Abstract
Our study assessed the cost-effectiveness of screening for hepatic fibrosis in cases suspected of metabolic dysfunction-associated steatotic liver disease (MASLD) using serological noninvasive tests like the fatty liver index (FLI) and hepatic steatosis index (HSI). We applied a decision tree and Markov model from a healthcare system perspective to estimate life-years, quality-adjusted life-years (QALYs), costs, and the incremental cost-effectiveness ratio (ICER) for screening versus no screening in the United States. Prevalence of advanced hepatic fibrosis in individuals suspected of having MASLD was significantly higher when defined by FLI (10.6% vs. 1.3%, P < 0.001) and HSI (8.6% vs. 2.2%, P < 0.001), compared to those without MASLD. Screening (base case) for suspected MASLD defined by FLI had an ICER of $78,647 per QALY and by HSI, $84,874 per QALY, both of which were considered cost-effective based on the implicit ICER threshold of $100,000/QALY in the United States. However, screening for other subgroups without evidence of MASLD was not deemed cost-effective. When applying medical costs and fibrosis distribution data from Korea, similar results were observed. Implementing a two-step screening algorithm for advanced hepatic fibrosis in patients with suspected MASLD based on HSI or FLI calculation is cost-effective in primary care settings.
Similar content being viewed by others
Introduction
The increasing prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) is a global concern1,2. Given that the stage of hepatic fibrosis is the most significant prognostic factor for MASLD, its assessment is critical for its effective management3,4. Previous studies indicate that the prevalence of significant hepatic fibrosis in the general population ranges from 7.3 to 11.4% in the United States and from 5.1 to 9.5% in Korea5,6,7,8. Consequently, establishing a screening strategy and identifying high-risk groups for hepatic fibrosis in primary care settings are necessary.
Screening for hepatic fibrosis in individuals with MASLD typically begins after a diagnosis of hepatic steatosis through sonographic assessment. However, several barriers exist when evaluating hepatic fibrosis following a sonographic diagnosis of steatosis. First, only a small proportion of patients with MASLD undergo ultrasonographic examinations owing to resource and cost constraints. Performing abdominal ultrasound for all MASLD cases, which make up nearly 30% of adults globally, would place a significant social and economic burden on most societies9. Second, in many primary care clinics, which play a critical role in diagnosing and managing MASLD, conducting abdominal ultrasound is often not feasible for various reasons, leading to the referral of patients to specialized centers or specialists for such examinations. Third, a recent study highlighted that a considerable proportion (approximately 40%) of individuals with significant hepatic fibrosis in a health checkup cohort did not exhibit evidence of hepatic steatosis on abdominal sonography10. This suggests that many patients with MASLD with advanced hepatic fibrosis may be missed during initial screenings. Therefore, in primary care settings, it may be more practical to screen for advanced hepatic fibrosis in individuals suspected of having MASLD using serological noninvasive tests (NITs) rather than relying solely on sonographic assessments.
The fatty liver index (FLI) and hepatic steatosis index (HSI) are well-validated indicators for diagnosing patients with hepatic steatosis with reasonable accuracy11,12. To date, no study has directly compared the diagnostic accuracy of abdominal ultrasound with that of FLI or HSI. Although these methods cannot replace imaging modalities such as ultrasound or MRI-PDFF for definitive diagnosis due to their indirect nature, recent reviews suggest that FLI and HSI exhibit reasonable performance in diagnosing MASLD, with reported AUROCs of 0.84 and 0.81, respectively13. First of all, because these indicators are calculated using simple data from blood chemistry and anthropometry, they can be easily utilized in primary care clinics. Additionally, FLI or HSI can be an effective predictor of liver- and MASLD-related mortalities, such as cardiovascular disease (CVD) or cancer14,15. Recent guidelines recommend hepatic fibrosis screening using the fibrosis 4 (FIB-4) index even in cases where MASLD is suspected (such as in individuals with obesity and other cardiometabolic risk factors, type 2 diabetes, or persistent elevation of liver enzymes), without necessarily requiring a prior sonographic diagnosis of steatosis16. This approach is likely motivated by the growing disease burden of MASLD and the need to overcome the limitations of ultrasound equipment as the initial method for hepatic fibrosis screening in MASLD, as mentioned earlier. In this context, both the FLI and HSI present attractive options as screening tools for identifying patients who require hepatic fibrosis screening, particularly in primary care clinics. This is due to their ability to assess hepatic steatosis, ease of application, and their capacity to predict the overall risk of clinical outcomes associated with MASLD.
Screening for hepatic fibrosis in patients with MASLD diagnosed through imaging or histology is cost-effective17. However, there is limited evidence regarding the cost-effectiveness of screening for advanced hepatic fibrosis in suspected MASLD cases using serologic hepatic steatosis calculators without abdominal ultrasonography. Therefore, this study aimed to evaluate the cost-effectiveness of implementing a two-step screening algorithm for advanced hepatic fibrosis in suspected cases of MASLD based on serological NITs within the primary care setting.
Methods
Overview of the cost-effectiveness analysis
A cost-utility analysis was performed to assess the cost-effectiveness of the two-step screening algorithm versus no-screening for detecting advanced hepatic fibrosis in the United States over a 20-year period with a 1-year cycle from a healthcare system perspective. A combined model of the decision tree and Markov model was used to estimate the long-term clinical and economic impact of screening, which was implemented using Microsoft Excel 2019. This study adhered to the Consolidated Health Economic Evaluation Reporting Standards reporting guidelines for economic evaluations18. This study was approved by the institutional review board of Hanyang University Hospital (IRB No. HY-2021-04-001-001) with a waiver of informed consent.
Study population and data sources
To identify the distribution of hepatic fibrosis among the study population, we used hepatic fibrosis data from the United States (US) National Health and Nutrition Examination Survey (NHANES) from March 2017 to March 202019. Adult participants (aged ≥ 20 years) with valid vibration-controlled transient elastography (VCTE) data (> 10 measurements with an interquartile range of < 30% from the median) were recruited. Participants with a risk of viral or alcoholic liver disease or those with missing data for calculating fatty liver scores at the time of testing were excluded. Finally, participants with FLI (n = 5908) and HSI (n = 6032) were analyzed to evaluate the distribution of hepatic fibrosis (Tables S1 and S2). FLI and HSI were calculated as previously described11,12. Patients with suspected MASLD were defined based on FLI ≥ 60 and HSI ≥ 36. Hepatic fibrosis stages were determined using VCTE. Sensitivity analyses were conducted by applying various cut-offs for advanced hepatic fibrosis, including a low cut-off (8.8–11.8 kPa) and a high cut-off (9.7–13.6 kPa) (Table S3). In the base-case analysis, the cut-off for advanced hepatic fibrosis was set at 9.6–13.0 kPa.
Model design
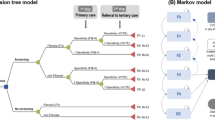
Our model began with a hypothetical cohort of 50-year-old suspected patients of MASLD. The cohort was assigned to either the “screening” group or the “no-screening” group. In the decision tree model, regarding the screening group, the cohort visiting primary care clinics was screened using the FIB-4 index (applied cut-off score: ≥ 1.3). Patients who tested positive were referred to a tertiary hospital, where VCTE was conducted to confirm fibrosis (Fig. 1A). The sensitivity and specificity of the performance of the FIB-4 index and VCTE were 69% and 64% and 80% and 74%, respectively20,21. After the screening, patients diagnosed with fibrosis (both true positive [TP] and false positive [FP]) participated in an intensive lifestyle intervention (ILI) program. And then, the simulated cohort in the no-screening group has the same risk profile and fibrosis stage distribution as the screening group. However, because advanced fibrosis remains undiagnosed in the absence of screening, individuals in this group do not have the opportunity to participate in ILI. The population categorized as having fibrosis distribution F0–F4 according to the diagnostic results was entered into the Markov model, reflecting the natural history of MASLD. The model included predefined health states, including fibrosis stages F0–F4, CVD with fibrosis stages (F0–F4), decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), and death (Fig. 1B). The modeled cohort could have regression or progression of fibrosis or develop liver-related complications (DC and HCC) or CVD. This could also result in liver-related, cardiovascular, and age-specific all-cause mortality22,23,24,25. In each cycle, patients either remained in their current health state (recursive arrow) or progressed to another health state (straight arrow) according to transition probabilities.
Cost-effectiveness model: (A) Decision tree model (B) Markov model. CVD cardiovascular disease, DC decompensated cirrhosis, FLI fatty liver index, FN false negative, FP false positive, HCC hepatocellular carcinoma, HSI hepatic steatosis index, ILI intensive lifestyle intervention, MASLD metabolic dysfunction-associated steatotic liver disease, TE transient elastography, TN true negative, TP true positive.
Input parameters, including transition probabilities, medical costs, and utility weights to input the analytic model, were obtained from a comprehensive review of published studies and our previous work (Tables S4–S7)26. We decided on utility weights applying to our analytic model from the literature review based on two criteria: (1) preferential inclusion of studies reporting a comprehensive set of utility values relevant to the health states required for our model, and (2) alignment of extracted values with the severity of each health state. All cost parameters were adjusted to the 2023 US dollars ($) using the Medical Care component of the US Consumer Price Index27. Some assumptions were made in the analytical model as follows: (1) All patients diagnosed with advanced fibrosis received an ILI program for 10 years—considering the nature of MASLD as a chronic disease and the relatively slow progression or regression of hepatic fibrosis—without waning treatment effect. While receiving ILI, patients were monitored for advanced fibrosis regardless of TP or FP. (2) Among individuals receiving ILI, only 40% are classified as responders and thus derive clinical benefit from the treatment. The net regression rate, including both responders and non-responders, among those receiving ILI in the screening group is 17.0%26. The responders in the first year maintain the efficacy––31.5% of fibrosis regression and no progression––during the period of ILI from the following year. For patients (non-responders) who have not achieved sufficient response to the ILI program or who have ended the ILI period, the same regression rate as the no-screening group was applied. (3) Even in patients who do not receive screening and ILI program in the no-screening group, 7.1–7.9% of fibrosis regression could be induced annually. (4) The model reflected the benefits of the ILI program as two ways to reduce CVD risk and hepatic fibrosis progression. (5) The risk of liver-related events in each fibrosis stage with CVD was the same as that of the fibrosis stage without CVD. (6) ILI effectiveness in reducing CVD risk is maintained during ILI treatment, even if the efficacy against fibrosis regression remains insufficient.
Analysis
The economic model estimated costs, life-years (LYs), and quality-adjusted life-years (QALYs) gained with screening versus no-screening over a 20-year period. Cost and outcomes were discounted at a rate of 3.0% per year. The primary results were presented as the incremental cost-effectiveness ratio (ICER) between the screening and no-screening strategies. When the estimated ICER was < $100,000/QALY28, the recommended willingness-to-pay (WTP) threshold in the US, the screening was deemed cost-effective. A result from the “middle cut-off” setting was presented as a base-case analysis. We also estimated ICERs for the other two scenarios adapted “high cut-off’” and “low cut-off” in Table S3. The ICER was calculated as follows:
One-way deterministic and probabilistic sensitivity analyses were conducted to explore the robustness of the results by varying values and assumptions. For one-way sensitivity analyses, the variables related to screening, ILI, medical cost, utility weight time horizon, and discount rate were changed. In addition, we conducted a scenario analysis of this model for the Korean setting. Owing to the differences in liver fibrosis prevalence and racial demographics between the United States (base-case setting) and Korea, both medical costs and data on the distribution of fibrosis in Korea were applied. The distribution of fibrosis in Korea was obtained from a nationwide, large-scale health checkup cohort. The Korean study population reflected real-world data from 5106 participants who had undergone magnetic resonance elastography (MRE) as part of their health checkup and had no risk for viral or alcoholic liver disease at the time6. The fibrosis distribution of the hepatic fibrosis stage, MRE cut-off values, and medical costs in Korea are presented in Tables S7 and S8. A probabilistic sensitivity analysis applying a second-order Monte Carlo simulation was conducted to assess the comprehensive effects of uncertainty. The simulation was conducted 1000 times using parameter values randomly drawn from the relevant distributions. We employed a beta distribution for the transition probabilities and utility weights and a gamma distribution for the costs. The results were shown as a cost-effectiveness acceptability curve.
Results
Base-case analysis
In patients with suspected MASLD based on FLI score (≥ 60), implementing a two-step algorithm of FIB-4 index and VCTE for screening yielded 13.620 LYs and 11.406 QALYs per patient, respectively, with a cost of $27,748 over a 20-year period. Moreover, the no-screening strategy resulted in 13.585 LYs and 11.369 QALYs per patient, respectively, costing $24,805. The incremental QALY was 0.037 at an additional cost of $2943, resulting in an ICER of $78,647/QALY (Table 1). In the low-risk population (FLI score < 60), both the incremental QALY and cost decreased (0.013 QALY and $2101) compared to those observed in the high-risk population, and while the reduction in cost was approximately 0.7 times, the reduction in QALY showed a greater reduction to approximately 0.35 times, resulting in an estimated ICER of $161,675/QALY. In addition, a similar tendency was observed in the results based on the HSI score. The estimated ICER was $84,874/QALY in the high-risk population (HSI score ≥ 36) and $150,458/QALY in the low-risk (HSI score < 36) population (Table 1). The two-step screening and ILI program for high-risk populations was shown to be cost-effective based on the WTP threshold of $100,000/QALY. When using two types of NITs to define suspected MASLD, the ICER difference based on the FLI score was greater than that classified by the HSI score.
Scenario analysis according to various VCTE cut-off
The distribution of hepatic fibrosis stages was determined using VCTE. Given the significant impact of fibrosis distribution (F0–F4) on the outcomes of the cost-effectiveness analysis, we conducted sensitivity analyses through scenario analyses, applying various VCTE cut-off values (Fig. 2 and Table S9). Regardless of cut-off values of VCTE and type of NITs (FLI and HSI) for diagnosing suspected MASLD, the screening strategy in suspected MASLD defined by serology-based NITs was cost-effective, as their ICERs were below the threshold. Applying a low cut-off suggests that the population risk can be established at a level higher than that at baseline. Applying a lower VCTE cut-off increased the proportion of F3 or F4 cases, which led to a higher number of patients undergoing VCTE at tertiary hospitals and receiving ILI. This change increased medical costs; however, it also enhanced the sensitivity of screening, thereby increasing QALYs gained through disease prevention. Consequently, analyses applying various VCTE cut-offs showed that a lower VCTE cut-off resulted in a lower ICER.
One-way sensitivity analyses
The results of one-way sensitivity analyses are presented in Table S10 and Fig. 3. The most influential parameter of the ICER was the regression rate of fibrosis. As the regression rate of the first year in the screening group increased, the ICER decreased remarkably. When the regression rate fell to 15% (Δ7.6%: 15% in screening group vs. 7.4% in no-screening group), the ICER exceeded $100,000/QALY. Regarding the duration of the ILI program, an extension of the program to 15 years increased the benefit of preventing poor outcomes associated with liver fibrosis and CVD progression, resulting in a decrease in ICER despite the increased cost of ILI. Conversely, shortening the duration to 5 years raised the ICER to $112,720. If the therapeutic effect of ILI was limited to a single effect on liver disease, excluding the effect on CVD in the analytical model without considering both aspects of liver and extrahepatic benefits, the ICER increased to $105,290. The time horizon of the simulation and discount rate were also influential factors in the cost-effectiveness. We examined the impact on the ICER by substituting utility weights drawn from recently published studies conducted in the MASLD population29,30,31,32. The utility weights for each health state used in these prior studies, along with the corresponding ICER estimates when applying those values, are summarized in Table S11. Overall, the ICERs estimated across the four alternative scenarios remained below the willingness-to-pay threshold. Notably, the ICER tended to decrease when higher utility weights were applied to mild health states and lower weights to severe health states. The service fee of ILI and the performance of diagnostic tools showed a small impact on ICER. The results of scenario analysis adapting parameters obtained from the Korean healthcare setting are presented in Table S12. Similar to the findings described above, screening and participation in the ILI program for the high-risk population was cost-effective based on $25,000/QALY, implicitly accepted as a WTP threshold in South Korea.
The findings of the probabilistic sensitivity analysis, conducted with 1000 simulations and depicted in the cost-effectiveness acceptability curve (Fig. S1), indicated that the screening strategy was cost-effective across various levels of ICER thresholds. The curve demonstrated that the likelihood of the screening being cost-effective exceeded that of the no-screening strategy at a threshold of $74,000 per QALY.
Health-related outcomes
Under the high-risk condition (FLI ≥ 60), a two-step screening algorithm for advanced hepatic fibrosis in suspected cases of MASLD, the number of preventable HCCs, CVDs, liver-related death, and CV-related death per 100,000 persons over 20-years was 241, 240, 519, and 143, respectively. The estimates under other conditions are presented in Table S13. After conversion to rate (calculated as the number of preventable cases divided by the total cases in the no screening), the rates obtained were 5.84%, 1.32%, 5.23%, and 1.57%, respectively.
To validate the clinical relevance of the presented analytic model, we conducted an evaluation by comparing the major clinical outcomes of suspected MASLD estimated by the Markov model with real-world data from the US general population and patients with biopsy-proven MASLD. Through the 10-year simulation with the current model, the estimated incidence rates of HCC, CVD, liver disease-related death, and CVD-related death in patients with suspected MASLD group were between those of the general population and patients with biopsy-proven MASLD or similar to those of patients with biopsy-proven MASLD (Table 2). Therefore, we believe that our analytical model results are within the expected range.
Discussion
Our findings demonstrate that implementing a two-step screening algorithm for advanced hepatic fibrosis in patients with suspected MASLD based on FLI or HSI is cost-effective in the primary care setting. Previous studies have consistently shown the cost-effectiveness of screening for advanced hepatic fibrosis in patients with confirmed MASLD using liver biopsy or ultrasonography17,33,34,35 and a two-step algorithm using FIB-4 followed by VCTE has been established as a fundamental screening tool for the MASLD population, particularly in primary care settings16. Subsequently, the cost-effectiveness of hepatic fibrosis screening has also been demonstrated in individuals with type 2 diabetes30. Most recently, a socio-economic evaluation within the US healthcare system (WTP: $50,000 per QALY) confirmed that this approach is cost-effective for individuals with suspected MASLD—those with type 2 diabetes (ICER: $26,913 to $27,884 per QALY) or medically complicated obesity (ICER: $23,265 to $24,992 per QALY)—even in the absence of a prior sonographic diagnosis of hepatic steatosis36. These findings suggest that the target population for hepatic fibrosis screening has expanded beyond those with MASLD to include individuals with other fibrosis-related risk factors. This shift is primarily driven by the presence of comorbidities, despite the potential risk of reduced accuracy in the assessment of hepatic steatosis. However, selecting the target population using FLI or HSI offers the advantage of simultaneously identifying individuals who not only have evidence of hepatic steatosis but are also at risk for MASLD-related complications. However, there is limited evidence regarding the cost-effectiveness of screening for advanced hepatic fibrosis in suspected MASLD based on these serologic hepatic steatosis calculators. More active screening for MASLD and reducing unnecessary referral rates in primary care clinics are crucial. Given that many primary care clinics have limited access to abdominal ultrasound or VCTE, utilizing FLI or HSI to more proactively screen for patients with suspected MASLD in these settings is regarded as a cost-effective strategy in the current MASLD era.
In our study, FIB-4 followed by VCTE in suspected patients with MASLD defined by FLI (ICER $78,647/QALY) and HSI (ICER $84,874/QALY) through base-case analysis was evaluated as a cost-effective method based on the implicit ICER threshold of $100,000/QALY in the US. However, screening in other subgroups without evidence of MASLD was not cost-effective. When applying the medical cost and fibrosis distribution in Korea, ICERs of $19,471/QALY in FLI and $22,469/QALY in HSI for suspected MASLD were obtained. The ICERs were < $25,000/QALY, which is the implicit threshold of the WTP in Korea. These findings provide clear evidence that the use of FIB-4 followed by VCTE, especially in patients with suspected MASLD, is a reasonable approach and can be an attractive option for the target population, especially in primary care settings. The most important factor in determining cost-effectiveness is the hepatic fibrosis burden in the target population. Individuals with suspected MASLD diagnosed by FLI (10.3–13.3% vs. 1.2–1.8% P < 0.001) and HSI (8.3–10.9% vs. 2.2–2.9% P < 0.001) in Table S3 showed distinctively higher fibrosis burden than those without MASLD, regardless of cut-off values of VCTE. Similar results were observed in a Korean MRE cohort. Advanced hepatic fibrosis prevalence in the suspected MASLD group was at least three times higher. We believe that this is the primary reason for the cost-effectiveness of hepatic fibrosis screening in these populations. No studies exist on the diagnostic capacity of these calculators (FLI and HSI) for direct diagnosis of hepatic fibrosis. This may result from the fact that other NITs, such as FIB-4 and NAFLD fibrosis score (NFS), which were originally developed to assess hepatic fibrosis, have been widely used. It is unlikely that hepatic steatosis calculators can better assess liver fibrosis than other hepatic fibrosis indicators. However, the fact that they can simultaneously identify individuals with hepatic steatosis and hepatic fibrosis burden is a very important factor in selecting the target population for fibrosis screening.
Although neither our model nor current guidelines support hepatic fibrosis screening for the general population using FLI or HSI if these tools are applied broadly, approximately 6–8% of the total population classified as positive by FIB-4-based screening would undergo ILI treatment. The proportion of suspected MASLD would represent 50–60% of the total population (Fig. S2). Assuming that the entire suspected MASLD group participates in FIB-4-based screening, approximately 17–23% of the population classified as positive by FIB-4 would receive VCTE in our model. Ultimately, approximately 6–8% of the total population would be classified into the high-risk group. However, hepatic fibrosis screening in the general population is not currently recommended, and further data is required to support this approach.
Special caution should be exercised when interpreting our results. We demonstrated the implementation of a two-step screening algorithm for advanced hepatic fibrosis and ILI as treatment options for patients with suspected MASLD when compared to the no-screening group. Therefore, a direct comparison of the cost-effectiveness between the use of ultrasonography and serological hepatic steatosis calculators cannot be discussed. Our results cannot be used as a basis to replace the use of ultrasound. However, our study supports the screening of patients with suspected MASLD for hepatic fibrosis in primary care settings, where ultrasound is not readily available. In addition, the recent approval of drugs for hepatic fibrosis in MASLD may have had a considerable impact on our analysis. If the new drug was included as therapeutic effects, more accurate results would be expected. However, there are still limited studies on the long-term therapeutic and adverse effects of new drugs, and the cost of the drugs remains unclear. Therefore, applying the effects of a new drug to the model in practice remains challenging. Moreover, the appropriate use of new drugs usually moves in the direction of favorable cost-effectiveness. Therefore, screening for hepatic fibrosis in patients with suspected MASLD may be cost-effective, regardless of the use of new agents. Lastly, this analysis did not evaluate the cost-effectiveness of repeated screening strategies, as it focused on a one-time screening approach using a two-step algorithm followed by intensive lifestyle intervention in individuals with suspected MASLD identified through FLI or HSI. Future economic evaluations are warranted to determine the optimal screening frequency and interval.
This study had some limitations. First, in our Markov model, most transition probabilities of liver disease, CVD, and extrahepatic malignancies in not only the suspected MASLD group but also low risk group defined by FLI and HSI were obtained from studies using the MASLD cohort. Although serological hepatic steatosis calculators are not the gold standard for diagnosing hepatic steatosis, they have reasonable performance in diagnosing MASLD. Therefore, the input parameters might not be significantly different between the two groups. However, the input parameters for the low-risk group can be a significantly lower risk compared to those for individuals with suspected MASLD (high risk group) or confirmed MASLD. Nevertheless, the primary focus of this study is the cost-effectiveness of implementing hepatic fibrosis screening in the suspected MASLD population. In addition, applying the same input parameters to the low-risk group could lead to an overestimation of disease burden and screening benefits. Given that these factors are more likely to bias the results conservatively, their influence on the overall conclusions of the study is expected to be minimal. Second, the distribution of hepatic fibrosis in suspected MASLD among the general population is unknown and may differ according to region and ethnicity. We attempted to reflect real-world hepatic fibrosis data from the US National Health and Nutrition Examination Survey (NHANES) from March 2017 to March 2020, where VCTE data were available. Moreover, the Korean MRE cohort was used independently as a sensitivity analysis. Because the above factors can vary according to region and ethnicity and can be an important factor affecting the cost-effectiveness of screening strategies, another cost-effective study in these populations using data from other ethnicities or countries will be needed in the future. Lastly, some assumptions in our model—such as (1) a 100% referral rate to tertiary care for ILI treatment, (2) full adherence to ILI over 10 years without waning effects, and (3) no fibrosis progression in responders—may overestimate the screening effect and yield optimistic results. A more detailed model incorporating these factors could provide a more precise analysis. However, the primary aim of our study was to assess the cost-effectiveness of initiating hepatic fibrosis screening in individuals with suspected MASLD identified by FLI or HSI, rather than to evaluate treatment efficacy assumptions in depth. Nonetheless, our model includes several conservative assumptions that may compensate these limitations. Notably, we assumed that all non-responders—representing 60% of those receiving ILI—would incur the full 10-year treatment cost despite deriving no clinical benefit. In addition, treatment efficacy parameters, including net fibrosis regression in responders and the progression gap between responders and non-responders, were set to be comparable to or more conservative than those used in prior studies30,36. In practical, several clinical studies have reported that 10 years after implementing ILI, participants maintained an average weight reduction of approximately 6–6.9% from baseline37,38,39. Based on these findings, the present study adopted a conservative approach by assuming a net fibrosis regression rate of 17.0% in the ILI group and 7.1% in the control group. Considering that liver fibrosis improvement is typically observed when weight loss exceeds 7%, the reported 10-year average weight reduction of 6–7% following ILI provides reasonable support for our assumption that 17% of the ILI group maintained a 7–10% weight loss over 10 years and achieved fibrosis improvement.
In conclusion, a hepatic fibrosis screening strategy for suspected MASLD, defined by serological NITs (FLI and HSI), was found to be cost-effective in primary care clinic settings. The use of serological hepatic steatosis calculators (FLI or HSI) to identify suspected MASLD cases for hepatic fibrosis screening presents an attractive option, particularly in primary care settings with limited access to ultrasound.
Data availability
All datasets generated and analyzed during the current study are available from Dae Won Jun or Hye-Lin Kim on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DC:
-
Decompensated cirrhosis
- DM:
-
Diabetes mellitus
- FIB-4:
-
Fibrosis-4 index
- FLI:
-
Fatty liver index
- FN:
-
False negative
- FP:
-
False positive
- HCC:
-
Hepatocellular carcinoma
- HIRA:
-
Health insurance review and assessment service
- HSI:
-
Hepatic steatosis index
- ICER:
-
Incremental cost-effectiveness ratio
- ILI:
-
Intensive lifestyle intervention
- LC:
-
Liver cirrhosis
- LYs:
-
Life-years
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- MRE:
-
Magnetic resonance elastography
- NAFLD:
-
Non-alcoholic fatty liver disease
- NIT:
-
Noninvasive test
- QALYs:
-
Quality-adjusted life-years
- SLD:
-
Steatotic liver disease
- T2D:
-
Type 2 diabetes
- TN:
-
True negative
- TP:
-
True positive
- VCTE:
-
Vibration-controlled transient elastography
- WTP:
-
Willingness-to-pay
References
Paik, J. M. et al. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology 75, 1204–1217. https://doi.org/10.1002/hep.32228 (2022).
Kalligeros, M. et al. Prevalence of steatotic liver disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017–2020. Clin. Gastroenterol. Hepatol. 22, 1330–1332. https://doi.org/10.1016/j.cgh.2023.11.003 (2024).
Vilar-Gomez, E. et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology 155, 443–457 (2018).
Oh, H., Jun, D. W., Saeed, W. K. & Nguyen, M. H. Non-alcoholic fatty liver diseases: Update on the challenge of diagnosis and treatment. Clin. Mol. Hepatol. 22, 327–335. https://doi.org/10.3350/cmh.2016.0049 (2016).
Kang, K. A., Jun, D. W., Kim, M. S., Kwon, H. J. & Nguyen, M. H. Prevalence of significant hepatic fibrosis using magnetic resonance elastography in a health check-up clinic population. Aliment. Pharmacol. Ther. 51, 388–396. https://doi.org/10.1111/apt.15626 (2020).
Nah, E.-H. et al. Prevalence of liver fibrosis and associated risk factors in the Korean general population: A retrospective cross-sectional study. BMJ Open 11, e046529 (2021).
Kim, H. Y. et al. Prevalence of clinically significant liver fibrosis in the general population: A systematic review and meta-analysis. Clin. Mol. Hepatol. 30, S199–S213. https://doi.org/10.3350/cmh.2024.0351 (2024).
Bhattacharyya, M. et al. Prevalence and determinants of undiagnosed liver steatosis and fibrosis in a nationally representative sample of US adults. Cureus 15, e46783 (2023).
Miao, L., Targher, G., Byrne, C. D., Cao, Y. Y. & Zheng, M. H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 35, 697–707. https://doi.org/10.1016/j.tem.2024.02.007 (2024).
Park, H. et al. Selecting the target population for screening of hepatic fibrosis in primary care centers in Korea. J. Clin. Med. 11, 1474. https://doi.org/10.3390/jcm11061474 (2022).
Bedogni, G. et al. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006).
Lee, J. H. et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42, 503–508. https://doi.org/10.1016/j.dld.2009.08.002 (2010).
Abdelhameed, F. et al. Non-invasive scores and serum biomarkers for fatty liver in the era of metabolic dysfunction-associated steatotic liver disease (MASLD): A comprehensive review from NAFLD to MAFLD and MASLD. Curr. Obes. Rep. 13, 510–531. https://doi.org/10.1007/s13679-024-00574-z (2024).
Chung, G. E. et al. Association of fatty liver index with all-cause and disease-specific mortality: A nationwide cohort study. Metabolism 133, 155222 (2022).
Olubamwo, O. O., Virtanen, J. K., Pihlajamaki, J. & Tuomainen, T. P. Association of fatty liver disease with mortality outcomes in an Eastern Finland male cohort. BMJ Open Gastroenterol. 6, e000219. https://doi.org/10.1136/bmjgast-2018-000219 (2019).
European Association for the Study of the Liver, European Association for the Study of Diabetes & European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 81, 492–542. https://doi.org/10.1016/j.jhep.2024.04.031 (2024).
Tapper, E. B., Sengupta, N., Hunink, M. G., Afdhal, N. H. & Lai, M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am. J. Gastroenterol. 110, 1298–1304. https://doi.org/10.1038/ajg.2015.241 (2015).
Husereau, D. et al. Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: A report of the ISPOR CHEERS II good practices task force. Value Health 25, 10–31. https://doi.org/10.1016/j.jval.2021.10.008 (2022).
Akinbami, L. J. et al. National health and nutrition examination survey, 2017–March 2020 prepandemic file: Sample design, estimation, and analytic guidelines (2022).
Han, S. et al. Accuracy of noninvasive scoring systems in assessing liver fibrosis in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gut Liver 16, 952–963. https://doi.org/10.5009/gnl210391 (2022).
Siddiqui, M. S. et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 17, 156-163.e152. https://doi.org/10.1016/j.cgh.2018.04.043 (2019).
Social Security Administration. Period Life Table, 2020, as Used in the 2023 Trustees Report. https://www.ssa.gov/oact/STATS/table4c6.html#fn1. Accessed 25 Sept 2023.
American Cancer Society. ACS Cancer Facts & Figures 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html. Accessed 25 Sept 2023.
Centers for Disease Control and Prevention. Age-Adjusted Death Rates for Selected Causes of Death, by Sex, Race, and Hispanic Origin: United States, Selected Years 1950–2019. https://www.cdc.gov/nchs/hus/data-finder.htm?year=2020-2021&table=Table%20SlctMort. Accessed 25 Sept 2023.
Our World in Data. Cardiovascular Disease Death Rate by Age Group, United States, 2020. https://ourworldindata.org/grapher/cardiovascular-disease-death-rate-age-group-who-mdb. Accessed 25 Sept 2023.
Park, H. et al. Cost-effectiveness study of FIB-4 followed by transient elastography screening strategy for advanced hepatic fibrosis in a NAFLD at-risk population. Liver Int. 44, 944–954. https://doi.org/10.1111/liv.15838 (2024).
Sohn, W. et al. Effect of direct-acting antivirals on disease burden of hepatitis C virus infection in South Korea in 2007–2021: A nationwide, multicentre, retrospective cohort study. EClinicalMedicine 73, 102671. https://doi.org/10.1016/j.eclinm.2024.102671 (2024).
Neumann, P. J., Cohen, J. T. & Weinstein, M. C. Updating cost-effectiveness—The curious resilience of the $50,000-per-QALY threshold. N. Engl. J. Med. 371, 796–797. https://doi.org/10.1056/NEJMp1405158 (2014).
Javanbakht, M., Fishman, J., Moloney, E., Rydqvist, P. & Ansaripour, A. Early cost-effectiveness and price threshold analyses of resmetirom: An investigational treatment for management of nonalcoholic steatohepatitis. Pharmacoeconomics 7, 93–110. https://doi.org/10.1007/s41669-022-00370-2 (2023).
Noureddin, M. et al. Screening for nonalcoholic fatty liver disease in persons with type 2 diabetes in the United States Is Cost-effective: A comprehensive cost-utility analysis. Gastroenterology 159, 1985–1987. https://doi.org/10.1053/j.gastro.2020.07.050 (2020).
Rustgi, V. K., Duff, S. B. & Elsaid, M. I. Cost-effectiveness and potential value of pharmaceutical treatment of nonalcoholic fatty liver disease. J. Med. Econ. 25, 347–355. https://doi.org/10.1080/13696998.2022.2026702 (2022).
Sangha, K., Chang, S. T., Cheung, R. & Deshpande, V. S. Cost-effectiveness of MRE versus VCTE in staging fibrosis for nonalcoholic fatty liver disease (NAFLD) patients with advanced fibrosis. Hepatology 77, 1702–1711. https://doi.org/10.1097/hep.0000000000000262 (2023).
Gruneau, L., Kechagias, S., Sandstrom, P., Ekstedt, M. & Henriksson, M. Cost-effectiveness analysis of noninvasive tests to identify advanced fibrosis in non-alcoholic fatty liver disease. Hepatol. Commun. 7, e00191. https://doi.org/10.1097/HC9.0000000000000191 (2023).
Zhang, E. et al. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur. Radiol. 25, 3282–3294. https://doi.org/10.1007/s00330-015-3731-2 (2015).
Tapper, E. B., Hunink, M. M., Afdhal, N. H., Lai, M. & Sengupta, N. Cost-effectiveness analysis: Risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS ONE 11, e0147237 (2016).
Younossi, Z. M., Paik, J. M., Henry, L., Stepanova, M. & Nader, F. Pharmaco-economic assessment of screening strategies for high-risk MASLD in primary care. Liver Int. 45, e16119. https://doi.org/10.1111/liv.16119 (2025).
The Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity 22, 5–13. https://doi.org/10.1002/oby.20662 (2014).
Tomah, S. et al. Long-term effect of intensive lifestyle intervention on cardiometabolic risk factors and microvascular complications in patients with diabetes in real-world clinical practice: a 10-year longitudinal study. BMJ Open Diabetes Res. Care 11, e003179. https://doi.org/10.1136/bmjdrc-2022-003179 (2023).
Diabetes Prevention Program Research Group et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 374, 1677–1686. https://doi.org/10.1016/S0140-6736(09)61457-4 (2009).
Rumgay, H. et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77, 1598–1606. https://doi.org/10.1016/j.jhep.2022.08.021 (2022).
Ralapanawa, U. & Sivakanesan, R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: A narrative review. J. Epidemiol. Glob. Health 11, 169–177. https://doi.org/10.2991/jegh.k.201217.001 (2021).
Murphy, S. L., Kochanek, K. D., Xu, J. & Arias, E. Mortality in the United States, 2020. NCHS Data Brief, 1–8 (2021).
Fujii, H. et al. Clinical outcomes in biopsy-proven nonalcoholic fatty liver disease patients: A multicenter registry-based cohort study. Clin. Gastroenterol. Hepatol. 21, 370–379. https://doi.org/10.1016/j.cgh.2022.01.002 (2023).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 385, 1559–1569. https://doi.org/10.1056/NEJMoa2029349 (2021).
Dulai, P. S. et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 65, 1557–1565. https://doi.org/10.1002/hep.29085 (2017).
Simon, T. G., Roelstraete, B., Hagström, H., Sundström, J. & Ludvigsson, J. F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut 71, 1867–1875 (2022).
Funding
This research was supported by the grant from the Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC23C0058). The funding source had no role in study design, implementation, data collection, analysis, and interpretation or the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Guarantor of the article: Dae Won Jun Concept and design: D.W.J. and H.-L.K.; data collection and management: H.P., E.L.Y., M.K., J-Y.C. and R.C.; interpretation of data: H.P., E.L.Y., J.H.P. and M.K.; writing of the manuscript: H.P., H.-L.K.; supervision: D.W.J. and H.-L.K. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, H., Yoon, E.L., Kim, M. et al. Cost-effectiveness of advanced hepatic fibrosis screening in individuals with suspected MASLD identified by serologic noninvasive tests. Sci Rep 15, 24186 (2025). https://doi.org/10.1038/s41598-025-08434-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08434-z