Abstract
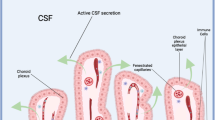
The choroid plexus (ChP) is crucial for most cerebrospinal fluid (CSF) secretion. However, few studies have explored the morphology of ChP in a natural upright posture. This study investigated the positional, areal, and volumetric changes of the ChP in response to gravity when comparing supine and upright scanning postures. Thirty-one healthy volunteers underwent MRI scans using an innovative 1.5T rotatable MRI at pitch angles of 0 and 90 employing a 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequence. Based on an in-house ChP segmentation pipeline, gap lengths between the ChP and lateral ventricles (LVEN), ChP surface area, volumes, and signal intensities in different directions were measured. The ChP exhibited a bottom gap decrease (-1.30 mm, P < 0.001), posterior gap increase (1.28 mm, P < 0.001), and bilateral gap growth (0.16 mm, P = 0.015) as well as downward centroidal shift (-1.31 mm, P < 0.001) relative to the LVEN when transitioning from lying to standing positions. Significant distance deviations were noted along the direction of gravity. Along with these positional changes, decreases in surface area (-5.30%, P = 0.021) and volume (-7.53%, P = 0.002) and an increase (11.46%, P < 0.001) in signal intensity of the ChP were observed from lying to standing. This study reveals the positional and volumetric change of the ChP within the LVEN with postural changes and demonstrates its morphology in a typical standing condition. These anatomic changes could provide additional evidence of CSF circulation and intracranial pressure in different postures.
Similar content being viewed by others
Introduction
The choroid plexus (ChP) is a vital organ that has received increasing attention. Cerebrospinal fluid (CSF) plays a crucial role in draining waste and delivering nutrition, and several studies investigated the correlation between Alzheimer’s disease and ChP which secrets CSF, featuring apical microvilli to increase the contact area with intraventricular space, and cilia to facilitate CSF circulation1,2,3,4. Many central nervous system diseases are related to alternation in CSF dynamics, whether through overproduction triggered by ChP papilloma or flow obstruction caused by ChP tumor, which can lead to hydrocephalus3,5,6.
Located in the body and inferior horn of the lateral ventricle (LVEN), the ChP clusters to be more conspicuous at the junction of these two portions, forming what is known as the choroid glomus7. It is also present along the roof of the third and fourth ventricles4,8. Given its soft leaf-like structure and the buoyancy provided by the surrounding CSF, its position and morphology within the ventricle may change with varying gravitational directions. However, this aspect has seldom been investigated9,10.
Previous studies have revealed that CSF flow and volume can increase by approximately 50% in the supine position compared with the upright position, potentially affected by variations in blood flow, intracranial pressure, and organ morphology11,12,13. Furthermore, lying down during sleep has been suggested to facilitate brain drainage clearance, which may influence the development of age-related dementia and neurodegenerative diseases14,15,16. Changes in intracranial structure have also been observed in weightless environments, such as those experienced in outer space, indicating gravity’s impact17.
However, the ChP under natural upright conditions that humans typically maintain in daily life has rarely been investigated and its differences from supine position are unknown. Traditionally, ChP research has predominantly utilized magnetic resonance imaging (MRI) due to its high resolution and excellent soft tissue contrast, with most imaging performed in the supine posture. Recent work on cryogen-free cooling techniques has made rotatable MRI systems feasible18. An in-house 1.5T rotatable, cryogen-free superconductive MRI was newly developed that enables scanning in both supine and upright mode, potentially enhancing our understanding of the relationship between cerebral fluid dynamics and human postures.
Against this background, the proposed study aims to explore the morphology of the ChP in the LVEN, including its position, shape, and volume in different postures, and to compare these characteristics between the supine and upright conditions.
Methods
Volunteer demographics
Thirty-three young and healthy adults were recruited in this study, undergoing scans on 15:02\(\:\pm\:\)2:25 in the afternoon between August 8th and September 1st, 2024. Age range out of 25–30 years, with a history of neurological or psychiatric pathology, brain abnormality, brain surgery, brain structure-related systemic disease, pregnancy, etc. were counted out of the inclusion criteria. Two subjects were excluded due to workstation data storage technical problems and the presence of uninformed brain lipoma, respectively. The remaining 31 volunteers (15 females) were included with a mean age of 26.5\(\:\pm\:\)3.7 years, an average height of 168.8\(\:\pm\:\)6.7 cm, and an average weight of 64.8\(\:\pm\:\)11.9 kg. This study received approval from the institutional ethics committees of University of Nottingham Ningbo China and Ningbo No. 2 Hospital in accordance with the Declaration of Helsinki. The informed consents were obtained from all participants, and the collected data were anonymized before processing to be kept confidential. Routine clinical exams on physiological signals were performed before and after scanning for all volunteers, including heat beat rate (HBR), systolic blood pressure (SBP), and diastolic blood pressure (DBP).
Image acquisition
All the experiments were conducted via a state-of-the-art, rotatable, cryogen-free superconductive 1.5T MRI scanner (XGY-Spin MRI-R001, Xingaoyi Medical Equipment Company, Ningbo, China) equipped with a 12-channel head coil as shown in Fig. 1. Scans were initially conducted in the supine position with the bed at the 0\(\:^\circ\:\) horizontal angle, followed by rotation of the scanner to a 90\(\:^\circ\:\) vertical angle for upright scanning. 3D sagittal T1-weighted images were acquired at each position using magnetization-prepared rapid gradient echo (MP-RAGE) sequences (inversion time: 970 ms, repetition time: 10 ms, echo time: 3.4 ms, flip angle: 12\(\:^\circ\:\), field-of-view: 230 mm\(\:\times\:\)230 mm\(\:\times\:\)187.2 mm, sampling matrix: 192\(\:\times\:\)192\(\:\times\:\)156, reconstruction matrix: 480\(\:\times\:\)480\(\:\times\:\)156, spatial resolution: 0.48 mm\(\:\times\:\)0.48 mm\(\:\times\:\)1.20 mm). Preparatory sequences of approximately 5 min before scanning in different postures were implemented. Foam pads and straps were used to immobilize the head and body to mitigate motion artifacts.
Image analysis
Since people might move between two scans, to facilitate comparison, automatic 3D rigid registration was employed to align each volunteer’s upright head image to the corresponding supine image using ITK-SNAP software (University of North Carolina, USA). Masks for the LVEN regions and the ChP within these regions were extracted using a human-in-the-loop ChP segmentation tool (https://github.com/princeleeee/ChP-Seg), which has demonstrated superior performance compared with the FreeSurfer and FastSurfer methods19. To quantify the overlapping rate of segmented volume, the Dice coefficient was calculated using Matlab 2024a (Mathwork, USA) for the segmented masks of both ChP and LVEN.
The centroid points were also determined in Matlab. Additionally, the positional deviations along three axes, head-foot (HF), anterior-posterior (AP), and left-right (LR), were calculated to investigate the positional changes between different postures of the LVEN and ChP.
The position shift of ChP relative to its encasing LVEN was investigated using the average distances by counting the number of pixels from the ChP surface to the ventricle boundary of the segmented volume masks in Matlab. These measurements included gaps of lower and upper portions in HF direction; back and front portions in AP direction; and the outer and inner portions in LR direction as illustrated in Fig. 2. The direction of gravity corresponded with the AP axis in the supine posture and with the HF axis in the upright posture. For instance, the HF lower gap distance was calculated by the average length between the bottom voxel of ChP and the bottom voxel of LVEN along each 3D image line in the HF direction. The variations in centroid positions of ChP relative to the LVEN were also quantified in these three directions to assess their relative center locations.
Additionally, the surface areas and volumes of the segmented 3D LVEN and ChP shapes were measured in Matlab. Because of the potential partial volume effects in the images, signal intensity should be proportional to ChP density. Therefore, the signal intensity of the ChP was also determined by averaging all the voxel signal intensities within the segmented ChP mask as an indicator of the ChP expansion.
Sagittal, transverse, and coronal registered images of a 30-year-old male. It shows segmented LVEN in blue and ChP in orange, scanned in upright (a) and supine (b) postures. The arrows labeled ‘G’ denote the direction of gravity. (c) A zoomed-in view of the segmentation, highlighting the gap distances along different directions. (d) A simplified 3D model of the right LVEN and ChP, illustrating their interspace hand-drawn by author using Procreate 5.3.15 (Savage Interactive Pty Ltd. Australia) and adapted from Human Anatomy Atlas 2026.00.019 (Cengage Learning, Inc. USA) retrieved on March 6, 2025 from www.visiblebody.com. (e) A 3D rendering of the LVEN and ChP in both postures in Matlab 2024a (Mathwork, USA) Volume Viewer toolbox.
Statistical analysis
Statistical significance analysis, including the two-tailed paired Student’s t-test or the non-parametric two-tailed signed-rank Wilcoxon test after the Levene and Shapiro-Wilk tests for variance homogeneity and distribution normality evaluations, was conducted to compare the centroid position deviations, intraventricular space gap lengths across six different directions, LVEN surface areas and volumes, ChP surface areas and volumes, and ChP densities between the upright and supine postures, using SPSS Statistics version 29 (IBM, USA). A P-value of 0.05 was established as the threshold for statistical significance.
Results
The mean values of subjects’ HBR were 79.0\(\:\pm\:\)14.0 before and 76.6\(\:\pm\:\)11.6 after (P=0.501); SBP was 115.2\(\:\pm\:\)12.8 mmHg before and 110.6\(\:\pm\:\)11.7 mmHg after (P=0.148); and DBP was 74.1\(\:\pm\:\)7.2 mmHg before and 70.9\(\:\pm\:\)7.5 mmHg after the scan (P = 0.101).
Influence on ChP position
The Levene and Shapiro-Wilk tests indicated that the centroid position changes of LVEN and ChP, their relative movements of three directions, and gap distance values for six portions demonstrated homogeneous variance and normal distribution (P > 0.05) and the Student’s t-test was conducted for them, except for the HF direction lower and upper gap data (P < 0.001) and the Wilcoxon test was applied.
As shown in Table 1, the position and shape of the LVEN did not exhibit significant differences across different postures, with a relatively high Dice similarity coefficient of 0.80, and its average centroid shift was less than one pixel in all three directions (P > 0.05). In contrast, the 3D regions of the ChP displayed much lower similarity, with a mean Dice similarity coefficient of only 0.31, and exhibited significant downward movements in upright scanning (P < 0.001).
Table 2, Fig. 3 illustrate the deviations in centroid locations of the segmented ChP and LVEN, using the statistically stable LVEN regions as reference. The centroids of the ChP were, positioned higher than those of the LVEN, but their distances decreased by 1.31 mm from supine to standing, indicating a downward shift. In addition, there was a slight and insignificant posterior movement of the ChP centroid relative to that of the LVEN in the supine position than upright one, and the LR directional change was negligible.
As demonstrated in Table 2, Fig. 4, in the HF direction, the ChP experienced a significant downward movement to the bottom of the LVEN when the volunteer was transitioned upright, especially for the lower portion, whose distance decreased by 1.30 mm (P < 0.001). As for the AP direction, the average length from ChP to the posterior LVEN surface dropped by 1.28 mm (P < 0.001), whereas the anterior interspace enlarged when volunteers were lying rather than standing. These shifts were consistent with the axis of gravity direction. The outer gap lengths also increased in the upright posture (0.16 mm, P = 0.015), and the corresponding inner ones did not have a significant change (0.08 mm, P > 0.05). Although the LR axis is not along the gravitational force direction, since the LVEN bottom is oblique, the ChP was closer to the LVEN inner lateral surface while standing as expected.
Boxplots of centroid position shift distances of ChP relative to LVEN. They are on the HF, AP, and LR directions of 31 healthy volunteers, comparing differences between supine and upright postures. A positive value indicates the centroid of ChP is in the anterior, top, or left directions of LVEN’s centroid. *** represents P-values less than 0.001.
Influence on ChP volume
The surface areas, volumes of LVEN and ChP, as well as the signal intensity of the ChP during lying and standing scans, all passed the Levene variance homogeneity and Shapiro-Wilk normality tests (P > 0.05), and their difference significances were calculated using the two-tailed paired Student’s t-test.
As depicted in Figs. 5 and 6, along with Table 3, there were no significant changes in the LVEN surface areas (-1.24%, P = 0.230) or volumes (-1.66%, P = 0.360) between postures. In contrast, the ChP surface area and volume exhibited a notable reduction of 5.30% (P = 0.021) and 7.53% (P = 0.002), respectively, when subjected to the vertical pitch angle. Additionally, the mean signal intensity of the ChP increased markedly by 11.46% (P < 0.001) in the standing position.
Discussion
Body posture can affect bodily functions, such as CSF secretion. These alterations may represent adaptive changes that occurred during the evolution of upright walking in humans. To our knowledge, this is the first study to explore structural changes in ChP across different postures.
In this study, our rotatable MRI system measured the positional and configurational changes of the ChP across different positions. We successfully measured and compared the relative centroid movement and mean distance in multidirectional interspace gaps between the ChP and LVEN, and determined the positional shift of ChP, as well as changes in its surface area, volume, and signal intensity at different scanning pitch angles.
Significant ChP positional shifts were observed between upright and supine postures, whereas the LVEN positions and shapes had no obvious change. In the upright position, the ChP had the most significant centroid shift towards the foot, and the gap length deviations indicated that ChP was closer to the bottom and interior boundaries of the LVEN due to the HF-directional gravitational force. The posterior space increased in upright mode as the ChP sank towards the back side of the head while lying down. The largest gap length percentage changes were noted at the HF lower portion and AP back portion of -29.98% and 19.96% as expected, prominently affected in 0 and 90-degree positions. These findings supported the notion that acute changes in the gravity axis can cause structural changes in the brain.
The mean LVEN surface area and volume exhibited a slight decrease of 1.24% and 1.66% respectively from lying down to standing up, although the change was not statistically significant (P = 0.230 and P = 0.360 respectively). This trend aligns with the measurements reported by Yokoyama, et al. who noted a 4.90% volume drop (P < 0.001) when shifting from a lying to a sitting position20. Our study’s contrasting finding of insignificance might be attributed to more consistent volunteer demographics and the use of the same scanner21.
The surface area (-5.30%, P = 0.021) and volume (-7.53%, P = 0.002) of ChP exhibited a significant reduction when volunteers were standing, likely due to the gravitational force pulling the ChP downwards, causing it to fold densely towards the LVEN bottom in a vertical pitch angle and stretch in a horizontal pitch angle. In addition, the possible cerebral blood volume reduction in the upright position might also contribute to this trend, which will be investigated in the future perfusion study. The significant increase in ChP signal intensity (11.46%, P < 0.001) in the standing position could indicate higher proton density, suggesting a denser structure from mechanical deformation and potential decreased blood in the ChP capillaries22. This increased surface and expanded volume in the supine posture might enhance the efficiency of fluid transportation from ChP epithelial cells to ventricles due to a larger area of microvillus and cilia contact, thus promoting CSF secretion and circulation. Additionally, reduced blood flow rates in the upright posture as observed by Alperin, et al. using a 0.5 T vertical gap MRI scanner, contrast with the engorged and dilated blood when lying down23, which could augment CSF production since CSF is derived from the filtered plasma of fenestrated ChP capillaries24. Alperin, et al. also suggested lower CSF flow and exchange while sitting, and sleep deprivation can possibly have a negative effect on CSF transportation, consistent with the conjecture23,25,26.
Previous research has examined brain structural alternation in different postures using vertical CT or permanent magnet upright MRI scanners. However, these studies often employed separate MRI scanners, potentially introducing biases related to different scanner models. Our novel rotatable MRI system helps mitigate this issue. Furthermore, the small ChP is typically overlooked or inaccurately segmented in the brain region due to the partial volume effect associated with the low magnetic field strength of conventional permanent magnet MRI27.
Despite these insights, several limitations and potential avenues for further research exist. Firstly, the imaging parameters can be further optimized to obtain isotropic image resolution. The current anisotropy might have adversely impacted segmentation accuracy, quantification precision, and overall interpretation of the imaging data. Additionally, suboptimal resolution could have introduced partial-volume effects, potentially limiting the reliability of quantitative analyses. Future studies should address these limitations through careful optimization of imaging parameters, specifically by achieving isotropic, higher-resolution imaging to enhance accuracy and robustness of the findings. Secondly, the chosen ChP segmentation tool does not account for the third or fourth ventricles, or the ChP within these regions due to insufficient image resolution. The morphological changes of ChP in these regions between different postures remain uninvestigated. Thirdly, current work focused only on healthy young adults, but the ChP morphology and function can be influenced by factors such as aging and diseases like hydrocephalus, Alzheimer’s, etc28,29. In future studies, the relationship between ChP elasticity and deformation in patients can be compared, and the upright mode might be more beneficial for brain structural pathology diagnosis. Its positional and volumetric impact across different sex, age, and other demographic groups can also be investigated, using varying scanning postures. Additionally, the possible LVEN regional change between postures in the old or ill subjects is not completely excluded so that more localized and detailed LVEN morphology should be investigated. Fourthly, the direct relationship between CSF secretion and gravity was not established. CSF production rates can be estimated using phase-contrast (PC) or arterial spin labeling (ASL) MRI techniques and compared with ChP changes30,31,32. With this rotatable MRI scanner, more factors related to intracranial blood and CSF dynamics can be investigated in the future9,33. Fifthly, since previous studies revealed diurnal CSF change and its highest production in the dark phase, this study was only conducted in the afternoon period which mitigated the time-of-day ChP change, but this can be measured in the future study to see the influence of circadian rhythms34,35. Lastly, only two pitch angles were examined, limited to only horizontal and vertical direction data. The gradual ChP modification with incremental intermediate pitch angles can be tested in further investigations.
Conclusions
In conclusion, significant positional changes in the ChP parallel to the direction of gravity were observed when comparing upright to supine MRI scans among healthy volunteers. Furthermore, it was observed that ChP surface area and volume decrease, and density increases in a standing posture. These findings offer valuable insights into the study of in-vivo brain structure and CSF flow across different postures. Since CSF exchange and flow are known to be affected by body positions, the positional and morphological changes of the CSF-producing ChP may be linked to alterations in cerebral hemodynamics and hydrodynamics.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Ningbo Number 2 Hospital.
Abbreviations
- AP:
-
Anterior-posterior
- ASL:
-
Arterial spin labeling
- ChP:
-
Choroid plexus
- CSF:
-
Cerebrospinal fluid
- DBP:
-
Diastolic blood pressure
- HBR:
-
Heat beat rate
- HF:
-
Head-foot
- LR:
-
Left-right
- LVEN:
-
Lateral ventricle
- MP-RAGE:
-
Magnetization-prepared rapid gradient echo
- MRI:
-
Magnetic resonance imaging
- PC:
-
Phase-contrast
- SBP:
-
Systolic blood pressure
References
Wolburg, H. & Paulus, W. Choroid plexus: biology and pathology. Acta Neuropathol. 119, 75–88 (2010).
Lun, M. P., Monuki, E. S. & Lehtinen, M. K. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 16 (8), 445–457 (2015).
Javed, K., Reddy, V., Lui, F. & Neuroanatomy choroid plexus. (2019).
Telano, L. N. & Baker, S. Physiology, cerebral spinal fluid. (2018).
Rickert, C. H. & Paulus, W. Tumors of the choroid plexus. Microsc. Res. Tech. 52 (1), 104–111 (2001).
Eisenberg, H. M., McComb, J. G. & Lorenzo, A. V. Cerebrospinal fluid overproduction and hydrocephalus associated with choroid plexus papilloma. J. Neurosurg. 40 (3), 381–385 (1974).
Koopmans, R., Li, D. & Paty, D. Glomus of the choroid plexus: the normal spin-echo appearance on magnetic resonance imaging. Can. Association Radiol. J. = J. L’association Canadienne Des. Radiologistes. 41 (4), 195–200 (1990).
Kratzer, I., Ek, J. & Stolp, H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim. Et Biophys. Acta (BBA)-Biomembranes. 1862 (11), 183430 (2020).
Kaur, C., Rathnasamy, G. & Ling, E-A. The choroid plexus in healthy and diseased brain. J. Neuropathology Experimental Neurol. 75 (3), 198–213 (2016).
Redzic, Z. B. & Segal, M. B. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv. Drug Deliv. Rev. 56 (12), 1695–1716 (2004).
Muccio, M. et al. Upright versus supine MRI: effects of body position on craniocervical CSF flow. Fluids Barriers CNS. 18 (1), 61 (2021).
Gehlen, M., Kurtcuoglu, V. & Daners, M. Is posture-related craniospinal compliance shift caused by jugular vein collapse? A theoretical analysis. Fluids Barriers CNS. 14, 5 (2017).
Sagirov, A. F., Sergeev, T. V., Shabrov, A. V., Yurov AYe, Guseva, N. L. & Agapova, E. A. Postural influence on intracranial fluid dynamics: an overview. J. Physiol. Anthropol. 42 (1), 5 (2023).
Hidaka, Y. et al. Association between choroid plexus volume and cognitive function in community-dwelling older adults without dementia: a population-based cross-sectional analysis. Fluids Barriers CNS. 21, 101 (2024).
Sun, S. et al. Elevated peripheral inflammation is associated with choroid plexus enlargement in independent sporadic amyotrophic lateral sclerosis cohorts. Fluids Barriers CNS. 21 (1), 83 (2024).
Eide, P. K., Vinje, V., Pripp, A. H., Mardal, K-A. & Ringstad, G. Sleep deprivation impairs molecular clearance from the human brain. Brain 144 (3), 863–874 (2021).
Van Ombergen, A. et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc. Natl. Acad. Sci. 116 (21), 10531–10536 (2019).
Wang, Y. et al. Design, construction and performance testing of a 1.5 T cryogen-free low-temperature superconductor whole-body MRI magnet. Supercond. Sci. Technol. 36 (4), 045002 (2023).
Li, J. et al. Associations between the choroid plexus and Tau in alzheimer’s disease using an active learning segmentation pipeline. Fluids Barriers CNS. 21 (1), 56 (2024).
Yokoyama, Y. et al. Effect of gravity on brain structure as indicated on upright computed tomography. Sci. Rep. 11 (1), 392 (2021).
Nakada, T. & Tasaka, N. Human brain imaging in the upright position. Neurology 57 (9), 1720–1722 (2001).
Higgins, C. B., Hricak, H., Helms, C. A. & Paulson, E. Magnetic resonance imaging of the body. AJR-American J. Roentgenol. 169 (2), 348 (1997).
Alperin, N., Lee, S. H., Sivaramakrishnan, A. & Hushek, S. G. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J. Magn. Reson. Imaging: Official J. Int. Soc. Magn. Reson. Med. 22 (5), 591–596 (2005).
Thompson, D., Brissette, C. A. & Watt, J. A. The choroid plexus and its role in the pathogenesis of neurological infections. Fluids Barriers CNS. 19 (1), 75 (2022).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342 (6156), 373–377 (2013).
Achariyar, T. M. et al. Glymphatic distribution of CSF-derived ApoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegeneration. 11, 1–20 (2016).
Zhao, L., Feng, X., Meyer, C. H. & Alsop, D. C. (eds) Choroid plexus segmentation using optimized 3D U-Net. 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI); : IEEE. (2020).
Delvenne, A. et al. Involvement of the choroid plexus in alzheimer’s disease pathophysiology: findings from mouse and human proteomic studies. Fluids Barriers CNS. 21 (1), 58 (2024).
Eisma, J. J. et al. Deep learning segmentation of the choroid plexus from structural magnetic resonance imaging (MRI): validation and normative ranges across the adult lifespan. Fluids Barriers CNS. 21 (1), 21 (2024).
Brinkmann, G., Harlandt, O., Muhle, C., Brossmann, J. & Heller, M. Quantification of fluid flow in magnetic resonance tomography: an experimental study of a flow model and liquid flow measurements in the cerebral aqueduct in volunteers. RoFo: Fortschr. Auf Dem Gebiete Der Rontgenstrahlen Und Der Nuklearmedizin. 172 (12), 1043–1051 (2000).
Zhao, L., Taso, M., Dai, W., Press, D. Z. & Alsop, D. C. Non-invasive measurement of choroid plexus apparent blood flow with arterial spin labeling. Fluids Barriers CNS. 17, 1–11 (2020).
Petitclerc, L. et al. Ultra-long-TE arterial spin labeling reveals rapid and brain-wide blood-to-CSF water transport in humans. Neuroimage 245, 118755 (2021).
Liu, G., Ladrón-de-Guevara, A., Izhiman, Y., Nedergaard, M. & Du, T. Measurements of cerebrospinal fluid production: a review of the limitations and advantages of current methodologies. Fluids Barriers CNS. 19 (1), 101 (2022).
Smets, N. G., Strijkers, G. J., Vinje, V. & Bakker, E. N. Cerebrospinal fluid turnover as a driver of brain clearance. NMR Biomed. 37 (7), e5029 (2024).
Edelbo, B. L., Andreassen, S. N., Steffensen, A. B. & MacAulay, N. Day–night fluctuations in choroid plexus transcriptomics and cerebrospinal fluid metabolomics. PNAS nexus. 2 (8), pgad262 (2023).
Funding
This work was supported by the National Key R&D Program of China: 2022YFC2408900 and 2023YFE0118900, Ningbo “S&T Innovation 2035” Major programs: 2022Z141 and 2023Z182.
Author information
Authors and Affiliations
Contributions
Y. W., C. W., S. K. and L. Z. conceived the experiment design. S. K., S. N., J. Z., J. Z. and C. Y. recruited volunteers and collected data. Y. W., J. L. and S. K. performed the image processing and data analysis. Y. W. wrote the main manuscript text. C. W., L. Z., T. M. and J. Z critically and substantively revised the manuscript. G. L. and H. Z. reviewed and edited the writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Human ethics and consent to participate declarations
All experiments of this study were ethically approved by the Research Ethics Panel of University of Nottingham Ningbo China and Ningbo No.2 Hospital Ethics Committee (Approval No.: YJ-NBEY-KY-2023-121-03), complied with the principles stated in the Declaration of Helsinki. Written informed consent was obtained from each participating subject before the experiment.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Ke, S., Li, J. et al. Assessing morphological changes in the choroid plexus between standing and supine positions using a rotatable MRI system. Sci Rep 15, 22329 (2025). https://doi.org/10.1038/s41598-025-07985-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07985-5