Abstract
Coronary microvascular disease (CMVD) is associated with abnormalities in glucose-lipid metabolism. And the triglyceride to high density lipoprotein (HDL) (TG/HDL) ratio can be used to characterize levels of glycolipid metabolism. Therefore, it is hypothesized that increased TG/HDL may trigger CMVD. This study enrolled patients with angina pectoris but negative coronary angiograms to explore inflammatory factor-mediated disorder of glycolipid metabolism triggers CMVD. Logistics regression model and subgroup analysis were constructed to explore the associations between TG/HDL and CMVD. Restricted cubic splines were applied to further the associations of TG/HDL with CMVD. Given inflammatory factors as intermediary factor, we investigate the mediating effects of TG/HDL on CMVD. 242 patients were eventually recruited and 150 patients were diagnosed with CMVD. In the multivariable-adjusted model, TG/HDL and inflammatory indexes including the C-reaction protein (CRP), C-reaction protein to lymphocyte ratio (CLR) and inflammatory burden index (IBI) were positively related to CMVD (Odds Ratio (OR) = 1.71, 95% CI = 0.69–4.25; OR = 1.89, 95% CI = 1.32–2.68; OR = 2.76, 95% CI = 1.56–4.89; OR = 1.22, 95% CI = 1.08–1.37, respectively). Mediation analysis indicated that CRP, CLR and IBI mediated 26.37%, 16.89% and 10.45% of the association of TG/HDL with CMVD. TG/HDL is positively associated with CMVD. And this association appeared to be partially mediated through inflammatory indices.
Similar content being viewed by others
Introduction
Coronary microvascular disease (CMVD) as a subtype of ischemia with non-obstructive coronary artery disease (INOCA) is a non-atherosclerotic disease leading to heart ischemia1. Some studies have shown that patients with have a significantly increased risk of developing heart failure with preserved ejection fraction (HFpEF)2. The GUSTO IIb study found that non-obstructive chest pain was present in 4.2–30.5% of 12,142 patients with ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) or unstable angina3. Moreover, a study using PET as a diagnostic tool to explore the incidence of CMVD found that myocardial perfusion abnormalities were present in 51% of male and 54% of female patients on the prevalence of CMVD using PET as a tool4. In recent years, the pathophysiology of CMVD has been attracted physician to extensive attention, and various hypotheses have emerged, platelet activation, capillary thinning, and inflammatory response5,6.
The coronary endothelium cells play a pivotal role in maintaining microcirculatory hemodynamics. Some risk factors such as diabetes, hyperlipidemia and insulin resistance (IR) can contribute to endothelial damage and consequently arteriolar vasomotor dysfunction. The TG/HDL-C ratio was initially regard as an indicator of atherosclerosis7 and the Triglyceride to high density lipoprotein (TG/HDL) was more valuable than other single lipid indices, due to strength of reflecting the complex interaction between lipoprotein metabolism, and better predicting the development of atherosclerosis8. Moreover, TG/HDL is considered a biomarker of IR, closely related to metabolic syndrome9. Elevated TG/HDL is not only strongly associated with IR, but is also often a concomitant phenomenon of centripetal obesity, both of which are key drivers of escalating cardiovascular disease risk10. IR can affect multiple systems throughout the body, including lipid metabolism, which in turn promotes the development of atherosclerosis. Recent studies have shown that inflammation not only directly contributes to vascular endothelial damage and promotes plaque formation and destabilization, but also has an intricate interaction with dyslipidemia11. Disturbances in lipid metabolism, especially the oxidation of low-density lipoprotein cholesterol (LDL-C) and the accumulation of triglycerides, can trigger the inflammatory response, and ultimately damage to endothelial cells12.
Emerging evidence underscores the pivotal role of inflammatory mechanisms in cardiovascular pathogenesis, notably in acute coronary syndromes (ACS) and heart failure progression13,14. Proinflammatory mediators - particularly C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) - induce coronary endothelial dysfunction through synergistic interactions between oxidative stress and structural endothelial damage15. Elevated CRP levels, reflecting systemic inflammation, correlate with impaired endothelial homeostasis in coronary microvascular disease (CMVD) patients16. Recent advances propose novel composite biomarkers - C-reactive protein/lymphocyte ratio (CLR) and inflammatory burden index (IBI) - as superior predictors of cumulative inflammatory load and adverse cardiovascular outcomes compared to single-parameter indices17,18.
The aim of this study is to investigate the association between lipid metabolism, especially changes in TG/HDL ratio, and CMVD, and to further analyze whether this association is mediated through the key pathway of inflammatory response. By elucidating these mechanisms, we can provide a scientific basis for clinical prevention and treatment.
Methods
Data source
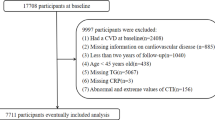
This is a prospective study to explore the correlation of lipid metabolism and CMVD mediated by inflammatory indicators. All clinical data of patients included in the study were sourced from inpatients admitted to the Cardiovascular Department of China Academy of Chinese Medical Sciences Xiyuan Hospital, China Academy of Traditional Chinese Medicine, between January 2022 and September 2024. The patient screening process was shown in Fig. 1 (Flowchart of study participants). The study passed the ethics committee of Xiyuan Hospital. Ethical approval was granted by the hospital’s ethics committee. Furthermore, all methods were performed in accordance with the relevant guidelines and regulations.
Diagnostic criteria for CMVD
Diagnostic criteria for CMVD adhered to the Chinese Multidisciplinary Expert Consensus on the Diagnosis and Treatment of Microvascular Diseases (2020)19, encompassing myocardial ischemia symptoms, objective evidence of myocardial ischemia, non-obstructive coronary artery disease (< 50% stenosis on CTA/CAG), and impaired coronary microcirculatory function, involving impaired coronary flow reserve (CFR) < 2.5 or < 2.0, or the slow coronary flow phenomenon (TIMI frames > 25), the index of microvascular resistance index (IMR) > 25.
Inclusion criteria
CMVD group: (i) no significant stenosis in 3 coronary arteries including left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery (RCA) by coronary angiography (CAG) (stenosis < 50%); (ii) 3 coronary arteries with normal quantitative flow ratio (QFR) > 0.8, and the angiographic microvascular resistance (AMR) > 250 mmHg*s/m20; Control group (non-CMVD): (i) no significant stenosis of coronary artery shown by CAG; (ii) 3 coronary arteries measured by QFR, QFR > 0.8, AMR < 250 mmHg*s/m; Furthermore, all recruited patients aged over 20, regardless of gender.
Exclusion criteria
(i) Undergoing coronary revascularization within 1 month before this CAG, including Percutaneous Coronary Intervention (PCI), Percutaneous Transluminal Coronary Angioplasty (PTCA) and coronary artery bypass grafting (CABG); (ii) organic heart disease, such as severe heart failure (HF), malignant arrhythmia, valvular disease, etc.; (iii) acute and chronic inflammation, immune diseases or malignant tumors; (iv) severe renal insufficiency: glomerular filtration rate < 45 mL/min; (v) severe coagulation disorders or bleeding; (vi) allergy to contrast media. In addition, the exclusion criteria of CAG included poor visualization during or overlapping of the contrasted vessels.
AMR examination
Patients who meet the inclusion criteria underwent QFR examination. Real-time CAG images were conducted to the QFR instrument (Pulse Medical, Shanghai) and analyzed under a single angle of a single vessel using the software AngioPlus Core (version V2). The process is performed by a trained cardiologist, who performed the analysis. After selecting the optimal contrast view with minimal vessel overlap, the software automatically outlined the detected vessel lumen. The contrast flow rate is derived by dividing the vessel length by the contrast fill time, which is then converted to the filling flow rate9. Subsequently, frames in which the lumen contour was fully exposed were selected as analysis frames to determine the main and side branches of the analyzed vessels. According to Murray’s bifurcation-principle, the reference vessel diameter needs to be reconstructed21. Finally, according to fluid dynamics, AMR was calculated following the blow equations (Supplemental Fig. 1)22,23,24,25,26.
Note
Pa is aortic pressure at baseline; Pd is distal coronary artery pressure; Velocityhyp indicates coronary blood flow velocity in the hyperemic status.
Definition of inflammatory indexes and lipid metabolism
The inflammatory index involving CRP, CLR and IBI were calculated using the below formulas:
-
The CLR is defined as C-reaction protein/lymphocyte count.
-
The IBI is formulated as C-reaction protein × neutrophil count/lymphocyte count27.
-
TG/HDL is used to characterize lipid metabolism levels, and it is expressed as serum triglycerides/ serum high-density lipoprotein28.
Statistical analysis
All data were analyzed via R statistical (version 4.2.2). According to the normal distribution, all continuous variables do not follow normal distribution, and thus were presented in the form of medians and interquartile ranges. Whereas, categorical variables were expressed as frequencies and percentages. To clarify the differences in variables among different TG/HDL levels, TG/HDL were categorized into quartile groups, ranging from the lowest quartile (maximally anti-inflammatory) to the highest quartile (maximally pro-inflammatory). The Chi-squared test or Kruskal-Wallis H test was used to analyze various DII four categories. One-way ANOVA test, Kruskal-Wallis H test or Chi-squared test were applied to compare continuous or categorical variables in different TG/HDL quartile groups29. A statistically significant result was determined as a two-sided P value < 0.05.
Multivariate logistics regression models were employed to estimate the associations of TG/HD, inflammatory indexes and CMVD, and to calculate hazard ratios (HRs) and 95% confidence interval (CI). Model 1 was unadjusted. Model 2 was adjusted for gender, age, smoking, and drinking; Model 3 was additionally modified to account for gender, age, smoking, drinking, hypertension, hyperlipidemia, glucose, cholesterol and triglyceride. Next, we performed the subgroup analyses to explore potential differences among specific populations including gender, smoking, drinking, hypertension, hyperlipidemia and diabetes. The restricted cubic spline (RCS) model was applied to investigate the non-linear relationship between TG/HDL and CMVD. Furthermore, to clarify the correlation of inflammatory indicators, TG/HDL and CMVD, we conducted a linear analysis and And the violin plots was applied to characterize the differences in these indicators between the CMVD and non-CMVD group. Afterwards, the receiver operating characteristic (ROC) curve with the area under the curve (AUC) was used to explore the cutoff value of TG/HDL, CRP, CLR and IBI to identify CMVD. Eventually, the presence of a mediating effect was defined as existing a significant indirect effect, direct effect and total effect30.
Results
Baseline characteristic
Table 1 presents the baseline characteristics of enrolled participants in this study with different TG/HDL quartiles. Within all included participants, the median TG/HDL values were 1.20, and ranging from 0.60 in Q1, 1.01 in Q2, 1.41 in Q3 and 2.45 in Q4. The median age of the 242 enrolled patients was 66, and about 57.44% sample were male. In comparison with those in the low-level TG/HDL group, participants with elevated levels of TG/HDL had an increasing proportion of male, drinking alcohol, inflammatory indexes (including CRP, CLR and IBI). Additionally, regarding laboratory indexes, participants in higher quartiles had a relatively higher level of creatine kinase isoenzyme-MB, brain natriuretic peptide, alanine aminotransferase, uric acid, triglyceride, HDL and AMR of RCA.
Associations of TG/HDL with CMVD
In this study, the incidence of CMVD was 38.02% among these participants with angina pectoris. Table 2 demonstrates the associations of TG/HDL with CMVD. TG/HDL was significantly associated with an increased risk of CMVD (OR = 1.62, 95%CI = 1.15–2.28) in the crude model. After multivariable adjustment, the results remained robust and statistically significant, with model 2 (OR = 1.53, 95%CI = 1.09–2.17), but the results showed no significance with model 3 (OR = 1.71, 95%CI = 0.69–4.25). Compared to the first quartile of TG/HDL, ORs for participants in the second, third and fourth quartile tend to be higher regardless of whether to adjust variables.
Results of nonlinear of TG/HDL and CMVD
By the RCS models with adjustment for above mentioned confounders (model 3), the results showed that there was the L-shaped association between TG/HDL and CMVD (Fig. 2 and the cut-off value for CMVD was 1.22.
Subgroup analysis
We performed subgroup analyses to explore the relationships of TG/HDL with CMVD and the detailed results were showed in Fig. 3 Most analyses showed no differences within groups, for example the association between TG/HDL and CMVD was irrelevant in the participants who were smoking, drinking alcohol, hypertension and hyperlipidemia.
Associations of inflammation with TG/HDL and CMVD
Table 3 displays the associations of TG/HDL and inflammatory indexes after multivariate logistic regression. As identical adjusted variables method mentioned above, DII was positively associated with CPR (OR = 1.25, 95% CI = 1.03–1.51), CLR (OR = 1.35, 95% CI = 1.04–1.76), and IBI (OR = 1.04, 95% CI = 0.98–1.11).
Furthermore, logistics regression results of inflammatory indexes with CMVD are demonstrated in Table 4. The results showed that all indicators were positively related to CMVD except IBI in model 3.
Evaluation of the impact of inflammatory parameters and TG/HDL on CMVD
A ROC analysis to determine whether TG/HDL, CRP, CLR and IBI can be a diagnostic and prognostic marker in CMVD patients revealed TG/HDL (AUC: 0.634, 95% CI = 0.56–0.71, P < 0.01), CRP (AUC: 0.714, 95% CI = 0.65–0.78, P < 0.01), CLR (AUC: 0.698, 95% CI = 0.63–0.76, P < 0.01) and IBI (AUC: 0.696, 95% CI = 0.63–0.76, P < 0.01) with cutoff valvule of 0.93, 1.59, 1.23 and 6.00, respectively (Fig. 4.
Receiver operating characteristic (ROC) curve of the TG/HDL-C, C-reaction protein, C-reaction protein to lymphocyte ratio and inflammatory burden index as high-risk indicators of CMVD. CMVD Coronary microvascular disease, CRP C-reaction protein, CLR C-reaction protein to lymphocyte ratio, IBI inflammatory burden index, TG/HDL triglycerides to high-density lipoprotein cholesterol ratio.
Linear correlation of inflammatory parameters and TG/HDL with AMR
The Spearman correlation analysis showed that TG/HDL, CRP, CLR and IBI have positive correlation with AMR (R = 0.17, P = 0.007; r = 0.24, P < 0.001; r = 0.22, P < 0.001; r = 0.19, P < 0.001; respectively) in the whole study population (Supplemental Figure).
Distribution differences of inflammatory parameters and TG/HDL
We conducted violin plot analysis to compare the expression of inflammatory parameters and TG/HDL between CMVD and non-CMVD group. The result showed the level of these indices in the CMVD group (Supplemental Figure).
Mediating role of inflammatory indexes
Figure 5 shows that CRP mediated 26.37% of the association between TG/HDL and CMVD, the CLR mediated 16.89% of the association between TG/HDL and CMVD, and the IBI mediated 10.45% of the association between TG/HDL and CMVD. (Fig. 5.
Discussions
In this study, we found a positive association of TG/HDL with CMVD among the angina pectoris population, and after the comprehensively adjusted model, these results remained significantly. Given that the positive correlations of inflammatory indexes with TG/HDL and CMVD were uncovered by logistic regression, we performed the mediation analysis to identify the significant roles of CRP, CLR, and IBI in linking TG/HDL with CMVD, further expounding inflammation as a potential underlying mechanism to trigger CMVD. Therefore, the level of blood lipid metabolism may be a potent approach for the onset of CMVD and this process may be mediated by inflammation.
Although CMVD is a non-atherosclerotic disease, the WISE sub-study utilized intravascular ultrasound (IVUS) to detect female patients with non-obstructive CAD and demonstrated that the increased risk in patients with CMVD is strongly associated with atherosclerosis-related risk factors, such as hypertension31, diabetes mellitus32,33, and hyperlipidemia34. It was found that TG levels were higher in the CMVD group in comparison. In binary logistic regression, TC promoted CMVD (OR:2.19, 95%CI = 1.12–4.25) 35. Hyperlipidemia is regard as one of the main risk factors for the development of atherosclerosis and epidemiologic studies suggest that elevated triglyceride levels are a biomarker of cardiovascular disease36. Michael Miller suggested impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial37. Notably, among the various lipoprotein classes, however, HDL is inversely associated with the incidence of CVD38,39. Because they can exert a series of atheroprotective functions40. Currently, many scholars have proposed that the ratio of the two is more reflective of lipid metabolism levels and is also more closely linked to the development of IR. RCSCD-TCM study reported TG/HDL is positively risk of prediabetes and Type 2 diabetes in patients with CAD in China (OR = 1.19, 95%CI = 1.16–1.23)41. A cross-sectional study suggested high TG/HDL with association of a higher risk of metabolic syndrome (OR = 3.07, 95% CI = 2.402 to 3.924, P < 0.001)42. The Jichi Medical School Cohort Study suggested increased TG/HDL ratio correlated with a significant increase in stroke risk in the healthy body mass index (BMI) participants43. Some studies also indicated that the TG/HDL is a marker of CAD44,45. Moreover, TG/HDL is an independent risk factor for the occurrence of CMVD. Li ping Liao, etc. indicated that the proportion of females, the incidence of hypertension and diabetes, the level of TG, and C-reactive protein, and the ratio of TG/HDL-C were higher in the CMVD group46. And they defined CMVD based on lactic acid levels. For this study CMVD is diagnosed based on AMR without pressure guidewires and adenosine injection to reach the maximum hyperemic state given that the accuracy of diagnosis has been clearly reported before, whereas our conclusions are similar in that TG/HDL promotes CMVD. Interestingly, there were more male patients in our study. CMVD is an abnormality of microvascular diastolic function due to endothelial damage caused by various injurious factors47. Hyperlipidemia and IR has been reported to directly exacerbate vascular endothelial cell injury48. A study concerning prevalence of CMVD showed hyperlipidemia (69.48% vs. 55.22%) was relatively more prevalent in CMVD group49. Moreover, the content of vasodilator factors decreases in response to insulin resistance may cause endothelial-dependent functional impairment50. IR mainly involve endothelium-independent mechanisms in which stimulation of both adenosine A1 and A2 receptors and the opening of ATP-sensitive potassium channels on smooth muscle cells play a role51. On the other hand, IR in cardiomyocytes results in decreased glucose uptake and glucose oxidation52 and activates the inflammatory pathway, in which the proinflammatory TNF-α/IL-6/C-reactive protein (CRP) pathway was significantly associated with CMVD53. Emerging evidence indicates that hypovitaminosis D in patients with microvascular dysfunction may exacerbate endothelial pathology through multifaceted mechanisms, including (1) potentiation of pro-inflammatory cytokine cascades (e.g., IL-1β, TNF-α), (2) dysregulation of immunomodulatory pathways (reduced Treg cell activity), and (3) facilitation of atherosclerotic plaque formation via lipid peroxidation and foam cell accumulation54,55. Several of the mechanisms of endothelial dysfunction for IR are shared with some signaling pathways, including formation of advanced glycation end products, activation of protein kinase C (PKC). With increased AGE level, the level of AGE receptors accordingly increases with expression on inflammatory T cells, leading to increased cytokine production and ROS generation. Meanwhile, AGE/RAGE signaling is involved in increased expression of TNF-α and oxidative stress markers including NOX-256. Moreover, IR also contributes to CMVD, particularly via impairment of the phosphatidylinositol-3-kinase pathway15.
CMVD as a result of both endothelium-dependent and non-endothelium-dependent injury, and endothelial injury in response to inflammation has been recognized as a major pathogenesis. A study showed a significantly higher level of IMR in the high CRP group (P = 0.02)57. Our research also showed that CRP, and CLR are positively correlated with CMVD (OR = 1.76,95% CI 1.29 to 2.41and OR = 2.48, 95%CI 1.50 to 4.09, respectively). The damage of CRP on endothelium-dependent dilation is mediated by activation of NADPH oxidase, as a catalyst for free radical production, and p38 kinase, an upstream activator of NADPH oxidase58. Karasu, M. showed that IL-34 is an independent predictor of slow coronary flow (OR: 1.044, 95% CI: 1.006–1.084)59. In addition, some studies have shown that in patients with CMVD, pro-inflammatory factors predispose to endothelial dysfunction, which is due to the presence of reactive oxygen species, of which the intermediary cytokines IL-1, IL-6, and TNF-α are key mediators of the inflammatory cascade response with reduced eNOS expression60.
This prospective cohort study yields three principal findings: (1) It provides novel epidemiological evidence supporting the positive correlation between triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-C) and coronary microvascular dysfunction (CMVD); (2) Mediation analysis confirms the intermediary role of systemic inflammation (CRP-mediated proportion: 28.6%, P < 0.01) in the TG/HDL-C–CMVD pathway; (3) Longitudinal monitoring of lipid ratios may serve as a cost-effective strategy for early CMVD risk stratification. Notably, the observational design precludes causal inference, and the single-center recruitment limits generalizability of findings.
Conclusions
This study provided evidence for the positive associations of TG/HDL with CMVD, while also indicating the significant mediating role of inflammatory indices in this association.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACS:
-
Acute coronary syndromes
- AMR:
-
Angiographic microvascular resistance
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CAG:
-
Coronary angiography
- CABG:
-
Coronary artery bypass grafting
- CFR:
-
Coronary flow reserve
- CMVD:
-
Coronary microvascular disease
- CRP:
-
C-reaction protein
- CLR:
-
C-reaction protein to lymphocyte ratio
- HFpEF:
-
Heart failure with preserved ejection fraction
- HDL:
-
High density lipoprotein
- IMR:
-
Index of microvascular resistance index
- IBI:
-
Inflammatory burden index
- IR:
-
Insulin resistance
- IL-6:
-
Interleukin-6
- IVUS:
-
Intravascular ultrasound
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- LDL-C:
-
Low-density lipoprotein cholesterol
- NSTEMI:
-
Non-ST-segment elevation myocardial infarction
- OR:
-
Odds Ratio
- PCI:
-
Percutaneous coronary intervention
- PTCA:
-
Percutaneous Transluminal Coronary Angioplasty
- QFR:
-
Quantitative flow ratio
- ROC:
-
Receiver operating characteristic
- RCS:
-
Restricted cubic spline
- RCA:
-
Right coronary artery
- STEMI:
-
ST-segment elevation myocardial infarction
- TG/HDL:
-
triglyceride to high density lipoprotein
- TNF-α:
-
tumor necrosis factor-alpha
References
Mileva, N. et al. Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: systematic review and Meta-Analysis. J. Am. Heart Assoc. 11 (7), e023207 (2022). Epub 2022 Mar 18. PMID: 35301851; PMCID: PMC9075440
Toya, T., Nagatomo, Y., Ikegami, Y., Masaki, N. & Adachi, T. Coronary microvascular dysfunction in heart failure patients. Front. Cardiovasc. Med. 10, 1153994. https://doi.org/10.3389/fcvm.2023.1153994 (2023). PMID: 37332583; PMCID: PMC10272355
Acute coronary syndromes. In the GUSTO-IIb trial: prognostic insights and impact of recurrent ischemia. GUSTO-IIb Investigators Circulation. 98 (18), 1860–1868 (1998).
Murthy, V. L. et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 129 (24), 2518–2527. https://doi.org/10.1161/CIRCULATIONAHA.113.008507 (2014). Epub 2014 Apr 30. PMID: 24787469; PMCID: PMC4076200
Siasos, G. et al. Role of local coronary blood flow patterns and shear stress on the development of microvascular and epicardial endothelial dysfunction and coronary plaque [J]. Curr. Opin. Cardiol. 33, 638–644 (2018).
Del Buono, M. G. et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC State-of-the-Art review [ J]. Am. Coll. Cardiol. 78, 1352–1371 (2021).
Gaziano, J. M., Hennekens, C. H., O’Donnell, C. J., Breslow, J. L. & Buring, J. E. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 96 (8), 2520–2525. https://doi.org/10.1161/01.CIR.96.8.2520 (1997).
Sambuceti, G., L’Abbate, A. & Marzilli, M. Why should we study the coronary microcirculation? Am. J. Physiol. Heart Circ. Physiol. 279 (6), H2581–H2584. https://doi.org/10.1152/ajpheart.2000.279.6.H2581 (2000).
Nguyen, H. H. et al. TG/HDL-C ratio is a risk factor associated with CKD: use in assessing the risk of progression of CKD. Pathophysiology 29 (3), 374–382. https://doi.org/10.3390/pathophysiology29030029 (2022). PMID: 35893599; PMCID: PMC9326757
Nosrati, M., Safari, M., Alizadeh, A., Ahmadi, M. & Mahrooz, A. The atherogenic index log (Triglyceride/HDL-Cholesterol) as a biomarker to identify type 2 diabetes patients with poor glycemic control. Int. J. Prev. Med. 12, 160 (2021).
Welty, F. K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 15, 400 (2013).
Rezapour, M. et al. Comparison of lipid ratios to identify metabolic syndrome. Arch. Iran. Med. 21, 572–577 (2018).
Hamdy, N. M. Relationship between pro-anti-inflammatory cytokines, T-cell activation and CA 125 in obese patients with heart failure. Med. Sci. Monit. 17 (3), CR174–CR179. https://doi.org/10.12659/MSM.881453 (2011). PMID: 21358606; PMCID: PMC3524732
El-Mesallamy, H. O., Hamdy, N. M., El-Etriby, A. K. & Wasfey, E. F. Plasma granzyme B in ST elevation myocardial infarction versus non-ST elevation acute coronary syndrome: comparisons with IL-18 and fractalkine. Mediators Inflamm. 2013, 343268. https://doi.org/10.1155/2013/343268 (2013). Epub 2013 Nov 6. PMID: 24307760; PMCID: PMC3836447
Sabe, S. A., Feng, J., Sellke, F. W. & Abid, M. R. Mechanisms and clinical implications of endothelium-dependent vasomotor dysfunction in coronary microvasculature. Am. J. Physiol. Heart Circ. Physiol. 322 (5), H819–H841. https://doi.org/10.1152/ajpheart.00603.2021 (2022). Epub 2022 Mar 25. PMID: 35333122; PMCID: PMC9018047
Teragawa, H. et al. Relation between C reactive protein concentrations and coronary microvascular endothelial function. Heart 90, 750–754. https://doi.org/10.1136/hrt.2003.022269 (2004).
Song, Z. et al. Inflammatory burden index: association between novel systemic inflammatory biomarkers and prognosis as well as in-Hospital complications of patients with aneurysmal subarachnoid hemorrhage. J. Inflamm. Res. 16, 3911–3921. https://doi.org/10.2147/JIR.S416295 (2023). PMID: 37692059; PMCID: PMC10488670
Xie, H. et al. Inflammatory burden as a prognostic biomarker for cancer. Clin. Nutr. 41 (6), 1236–1243 (2022).
Chen Hao, G. & Tao, W. Expert consensus on diagnosis and treatment of multidisciplinary microvascular diseases in China. Chin. J. Circulation. 35 (12), 1149–1165 (2020).
Zhang, Y. et al. B. Automatic coronary blood flow computation: validation in quantitative flow ratio from coronary angiography. Int. J. Cardiovasc. Imaging. 35, 587–595 (2019).
Suda, A. et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J. Am. Coll. Cardiol. 74, 2350–2360 (2019).
Shaw, L. J. et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and In-Hospital mortality in the American college of Cardiology-National cardiovascular data registry. Circulation 117, 1787–1801 (2008).
Zhang Yun, C. & Yundai Fu xianghua, etc. Chinese expert consensus on the diagnosis and treatment of coronary microvascular disease [J]. Chin. J. Circulation. 32 (05), 421–430 (2017).
Mileva, N., Nagumo, S. & Mizukami, T. etc. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients With Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J Am Heart Assoc. ;11(7): e023207. (2022). https://doi.org/10.1161/JAHA.121.023207. Epub 2022 Mar 18. PMID: 35301851; PMCID: PMC9075440
Huang, F-Y., Huang, B-T. & Lv, W-Y. etc. The prognosis of patients with nonobstructive coronary artery disease versus normal arteries determined by invasive coronary angiography or computed tomography coronary angiography: a systematic review. Medicine ;95: e3117 (2016).
Geng, L. et al. Association of quantitative flow ratio-derived microcirculatory indices with anatomical-functional discordance in intermediate coronary lesions. Int. J. Cardiovasc. Imaging. 37 (10), 2803–2813. https://doi.org/10.1007/s10554-021-02292-2 (2021). Epub 2021 May 31. PMID: 34059977.
Xie, H. et al. Inflammatory burden as a prognostic biomarker for cancer. Clin. Nutr. 41 (6), 1236–1243 (2022). Epub 2022 Apr 22. PMID: 35504166.
Kosmas, C. E. et al. The Triglyceride/High-Density lipoprotein cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics (Basel). 13 (5), 929. https://doi.org/10.3390/diagnostics13050929 (2023). PMID: 36900073; PMCID: PMC10001260
Cao, Y. et al. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: results from NHANES. Front. Immunol. 14, 1087345. https://doi.org/10.3389/fimmu.2023.1087345 (2023). PMID: 36817427; PMCID: PMC9932782
Xu, B. et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc. Diabetol. 23 (1), 212. https://doi.org/10.1186/s12933-024-02251-w (2024). PMID: 38902748; PMCID: PMC11191290
Rizzoni, D. et al. Relationships between coronary flow vasodilator capacity and small artery remodelling in hypertensive patients. J. Hypertens. 21 (3), 625–631. https://doi.org/10.1097/00004872-200303000-00030 (2003).
Nitenberg, A. et al. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes 42 (7), 1017–1025. https://doi.org/10.2337/diab.42.7.1017 (1993).
Dagres, N. et al. Insulin sensitivity and coronary vasoreactivity: insulin sensitivity relates to adenosine-stimulated coronary flow response in human subjects. Clin. Endocrinol. (Oxf). 61 (6), 724–731. https://doi.org/10.1111/j.1365-2265.2004 (2004). 02156.x.
Kaufmann, P. A., Gnecchi-Ruscone, T., Schäfers, K. P., Lüscher, T. F. & Camici, P. G. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J. Am. Coll. Cardiol. 36 (1), 103–109. https://doi.org/10.1016/S0735-1097(00)00697-5 (2000).
Tong, D. C. et al. Protein is a predictor of coronary microvascular dysfunction in patients with ischemic heart disease. Front. Cardiovasc. Med. 4, 81. https://doi.org/10.3389/fcvm.2017.00081 (2018). PMID: 29376057; PMCID: PMC5770395
Duran, E. K. et al. Triglyceride-Rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J. Am. Coll. Cardiol. 75 (17), 2122–2135. https://doi.org/10.1016/j.jacc.2020.02.059 (2020). PMID: 32354380; PMCID: PMC8064770
Miller, M. et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. ;51(7):724 – 30. (2008). https://doi.org/10.1016/j.jacc.2007.10.038. PMID: 18279736.
Miller, N. E., Thelle, D. S., Forde, O. H. & Mjos, O. D. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. ;1(8019):965-8. (1977). https://doi.org/10.1016/s0140-6736(77)92274-7. PMID: 67464.
Barter, P. et al. Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. ;357(13):1301-10. (2007). https://doi.org/10.1056/NEJMoa064278. PMID: 17898099.
Ossoli, A., Pavanello, C., Giorgio, E., Calabresi, L. & Gomaraschi, M. Dysfunctional HDL as a Therapeutic Target for Atherosclerosis Prevention. Curr Med Chem. ;26(9):1610–1630. (2019). https://doi.org/10.2174/0929867325666180316115726. PMID: 29546829.
Yang, T. et al. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and type 2 diabetes in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc. Diabetol. 21 (1), 93. https://doi.org/10.1186/s12933-022-01531-7 (2022). PMID: 35659300; PMCID: PMC9166647
Nie, G., Hou, S., Zhang, M. & Peng, W. High TG/HDL ratio suggests a higher risk of metabolic syndrome among an elderly Chinese population: a cross-sectional study. BMJ Open. 11 (3), e041519. https://doi.org/10.1136/bmjopen-2020-041519 (2021). PMID: 33753431; PMCID: PMC7986938
Sato, F., Nakamura, Y., Kayaba, K. & Ishikawa, S. TG/HDL-C ratio as a predictor of stroke in the population with healthy BMI: the Jichi medical school cohort study. Nutr. Metab. Cardiovasc. Dis. 32 (8), 1872–1879 (2022). Epub 2022 May 16. PMID: 35753859.
McLaughlin, T. et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am. J. Cardiol. 200596399–200596404 https://doi.org/10.1016/j.amjcard.2005.03.085
McLaughlin, T. et al. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann. Intern. Med. 2003139802–2003139809. https://doi.org/10.7326/0003-4819-139-10-200311180-00007
Liao, L. P., Wu, L. & Yang, Y. The relationship between triglyceride/high-density lipoprotein cholesterol ratio and coronary microvascular disease. BMC Cardiovasc. Disord. 23 (1), 228. https://doi.org/10.1186/s12872-023-03229-4 (2023). PMID: 37131145; PMCID: PMC10155446
Sabe, S. A., Feng 」, Sellke, F. W. & Abid, M. R. Mechanisms and clinical implications ofendothelium-dependent vasomotor dysfunction in coronary microvasculature. Am 」 physiol heart circ physiol.2022 May 1:322(5): H819-H841.https://doi.org/10.1152/aipheart.00603.2021.Epub 2022 Mar 25.PMID:35333122:PMCID: PMC9018047
Muris, D. M., Houben, A. J., Schram, M. T. & Stehouwer, C. D. Microvascular dysfunction: an emerging pathway in the pathogenesis of obesity-related insulin resistance. Rev. Endocr. Metab. Disord. 14 (1), 29–38. https://doi.org/10.1007/s11154-012-9231-7 (2013).
Sara, J. D. et al. Prevalence of Coronary Microvascular Dysfunction Among Patients with Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. ;8(11):1445–1453. doi: 10.1016/j.jcin.2015.06.017. PMID: 26404197. (2015).
Ikonomidis, I. et al. Insulin resistance and acute glucose changes determine arterial elastic properties and coronary flow reserve in dysglycaemic and first-degree relatives of diabetic patients. Atherosclerosis 241 (2), 455–462. https://doi.org/10.1016/j.atherosclerosis.2015.06.006 (2015).
Zhang, Y. et al. Adenosine and adenosine receptor-mediated action in coronary microcirculation. Basic. Res. Cardiol. 116, 22 (2021).
Chatham, J. C. & Seymour, A. M. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc. Res. 55, 104–112 (2002).
Schroder, J. et al. Pro-inflammatory biomarkers in women with non-obstructive angina pectoris and coronary microvascular dysfunction. Int. J. Cardiol. Heart Vasc. 24, 100370. https://doi.org/10.1016/j.ijcha.2019.100370 (2019).
Turhan Caglar, F. N. et al. Evaluation of serum vitamin D levels in patients with X syndrome. Eur. Rev. Med. Pharmacol. Sci. 20 (6), 1155–1160 (2016). PMID: 27049271.
Chen, Y. C. et al. Effect of vitamin D supplementation on primary dysmenorrhea: A systematic review and Meta-Analysis of randomized clinical trials. Nutrients 15 (13), 2830. https://doi.org/10.3390/nu15132830 (2023). PMID: 37447156; PMCID: PMC10343446
Gao, X., Zhang, H., Schmidt, A. M. & Zhang, C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 295, H491–H498. https://doi.org/10.1152/ajpheart.00464.2008 (2008).
Tong, D. C. et al. Protein is a predictor of coronary microvascular dysfunction in patients with ischemic heart disease. Front. Cardiovasc. Med. 12, 4:81. https://doi.org/10.3389/fcvm.2017.00081 (2018 Jan). PMID: 29376057; PMCID: PMC5770395
Qamirani, E., Ren, Y., Kuo, L. & Hein, T. W. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler. Thromb. Vasc Biol. 25, 995–1001. https://doi.org/10.1161/01.ATV.0000159890.10526.1e (2005).
Karasu, M. & Bolayır, H. A. Cut-off value for interleukin-34 as an additional potential inflammatory biomarker for Estimation of slow coronary flow risk. BMC Cardiovasc. Disord. 24 (1), 2. https://doi.org/10.1186/s12872-023-03677-y (2024). PMID: 38166811; PMCID: PMC10762812
Oikonomou, E. et al. Tousoulis D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: clinical and therapeutic implications.
Acknowledgements
No Applicable.
Funding
This work was supported by Hospital capability enhancement project of Xiyuan Hospital, CACMS. (No.CI2021A00901). Beijing Clinical Research Ward (BCRW202108). Beijing Traditional Chinese Medicine Technology Development Fund Project (BJZYZD-2023-11).
Author information
Authors and Affiliations
Contributions
W.W., Y.C., and Y-Q Z. collected the data and drafted the manuscript; M-W L., B-L X., M-J G., Z-D J. and L-L J. performed the statistical analyses; F-H Z and K-J C reviewed the manuscript and guided the methodology.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Xiyuan Hospital(2024XLA031-2). We informed the patient that the clinical data would be used for publication and obtained consent from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wen, W., Chi, Y., Liu, M. et al. Inflammation mediated glycolipid dysregulation in coronary microvascular disease pathogenesis. Sci Rep 15, 22694 (2025). https://doi.org/10.1038/s41598-025-08057-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08057-4