysis, but also to other specialties including cardiology when assessing the risk of mortality following an acute ischemic cardiac event, confirming the predictive value of this biomarker as a proxy of endothelial dysfunction and more broadly vascular damage. Furthermore, ß2M kinetic modeling has recently been revisited by Ward et al. in an interesting approach based on real-world data71. They provided simulated clearances of ß2M and projected levels of time-averaged concentration of ß2M according to treatment modalities, comparing high flux HD and HDF, relying on various convection doses and sieving properties of membranes. Their kinetic modeling showed that the percent reduction of ß2M increased parabolically with increased substitution volume in a dose-dependent manner, consequently leading to a decrease in ß2MTAC. Additionally, using the CONVINCE simulated data, they forecasted that high-volume HDF would have decreased ß2MTAC to around 14 mg/l, compared to 16 mg/l in high-flux HD relying on the 26 L convection volume used, given that patients generate up to 240 mg of ß2M daily. Interestingly, their predictions for ß2M concentrations closely align with our current findings.
The strength of this study lies in the novel development of a new metric for dialysis efficiency closely aligned with native glomerular filtration rate, and by providing a precise method to compare dialysis modalities. As shown, in this analysis, HDF conducted within the same timeframe and virtually identical operating conditions, with a median convection volume of 23 L yielded a + 1.5ml/min equivalent glomerular filtration rate compared to high flux HD. This finding may align with previous studies that have emphasized the significant importance of residual kidney function on clinical benefits and improved patient outcomes. As indicated, survival benefits rely on an eGFR greater than 13.2 ml/min which can be achieved with a convection volume of 23 L per session of. Additionally, it has been demonstrated that increasing the convection volume to more than 25 L provides additional benefits, as it is associated with a higher eCDC. It can then be speculated that the 26,5 L of convection volume delivered in the CONVINCE study will correspond to about 16 ml/min and this may explain the survival benefit observed. Such an eGFR value may reflect the upper convective doses delivered in the ESHOL study, so explaining their reported clinical benefits.
The limitations of this study must be acknowledged. Firstly, it is a post hoc analysis of the FRENCHIE study, where the intended convective dose of 24 L per session was not uniformly achieved, potentially impacting the ability to show improved survival with HDF in the elderly population. Second, the narrow range of convective doses delivered constrained the precision of eCDCß2M estimates at higher convection volumes, particularly beyond 25 L per session. Third, the relatively limited number of cardiac events observed (as shown in Table 6) limited the statistical power to assess the relationship between eCDCß2M and cardiovascular mortality or hospitalization, precluding any definitive conclusion on this outcome. Fourth, plasma ß2M concentrations can be influenced by factors beyond dialysis efficiency and residual kidney function, such as infections and systemic inflammation. For this reason, regular ß2M measurements are recommended to establish a stable baseline and accurately assess treatment efficacy. Nevertheless, in our study, CRP levels did not suggest significant inflammatory activity, as reflected by their stable distribution across the patient quartiles. Fifth, residual kidney function was not factored into the analysis, as it was considered negligible due to the advanced dialysis vintage (mean 5 years) in this cohort. Nevertheless, it is important to note that in patients with preserved residual function, additional native ß2M clearance would likely contribute to higher eCDCß2M values and potentially better outcomes.
From a clinician’s perspective, as developed in this study, the use of eCDCß2M offers a novel integrated and goal-directed approach to assess effective dialysis efficiency across dialysis modalities, considering native glomerular filtration rate as reference, with prognostic value for patient outcomes. However, further and larger studies are necessary to validate the proposed concept of estimating eCDCß2M from circulating levels of ß2M in end-stage chronic kidney disease patients on maintenance dialysis. Additionally, comparing the effective eCDCß2M delivered in intensive dialysis such as nocturnal, alternate day, or daily treatment programs would be essential to link their better outcomes to some tangible biomarker. Despite its limitations, eCDCß2M may already provide a relatively simple and reliable metric to assess the treatment efficiency across all modalities of kidney replacement therapies, including the potential effect of residual kidney function.
Conclusion
ß2M-based eCDC provides a new tool that facilitates the comparison of dialysis efficiency across different modalities, incorporating residual kidney function. As demonstrated in our study, HDF offers higher treatment efficiency compared to high-flux HD, which can be quantified as an increase of + 1.5 ml/min in eCDCß2M for a substitution volume of 23 L. This novel approach aligns more closely with native kidney function, enhancing and allowing personalizing patient management as part of a goal-oriented strategy, and potentially improving patient outcomes. However, this concept requires further validation and exploration of its predictive value across diverse dialysis modalities and patient cohorts.
Data availability
The data supporting the findings of this study are not publicly available due to privacy concerns that could compromise the confidentiality of research participants. However, the data can be obtained from the corresponding author [BC] upon reasonable request, subject to appropriate ethical and privacy considerations.
References
Peters, S. A. et al. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol. Dial Transplant. 31(6), 978–984 (2016).
Davenport, A. et al. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: the effect of adjustment for body size. Kidney Int. 89(1), 193–199 (2016).
Blankestijn, P. J. et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 389(8), 700–709 (2023).
Maduell, F. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 24(3), 487–497 (2013).
Maduell, F. et al. Hemodiafiltration Reduces All-Cause and Cardiovascular Mortality in Incident Hemodialysis Patients: A Propensity-Matched Cohort Study. Am. J. Nephrol. 46(4), 288–297 (2017).
Canaud B, Blankestijn PJ, Grooteman MPC, Davenport A. Why and how high volume hemodiafiltration may reduce cardiovascular mortality in stage 5 chronic kidney disease dialysis patients? A comprehensive literature review on mechanisms involved. Semin Dial. (2021).
Daugirdas JT, Chan CT. Survival Benefit with Hemodiafiltration: Are We Convinced, and If So, What Might Be the Mechanism? Clin. J. Am. Soc. Nephrol. (2023).
Carney, E. F. CONVINCE trial reports a survival benefit of haemodiafiltration compared with haemodialysis. Nat. Rev. Nephrol. 19(8), 478 (2023).
Mayne, K. J. & Ronco, C. Will another trial CONVINCE nephrologists to adopt high-dose haemodiafiltration over conventional haemodialysis?. Clin. Kidney J. 16(12), 2393–2395 (2023).
Meena P, Locatelli F. Unmasking the CONVINCE trial: is hemodiafiltration ready to steal the spotlight in real-world practice? Clin. Kidney J. (2024).
Daugirdas, J. T. et al. Standard Kt/Vurea: a method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 77(7), 637–644 (2010).
Daugirdas JT, Leypoldt JK, Akonur A, Greene T, Depner TA, Group FHNT. 2013 Improved equation for estimating single-pool Kt/V at higher dialysis frequencies. Nephrol. Dial Transplant.. (2013).
Greene, T. et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias”. J. Am. Soc. Nephrol. 16(11), 3371–3380 (2005).
Bowry, S. K. & Canaud, B. Achieving high convective volumes in on-line hemodiafiltration. Blood Purif. 35(Suppl 1), 23–28 (2013).
Canaud, B. et al. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int. 88(5), 1108–1116 (2015).
Canaud, B. & Bowry, S. K. Emerging clinical evidence on online hemodiafiltration: does volume of ultrafiltration matter?. Blood Purif. 35(1–3), 55–62 (2013).
Canaud, B., Koehler, K., Bowry, S. & Stuard, S. What Is the Optimal Target Convective Volume in On-Line Hemodiafiltration Therapy?. Contrib. Nephrol. 189, 9–16 (2017).
Chapdelaine I, de Roij van Zuijdewijn CL, Mostovaya IM, Lévesque R, Davenport A, Blankestijn PJ, et al. Optimization of the convection volume in online post-dilution haemodiafiltration: practical and technical issues. Clin. Kidney J. (2015).
Maduell, F. Optimizing the prescription of hemodiafiltration. Contrib. Nephrol. 158, 225–231 (2007).
Maduell, F. Is There an “Optimal Dose” of Hemodiafiltration?. Blood Purif. 40(Suppl 1), 17–23 (2015).
Jaffrin, M. Y. Convective mass transfer in hemodialysis. Artif. Organs. 19(11), 1162–1171 (1995).
Jaffrin, M. Y., Ding, L. H. & Laurent, J. M. Simultaneous convective and diffusive mass transfers in a hemodialyser. J. Biomech. Eng. 112(2), 212–219 (1990).
Argyropoulos, C. P. et al. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front Med. (Lausanne). 4, 73 (2017).
Roumelioti, M. E., Nolin, T., Unruh, M. L. & Argyropoulos, C. Revisiting the Middle Molecule Hypothesis of Uremic Toxicity: A Systematic Review of Beta 2 Microglobulin Population Kinetics and Large Scale Modeling of Hemodialysis Trials In Silico. PLoS ONE 11(4), e0153157 (2016).
Achakzai MI, Argyropoulos C, Roumelioti ME. Predicting Residual Function in Hemodialysis and Hemodiafiltration-A Population Kinetic, Decision Analytic Approach. J. Clin. Med. (2019).
Roumelioti, M. E. et al. Beta-2 microglobulin clearance in high-flux dialysis and convective dialysis modalities: a meta-analysis of published studies. Nephrol. Dial Transplant. 33(6), 1025–1039 (2018).
Liabeuf, S. et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 82(12), 1297–1303 (2012).
Shi, F., Sun, L. & Kaptoge, S. Association of beta-2-microglobulin and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 320, 70–78 (2021).
Gong, S. et al. Elevated serum beta-2 microglobulin level predicts short-term poor prognosis of patients with de novo acute omicron variant COVID-19 infection. Front Cell Infect Microbiol. 13, 1204326 (2023).
Bethea, M. & Forman, D. T. Beta 2-microglobulin: its significance and clinical usefulness. Ann. Clin. Lab Sci. 20(3), 163–168 (1990).
Bataille R, Grenier J. Serum beta 2 microglobulin in multiple myeloma. A critical review. Eur J Cancer Clin. Oncol. (1987).
Morena, M. et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 91(6), 1495–1509 (2017).
Zhang, W. R. & Parikh, C. R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 81, 309–333 (2019).
Inker, L. A. et al. A New Panel-Estimated GFR, Including β(2)-Microglobulin and β-Trace Protein and Not Including Race, Developed in a Diverse Population. Am. J. Kidney Dis. 77(5), 673–83.e1 (2021).
Ward, R. A. & Daugirdas, J. T. Kinetics of β -2-Microglobulin with Hemodiafiltration and High-Flux Hemodialysis. Clin. J. Am. Soc. Nephrol. 19(7), 869–876 (2024).
Bergström, J. & Wehle, B. No change in corrected beta 2-microglobulin concentration after cuprophane haemodialysis. Lancet 1(8533), 628–629 (1987).
Tattersall, J. Clearance of beta-2-microglobulin and middle molecules in haemodiafiltration. Contrib. Nephrol. 158, 201–209 (2007).
Tattersall, J. E. & Ward, R. A. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol. Dial Transplant. 28(3), 542–550 (2013).
Ward, R. A., Greene, T., Hartmann, B. & Samtleben, W. Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int. 69(8), 1431–1437 (2006).
Paats, J. et al. Time-averaged concentration estimation of uraemic toxins with different removal kinetics: a novel approach based on intradialytic spent dialysate measurements. Clin. Kidney J. 16(4), 735–744 (2023).
Lopot, F., Nejedly, B. & Sulkova, S. Physiology in daily hemodialysis in terms of the time average concentration/time average deviation concept. Hemodial Int. 8(1), 39–44 (2004).
Castro, I. & Rodrigues, A. Estimating Residual Kidney Function: Present and Future Challenge. SN Comprehensive Clin. Med. 2(2), 140–148 (2020).
Murea, M., Deira, J., Kalantar-Zadeh, K., Casino, F. G. & Basile, C. The spectrum of kidney dysfunction requiring chronic dialysis therapy: Implications for clinical practice and future clinical trials. Semin. Dial. 35(2), 107–116 (2022).
Shafi, T. & Levey, A. S. Measurement and Estimation of Residual Kidney Function in Patients on Dialysis. Adv. Chronic Kidney Dis. 25(1), 93–104 (2018).
Steubl, D. et al. Development and Validation of Residual Kidney Function Estimating Equations in Dialysis Patients. Kidney Med. 1(3), 104–114 (2019).
Okazaki, M. et al. Residual Kidney Function and Cause-Specific Mortality Among Incident Hemodialysis Patients. Kidney Int. Rep. 8(10), 1989–2000 (2023).
Daugirdas, J. T. Residual Kidney Function and Cause-Specific Mortality. Kidney Int. Rep. 8(10), 1914–1916 (2023).
Vilar, E. et al. Plasma Levels of Middle Molecules to Estimate Residual Kidney Function in Haemodialysis without Urine Collection. PLoS ONE 10(12), e0143813 (2015).
Vilar, E. & Farrington, K. Emerging importance of residual renal function in end-stage renal failure. Semin. Dial. 24(5), 487–494 (2011).
Vilar, E. et al. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin. J. Am. Soc. Nephrol. 4(12), 1944–1953 (2009).
Wong, J., Kaja Kamal, R. M., Vilar, E. & Farrington, K. Measuring Residual Renal Function in Hemodialysis Patients without Urine Collection. Semin. Dial. 30(1), 39–49 (2017).
Wong, J. et al. Predicting residual kidney function in hemodialysis patients using serum β-trace protein and β2-microglobulin. Kidney Int. 89(5), 1090–1098 (2016).
Wong, J., Vilar, E., Davenport, A. & Farrington, K. Incremental haemodialysis. Nephrol. Dial Transplant. 30(10), 1639–1648 (2015).
Davenport, A. Will incremental hemodialysis preserve residual function and improve patient survival?. Semin Dial. 28(1), 16–19 (2015).
Davenport, A. Measuring residual renal function for hemodialysis adequacy: Is there an easier option?. Hemodial. Int. 21(Suppl 2), S41–S46 (2017).
Canaud, B., Morena, M., Cristol, J. P. & Krieter, D. Beta2-microglobulin, a uremic toxin with a double meaning. Kidney Int. 69(8), 1297–1299 (2006).
Maruyama, Y. et al. Association between serum beta2-microglobulin and mortality in Japanese peritoneal dialysis patients: A cohort study. PLoS ONE 17(4), e0266882 (2022).
Casino, F. G. & Lopez, T. The equivalent renal urea clearance: a new parameter to assess dialysis dose. Nephrol. Dial Transplant. 11(8), 1574–1581 (1996).
Casino, F. G. et al. A simple approach for assessing equilibrated Kt/V beta 2-M on a routine basis. Nephrol. Dial Transplant. 25(9), 3038–3044 (2010).
Leypoldt, J. K., Cheung, A. K. & Deeter, R. B. Rebound kinetics of beta2-microglobulin after hemodialysis. Kidney Int. 56(4), 1571–1577 (1999).
Leypoldt, J. K., Holmes, C. J. & Rutherford, P. Clearance of middle molecules during haemodialysis and haemodiafiltration: new insights. Nephrol. Dial Transplant. 27(12), 4245–4247 (2012).
Leypoldt, J. K. et al. Intradialytic kinetics of middle molecules during hemodialysis and hemodiafiltration. Nephrol. Dial Transplant. 34(5), 870–877 (2019).
Lornoy, W., Becaus, I., Billiouw, J. M., Sierens, L. & van Malderen, P. Remarkable removal of beta-2-microglobulin by on-line hemodiafiltration. Am. J. Nephrol. 18(2), 105–108 (1998).
Lornoy W, Becaus I, Billiouw JM, Sierens L, Van Malderen P, D'Haenens P. On-line haemodiafiltration. Remarkable removal of beta2-microglobulin. Long-term clinical observations. Nephrol. Dial Transplant. (2000).
Maduell, F. et al. Comparison of Solute Removal Properties Between High-Efficient Dialysis Modalities in Low Blood Flow Rate. Ther. Apher. Dial. 24(4), 387–392 (2020).
Maduell, F. et al. Medium Cut-Off Dialyzer versus Eight Hemodiafiltration Dialyzers: Comparison Using a Global Removal Score. Blood Purif. 48(2), 167–174 (2019).
Cheung, A. K. et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J. Am. Soc. Nephrol. 17(2), 546–555 (2006).
Eknoyan, G. et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 347(25), 2010–2019 (2002).
Locatelli, F. et al. Effect of membrane permeability on survival of hemodialysis patients. J. Am. Soc. Nephrol. 20(3), 645–654 (2009).
Watanabe, Y. et al. Japanese society for dialysis therapy clinical guideline for “hemodialysis initiation for maintenance hemodialysis”. Ther. Apher. Dial. 19(Suppl 1), 93–107 (2015).
Ward RA, Daugirdas JT. Kinetics of Beta2-Microglobulin with Hemodiafiltration and High-Flux Hemodialysis. Clin. J. Am. Soc. Nephrol. (2024).
Acknowledgements
The authors would like to thank all the contributing centers and collaborators involved in the original FRENCHIE study: Marion Morena, Anne-Marie Dupuy, Jean-Paul Cristol (Laboratoire de Biochimie, CHU de Montpellier, Montpellier, France); Audrey Jaussent and Marie-Christine Picot (Département de l’Information Médicale, CHU de Montpellier, Montpellier, France); Hélène Leray-Moragues and Leila Chenine (Service de Néphrologie, CHU de Montpellier, Montpellier, France); Lotfi Chalabi and Laure Patrier (AIDER – Association pour l’Installation à Domicile des Epurations Rénales, Montpellier and Nîmes, France); Alain Debure (ATS, Saint-Denis, France); Damien Thibaudin (Service de Néphrologie, CHU de Saint-Étienne, Saint-Étienne, France); Lynda Azzouz (Association Régionale pour le Traitement de l’Insuffisance Rénale Chronique, Saint-Priest-en-Jarez, France); François Maurice (Centre Hémodialyse du Lez, Castelnau-le-Lez, France); Philippe Nicoud (Centre de Néphrologie du Mont Blanc, Sallanches, France); Claude Durand (Polyclinique Saint Martin, Pessac, France); Bruno Seigneuric (Service de Néphrologie, CHU de Toulouse, Toulouse, France); and Bernard Canaud (Université de Montpellier, Néphrologie, Montpellier, France).
Author information
Authors and Affiliations
Contributions
BC, MMC, JPC, AD wrote the main manuscript text and prepared figures and tables. MA, NM made statistical analysis. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Canaud, B., Davenport, A., Morena-Carrere, M. et al. Continuous beta-2 microglobulin–based clearance highlights superiority of high-Dose HDF over high-flux HD in predicting outcomes. Sci Rep 15, 20421 (2025). https://doi.org/10.1038/s41598-025-07497-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07497-2
Keywords
This article is cited by
-
Selected tryptophan metabolites and inflammatory molecules in hemodialyzed patients
Pharmacological Reports (2026)