Abstract
Urinary extracellular vesicles (uEVs) and immune mediators have emerged as potential minimally invasive renal biomarkers. Even though active lupus nephritis (LN) is associated with immune complex deposition, tissue inflammation, and podocyte damage, it remains unclear how these parameters are simultaneously altered in systemic lupus erythematosus (SLE). Thus, we aimed to evaluate uEVs as biomarkers in LN, in association with urinary immune mediators. In this cross-sectional study, uEVs were isolated from SLE patients and healthy donors by differential centrifugation and characterized and/or quantified by electron microscopy, nanoscale flow cytometry, and nanoparticle tracking analysis (NTA). Urinary immune mediators were assessed by a multiplex assay. We included 82 patients (42.6 ± 11.3 years-old, 91.4% female), of whom 56.1% (n = 46) had LN, and 18 healthy donors (37.5 ± 8.2 years-old, 83.3% female). No differences were found for particle size/concentration by NTA, but higher counts of total (P = 0.03) and podocyte-derived (P = 0.01) uEVs were observed in SLE patients, especially in active LN (P = 0.02; P = 0.03). We also identified higher urinary levels of cytokines such as IL-6, IL-8, and CCL-2 according to SLE activity and LN (P < 0.05). Significant correlations were observed between uEVs, immune mediators, R-SLEDAI-2K, proteinuria, and albuminuria in active LN. Lastly, the combinatory analysis of podocyte uEVs, IL-6, IFN-γ, IL-8, uCCL-2 and CCL-3 showed a good predictive power to detect active LN (AUC = 0.88, P = 0.0009). Our results suggest that urinary podocyte-derived uEVs and cytokines are associated with LN activity, which may reflect podocyte injury mediated by inflammation. Thus, the combined application of these biomarkers could help to identify patients with podocyte damage and renal inflammation.
Similar content being viewed by others
Introduction
Lupus nephritis (LN) affects 30–60% of adult patients with systemic lupus erythematosus (SLE) in the first five years after diagnosis, with possible progression to kidney failure during the first decade1,2. The pathogenesis of LN is multifactorial, involving different mechanisms such as inflammation triggered by immune complex deposition in the glomeruli, complement system activation, and the release of immune mediators resulting from the abnormal innate and adaptive immune response, which directly contributes to the development of glomerular lesions3.
Podocytes are specialized glomerular epithelial cells responsible for the regulation of glomerular filtration. Lupus podocytopathy is directly caused by lymphocyte activation and/or indirectly by the immune complex deposition, which leads to diffuse epithelial foot process effacement and damaging of the glomerular filtration barrier, allowing the “leakage” of proteins, red blood cells and leukocytes3,4. Also, the podocyte can be involved in antigen presentation to immune cells and cytokine release, being a key cell in the pathophysiology of LN5,6,7. However, lupus podocytopathy represents approximately 1% of biopsy-proven cases of LN, being a unique subtype that it is clinically presented as nephrotic syndrome3,7.
In addition to kidney biopsy, which is used to assess the extent of renal damage using activity and chronicity indices8, conventional biomarkers such as serum creatinine, glomerular filtration rate (GFR) and proteinuria, can also be used to predict/monitor lupus renal crisis9. In addition, other biomarkers such as serum and urinary cytokines, anti-nucleosome antibodies, and serum anti-C1q antibodies have been investigated as alternative methods to assess LN2,10. Nevertheless, in recent years, studies have been focusing on the role of urinary extracellular vesicles (uEVs) as potential non-invasive biomarkers for the diagnosis and monitoring of kidney diseases, since urine is a rich source of EVs derived from urinary tract cells11,12. EVs are responsible for intercellular communication through the delivery of mRNA, DNA, proteins, lipids, and other cellular content to other cells13. In the context of SLE, EVs have been reported to carry self-antigens, promoting complement activation and amplification of the inflammatory response14.
Despite the increasing interest on EVs in different pathological scenarios, few studies have investigated the role of uEVs in SLE as possible biomarkers of podocyte injury. Little is also known about the combined detection of uEVs and urinary immune mediators in LN. Thus, our aim was to analyze the concentration of total and podocyte-derived uEVs in association with urinary immune mediators in patients with SLE according to disease activity, kidney involvement with LN diagnosis/activity and immunosuppression status.
Methods
Study design and data collection
We conducted a cross-sectional study that included adult SLE patients that attended the rheumatology outpatient clinic of the Hospital Universitário Antônio Pedro (HUAP – EBSERH - Niterói, Rio de Janeiro, Brazil) who were recruited from March 2022 to February 2024. The study was approved by the Ethics Committee of the Faculty of Medicine (UFF) (IRB#12125219.80000.5243 and 60747722.20000.5243) and all the activities were performed according to the Declaration of Helsinki after obtaining written informed consent.
SLE diagnosis was confirmed by the American College of Rheumatology and the European League Against Rheumatism criteria15,16. As a control group, we also included healthy donors with no evidence of autoimmune disease or other comorbidities of equivalent age and sex (n = 18). Exclusion criteria were: pregnancy at the time of collection, current infection, chronic kidney disease (CKD) in advanced stages (estimated GFR < 30 mL/min/1.73m2) or missing clinical and/or laboratory data needed to assess SLE activity.
Patient data (e.g. gender, ethnicity, age, time of lupus diagnosis, therapeutic regimens, comorbidities, and routine laboratory tests) were obtained from medical records. Standard immunosuppressive treatment consisted of using prednisolone ≥ 15 mg/day, mycophenolate mofetil ≥ 1 g/day, or azathioprine > 2 mg/kg/day, any dose of cyclophosphamide and methotrexate ≥ 20 mg/day17.
SLE activity was estimated using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K, range 0–105) and LN activity was assessed by the SLEDAI Renal Domain (R-SLEDAI-2K, range 0–16), which evaluates the presence of hematuria (> 5 red blood cells per field), pyuria (> 5 white blood cells per field), proteinuria (> 0.5 g/24 h) and the presence of urinary casts18. According to SLEDAI-2K and R-SLEDAI-2K, patients were divided into groups as follows: “Inactive no renal” - SLEDAI-2K = 0 and no renal involvement; “Active no renal” - SLEDAI-2K ≥ 1 and no renal involvement; “Inactive LN” - patients with a history of LN and R-SLEDAI-2K = 0; and “Active LN” - patients with a history of severe LN, systemic disease activity (SLEDAI-2K ≥ 1), and R-SLEDAI-2K ≥ 4 (which reflects the presence of proteinuria, pyuria, haematuria and/or urinary casts).
Sample collection and routine laboratory tests
Peripheral venous blood samples were collected in serum clot activator tubes (BD Vacutainer®) for routine laboratory tests. Urine samples were obtained from the first-morning void in a sterile polypropylene tube (40mL) and processed within four hours of collection. Initial processing was performed with centrifugation at 600 × g for 10 min at room temperature to eliminate cells and debris. Posteriorly, the supernatant was immediately frozen at − 80 °C. Aliquots were only defrosted once at the time of analysis. Measurements of urinary creatinine, urinary protein, albumin-to-creatinine ratio (ACR), serum creatinine, serum anti-ds-DNA, C3, and C4 were carried out by the Clinical Analysis and Pathology Unit (UACAP-HUAP). GFR was estimated using the CKD-EPI 2021 equation (eGFR)19.
Transmission electron microscopy
In accordance with the recommendations of the International Society for Extracellular Vesicles (ISEV), we used three different methods to detect and characterize EVs20. In this regard, transmission electron microscopy (TEM) was carried out using the protocol described by Corona et al. (2023) with some modifications21. Urine supernatant was thawed at room temperature and centrifuged at 20,000 × g for 20 min at 4 °C to obtain large EVs (200–1000 nm), which were the focus of our study. The uEV isolate was fixed with 4% paraformaldehyde (1:1; cat. P6148, Sigma Aldrich, Darmstadt, Germany) for 1 h at 4 °C. Subsequently, the fixed isolate was adhered to a 300 mesh nickel grid (TED PELLA Inc., Canada) previously treated with polylysine for 20 min. The grid was washed six times with Milli-Q® water to remove the excess of solution and possible contaminants. For staining and contrast, 2% uranyl acetate (ACS Científica, São Paulo, Brazil) was used and a 13 min incubation was performed at room temperature. The analysis was carried out using the Hitachi 7800 TEM Electron Microscope of the National Center for Structural Biology and Bioimaging (Cenabio) located at the Universidade Federal do Rio de Janeiro (UFRJ).
Nanoparticle tracking analysis
After the 20,000 × g centrifugation (20 min at 4 °C), the uEV isolate was diluted 1:50 in filtered PBS (0.22 μm) to a final volume of 1 mL and inserted into the ZetaView Twin PMX220 (Particle Metrix, Germany). Experiments were conducted in a room temperature of 20ºC and software settings were selected according to the manufacturer’s guidelines for EVs or nanospheres. After capture, the videos were analyzed by the built-in software ZetaView 8.02.31 with specific analysis parameters, with a minimum and maximum particle size of 5 and 1000 nm, respectively.
Nanoscale flow cytometry
For the detection of total and podocyte-derived uEVs, each urine sample was initially submitted to differential centrifugation as mentioned above. The pellet was resuspended in Annexin V binding buffer (cat. V13246, Invitrogen, Waltham, Massachusetts, USA) with Annexin-V FITC (1:50, Biolegend, San Diego, California, USA) and Anti-human Podoplanin APC (1:100, Biolegend, San Diego, California, USA). After a second centrifugation and resuspension, samples were acquired using the CytoFLEX S flow cytometer (Beckman Coulter, Brea, California, USA). uEV identification was based on size using calibration beads (100–900 nm, MEGAMIX-Plus FSC, and SSC, Biocytex, Marseille, Provence, France) and antibody positivity, as previously described22. We performed five experiments to quantify uEVs in all samples and the control batch effect between experiments was minimized by using Cytometer Setup and Tracking beads to adjust gain and signal readings (cat. B53230, Cytoflex Daily QC Fluorespheres, Beckman Coulter Inc, USA) in addition to maintaining the same acquisition parameters (volume, time, flow rate, number of events). The gating strategy and controls (0.1% Triton-X, Milli Q water, isotype, Annexin V buffer, and antibody mixture) are shown in Supplementary Fig. 1. We have also included a summary of our experiments using the MIFlowCyt-EV reporting table template23 (Supplementary Table 1). Data analysis was performed using the FlowJo version 9/10 software (FlowJo LLC, Becton Dickinson, Franklin Lakes, NJ, USA). It is important to mention that uEV levels were normalized to urinary creatinine for the correction of differences in urine concentration24.
Multiplex assay
Urinary levels of immune mediators were assessed by a multiparametric assay using the Bio-Plex Pro Human Cytokine Screening Panel 27 Plex high-throughput screening kit (Bio-Rad, Hercules, California, USA) in a single experiment. We analyzed: (i) chemokines [C-C chemokine ligand (CCL)−2/chemokine protein 1/MCP-1, CCL3/MIP-1 α macrophage inflammatory protein 1α, CCL-4/MIF-1β macrophage inflammatory protein 1β, CCL5/RANTES, CCL11/eotaxin, C-X-C interferon chemokine ligand (CXCL)−8 (IL-8) and CXCL-10/IP-10 gamma interferon-induced protein 10; (ii) cytokines [tumor necrosis factor-α/TNF-α, interferon γ/IFN-γ, interleukin 1 receptor antagonist/IL-1Ra, and interleukins (IL)−1β, −2, −4, −5, −6, −7, −8,−9, −10,−12p70, −13, −15, −17]; and (iii) growth factors granulocyte-macrophage colony-stimulating factor/GM-CSF; granulocyte colony-stimulating factor/G-CSF; platelet-derived growth factor-BB/PDGF-BB; vascular endothelial growth factor/VEGF]. Reading was performed using the MAGPIX Luminex instrument (Luminex, Austin, TX, USA), according to the manufacturer’s instructions. Urinary levels of immune mediators were also normalized to urinary creatinine and expressed as pg/mg of urinary creatinine.
Statistical analysis
Data was expressed as median and interquartile range (IQR) or frequencies (%). T-student or Mann-Whitney tests were applied to assess differences between two independent groups according to variable’s distribution. For more than two groups, we used Kruskal-Wallis with Dunn’s post-test or ANOVA with Tukey’s post-test. Differences between frequencies were analyzed with the Chi-square test. Spearman’s coefficients were assessed to investigate correlations between variables. To identify the most relevant biomarkers associated with active LN, a random forest classification model was used due to its robustness in handling nonlinear relationships and complex interactions between predictors, as well as its effectiveness in selecting variables in high-dimensional datasets. The response variable, active LN, was treated as a binary factor. The model was implemented with 500 trees (ntree = 500) and a random subset of four variables sampled at each split (mtry = 4). The relevance of each variable was assessed using the mean decrease accuracy (MDA) metric, which quantifies the average reduction in model accuracy after randomly permuting the values of each variable, reflecting its predictive contribution. Posteriorly, receiver operating characteristic (ROC) analyses were performed and sensitivity, specificity, and the area under the curve (AUC) > 0.70 were used to identify the best combination panel of uEVs and immune mediators as predictors of active LN. P-values < 0.05 were considered significant and statistical analyses were conducted using the Prism software (GraphPad Software 8.0, San Diego, CA, USA) and R.3.2.2 (R Core Team).
Results
Patient’s characteristics
This study included 82 SLE patients with a mean age of 42.6 ± 11.3 years, 91.6% female. Of these, 46 patients (56.1%) had LN. The mean age of healthy donors was 37.5 ± 8.2 years and 83.3% were female. The analysis of clinical and laboratory characteristics was performed according to the presence of renal involvement (Table 1). As expected, patients with active LN (n = 19) presented higher SLEDAI-2K (P < 0.0001), R-SLEDAI-2K (P < 0.0001), proteinuria (P < 0.001), and ACR (P < 0.0001). Of these individuals, 68.4% (n = 13) were immunosuppressed (P = 0.01), with 36.8% (n = 7) under prednisone dosage ≥ 15 mg/day (P = 0.01). These patients also presented higher levels of anti-dsDNA (P < 0.005) in addition to lower levels of C3 (P = 0.04).
Assessment of uEVs in SLE patients
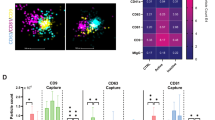
The characterization of urinary particles is shown in Fig. 1. NTA showed no differences in the number of particles/mL and medium diameter (nm) between groups. However, when we analyzed the results of uEV detection by nanoscale flow cytometry (Fig. 2), we observed that SLE patients presented higher counts of total urinary and podocyte-derived uEVs in comparison to healthy donors (P = 0.03 and P = 0.01, respectively), but uEVs were not associated with SLE activity (SLEDAI-2K < 4 vs. ≥ 4, P = 0.92 and P = 0.19). Moreover, we identified that patients with active LN showed significantly elevated uEVs when compared to patients with active SLE but without renal involvement (P = 0.02 and P = 0.03 for total and podocyte uEVs, respectively).
Characterization of urinary extracellular vesicles in patients with systemic lupus erythematosus (SLE) and healthy donors (HD). Electron micrograph of the uEV isolate (scale bars 250 nm) (A). Comparison of particle’s mean diameter and counts by NTA between SLE and HD (B), according do SLE activity (SLEDAI-2K) (C) and inactive/active LN (D). HD: healthy donors; LN: lupus nephritis; SLE: systemic lupus erythematosus.
Analysis of uEVs in SLE patients by nanoscale flow cytometry. (A) Representative dot plot flow cytometry charts. (B) Comparison of uEVs in SLE patients and healthy donors (C) and according to disease activity (SLEDAI-2 K). (D) Comparison renal involvement (E) and number of immunosuppressive drugs administered to SLE patients. P-values were assessed using Manny-Whitney test. HD: healthy donors; LN: lupus nephritis; SLE: systemic lupus erythematosus; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index; uCr: urinary creatinine; uEVs: urinary extracellular vesicles.
uEVs were also analyzed according to the status of immunosuppression, but no significant results were found for total (P = 0.99) and podocyte uEVs (P = 0.21). Similar results were observed when uEV levels were analyzed according to daily use and dosage of medications (data not shown). However, it was observed that SLE patients under two or more immunosuppressive drugs presented significantly higher counts of podocyte-derived uEVs (P = 0.02), as shown in Fig. 2. Of note, no differences in uEV counts were observed according to sex (P = 0.44; P = 0.85) and the presence of comorbidities such as hypertension (P = 0.88; P = 0.39) and diabetes (P = 0.27; P = 0.23) (data not shown).
Analysis of urinary immune mediators and correlations with uEVs
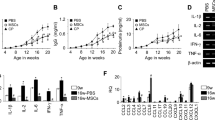
Of the 27 immune mediators examined in this study, eight were below the lower limit of detection (IL-1RA, IL-2, IL-4, L-7, FGF-b, PDGF-BB, G-CSF, VEGF). Thus, the concentrations of 19 immune mediators were considered for further analysis. Overall, we identified that patients with SLEDAI-2K > 5 (moderate-to-high disease activity) presented significantly higher levels of uIL-6, uIFN-γ, uCCL-2, and uCCL-5 (P < 0.05). In addition, patients with active LN presented higher uIL-6, uIL-8, and uCCL-2 when compared to SLE without renal involvement (P < 0.05). These results are shown in Fig. 3. Subsequently, we analyzed the correlations between urinary levels of immune mediators and uEVs in patients with active LN (Table 2). We observed moderate positive correlations between total uEVs with R-SLEDAI-2K, uCCL-2, uCCL-3, and uCCL-4; and a negative correlation with C3 levels. Moreover, podocyte-derived uEVs were significantly correlated with proteinuria, ACR, and uIL-10.
Analysis of urinary immune mediators in SLE patients according to disease activity based on SLEDAI-2 K (A) and renal involvement/lupus nephritis (B). P-values were assessed using Kruskal Wallis with Dunn post-test. CCL: Chemokine ligand; IFN: interferon; IL: interleukin; LN: lupus nephritis; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index; u: urinary.
Performance of uEVs and immune mediators in distinguishing active LN
In order to evaluate variable’s predictive power to identify active LN, we initially performed individual ROC curves for immune mediators and uEVs, using the variables that were selected by the random forest classification model. Our aim was to investigate if these parameters were able to distinguish active LN among patients with active SLE (SLEDAI-2K ≥ 1). The model produced an out-of-bag error rate of 27.27%. The confusion matrix indicated class-specific error rates of 27.78% and 26.67% for negative and positive classes, respectively. Biomarkers that demonstrated the highest importance included uIL-6 (MDA = 13.17), uIL-8 (MDA = 5.02), and uCCL2 (MDA = 4.93). Additional contributors with moderate positive importance were podocyte-derived uEVs (MDA = 2.06), uIFN-γ (MDA = 2.46), and uCCL-3 (MDA = 2.89). On the other hand, several variables, such as total uEVs (MDA=−1.68), uCCL-4 (MDA=−0.88) and uTNF-α (MDA=−2.60), exhibited negative MDA values, indicating insignificant or no contribution to the predictive performance of the model. As shown on Table 3, when evaluated individually, urinary IL-6, IL-8, IFN-γ, and CCL-2 showed values of AUC values between 0.66 and 0.76 (P < 0.05), indicating a mild ability to discriminate groups. However, when we performed a multiparametric analysis, we identified that combining uEVs and immune mediators improved the diagnostic power. The combination of podocyte uEVs with uIL-8 and uCCL-2 demonstrated an AUC = 0.85 and sensitivity and specificity ≥ 80% (P = 0.001), and the combination of podocyte uEVs with all immune mediators showed an AUC = 0.88, sensibility of 80% and specificity of 94.4% (P = 0.0009).
Discussion
LN development involves complex processes directly involved to the immune response4. Once in the kidneys, immune mediators stimulate the recruitment of inflammatory cells, causing damage to podocytes and glomerular dysfunction1,10. Renal biopsy is the gold standard for LN diagnosis and classification, but it may not always be indicated because the procedure is associated with a high risk for complications such as hemorrhage and infections25. In addition, 14% of kidney biopsies may obtain inadequate or insufficient material for analysis26. Thus, the study of non-invasive or minimally invasive biomarkers in these scenarios could be a new direction for LN early diagnosis, monitoring and therapeutical guidance, preventing the risks of repeated biopsies27,28. In this context, our aim was to evaluate the role of urinary EVs and immune mediators as biomarkers of LN.
Our study identified that 54.3% of patients treated at a quaternary hospital in the metropolitan region of Rio de Janeiro had a clinical history of LN with presentation after around 10 years of SLE onset. Some studies have described that 30–70% of SLE patients can rapidly progress to class IV after five years of diagnosis29,30. In juvenile SLE, this rate can reach up to 80% with progression to advanced-stage renal disease in 30% of cases31. Some prognostic factors of LN activity include male gender, proteinuria > 4 g/day, thrombocytopenia, frequent relapses, presence of antiphospholipid antibodies, high titers of anti-dsDNA, anti-C1q and persistent complement consumption30. In our study, we identified some of these alterations in this group, such as higher levels of anti-dsDNA and lower C3, which is consistent with the literature32; in addition to proteinuria and albuminuria, as expected.
Previous studies highlight uEVs as emerging tools for diagnosis, prognosis, and monitoring of kidney disease. Due to their ability to transport proteins related to lipid metabolism, oxidative stress, inflammation and fibrosis, alterations in these biomarkers can be a reflection of kidney disease progression11,12. Our results demonstrated that total uEVs, as well as podocyte-derived uEVs, are significantly elevated in SLE, and the highest counts were observed in patients with active LN. In the literature, few studies were focused on evaluating uEVs in SLE. Our results agree with Lu et al. (2019), which also observed alterations in podocyte uEVs in a cross-sectional study with 34 patients. However, they found increased levels of uEVs according to SLEDAI-2K33, which was not observed in our study. Recently, Navarro-Hernandez et al. (2024) showed that EVs sized 100–500 nm are increased in the urine of LN patients, but the size profile was similar between groups32. Corroborating with this finding, we also did not find differences in the median particle size by NTA.
Urinary EVs from different cellular origins were also identified in SLE. Burbano et al. (2019) showed that EVs expressing platelet and leukocyte markers can be detected in urine samples and that LN patients present higher counts of uEVs positive for the high-mobility group box 1 (HMGB1) protein, which was associated with proteinuria34. In our study, these type of EVs could be among the pool of “total uEVs”, and further analysis is necessary to better explore these urinary EV populations. Importantly, in addition to uEV characterization using surface markers, research is also focused on the analysis of EV cargo in SLE, especially the investigation of microRNAs in small EVs35,36. Taken together, these findings suggest that kidney injury may be assessed by the analysis of EVs in urine samples.
Regarding the analysis of uEV counts according to immunosuppression, we identified that patients under two or more immunosuppressant drugs presented higher concentrations of podocyte uEVs. It is known that podocytes have the potential to synthesize inflammatory mediators, such as IL-1α and IL-1β6. Thus, it is possible that, clinically, patients with active LN, and consequently elevated podocyte-derived uEVs, required more intensive therapeutic schemes (prednisone ≥ 15 mg/day in 31.6% of cases) because of the role of these cells in the amplification of renal inflammation. Also, it is known that podocytes express nuclear glucocorticoid receptors and the activation of these receptors lead to beneficial effects on podocyte biology37. Considering this, further studies are required to clarify if these cells respond in vitro to glucocorticoid treatment by increasing the release of EVs. Moreover, previous studies have investigated variations in plasma EVs according to immunosuppression in autoimmune disease38,39; however, the data is scarce for the assessment of uEVs. We highlight the study of Troyer et al. (2022), which demonstrated that increased levels of glycosphingolipids within uEVs can predict a poor treatment response to mycophenolate40. It is important to mention that not only EV counts can be altered in response to drugs, but also their activity, as increased expression of activation markers and cytokines have been found in immune cells exposed to EVs from SLE patients under glucocorticoid therapy41.
In addition to exploring uEVs as biomarkers in SLE, we also investigated urinary levels of immune mediators. Overall, we observed elevation of uIL-6, uIL-8, uIFN-γ and uCCL-2/MCP-1 according to SLE activity. Moreover, higher uIL-6, uIL-8, and uCCL-2/MCP-1 were found in patients with active LN. In addition, we identified that some mediators (uIL-10, uCCL-2/MCP-1, uCCL-3 and uCCL-4) were associated with total and podocyte uEVs. Lastly, total uEVs were negatively correlated with C3 levels, which agrees with previous findings32,33. In this context, studies have reported alterations in immune mediators such as CCL-2/MCP-1, IL-6, CXCL-10 in the urine of patients with active LN42,43. Rovin et al. (2005) monitored uCCL-2/MCP-1 levels every two months, which remained elevated only in patients with LN, suggesting a possible prediction of LN activity before the crisis44. Once again, these findings could reflect the renal inflammatory response in SLE, since it is known that EVs, especially microvesicles/microparticles (large EVs), can carry high levels of chromatin from apoptosis, immunoglobulins (IgG, IgA, and IgM), and components of the complement system (C1q, C1s, C3, C4b and C9), thus being sources for the formation of immunocomplexes45,46. Also, studies have shown that cytokines can be “packaged” into EVs and transmitted to neighboring cells for communication47. Thus, EVs present important pro-inflammatory properties in autoimmune disease.
As evidenced in previous works, uEVs can be a promising analytical parameter to distinguish SLE activity and/or LN32,33. In our study, we aimed to investigate if total and podocyte-derived uEVs were able to identify LN among patients with active SLE, but the individual analysis of ROC curves did not show significant results. However, a combinatory analysis with immune mediators, specially uIL-8 and uCCL-2/MCP-1, showed a good predictive power to distinguish LN. In this context, studies have shown significant results of ROC curve analysis for uCCL-2/MCP-1 to detect LN with high sensitivity and specificity values10,48, but as far as we know, a combinatory analysis with uEVs have not been yet demonstrated. The evaluation of models combining podocyte-derived uEVs with urinary immune mediators provides a clear view of the potential that these biomarkers may have in clinical practice for detecting patients with active LN. Importantly, this combination approach could be more useful since it provides measurements of renal inflammation (cytokine release by kidney cells or infiltrating immune cells), glomerular filtration barrier dysfunction (also allowing cytokine release in urine) and podocyte stress.
Our study has some limitations. Besides the relatively small sample size, we were not able to compare our results with histological findings since few kidney biopsies were performed during the study. Due to the cross-sectional study design, we were not able to look at changes in response to therapy over time and clinical outcomes such as hospitalization and death, to investigate if uEVs can predict LN prognosis. Longitudinal studies are required to clarify this hypothesis. Considering the heterogeneity of renal manifestations, further studies are necessary to investigate other uEV subpopulations (e.g. tubular and endothelial cells) and the association of these parameters with classical and immunological urinary biomarkers. Despite of presenting significant results for SLE patients, we were not able to measure urinary immune mediators in healthy donors, but previous findings have shown that urinary cytokines are significantly increased in SLE when compared to individuals without the disease10.
Conclusion
Our results showed that SLE patients present higher counts of uEVs, as well as elevated urinary levels of immune mediators such as uIL-8, uCCL-2 and uIL-6, especially those with active LN. In addition, uEVs were significantly associated with immune mediators and other laboratory parameters indicative of inflammation. These findings suggest that there is local inflammation and podocyte damage in active LN, and this may be reflected by the non-invasive assessment of both urinary EVs and cytokines, even if the disease activity is moderate. Therefore, the combined application of these biomarkers could help to identify patients with podocyte damage, which could be applied for other glomerulopathies. However, only a prospective study could confirm the use of podocyte damage detection as an early marker of a severe manifestation of LN.
Data availability
Data may be available upon request. Please contact the correspondent authors (thaliamedeiros@id.uff.br, aasilva@id.uff.br).
References
Davidson, A. What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. https://doi.org/10.1038/nrrheum.2015.159 (2016).
Wang, Y. et al. Clinical features, and outcomes of patients with antineutrophil cytoplasmic antibody-positive systemic lupus erythematosus. Ren. Fail. https://doi.org/10.1080/0886022X.2023.2235431 (2023).
Khan, O. et al. Lupus podocytopathy: A rare cause of nephrotic syndrome in patients with systemic lupus erythematosus. J. Community Hosp. Intern. Med. Perspect. https://doi.org/10.55729/2000-9666.1218 (2023).
Chernova, I. Lupus nephritis: immune cells and the kidney microenvironment. Kidney360 https://doi.org/10.34067/KID.0000000000000531 (2024).
Machida, H. et al. Expression of Toll-like receptor 9 in renal podocytes in childhood-onset active and inactive lupus nephritis. Nephrol. Dial Transpl. https://doi.org/10.1093/ndt/gfq058 (2010).
Wright, R. D. & Beresford, M. W. Podocytes contribute, and respond, to the inflammatory environment in lupus nephritis. Am. J. Physiol. Ren. Physiol. https://doi.org/10.1152/ajprenal.00512.2017 (2018).
Sakhi, H. et al. Podocyte injury in lupus nephritis. JCM https://doi.org/10.3390/jcm8091340 (2019).
Bajema, I. M. et al. Revision of the international society of nephrology/renal pathology society classification for lupus nephritis: clarification of definitions, and modified National institutes of health activity and chronicity indices. Kidney Int. https://doi.org/10.1016/j.kint.2017.11.023 (2018).
Mahajan, A. et al. Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression. Lupus https://doi.org/10.1177/0961203320932219 (2020).
Elsaid, D., Abdel Noor, R., Shalaby, K. & Haroun, R. H. Urinary tumor necrosis Factor-Like weak inducer of apoptosis (uTWEAK) and urinary monocyte Chemo-attractant Protein-1 (uMCP-1): promising biomarkers of lupus nephritis activity?? Saudi J. Kidney Dis. Transpl. https://doi.org/10.4103/1319-2442.318522 (2021).
Medeiros, T., Myette, R. L., Almeida, J. R., Silva, A. A. & Burger, D. Extracellular vesicles: Cell-Derived biomarkers of glomerular and tubular injury. Cell. Physiol. Biochem. https://doi.org/10.33594/000000207 (2020).
Erdbrügger, U. & Le, T. H. Extracellular vesicles in renal diseases: more than novel biomarkers?? J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2015010074 (2016).
Kalra, H., Drummen, G. P. C. & Mathivanan, S. Focus on extracellular vesicles: introducing the next small big thing. Int. J. Mol. Sci. https://doi.org/10.3390/ijms17020170 (2016).
Nielsen, C. T., Rasmussen, N. S., Heegaard, N. H. H. & Jacobsen, S. Kill the messenger: targeting of cell-derived microparticles in lupus nephritis. Autoimmun. Rev. https://doi.org/10.1016/j.autrev.2016.03.009 (2016).
Aringer, M. et al. 2019 European league against rheumatism/american college of rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2018-214819 (2019).
Hochberg, M. C. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthr. Rhuem. https://doi.org/10.1002/art.1780400928 (1997).
Athanassiou, P. & Athanassiou, L. Current treatment approach, emerging therapies and new horizons in systemic lupus erythematosus. Life https://doi.org/10.3390/life13071496 (2023).
Gladman, D. D., Ibañez, D. & Urowitz, M. B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29 (2), 288–291 (2002).
Delgado, C. et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. JASN https://doi.org/10.1681/ASN.2021070988 (2021).
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicle. https://doi.org/10.1002/jev2.12404 (2024).
Corona, M. L., Hurbain, I., Raposo, G. & Van Niel, G. Characterization of extracellular vesicles by transmission Electron microscopy and Immunolabeling Electron microscopy. In Cell-Secreted Vesicles [Internet] (ed. Vainio, S.) (Springer US, 2023). https://doi.org/10.1007/978-1-0716-3203-1_4.
Medeiros, T. et al. Exploring urinary extracellular vesicles and immune mediators as biomarkers of kidney injury in COVID-19 hospitalized patients. Diagnostics https://doi.org/10.3390/diagnostics12112600 (2022).
Welsh, J. A. et al. MIFlowCyt-EV: a framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicle. https://doi.org/10.1080/20013078.2020.1713526 (2020).
Erdbrügger, U. et al. Urinary extracellular vesicles: A position paper by the urine task force of the international society for extracellular vesicles. J. Extracell. Vesicle. https://doi.org/10.1002/jev2.12093 (2021).
Rovin, B. H., Parikh, S. V. & Alvarado, A. The kidney biopsy in lupus nephritis. Rheumatic Disease Clin. North. Am. https://doi.org/10.1016/j.rdc.2014.04.004 (2014).
Nissen, C. J., Moreno, V., Davis, V. G. & Walker, P. D. Increasing incidence of inadequate kidney biopsy samples over time: A 16-Year retrospective analysis from a large National renal biopsy laboratory. Kidney Int. Rep. https://doi.org/10.1016/j.ekir.2021.11.026 (2022).
Klumb, E. M. et al. The landscape of systemic lupus erythematosus in brazil: an expert panel review and recommendations. Lupus https://doi.org/10.1177/09612033211030008 (2021).
Liu, T., Yang, Y., Zhou & Jiang, Y. mei. Noninvasive biomarkers for lupus nephritis. Lab. Med. https://doi.org/10.1093/labmed/lmae015 (2024).
Fanouriakis, A., Tziolos, N., Bertsias, G. & Boumpas, D. T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2020-218272 (2021).
Kaul, A. et al. Systemic lupus erythematosus. Nat. Rev. Dis. Primers. https://doi.org/10.1038/nrdp.2016.39 (2016).
Reis-Neto, E. T. D. et al. II Brazilian society of rheumatology consensus for lupus nephritis diagnosis and treatment. Adv. Rheumatol. https://doi.org/10.1186/s42358-024-00386-8 (2024).
Navarro-Hernandez, I. C. et al. Urine extracellular vesicles size subsets as lupus nephritis biomarkers. Diagnostics https://doi.org/10.3390/diagnostics14202271 (2024).
Lu, J. et al. Urinary podocyte microparticles are associated with disease activity and renal injury in systemic lupus erythematosus. BMC Nephrol. https://doi.org/10.1186/s12882-019-1482-z (2019).
Burbano, C. et al. HMGB1 + microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. Eur. J. Immunol. https://doi.org/10.1002/eji.201847747 (2019).
Perez-Hernandez, J. et al. Urinary Exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J. Nephrol. https://doi.org/10.1007/s40620-020-00832-y (2021).
Solé, C., Moliné, T., Vidal, M., Ordi-Ros, J. & Cortés-Hernández, J. An Exosomal urinary MiRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells https://doi.org/10.3390/cells8080773 (2019).
Agrawal, S., He, J. C. & Tharaux, P. L. Nuclear receptors in podocyte biology and glomerular disease. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-00339-6 (2021).
Viñuela-Berni, V. et al. Proportions of several types of plasma and urine microparticles are increased in patients with rheumatoid arthritis with active disease. Clin. Exp. Immunol. https://doi.org/10.1111/cei.12598 (2015).
Sellam, J. et al. Increased levels of Circulating microparticles in primary sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. https://doi.org/10.1186/ar2833 (2009).
Troyer, B. et al. Glycosphingolipid levels in urine extracellular vesicles enhance prediction of therapeutic response in lupus nephritis. Metabolites https://doi.org/10.3390/metabo12020134 (2022).
López, P., Rodríguez-Carrio, J., Martínez-Zapico, A., Caminal-Montero, L. & Suárez, A. Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity. Int. J. Cardiol. https://doi.org/10.1016/j.ijcard.2017.02.107 (2017).
Aragón, C. C. et al. Urinary biomarkers in lupus nephritis. J. Transl Autoimmun. https://doi.org/10.1016/j.jtauto.2020.100042 (2020).
Wen, S. et al. IFN-γ, CXCL16, uPAR: potential biomarkers for systemic lupus erythematosus. Clin. Exp. Rheumatol. 36 (1), 36–43 (2018).
Rovin, B. H. et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2004080658 (2005).
Rother, N., Yanginlar, C., Pieterse, E., Hilbrands, L. & van der Vlag, J. Microparticles in autoimmunity: cause or consequence of disease?? Front. Immunol. https://doi.org/10.3389/fimmu.2022.822995 (2022).
Zhao, Y., Wei, W. & Liu, M. L. Extracellular vesicles and lupus nephritis - New insights into pathophysiology and clinical implications. J. Autoimmun. https://doi.org/10.1016/j.jaut.2020.102540 (2020).
Barnes, B. J. & Somerville, C. C. Modulating cytokine production via select packaging and secretion from extracellular vesicles. Front. Immunol. https://doi.org/10.3389/fimmu.2020.01040 (2020).
Mirfeizi, Z. et al. Urine monocyte chemoattractant Protein-1(UMCP-1) as a biomarker of renal involvement in systemic lupus erythematosus. Iran. J. Basic. Med. Sci. 15 (6), 1191–1195 (2012).
Acknowledgements
We are grateful to all professionals from Hospital Universitario Antônio Pedro who contributed to routine laboratory assessment and clinical follow-up. We would like to thank the members of Multiuser Laboratory for Research Support in Nephrology and Medical Science (LAMAP/UFF) and the Clinical Research Unit (UPC) for granting us the use of Cytoflex S. We also thank the partnership of D’Or Institute for Research and Education (IDOR) for supporting the use of ZetaView and Cenabio (UFRJ) for Transmission Electron Microscope equipments.
Funding
This study was financed in part by the Brazilian National Council for Scientific and Technological Development/CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, #001), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, E-26/211.044/2019). TM is recognized as a Young Scientist of the State of Rio de Janeiro (E-26.204.541/2024) and AAS is recognized as a Scientist of the State of Rio de Janeiro (E-26/201.155/2022). The funders had no role in the study design, data collection, analysis, the decision to publish, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization - LSA, JRA, TM, AAS; Methodology - LSA, JM, APL, SCF, GSL, PBF, FGDF; Formal analysis - LSA, AR, MJCC; Data curation - DB, TM, AAS; Investigation - LSA, CC, HL, SCF, TM, AAS; Writing (original draft preparation) - LSA, TM, AAS; Writing (review and editing): MJCC, JPSM, JRA, DB, TM, AAS; Supervision/Funding acquisition: TM, AAS. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alves, L., Lemos, A.P., Martins, J. et al. Podocyte extracellular vesicles and immune mediators as urinary biomarkers in active lupus nephritis. Sci Rep 15, 28630 (2025). https://doi.org/10.1038/s41598-025-08236-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08236-3