Abstract
The parabrachial nucleus (PBN) plays a crucial role in transmitting itch and affective pain signals to the brain regions such as the central amygdala (CeA). While CGRP+ PBN neurons have been implicated in itch processing, the specific projections involved remain unclear. This study aimed to determine the proportion of itch-responsive PBN-CeA projections that express CGRP and to assess their functional role in itch and anxiety behaviors in mice. Using the Targeted Recombination in Active Populations system, we labeled itch-responsive PBN neurons with serotonin. Retrograde tracing revealed that approximately half of serotonin-responsive PBN neurons projecting to the CeA are CGRP+. Optogenetic stimulation of these PBN-CeA neurons elicited scratching behavior but did not enhance pruritogen-induced scratching or affect anxiety-like behaviors. In a mouse model of chronic itch, the inhibition of PBN-CeA neurons significantly reduced spontaneous scratching without impacting anxiety-like behaviors. These findings suggest that serotonin-responsive PBN-CeA neurons include both CGRP+ and non-CGRP+ populations and selectively mediate itch signaling.
Similar content being viewed by others
Introduction
The parabrachial nucleus (PBN) functions as a central hub for relaying itch and affective pain signals to various brain regions1,2,3,4. It predominantly comprises glutamatergic neurons, with a smaller subset of GABAergic neurons5. Previous studies have confirmed the role of PBN in itch signaling: optogenetic activation of spinal gastrin-releasing peptide receptor expressing neurons, crucial for itch processing, robustly activates PBN-projecting spinal neurons6. Additionally, silencing CGRP+ neurons within the PBN reduces itch-induced scratching behaviors, indicating a functional role for CGRP+ PBN neurons in itch7. While CGRP+ PBN neurons project to multiple brain areas5the specific projections that mediate itch have yet to be identified.
The central amygdala (CeA), which contains a heterogeneous population of GABAergic neurons, receives diverse inputs, including those from the PBN8. Nociceptive information reaches the CeA via dynorphin-expressing interneurons in the PBN, which in turn activate CeA-projecting neurons9. Recent studies have begun to explore the role of CeA in itch regulation, particularly through the activation of certain CeA neuron subsets that induce itch and related affective behaviors in mice10,11,12,13. Although PBN-CeA projections are presumed to contribute to itch, direct evidence is lacking.
In this study, we investigate the proportion of itch-responsive PBN-CeA projections that express CGRP. Using recently developed genetic and optogenetic techniques14we selectively activated itch-responsive neurons in the PBN to assess whether this activation elicits itch- and anxiety-related behaviors in mice. We also examined whether inhibiting itch-responsive neurons in the PBN could suppress spontaneous itch in a chronic itch mouse model.
Methods
Mice
All mouse procedures were approved by Institutional Animal Care and Use Committees of the University of Miami (protocol number: 21–111) and were performed in accordance with the guidelines for the Care and Use of Laboratory Animals. The study was carried out in compliance with the ARRIVE guidelines. At the experimental endpoint, mice were euthanized using methods consistent with the recommendations of the American Veterinary Medical Association Guidelines (e.g., CO2 chamber or exsanguination under anesthesia).
TRAP2 (Fos2A − iCreER; JAX stock #030323)14Ai14 mice (JAX stock #007908), and C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). TRAP2 mice were mated with Ai14 mice to generate TRAP2:Ai14 mice. All experimental procedures followed our previously established protocols with minor midifications10,12,13. The mice were group housed (2–5 per cage) in a 12–12 light-dark cycle and had access to food and water ad libitum. Prior to all testing mice were habituated for 5 days to the testing room, handling, intradermal (i.d.) injections, and cable attachment. A small patch of fur on the nape of the neck was shaved for i.d. injections in all mice. Adult male and female mice were randomly assigned to experimental groups. Sexes of the same genotype were pooled for analysis. All mice included in the study were over 8 weeks old at the time of surgery. Following surgery, mice were allowed a two-week recovery period prior to starting behavior testing. Mice were typically used for a battery of behavioral tests, with a one-week break between each test. However, in some instances, the cement securing the optic fiber to the head became detached. Additionally, in certain behavioral experiments, the optic cable either detached or became tangled. Consequently, we were unable to complete the full battery of behavioral tests in some mice. After behavioral testing, mice were deeply anesthetized and euthanized by perfusion.
TRAP procedure
To obtain genetic access to Serotonin- or PBS-responsive PBN or amygdala neurons, we used the Targeted Recombination in Active Populations (TRAP2) system14. TRAP2:Ai14 mice received an i.p. injection of 4-hydroxytamoxifen (50 mg/kg in Chen oil; Sigma-Aldrich) followed 10 min later by an injection of PBS vehicle or serotonin (10 µg/10 µl, i.d.) as a pruritogen15,16,17.
Adeno-associated virus injection
A week after TRAP procedure, mice were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) or isoflurane (3%) and positioned in a stereotaxic frame. A cre-dependent AAV5 (pAAV-EF1a-double floxed-hChR2(H134R)-EYFP (1.0 × 1013 vector genomes/ml); a gift from Karl Deisseroth; Addgene viral prep # 20298-AAV5; RRID: Addgene_ 20298) was bilaterally injected into the PBN (coordinates: DV -3.6 mm, ML ± 1.4 mm, AP -4.92 mm). The injection volume was 0.25 µl, injected over 1 min using a glass needle and plunger. For CeA stimulation, optic fibers (200 μm diameter, 3.5 mm length, numerical aperture: 0.39) were implanted using stereotaxic coordinates (AP − 1.34 mm; ML ± 2.7 mm)10with the dorsal-ventral depth determined by the fiber length to target the CeA. Fibers were secured to the skull using dental cement. Mice were allowed 2 weeks to recover from surgery before behavior testing.
Retrograde tracing and CGRP staining
A week after TRAP procedure, mice were anesthetized with isoflurane (3%) and placed into the stereotaxic frame. Cholera toxin subunit B Alexa Fluor 647 conjugate (0.25 µl; CT-B; Thermo Fisher Scientific, Waltham, MA) was injected unilaterally into the left CeA (coordinates from Bregma: DV: -4.9 mm, ML: +2.8 mm, AP: -1.2 mm)18,19 over 60 s using a glass needle and plunger. Under anesthesia, mice were perfused with 20 mL PBS followed by 20 mL 4% paraformaldehyde (PFA) 3- or 5-days post-surgery. Brains were dissected and kept in 4% PFA overnight and then in 30% sucrose for 48 h prior to freezing in Optimal Cutting Temperature compound (OCT; Sakura Finetek, Torrance, CA), and coronally sectioned at 30-µm thickness on a cryostat10. Sections were stored in antifreeze solution at − 20 °C until staining.
For free-floating immunohistochemistry10the sections at around AP -5.20 mm were stained. Sections were washed with PBS and blocked with 5% donkey 0.2% Triton X-100 for 2 h at room temperature. Then they were incubated overnight at 4 °C in Goat-anti-Rat Calcitonin-Gene-Related-Peptide (1:1000; Cat#1720–9007; Bio-Rad Laboratories, Inc., Hercules, CA; AB_2290729) in blocking buffer. Following this, the sections were washed with PBS and then incubated for 2 h at room temperature in donkey-anti-goat secondary antibody conjugated with Alexa Fluor 488 (1:300, Invitrogen, Waltham, MA) in blocking buffer for 2 h at room temperature, washed again, and mounted on slides with Vectashield Mediumset Antifade Mounting Medium with DAPI (Vector Laboratories Inc, Newark, CA).
Stitched photomicrographs of whole sections were obtained using fluorescence microscopy (Leica Microsystems) at 10× and 20× objective magnification10. Structural boundaries were delineated using ImageJ software, guided by established atlases20. The number of CGRP+ neurons, TdTomato+ neurons, and CT-B+ neurons in each region of interest were counted manually by a trained observer.
Optogenetic stimulation
TRAP2:Ai14 mice were anesthetized with isoflurane (3%), and flexible fiber patch cords were attached to the external ends of the optic fibers10. Then, mice underwent a habituation period of 1 h to acclimate to the cables. These patch cords were subsequently connected to an LED light source (Thorlabs Inc, Newton, NJ) that delivered blue light (460 nm wavelength) capable of activating hChR2(H134R). The light pulses were maintained at consistent intensity and frequency (2.0 mW, 2 Hz) via an LED driver connected to a waveform generator13,21. Prior to implantation, the intensity of light was measured in each fiber using an optical power meter (Thorlabs).
Scratch testing
For scratching assessments in naive mice without peripheral pruritogen injection, mice were placed into the testing box and recorded for 30 minutes10. The session was divided into three equal 10-minute phases: the first and last phases were conducted with lights OFF, while the middle phase was conducted with lights ON.
For pruritogen-induced scratching assessments, mice were administered i.d. injections of 10 µL of histamine (50 µg, Sigma-Aldrich, St. Louis, MO), chloroquine (100 µg, Sigma-Aldrich), or serotonin (10 µg, Sigma-Aldrich) and then recorded for 30 min under conditions of either light ON or OFF12. Each mouse underwent two trials for each pruritogen, with a month-long interval between repetitions of the same chemical. Prior to each testing session, the experimental chamber was sanitized with 70% ethanol, both at the beginning of the day and after completion of testing for each group of mice to minimize odor effects. The number of scratch bouts was analyzed in 5-min bins by a trained observer blinded to the treatment condition. One scratch bout was defined as one or more rapid back-and-forth hind paw motions directed toward and contacting the injection site, ending with licking or biting of the toes or placement of the hind paw on the floor. Hind paw movements directed away from the injection site (e.g., ear-scratching) and grooming movements were not counted22.
Elevated plus maze
The elevated plus maze (EPM) procedure followed established protocols10,12. Briefly, each mouse was placed into the center square of the EPM, facing an open arm, and video recorded for 9 min with recording divided into three 3-min epochs (OFF-ON-OFF or OFF-OFF-OFF). During the ON epoch, mice were stimulated with blue light (2.0 mW, 2 Hz).
The time interval between experiments with OFF-ON-OFF and OFF-OFF-OFF conditions was four weeks. Videos were analyzed by a trained observer blinded to the treatment group. A mouse was considered to have entered an arm when all four paws were placed on the floor in that arm. Decreased time in open arms was a considered measure of anxiety-like behavior, and the total number of entries was used as a general measure of locomotion23.
Open field test
The open field test (OFT) procedure followed established protocols10,12,24,25. Briefly, each mouse was placed into a corner of the OFT, facing parallel to a wall, and video recorded for 9 min with recording divided into three 3-min epochs (OFF-ON-OFF or OFF-OFF-OFF). The time interval between experiments with OFF-ON-OFF and OFF-OFF-OFF conditions was four weeks. Videos were analyzed by a trained observer blinded to the treatment group. The number of entries into central and peripheral squares was counted. A mouse was considered to have entered a square when all four paws were placed on the floor in that square. Decreased percent entries into central squares (central/total) were considered a measure of anxiety-like behavior26.
Von Frey test
The von Frey test procedure followed established protocols12,13. Mice were acclimated to a perforated metal floor for 120 min before testing. The plantar surface of the hind paws was tested with a series of von Frey filaments to determine the paw withdrawal threshold (PWT). This baseline PWT assessment was conducted prior to any light stimulation. Following a 30-minute interval, the mice were exposed to blue light (2.0 mW, 2 Hz), and their hind paws were once again subjected to testing. The light stimulation was sustained throughout the testing session. The PWT was reassessed 60 min post-light stimulation. The strength (g) of the von Frey filament which induced paw withdrawal was noted for each stimulus.
Hargreaves test
The Hargreaves test procedure followed established protocols12,13. Mice were habituated to the Hargreaves arena for 120 min before testing. To determine the paw withdrawal latencies (PWLs), the plantar surface of the hind paws was exposed to five heat trials at 5-min intervals. PWL was measured both at baseline and during blue light stimulation (2.0 mW, 2 Hz). The light was turned on during each trial of the Hargreaves’ test. PWL was measured again 60 min after light stimulation. The beam active and idle intensities were 38 and 5, respectively. A cutoff of time of 10 s was used to prevent excessive tissue damage.
MC903-induced atopic dermatitis model
To prepare for Atopic Dermatitis (AD) induction, mice had their dorsal fur shaved. AD-like lesions were induced by applying 40 µL of 0.2 mM calcipotriol (MC903, MedChemExpress LLC, Monmouth Junction, NJ), a vitamin D3 analog, dissolved in 100% ethanol27. Mice were treated with MC903 or ethanol as a control for 14 days. Behavioral assessments included 30-minute recordings on day 10 to measure spontaneous scratching, a 5-minute test on the EPM on day 12, and a 5-minute test in the OFT on day 14. Mice were perfused under anesthesia on day 15. All behaviors were scored by a trained observer.
MC903-induced AD model with optogenetics
One week after TRAP procedure, mice were anesthetized with isoflurane (3%) and positioned in a stereotaxic frame. A cre-dependent AAV5 (pAAV-CAG-flex-ArchT-GFP (8.5 × 1011 vector genomes/ml); a gift from Edward Boyden; Addgene viral prep # 22222-AAV5; RRID: Addgene_2222228) was bilaterally injected into the PBN (coordinates: DV -3.6 mm, ML ± 1.4 mm, AP -4.92 mm) as described above. For CeA stimulation, optic fibers (200 μm in diameter, 3.5 mm length) were implanted following stereotaxic coordinates (AP − 1.34 mm; ML ± 2.7 mm) and fixed to the skull with dental cement. Mice were allowed 2 weeks to recover from surgery before behavior testing.
For AD induction, mice had their backs shaved, and 40 µL of 0.2 mM MC903 in ethanol was applied daily for 14 days27. On day 10, mice were recorded for 60 min to assess scratching behavior, with alternating 5-minute intervals of light “OFF” and “ON”. For ArchT activation, continuous yellow light (554 nm wavelength) was delivered at 4 mW29. The EPM and OFT tests were conducted under continuous “ON” light conditions on days 12 and 14, respectively, and mice were perfused under anesthesia on day 15. All behaviors were analyzed by a trained observer.
Statistical analysis
All statistical analyses and graphs were made using GraphPad Prism9 (GraphPad Software, San Diego, CA).
Analysis of Serotonin-responsive neurons in the amygdala and PBN
Data from serotonin- or PBS-TRAPed neurons in the amygdala (n = 3–4 mice) and PBN (n = 4–7 mice) were compared using a two-tailed unpaired t-test. P-values were corrected for multiple comparisons using Bonferroni correction. Each data point represents the average 2–3 sections from a single mouse.
Analysis of itch behavior in Naive mice
Scratching behaviors were assessed in serotonin- and PBS-TRAPed naive mice. Sample sizes were as follows: scratching without peripheral pruritogen injection (n = 16), histamine-evoked (n = 10–16/group), chloroquine-evoked (n = 11–16/group), and serotonin-evoked scratching (n = 9–14/group). Data were analyzed using a two-way repeated-measures ANOVA followed by Bonferroni’s post hoc multiple comparisons test.
Analysis of anxiety-like behavior in Naive mice
Anxiety-like behaviors were assessed in serotonin- and PBS-TRAPed naive mice (n = 10). Data were analyzed using a two-way repeated-measures ANOVA followed by Bonferroni’s post hoc multiple comparisons test.
Analysis of paw withdrawal behaviors in Naive mice
Paw withdrawal behaviors were assessed in serotonin- and PBS-TRAPed naive mice (n = 14–17). Data were analyzed using a one-way repeated-measures ANOVA followed by Tukey’s post hoc multiple comparisons test.
Analysis of behaviors in MC903-treated mice
Behavioral comparisons were made between MC903- and ethanol-treated mice (n = 8–9) or between serotonin- and PBS-TRAPed mice (n = 7–9) using two-tailed unpaired t-tests, except for the analysis of optogenetic inhibition effects on itch behavior. For that comparison, data were analyzed using two-way repeated-measures ANOVA followed by Bonferroni’s post hoc test.
Results
TRAP2 system labels itch-responsive neurons in the PBN
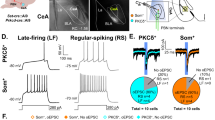
To study the itch-responsive neurons which project from the PBN to the CeA, we employed the TRAP2 system, which utilizes a Cre-lox method under the control of the Fos promoter (Fig. 1A). In this system, the lox cassette is only excised in the presence of 4-hydroxytamoxifen (4-OHT), enabling the expression of tdTomato. By pairing an i.d. injection of serotonin (or PBS for controls) with 4-OHT, we were able to label itch-responsive neurons through tdTomato expression. We observed a significant increase in serotonin-TRAPed neurons in the CeA (n = 3–4; two-tailed unpaired t-test, Bonferroni-corrected p = 0.0108), but not in the basolateral amygdala (BLA; n = 3–4; two-tailed unpaired t-test, Bonferroni-corrected p = 0.3494) (Fig. 1B-D). This result aligns with previous findings, which demonstrated a significant increase in histamine-TRAPed neurons in the CeA but not in the BLA10,11. Furthermore, the number of serotonin-TRAPed neurons in the lateral external PBN (PBNLE) was significantly greater than that of PBS-TRAPed neurons (n = 4–7; two-tailed unpaired t-test, Bonferroni-corrected p = 0.01), whereas no significant difference was observed in the lateral PBN population (n = 4–7; two-tailed unpaired t-test, Bonferroni-corrected p = 0.766) (Fig. 1E-G).
Serotonin-responsive neurons in the CeA and PBNLE are labeled in TRAP2 mice. (A) Schematic illustrating the TRAP system used for selective labeling of serotonin-responsive neurons. (B–D) In TRAP2 mice, intradermal serotonin injection significantly increased the number of tdTomato+ (red) neurons in the CeA compared to PBS injection (n = 2–3 sections from 3–4 mice). Scale bar, 200 μm. (E–G) Similarly, intradermal serotonin injection in TRAP2 mice resulted in a significant increase in tdTomato+ (red) neurons in the PBNLE compared to PBS injection (n = 2–3 sections from 4–7 mice). Scale bar, 200 μm. Data are presented as mean ± SEM. Statistical significance: *p < 0.05 (two-tailed unpaired t-test). P-values were corrected for multiple comparisons using Bonferroni correction.
The itch-responsive projection neurons from the PBN to the CeA are predominantly non-CGRP⁺ neurons
To quantify the CeA projecting neurons within itch-responsive PBN neurons, we injected a retrograde tracer (cholera toxin subunit B, CBT) into the CeA and assessed the overlap with TRAPed neurons (tdTomato) (Fig. 2). Out of the 727 total tdTomato neurons in the lateral PBN (from 3 mice, 3 sections per mouse), 28.3% (206) were found to project to the CeA (Fig. 2E).
Serotonin-responsive projection neurons from the PBN to the CeA are predominantly non-CGRP⁺. (A) Schematic timeline outlining the experimental protocol. (B) Schematic representation of the experimental procedure. Serotonin-TRAPed mice received unilateral intra-CeA injections of cholera toxin subunit B (CTB). Coronal sections including the PBN were collected for analysis. (C) Representative coronal section of the PBN, showing serotonin-TRAPed neurons (red), CGRP⁺ neurons (green), and CeA-projecting neurons (cyan). Scale bar, 100 μm. (D) Magnified view of the region outlined in panel C, focusing on the PBN lateral external (PBNLE) subregion. Scale bar, 100 μm. (E) Pie chart: Of 727 total TRAPed (tdTomato⁺) neurons identified in the lateral PBN (from 3 mice, 3 sections per mouse), 28.3% (206 neurons) projected to the CeA. (F) Pie chart: Among 542 CGRP⁺ neurons in the PBNLE, 8% (44 neurons) were TRAPed. (G) Pie chart: Of the 121 TRAPed neurons within the PBNLE, 36.3% (44 neurons) co-expressed CGRP. (H) Pie chart: Among the 67 TRAPed neurons that projected to the CeA within the PBNLE, 47.8% (32 neurons) were CGRP⁺.
To further characterize the itch-responsive PBN-CeA projection neurons, we analyzed the proportion of CGRP+ PBN neurons, which are implicated in affective pain and itch processing7. CGRP staining revealed that CGRP+ neurons were primarily localized to the lateral external parabrachial nucleus (PBNLE). Within the PBNLE, 542 CGRP+ neurons were identified, of which 8% (44 neurons) were TRAPed (Fig. 2F). Among these TRAPed CGRP+ neurons, the majority (72%, 32/44) projected to the CeA.
Within the PBNLE, 36.3% (44/121) of TRAPed neurons were CGRP+ (Fig. 2G), and among TRAPed projection neurons in this subregion, 47.8% (32/67) expressed CGRP (Fig. 2H). Across the entire lateral PBN, however, only 17% (36/206) of all TRAPed projection neurons were CGRP+.
Optogenetic stimulation of itch-responsive PBN-CeA projection neurons induces scratching behavior without altering pruritogen-induced scratching
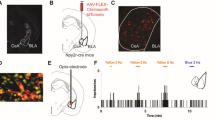
To control itch-responsive PBN-CeA projection neurons, we injected a Cre-dependent AAV encoding hChR2(H134R)-EYFP into the PBN and implanted an optic fiber into the CeA (Fig. 3A). Of the 202 TRAPed neurons counted across 4 mice (3 sections per mouse), 75% were EYFP-positive, indicating high viral transduction efficiency (Fig. 3B-C). Among the 157 EYFP-positive neurons, 97% were also tdTomato-positive (Fig. 3B-C), confirming the virus’s precise targeting.
Optogenetic stimulation of serotonin-responsive PBN-CeA projections induced itch-related behavior. (A) Schematic illustrating the strategy for optogenetic stimulation of serotonin-responsive projections from the parabrachial nucleus (PBN) to the central amygdala (CeA). (B) Representative coronal section of the lateral parabrachial nucleus (PBNLE) showing serotonin-TRAPed neurons labeled in red and hChR2(H134R)-EYFP-expressing neurons labeled in green, highlighting the overlap between targeted populations. Scale bar, 50 μm. (C) Pie chart showing that 75% of TRAPed neurons (202 total counted across 4 mice, 3 sections per mouse) were EYFP-positive. (D) Scratch bouts per 10 min in serotonin-TRAPed or PBS-TRAPed mice expressing AAV-DIO-hChR2(H134R)-EYFP, with or without concurrent light stimulation (n = 16/group). (E) Pie chart showing that optogenetically evoked scratching was predominantly directed toward the head and facial region. (F) Scratch bouts per 30 min following intradermal histamine injection, with or without concurrent light stimulation, in serotonin- or PBS-TRAPed mice (n = 10–16/group). (G) As in F, for intradermal chloroquine (n = 11–16/group). (H) As in F, for i.d. serotonin (n = 9–14/group). Data are shown as mean ± SEM. *p < 0.05, ****p < 0.0001 (two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparisons versus ON (D).
For the behavioral experiments, we first assessed whether optogenetic stimulation alone could induce scratching behavior. Light stimulation of serotonin-responsive PBN-CeA projection neurons, in the absence of a pruritogen injection, led to a significant increase in scratching, which returned to baseline once stimulation ceased (F(1, 30) = 12.25, p = 0.0015; Fig. 3D). Interestingly, the evoked scratching was predominantly directed toward the head and facial region (Fig. 3E). In contrast, the same stimulation had no effect on scratching behavior in PBS-TRAPed mice. We then evaluated scratching behavior in response to three pruritogens (histamine, chloroquine, serotonin) with and without optogenetic stimulation. No significant changes were observed in scratching behavior for either serotonin- or PBS-TRAPed mice (Fig. 3F-H).
Optogenetic stimulation of itch-responsive PBN-CeA projection neurons shows no impact on anxiety-like behavior
The CeA plays a crucial role in anxiety, as activation of itch-responsive neurons within the CeA has been shown to increase anxiety-like behavior10,11. Here, we investigate whether itch-responsive PBN-CeA projection neurons contribute to anxiety. To assess anxiety-like behavior, we used the EPM and OFT that measure thigmotaxis: a preference toward contact with walls versus exploring unprotected spaces.
Light stimulation of PBN-CeA projection neurons did not significantly alter anxiety-like behaviors compared to the control condition (OFF state) in either serotonin- or PBS-TRAPed mice (Fig. 4A, B,E, F). Furthermore, this stimulation had no significant effect on locomotor activity in either group (Fig. 4C, D,G, H).
Optogenetic stimulation of serotonin-responsive PBN-CeA projections does not affect anxiety-like behavior. Serotonin- or PBS-TRAPed mice received bilateral intra-PBN injections of AAV-DIO-hChR2(H134R)-EYFP, and optic fibers were implanted above the CeA. Anxiety- and locomotion-related behaviors were assessed using the elevated plus maze (EPM) and open field test (OFT) under light-off and light-on conditions. (A, B) In the EPM test, time spent in the open arms (a measure of thigmotaxis) was recorded for serotonin-TRAPed (A) and PBS-TRAPed (B) mice (n = 10/group). (C, D) Total arm entries (a measure of locomotion) were recorded for serotonin-TRAPed (C) and PBS-TRAPed (D) mice (n = 10/group). (E, F) Percentage of center square entries (another measure of thigmotaxis) was assessed in serotonin-TRAPed (E) and PBS-TRAPed (F) mice (n = 10/group). (G, H) In the OFT, total square entries (a measure of locomotion) were recorded for serotonin-TRAPed (G) and PBS-TRAPed (H) mice (n = 10/group). Data were analyzed using two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test. No significant differences were observed (p > 0.05). Error bars represent SEM.
Optogenetic stimulation of itch-responsive PBN-CeA projection neurons shows no impact on paw-withdrawal latency or threshold
To investigate whether itch-responsive PBN-CeA projection neurons are involved in other sensory processes, we conducted mechanical and thermal sensitivity tests. Mechanical sensitivity was assessed using von Frey filaments to evaluate whether light stimulation of serotonin-responsive PBN-CeA neurons affected withdrawal thresholds. Thermal sensitivity was measured with the Hargreaves test to examine the impact of light stimulation on withdrawal latency. Light stimulation did not significantly alter paw withdrawal thresholds or latencies in either serotonin- or PBS-TRAPed mice (Fig. 5).
Optogenetic stimulation of serotonin-responsive PBN-CeA projections does not alter basal thermal or mechanical sensitivities. (A) Paw withdrawal latency in Serotonin-TRAPed mice injected bilaterally in the PBN with AAV-DIO-hChR2(H134R)-EYFP. The Hargreaves test was conducted before, during, and after bilateral light stimulation (n = 17/group). (B) As in A for PBS-TRAPed mice (n = 10/group). (C) Paw withdrawal threshold in Serotonin-TRAPed mice received bilateral intra-PBN injections of AAV-DIO-hChR2(H134R)-EYFP. The von Frey filament test was performed before, during, and after light stimulation (n = 17/group). (D) As in C for PBS-TRAPed mice (n = 10/group). Data were analyzed using one-way repeated measure ANOVA followed by Tukey’s multiple comparison test. No significant differences were observed (p > 0.05). Error bars represent SEM.
Optogenetic inhibition of itch-responsive PBN-CeA neurons reduces spontaneous scratching without altering anxiety-like behavior in a chronic itch model
Given that activating itch-responsive PBN-CeA projection neurons increases spontaneous scratching, we hypothesized that inhibiting these neurons could reduce scratching in a chronic itch model. For this study, we used a vitamin D3 analog, MC903, known to induce AD-like skin lesions when applied topically to mouse skin over 7 days27,30,31. To validate this model, we compared the scratching and anxiety-like behaviors of mice treated with MC903 versus vehicle control as shown in the time course in Fig. 6A. MC903-treated mice showed a significant increase in scratching behavior (Fig. 6B; ***p < 0.001, n = 8–9, two-tailed unpaired t-test) and a significant increase in anxiety-like behavior, as evidenced by reduced time spent in the open arms of the EPM (Fig. 6C; **p < 0.01, n = 8–9, two-tailed unpaired t-test). There were no significant changes in locomotion (Fig. 6D) or anxiety-like behavior in the OFT (Fig. 6E).
Repeated application of MC903 induces itch-related and anxiety-like behaviors. (A) Schematic representation of the experimental timeline. (B) Scratch bouts recorded over 30 min in MC903- or ethanol-treated mice (n = 8–9 per group). (C) Time spent in the open arms during the elevated plus maze (EPM) test (n = 8–9 per group). (D) Total arm entries in the EPM test (n = 8–9 per group). (E) Percentage of center square entries in the OFT test (n = 8–9 per group).
Data are presented as mean ± SEM. Statistical significance: **p < 0.01, ***p < 0.001 (two-tailed unpaired t-test).
To inhibit itch-responsive PBN-CeA neurons, serotonin- or PBS-TRAPed mice received injections of a Cre-dependent AAV encoding ArchT-GFP into the PBN, with optic fibers implanted in the CeA (Fig. 7A-B). Of the 182 TRAPed neurons counted across 4 mice (3 sections per mouse), 79% were GFP-positive, indicating high viral transduction efficiency (Fig. 7C-D). Among the 148 GFP-positive neurons, 97% were also tdTomato-positive, confirming the virus’s precise targeting. Two weeks post-surgery, all mice were treated with MC903, followed by behavior tests including spontaneous scratching, EPM, and OFT. Because 4-OHT was administered only at the time of serotonin injection and not during the subsequent MC903 treatment, the TRAPed population reflects neurons activated specifically by serotonin, rather than the broader set of neurons activated during chronic inflammation.
Optogenetic inhibition of serotonin-responsive PBN-CeA projections reduces itch-related behavior in an MC903-induced atopic dermatitis mouse model. (A) Schematic timeline of the experimental protocol. (B) Schematic representation of the experimental procedure. Serotonin- or PBS-TRAPed mice received bilateral intra-PBN injections of AAV-FLEX-ArchT-GFP, followed by implantation of optic fibers above the CeA. (C) Representative coronal section of the PBNLE showing serotonin-TRAPed neurons (red) and ArchT-GFP-expressing neurons (green). Scale bar, 50 μm. (D) Pie chart showing that 79% of TRAPed neurons (182 total, across 4 mice with 3 sections per mouse) were GFP-positive. (E) Number of scratch bouts per 30 min with or without concurrent light stimulation, in serotonin- or PBS-TRAPed mice (n = 7–9/group) (F) Time spent in the open arms during the elevated plus maze (EPM) test during light stimulation (n = 7–9 per group). (G) Total arm entries in the EPM test during light stimulation (n = 7–9 per group). (H) Percentage of center square entries in the open field test during light stimulation (OFT) (n = 7–9 per group). Data are presented as mean ± SEM. *p < 0.05. Statistical analysis was performed using two-way repeated measures ANOVA followed by Bonferroni post hoc test for (E) or two-tailed unpaired t-tests (F-H).
To investigate the effect of inhibition of itch-responsive PBN-CeA neurons on spontaneous scratching, mice were recorded for 60 min with alternating 5-minute intervals of light “OFF” and “ON”. The experimental group showed a significant reduction in scratching behavior during light ON periods (Fig. 7E; p* < 0.05, n = 7–9, two-way repeated measure ANOVA, F (1, 14) = 5.045, p = 0.0116). To investigate the effect of the inhibition of itch-responsive PBN-CeA neurons on anxiety-like behaviors or locomotion, the EPM and OFT tests were conducted under continuous “ON” light conditions on days 12 and 14, respectively. No significant changes were observed in anxiety-like behaviors or locomotion (Fig. 7F-H). These findings indicate that inhibiting itch-responsive PBN-CeA neurons can alleviate spontaneous itching in a chronic itch model without affecting anxiety levels. Therefore, itch-responsive PBN-CeA neurons appear to selectively mediate itch under chronic conditions.
Discussion
This study provides compelling evidence that itch-responsive PBN-CeA neurons play a crucial role in mediating itch-related behavior. Optogenetic stimulation of these neurons triggered itch-related behaviors, while their optogenetic inhibition reduced such behaviors in a chronic itch model. Interestingly, retrograde tracing from the CeA revealed that about half of CeA-projecting itch-responsive PBN neurons expressed CGRP, despite itch-responsive PBN neurons representing a small fraction (8.1%) of all CGRP+ neurons in the PBN. This suggests that non-CGRP⁺ neurons may also contribute to this pathway.
These findings align with recent single-cell RNA-seq data showing extensive heterogeneity within the PBN, including 21 transcriptionally distinct subclusters5. Among the 10 subclusters projecting to the CeA, only one corresponds to the CGRP⁺ (Calca⁺) population. While a CGRP⁺ population has been implicated in encoding aversive signals32our findings suggest that non-CGRP⁺ PBN-CeA neurons, likely arising from other subclusters, may also contribute to itch signaling. This raises the possibility that specific non-CGRP⁺ PBN–CeA pathways selectively convey itch signals.
Interestingly, optogenetic activation of serotonin-TRAPed neurons primarily evoked head-directed scratching, despite the original serotonin injection being localized to the back. This finding suggests that the PBN–CeA pathway does not convey somatotopic (i.e., spatially localized) information about itch stimuli. Instead, somatotopic representation is primarily relayed via the thalamus to the primary somatosensory cortex33,34. This observation aligns with a previous finding showing that inhibition of NPY2R⁺ CeA neurons preferentially induced head scratching over truncal scratching13. These data raise the possibility that facial itch is under stronger modulatory control by CeA-derived inhibitory circuits.
Interestingly, while optogenetic stimulation of itch-responsive PBN-CeA neurons triggered scratching behavior, it did not enhance pruritogen-evoked scratching. This observation could be explained by two possibilities: (1) pruritogen stimuli might already maximally activate the PBN-CeA pathway, resulting in a ceiling effect, or (2) excessive activation of this pathway may trigger feedback inhibition mediated by Pdyn+ CeA neurons, which we recently identified as projecting back to the PBN to modulate itch signaling12.
Anxiety is a key component of the itch-affective circuit, known to exacerbate patients’ conditions35. The CeA is highly involved in both itch and anxiety, as demonstrated by optogenetic activation of CeA itch-responsive neurons increasing anxiety-like behaviors on the EPM and OFT10,11. In contrast, this study found that stimulating itch-responsive PBN-CeA neurons did not affect anxiety-like behaviors. This may be due to insufficient activation of the CGRP⁺ PBN-CeA pathway, which is implicated in negative valence, including affective pain36,37. Indeed, we observed that only 8% of CGRP⁺ neurons were TRAPed, suggesting limited engagement of this specific pathway.
Although PBN-CeA neurons are implicated in pain-related aversion, they do not mediate escape or withdrawal behaviors9,36. Consistent with this, optogenetic stimulation of itch-responsive PBN-CeA neurons did not alter withdrawal thresholds to thermal or mechanical stimuli.
Chronic itch is a significant source of stress for affected individuals, making it crucial to understand the pathways involved. AD is a common cause of chronic itch and can be modeled in mice using MC903 applied to the skin for over 8 days38,39,40. Mice treated with MC903 exhibited increased spontaneous scratching and anxiety-like behaviors compared to controls. However, inhibiting itch-responsive PBN-CeA neurons significantly reduced spontaneous scratching without affecting anxiety-like behaviors, further confirming that this pathway is involved in itch but not anxiety.
Importantly, the use of the TRAP system allowed us to label and manipulate serotonin-activated neurons selectively. As 4-OHT was administered prior to the onset of chronic inflammation, the silenced population represents neurons engaged during the initial serotonin response rather than the entire chronic inflammatory state. This temporal precision emphasizes that serotonin-activated PBN–CeA neurons, rather than a broader inflammatory ensemble, are sufficient and necessary to drive chronic itch behavior.
Overall, our findings indicate that itch-responsive PBN-CeA neurons include both CGRP+ and non-CGRP⁺ populations and are involved in itch processing without contributing to anxiety or mechanical/thermal sensitivities. These neurons represent a promising target for circuit-based interventions aimed at alleviating chronic itch without affecting other affective or sensory modalities. Future work should focus on resolving the molecular identity of these neurons and elucidating the broader network dynamics that govern itch sensation and its modulation.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Jansen, N. A. & Giesler, G. J. Jr. Response characteristics of pruriceptive and nociceptive trigeminoparabrachial tract neurons in the rat. J. Neurophysiol. 113, 58–70. https://doi.org/10.1152/jn.00596.2014 (2015).
Akiyama, T., Curtis, E., Nguyen, T., Carstens, M. I. & Carstens, E. Anatomical evidence of pruriceptive trigeminothalamic and trigeminoparabrachial projection neurons in mice. J. Comp. Neurol. 524, 244–256. https://doi.org/10.1002/cne.23839 (2016).
Piyush Shah, D. & Barik, A. The Spino-Parabrachial pathway for itch. Front. Neural Circuits. 16 https://doi.org/10.3389/fncir.2022.805831 (2022).
Chiang, M. C. et al. Parabrachial complex: A hub for pain and aversion. J. Neurosci. 39, 8225–8230. https://doi.org/10.1523/JNEUROSCI.1162-19.2019 (2019).
Pauli, J. L. et al. Molecular and anatomical characterization of parabrachial neurons and their axonal projections. Elife 11 https://doi.org/10.7554/eLife.81868 (2022).
Mu, D. et al. A central neural circuit for itch sensation. Science 357, 695–699. https://doi.org/10.1126/science.aaf4918 (2017).
Campos, C. A., Bowen, A. J., Roman, C. W. & Palmiter, R. D. Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622. https://doi.org/10.1038/nature25511 (2018).
Wang, Y. et al. Multimodal mapping of cell types and projections in the central nucleus of the amygdala. Elife 12 https://doi.org/10.7554/eLife.84262 (2023).
Chiang, M. C. et al. Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron 106, 927–939 e925, (2020). https://doi.org/10.1016/j.neuron.2020.03.014
Sanders, K. M., Sakai, K., Henry, T. D., Hashimoto, T. & Akiyama, T. A subpopulation of amygdala neurons mediates the affective component of itch. J. Neurosci. 39, 3345–3356. https://doi.org/10.1523/JNEUROSCI.2759-18.2019 (2019).
Samineni, V. K. et al. Cellular, circuit and transcriptional framework for modulation of itch in the central amygdala. Elife 10 https://doi.org/10.7554/eLife.68130 (2021).
Funahashi, H. et al. Dynorphinergic projections from the central amygdala to the parabrachial nucleus regulate itch. J. Neurosci. 43, 5340–5349. https://doi.org/10.1523/JNEUROSCI.0726-23.2023 (2023).
Pavlenko, D. et al. Activation of NPY2R-expressing amygdala neurons inhibits itch behavior in mice without lateralization. Sci. Rep. 14, 22125. https://doi.org/10.1038/s41598-024-73483-9 (2024).
Allen, W. E. et al. Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155. https://doi.org/10.1126/science.aan6747 (2017).
Weisshaar, E., Ziethen, B. & Gollnick, H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm. Res. 46, 412–416 (1997).
Hosogi, M., Schmelz, M., Miyachi, Y. & Ikoma, A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain 126, 16–23. https://doi.org/10.1016/j.pain.2006.06.003 (2006).
Akiyama, T., Carstens, M. I. & Carstens, E. Differential itch- and pain-related behavioral responses and micro-opoid modulation in mice. Acta Derm Venereol. 90, 575–581. https://doi.org/10.2340/00015555-0962 (2010).
Tokita, K., Inoue, T. & Boughter, J. D. Jr. Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience 171, 351–365. https://doi.org/10.1016/j.neuroscience.2010.08.026 (2010).
Douglass, A. M. et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 20, 1384–1394. https://doi.org/10.1038/nn.4623 (2017).
Franklin, K. B. & Paxinos, G. The mouse brain in stereotaxic coordinates, ed 3 San diego: academic. Brain Struct. & Function (2008).
Winke, N. et al. Brainstem somatostatin-expressing cells control the emotional regulation of pain behavior. bioRxiv, 2022.2001.2020.476899, (2022). https://doi.org/10.1101/2022.01.20.476899
Akiyama, T., Merrill, A. W., Zanotto, K., Carstens, M. I. & Carstens, E. Scratching behavior and Fos expression in superficial dorsal Horn elicited by protease-activated receptor agonists and other itch mediators in mice. J. Pharmacol. Exp. Ther. 329, 945–951. https://doi.org/10.1124/jpet.109.152256 (2009).
Rodgers, R. J. & Johnson, N. J. Factor analysis of Spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 52, 297–303. https://doi.org/10.1016/0091-3057(95)00138-m (1995).
Nishitani, N. et al. Manipulation of dorsal Raphe serotonergic neurons modulates active coping to inescapable stress and anxiety-related behaviors in mice and rats. Neuropsychopharmacology 44, 721–732. https://doi.org/10.1038/s41386-018-0254-y (2019).
Felix-Ortiz, A. C., Burgos-Robles, A., Bhagat, N. D., Leppla, C. A. & Tye, K. M. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209. https://doi.org/10.1016/j.neuroscience.2015.07.041 (2016).
Takahashi, A., Kato, K., Makino, J., Shiroishi, T. & Koide, T. Multivariate analysis of Temporal descriptions of open-field behavior in wild-derived mouse strains. Behav. Genet. 36, 763–774. https://doi.org/10.1007/s10519-005-9038-3 (2006).
Pavlenko, D. et al. Crisaborole inhibits itch and pain by preventing neutrophil infiltration in a mouse model of atopic dermatitis. Acta Derm Venereol. 103, adv13382. https://doi.org/10.2340/actadv.v103.13382 (2023).
Chow, B. Y. et al. High-performance genetically targetable optical neural Silencing by light-driven proton pumps. Nature 463, 98–102. https://doi.org/10.1038/nature08652 (2010).
Lu, Y. C. et al. ACC to dorsal medial striatum inputs modulate histaminergic itch sensation. J. Neurosci. 38, 3823–3839. https://doi.org/10.1523/JNEUROSCI.3466-17.2018 (2018).
Walsh, C. M. et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. Elife 8 https://doi.org/10.7554/eLife.48448 (2019).
Oetjen, L. K. et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171, 217–228 e213, (2017). https://doi.org/10.1016/j.cell.2017.08.006
Palmiter, R. D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293. https://doi.org/10.1016/j.tins.2018.03.007 (2018).
Omori, S. et al. Somatotopic representation of pain in the primary somatosensory cortex (S1) in humans. Clin. Neurophysiol. 124, 1422–1430. https://doi.org/10.1016/j.clinph.2013.01.006 (2013).
Leplus, A. et al. Somatotopy of the sensory thalamus: inputs from directional deep brain stimulation in pain patients. Ann. Clin. Transl Neurol. 11, 1502–1513. https://doi.org/10.1002/acn3.52067 (2024).
Sanders, K. M. & Akiyama, T. The vicious cycle of itch and anxiety. Neurosci. Biobehav Rev. 87, 17–26. https://doi.org/10.1016/j.neubiorev.2018.01.009 (2018).
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374. https://doi.org/10.1016/j.cell.2015.05.057 (2015).
Kang, S. J. et al. A central alarm system that gates multi-sensory innate threat cues to the amygdala. Cell. Rep. 40, 111222. https://doi.org/10.1016/j.celrep.2022.111222 (2022).
Li, M. et al. Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. J. Invest. Dermatol. 129, 498–502. https://doi.org/10.1038/jid.2008.232 (2009).
Li, M. et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. U S A. 103, 11736–11741. https://doi.org/10.1073/pnas.0604575103 (2006).
Weidinger, S. & Novak, N. Atopic dermatitis. Lancet 387, 1109–1122. https://doi.org/10.1016/S0140-6736(15)00149-X (2016).
Acknowledgements
This project was supported by grants from the National Institutes of Health (R01AR074062) and Stanley Glaser Foundation Research Award (UM SJG 2019-18) to T.A. The authors are grateful to Kevin Johnson (University of Miami) for his generous technical support.
Author information
Authors and Affiliations
Contributions
Conception of the work: T.A.; Acquisition, analysis, or interpretation of data: D.P., H.I., A.M., T.A.; Writing-Original Draft Preparation: D.P.; Writing-Review and Editing: T.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pavlenko, D., Ishida, H., Markan, A. et al. A subpopulation of projections from the parabrachial nucleus to the central amygdala mediates itch. Sci Rep 15, 26432 (2025). https://doi.org/10.1038/s41598-025-08612-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-08612-z