Abstract
Congenital toxoplasmosis is a significant public health issue caused by the transplacental passage of Toxoplasma gondii to the embryo/fetus. The standard treatment involves a combination of sulfadiazine and pyrimethamine, drugs often associated with adverse effects and high toxicity. The current study aimed to investigate the potential of prenylated chalcones (C2, C4 and C9) in controlling T. gondii infection in human trophoblast cells (BeWo) and human placental explants. As results, non-cytotoxic doses of C2, C4 and C9 impaired parasite invasion and subsequent intracellular proliferation in BeWo cells. Scanning and transmission electron microscopies evidenced the direct effect of chalcones on tachyzoites, which presented irregular rough surface, membrane with hole-like structures, torsion and shape substantial changes after pretreatment. C4 and, especially C9, caused notable ultrastructural damages due to the formation of vacuole-like structures in the parasite cytoplasm and surrounding the parasitophorous vacuole. Additionally, chalcones modulated the cytokine profile by increasing IL-8 and downmodulating MIF and ROS levels in BeWo cells and downregulating TNF-α release in villous explants. These findings highlight C2, C4, and C9 as promising candidates for the development of alternative therapies to prevent congenital toxoplasmosis, as well as chalcones as a valuable scaffold for the design of new anti-T. gondii agents.
Similar content being viewed by others
Introduction
Toxoplasma gondii is an obligatory intracellular parasite belonging to the phylum Apicomplexa, capable of infecting a broad spectrum of warm-blooded animals and cellular types1,2. Although most human toxoplasmosis cases are asymptomatic, the infection concern arises from the establishment of the parasite cysts in host tissues (bradyzoites), which is lifelong and can be reactivated in immune system suppression cases or even during pregnancy3. When T. gondii is acquired or reactivated during or just before gestation, the infection may result in vertical transmission2,4.
Congenital toxoplasmosis can lead to severe complications in the fetus and/or neonate, including miscarriages, stillbirth, growth restriction, malformations, neurological damage, encephalitis, chorioretinitis and brain calcifications, emphasizing the importance of early diagnosis and medical handling5,6. During the first 18 weeks of gestation, the treatment consists of spiramycin administration, as an attempt to prevent vertical transmission. From mid-gestation onwards, it is recommended the combination of sulfadiazine and pyrimethamine with folinic acid supplementation7. However, these drugs are ineffective during the latent phase of infection and can trigger serious adverse effects including bone marrow suppression, gastrointestinal disorders, allergies, teratogenicity and frequently lead to treatment discontinuation6,7,8. Furthermore, the increasing of drug-resistant T. gondii strains9,10,11 and the challenges of early diagnosis due to nonspecific initial symptoms emerged the urgent need for safer and more effective therapeutic alternatives12.
In this context, the current literature has highlighted natural products as a promising source of biologically-active compounds with antiparasitic properties13,14,15. Among them, chalcones, compounds belonging to the flavonoid family, have gained attention due to their biological properties, including anticancer16antibacterial17,18anti-inflammatory and antiparasitic19,20. Structurally characterized by a 1,3-diphenylprop-2-en-1-one skeleton, chalcones are easily accessible through dietary sources such as fruits, legumes and wines and are considered versatile scaffolds to synthetic structural modifications21,22,23. Previous studies have demonstrated the efficacy of chalcones and other types of flavonoids against bacteria and Trypanosoma, Plasmodium and Leishmania species. Zheoat et al. (2021) observed a significant effect of chalcones against infections by T. brucei, T. congolense and L. mexicana in human fibroblast cells24. Also, several synthetic chalcone derivatives have shown ability to control infections caused by T. cruzi, L. braziliensis, L. panamensis and P. falciparum with a prospect therapeutical index25,26,27. Furthermore, studies have demonstrated the significant influence of chalcones against periodontopathogenic bacteria, as well as Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa28,29. In the scenario of toxoplasmosis, Si et al. (2018) showed that licochalcones induced a potent control of T. gondii proliferation in human fibroblast cells, promoting significant damages in the parasite ultrastructure30. Also, several derivates of chalcones induced Toxoplasma growth control in vitro and in vivo, when HFF and murine peritoneal cells were observed with a lower number of parasites31.
Despite their reported biological functions, there are still no studies that investigate the effects of chalcones at the human maternal-fetal interface in the context of toxoplasmosis, so this is the first study showing the potential of chalcones in modulate T. gondii infection in maternal-fetal environment. Considering that our research group has reported distinct natural compounds with promising activity against T. gondii through in vitro and ex vivo experimental models of maternal-fetal interface32,33,34,35the main objective of this work was to evaluate the possible anti-T. gondii properties of synthetic chalcone analogs, as well as to unravel the possible underlying mechanisms related to parasitism control. Here, we employed two well-established experimental models: an in vitro model using human villous trophoblast cells (BeWo cells) and an ex vivo model using human villous explants from the third trimester of pregnancy36,37,38,39,40,41.
Results
Low doses of chalcones reduced T. gondii intracellular proliferation in bewo cells
Firstly, BeWo cells were exposed to serial dilutions of C2, C4 and C9 during 24 h to evaluate the effect of chalcones on cell viability. As shown in Fig. 1, cells treated with C2 and C4 demonstrated loss of cell viability when treated from 16 µg/mL onwards (Fig. 1A, B), while those treated with C9 presented lower viability only in the highest concentrations (64 and 128 µg/mL) in comparison to untreated cells (medium) (****P < 0.0001) (Fig. 1C). The vehicle control (0.25% DMSO) did not reduce cell viability compared with the untreated group (Fig. 1A–C). Next, we verified the parasite intracellular proliferation when T. gondii-infected BeWo cells were exposed to the less-cytotoxic concentrations of C2 and C4 (0.5–16 µg/mL) or C9 (0.5–32 µg/mL). As expected, S + P treatment was able to reduce the parasite replication by approximately 50% when compared to infected/untreated cells (medium) (Fig. 1D–F) (****P < 0.0001). Interestingly, it was observed that non-cytotoxic concentrations of chalcones (4 and 8 µg/mL for C2, 8 µg/mL for C4, 16 and 32 µg/mL for C9) were also effective in controlling the parasite growth in comparison to untreated/infected cells (medium) (P < 0.05) (Fig. 1D–F).
Cell viability and T. gondii intracellular proliferation assays. (A–C): BeWo cells were treated with different concentrations of C2 (A), C4 (B) and C9 (C) (0.5–128 µg/mL) for cell viability analysis (MTT colorimetric assay). Cells were also treated only with culture medium (M), representing the negative control (100% of viability), and with DMSO 0.25%, representing the vehicle control (D). The absorbance was measured at 570 nm and cell viability was expressed in percentages (cell viability %). (D–F): β-galactosidase colorimetric assays were performed to quantify T. gondii intracellular proliferation in infected BeWo cells treated with non-cytotoxic concentrations of chalcones. Infected cells treated only with culture medium (M) represented the negative control (100% parasite proliferation), and treatment with a combination of 200 µg/mL of sulfadiazine and 8 µg/mL of pyrimethamine (S + P) represented the positive control. The absorbance was measured at 570 nm and T. gondii intracellular proliferation was expressed in percentages (% of T. gondii proliferation). (A–C): *Comparison between untreated and treated cells. (D–F): *Comparison between infected/treated and infected/untreated cells; #Comparison between infected/treated and S + P/infected cells. Data are presented as means ± SEM from three independent experiments performed in eight replicates. Significant differences (P < 0.05) were detected by the One-Way ANOVA with Dunnett’s multiple comparison post-test.
Our results demonstrated that C2, C4 and C9 were all capable of controlling T. gondii intracellular proliferation at non-cytotoxic doses for BeWo cells. Upon calculating the half-maximal inhibitory concentration (IC50) against T. gondii and the half-maximal cytotoxic concentration (CC50) for BeWo cells, C2 exhibited an IC50 = 9.35 µg/mL ± 1.48, a CC50 = 18.62 µg/mL ± 1.43 and a selectivity index (SI) of 1.99. Similarly, C4 showed an IC50 = 7.18 µg/mL ± 0.40, a CC50 = 18.23 µg mL ± 0.31 and an SI of 2.53. In contrast, C9 demonstrated a higher CC50 value of 75.92 ± 4.62 µg/ml, with an IC₅₀ of 15.95 ± 2.40 µg/ml and an SI of 4.76 (Table 1). The SI value, also known as therapeutic index, was calculated as the ratio between CC50 and IC50 (SI = CC50/IC50) and provides an estimate of how selectively a compound targets a pathogen relative to its toxicity to cells. In this case, C9 was the compound with highest SI value (4.76) (Table 1), suggesting a more favorable therapeutic profile due to its greater ability to inhibit T. gondii proliferation, maintaining lower toxicity to BeWo cells. Thus, we observed that chalcones are potentially able to control the T. gondii intracellular proliferation in non-cytotoxic doses in BeWo cells.
Chalcones maintained their antiproliferative effect on T. gondii even after treatment removal
To further investigate whether the chalcone treatment could be reversible, the parasite quantification was performed in two experimental conditions: 24 h hours after treatment and 24 h after treatment removal. In agreement with Fig. 1, after 24 h of treatment with C2, C4, C9 or S + P, T. gondii intracellular proliferation had a significant decrease when compared to the infected/untreated cells (medium) (****P < 0.0001), exhibiting also greater reduction than the classical treatment with S + P (####P < 0.0001) (Fig. 2). Interestingly, 24 h after treatment removal, the antiproliferative effect of these treatments maintained their effectiveness in relation to both infected/untreated cells (medium) (****P < 0.0001) and S + P (####P < 0.0001) (Fig. 2). Comparing both situations (&), we noticed that even in the absence of treatments, the parasite growth was significantly lower 24 h after treatment removal condition (Fig. 2). In summary, S + P and chalcones presented an irreversible influence on T. gondii replication at least 24 h after the absence of treatment.
Reversibility assay. Infected BeWo cells were exposed to C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL) or only culture medium (M) for 24 h, followed or not by treatment removal for an additional 24 h. The β-galactosidase assay was performed and T. gondii intracellular proliferation was expressed in percentages (% of T. gondii proliferation). Treatment reversibility was deliberate by comparing the conditions of 24 h of treatment with the 24 h after treatment removal. The negative control group (M) was considered as 100% reversible. Data are expressed as means ± SEM from three experiments performed in eight replicates. (*) Comparisons between infected/untreated and infected/treated cells; (#) S + P and chalcone-treated cells; and (&) between infected/treated cells 24 h after treatment with the infected/treated cells 24 h after treatment removal. Significant differences were analyzed using (*;#) One-Way ANOVA with Dunnett’s multiple comparison post-test and (&) Unpaired Student´s t-test (two-tailed). Differences were considered as statistically significant when P < 0.05.
Chalcones induced damages on parasite ultrastructure
We also investigated the possible direct effect of C2, C4 and C9 on tachyzoites by electron microscopy. As shown in Fig. 3A, the infected/untreated BeWo cells showed a normal parasitophorous vacuole (PV) containing tachyzoites with unchanged ultrastructure: double membrane (arrowhead), rhoptries (Rp), nucleus (PNu) and dense granules (Dg). We did not observe any ultrastructural alteration on intracellular parasites exposed to C2 treatment (Fig. 3B), compared to the untreated group (medium) (Fig. 3A). On the other hand, C4 and especially C9 promoted the formation of vacuole-like structures (black arrows) surrounding the parasitophorous vacuole and in the parasite cytoplasm (Fig. 3C–E). Qualitative analysis showed that infected cells treated with C9 exhibited the most significant damage on tachyzoites, showing the largest density of vacuole-like structures and organelle disruption (Fig. 3D, E).
Ultrastructure analyses of T. gondii tachyzoites in the presence of chalcones. Transmission electron microscopy (TEM) micrographs showing T. gondii tachyzoites in BeWo cells treated for 24 h with: (A) only culture medium, (B) C2 (8 µg/mL), (C) C4 (8 µg/mL) and (D, E) C9 (32 µg/mL). Arrowhead: double membrane of the parasite. Black arrows: Vls (vacuole-like structures). Black asterisks: host cell mitochondria. Dg: dense granule. Nu: host cell nucleus. Rp: rhoptries. PNu, parasite nucleus; PV, parasitophorous vacuole. Scale bars (bottom left) 2 μm.
Pre-treatment of T. gondii tachyzoites with chalcones impaired the parasite adhesion to bewo cells
The initial experiments demonstrated that C2, C4 and C9 irreversibly inhibit T. gondii intracellular proliferation without compromising host cell viability and inducing significant ultrastructural alterations in the parasites. Thus, we sought to observe the influence of these chalcones during the parasite adhesion process on host cells. For this purpose, pretreated T. gondii tachyzoites were submitted to an adhesion assay. Our data on immunofluorescence analysis showed that S + P decreased both number of BeWo cells with adhered parasites and the total number of adhered parasites per field in comparison to the untreated parasites (medium) (*P = 0.0241; *P = 0.0402, respectively) (Fig. 4A, B). The same phenomenon was observed in parasites previously treated with C4 and C9 in comparison to untreated parasites (medium) (****P < 0.0001). The pretreatment with C2 did not induce significant changes in the number of adhered parasites or in the total number of adhered parasites per field (Fig. 4A, B). Representative immunofluorescence images illustrating the differences in the number of parasites adhered in BeWo cells according to the type of treatment were demonstrated in Fig. 4C–G.
Chalcones-pretreated parasites adhesion in BeWo cells. T. gondii tachyzoites were pre-incubated for 1 h with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL) or only culture medium (M) and then allowed to adhere in previously fixed BeWo cells for 3 h. Cells and parasites were fixed and labeled with DAPI (nuclei - blue), Phalloidin-Atto 565 (F-actin - red) and Alexa Fluor 488-conjugated anti-mouse IgG (tachyzoites – green). Images were captured using a confocal microscopy and approximately 20 fields were examined randomly to obtain: (A): the number of cells with adhered parasites. (B): the total number of adhered parasites per field analyzed. Significant differences (P < 0.05) were analyzed using Kruskal–Wallis test and Dunn’s multiple comparison post-test. Representative images highlighting the tachyzoites-host cell interaction of the different experimental conditions, as follow: (C) untreated parasites, (D) S + P-pretreated parasites, and (E, F, G) parasites pretreated with C2, C4 and C9, respectively. Data are expressed as means ± SEM from two independent experiments performed in four replicates. *Comparison between infected/untreated and infected/treated cells. #Comparison to S + P-treated cells. Scale bar: 40 μm. Tachyzoites were indicated by white arrowheads.
Chalcone-pretreated parasites presented compromised invasion and proliferation rates, as well as potential surface damages
Subsequently, we verified whether the pretreatment of T. gondii tachyzoites would be able to harm the invasion and intracellular proliferation. For this purpose, tachyzoites were pretreated for 1 h with chalcones and submitted to invasion or intracellular proliferation assays. Our findings showed that the conventional treatment (S + P) had no effect on T. gondii invasion when compared to untreated parasites (medium). However, C2, C4 and C9 significantly reduced the rate of parasite invasion in relation to the untreated parasites (medium) (****P < 0.0001 for all) and positive control (S + P) (####P < 0.0001 for all) (Fig. 5A). Regarding the intracellular proliferation assay, S + P, C2, C4 and C9 were effective in reducing intracellular growth when compared to untreated parasites (medium) (***P = 0.0001 for S + P; ****P < 0.0001 for C2, C4 and C9) (Fig. 5B). All chalcones showed more than 50% of inhibition in the parasite proliferation with C2 and C9 being able to control parasitism statistically better than the S + P pretreatment, as follows: C2 (##P = 0.0082) and C9 (#P = 0.0241) (Fig. 5B). These data suggest that the pretreatment of tachyzoites with C2, C4 and C9 impaired both the invasion and the subsequent T. gondii intracellular proliferation.
Chalcones-pretreated parasites. A pretreatment was carried out by incubating T. gondii tachyzoites with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL) or only culture medium for 1 h. (A): β-galactosidase assay was performed 3 h later to obtain T. gondii invasion rate (% of T. gondii invasion). Significant differences (P < 0.05) were analyzed using one-way ANOVA and Dunnett´s multiple comparisons post-test. (B): β-galactosidase assay was performed 24 h later and T. gondii proliferation was expressed in percentages (% of T. gondii proliferation). Significant differences (P < 0.05) were analyzed using Kruskal–Wallis test and Dunn’s multiple comparison post-test. Untreated parasites (M) represent the negative control and were considered as 100% of invasion and proliferation, respectively. Data are expressed as means ± SEM from three experiments performed in eight replicates. *Comparison between untreated/infected and infected/treated cells. #Comparison to S + P-treated cells. Representative scanning electron microscopy (SEM) images showing: (C) untreated T. gondii tachyzoite, (D) tachyzoite pretreated with S + P (200 + 8 µg/ml), (E) C2 (8 µg/mL), (F) C4 (8 µg/mL) and (G) C9 (32 µg/mL). Scale bars (bottom right) 2 μm. Loss of tachyzoite surface integrity or hole-like structures was indicated by white arrows.
Next, we performed SEM in these chalcone-pretreated parasites. The C2, C4 and C9 treatments were capable of triggering changes in parasite surfaces in comparison to untreated parasites or S + P-treated parasites (Fig. 5C–G). Morphological analysis of untreated T. gondii tachyzoites exhibited a characteristic arc-like shape with smooth and regular surface (Fig. 5C). Tachyzoites pretreated with S + P also showed a normal shape, although their surfaces presented a rougher aspect (Fig. 5D). Meanwhile, T. gondii tachyzoites treated with C2, C4 and C9 displayed irregular rough surface, plasma membrane rupture with hole-like structures, torsion and substantial changes in their characteristic shape (Fig. 5E–G), demonstrating a direct effect on the parasites.
Chalcones modulated the cytokine and ROS production in bewo cells
In sequence, we explored whether these compounds were capable of modulating the host cell production of intracellular reactive oxygen species (ROS) and IL-4, IL-6, IL-8, IL-10, MIF and TNF-α cytokines.
The T. gondii infection induced an increment of ROS production in all experimental conditions, regardless of the use of treatments, in comparison to their respective uninfected controls (Fig. 6A). In the absence of infection, C9 demonstrated an antioxidant effect, reducing the ROS production in cells in relation to untreated and S + P-treated cells (**P = 0.0037; ####P < 0.0001, respectively). This same effect was repeated in the infected conditions in relation to untreated and infected group (*P = 0.0234) (Fig. 6A).
ROS and cytokine production in BeWo cells treated with chalcones. BeWo cells were infected or not with T. gondii tachyzoites, followed by a 24 h treatment with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL) or only culture medium (M). (A): BeWo cells were incubated with the probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) and the ROS production was measured by fluorescence using a spectrophotometer. Data were expressed as MFI. After treatments, the supernatants were collected for measurement of (B) MIF, (C) IL-6 and (D) IL-8 cytokines. Data are expressed as means ± SEM from four independent experiments performed in twelve replicates. *Comparison between untreated and treated-cells. #Comparison between S + P and chalcone-treated cells. &Comparison between infected/treated cells with the respective control in the absence of infection. (A, C): significant differences (P < 0.05) were analyzed using (*;#) One-Way ANOVA with Dunnett’s multiple comparison post-test and (&) Unpaired Student´s t-test (two-tailed). (B, D): significant differences were analyzed using (*;#) Kruskal–Wallis test and Dunn’s multiple comparison post-test and (&) Mann-Whitney test (two-tailed).
Regarding MIF production, we noticed that T. gondii infection upregulated MIF levels in comparison to the uninfected cells, except for the S + P group. Data from infected/untreated cells (M) (&&P = 0.0079), C2, (&&P = 0.0047), C4 (&&P = 0.0027) and C9 (&&&P = 0.0002) are shown in Fig. 6B. Comparisons between the infected conditions revealed that S + P and C4 decreased MIF secretion in relation to infected/untreated cells (**P = 0.0079; *P = 0.0105, respectively). On the other hand, C9 showed higher levels of MIF when compared to the S + P treatment (##P = 0.0082) (Fig. 6B), maintaining similar levels to those found in infected/untreated group. The levels of MIF did not change significantly in uninfected cells (Fig. 6B).
In relation to IL-6, a similar release was observed in both experimental groups (uninfected/treated and infected/treated). However, in the presence of infection, BeWo cells treated with chalcones displayed higher IL-6 levels compared to those treated with S + P (##P = 0.0034; #P = 0.0209; ##P = 0.0064, respectively) (Fig. 6C). Finally, we analyzed the IL-8 levels produced by BeWo cells. When infection was absent, an IL-8 increment was detected in BeWo cells treated with C2 and C4 in comparison to untreated (***P = 0.0004; ****P < 0.0001, respectively) and S + P-treated cells (#P = 0.0479; ##P = 0.0024, respectively) (Fig. 6D). Similarly, in the presence of infection, C2 and C4 induced high levels of IL-8 in relation to infected/untreated cells (*P = 0.0241; *P = 0.0381, respectively). Interestingly, the T. gondii infection caused the upregulation of IL-8 by cells under S + P treatment in relation to infected/untreated group (*P = 0.0154) (Fig. 6D). The IL-4, IL-10 and TNF-α release by BeWo cells was also measured, but IL-4 and TNF-α levels were not detected, and IL-10 levels did not present significant differences (data not shown).
Therefore, our data suggest that C2, C4 and C9 had a modulatory effect in the cytokine profile and in ROS production in BeWo cells, especially downmodulating MIF (C4), upregulating IL-8 (C2 and C4) and decreasing ROS levels (C9).
Chalcones were unable to induce toxicity in human villous explants
To complement our in vitro findings, we also used human chorionic villous explants from the third trimester, since this experimental model is the gold pattern of human maternal-fetal interface. Firstly, human villous explants were collected and exposed to serial dilutions of C2, C4 and C9 during 24 h to evaluate the effect of chalcones on tissue viability by using MTT and LDH assays. Results from the MTT incorporation assay indicated that none of the tested concentrations of C2, C4 and C9 affected villi viability when compared to untreated villi (medium) (Fig. 7A–C), respectively. Also, the measurement of LDH in the supernatants revealed that only the highest concentration of C2 (128 µg/mL) caused a significant increase in the LDH levels when compared to untreated villi (****P < 0.0001), suggesting a reduction in villous integrity, whereas all other experimental conditions did not show evidence of tissue injury (Fig. 7D–F). In addition, the vehicle control (0.25% DMSO) did not show toxicity. To corroborate with these data, we evaluated the morphology of chalcone-treated villi using HE stained histological sections. The integrity of the chorionic villi treated with all types of chalcones remained intact, exhibiting typical morphology with a continuous syncytiotrophoblast layer surrounding the mesenchyme (M), similarly to the control condition (Fig. 7G–J).
Tissue viability assays in human chorionic villous explants treated with chalcones. Villous explants were treated for 24 h with different concentrations of C2, C4 and C9 (0.5–128 µg/mL) for tissue viability analysis. In parallel, tissues were also treated with culture medium only (M), representing the negative control (100% of viability) and with DMSO 0.25% (D), representing the vehicle control. (A, B, C): MTT colorimetric assay was performed and expressed in percentages (viability % by MTT incorporation). (D, E, F): supernatants were collected and used to measure LDH (U/L) levels (LDH assay). Data are means ± SEM from eight experiments performed in eight replicates. *Comparison between untreated and treated villi. Significant differences (P < 0.05) were determined using One-Way ANOVA and Dunnett’s multiple comparisons post-test. Representative photomicrographs of hematoxylin–eosin (HE) stained histological sections of villi incubated with culture medium (G), C2 (H), C4 (I) and C9 (J) (all 64 µg/mL). Syncytiotrophoblast cells are indicated by black arrows and mesenchyme by letter M. Scale bar: 60 μm.
Chalcones reduced T. gondii intracellular proliferation and modulated TNF-α in human villous placental explants
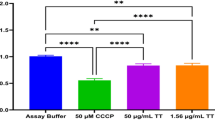
After verifying that chalcones were not cytotoxic for human villi, we assessed the T. gondii intracellular proliferation in these treated tissues. Our results confirmed that all types of chalcones were non-toxic to placental tissue in equal or lower doses of 64 µg/mL. Thus, the subsequent step was to evaluate their anti-T. gondii potential effect. In accordance to previous data, the concentrations of 150 and 200 µg/mL of S + P, respectively, were capable to reduce the parasitism in villous explants (****P < 0.0001; ~44%) (Fig. 8A). Similar to the positive control (S + P), both concentrations of 32 and 64 µg/mL of C2, C4 and C9 significantly decreased the parasite intracellular proliferation when compared to negative control (untreated villi) (****P < 0.0001) (Fig. 8A).
T. gondii intracellular proliferation and cytokine production in human villous explants treated with chalcones. (A) Villous explants were infected with T. gondii tachyzoites and treated with C2, C4, C9 (32 and 64 µg/mL), S + P (150 + 200 µg/mL, respectively) or culture medium only for 24 h. The percentages of T. gondii intracellular proliferation were measured using the β-galactosidase assay and compared with infected/untreated explants (M), considered as 100% of parasite proliferation, and positive control (S + P). B, C: After treatments, supernatants were collected for measurement of (B) MIF and (C) TNF-α. Cytokine levels were expressed in pg/mg of tissue. Data are expressed as means ± SEM of three independent experiments performed in eight replicates. *Comparison between untreated and treated villi. #Comparison to S + P-treated cells. &Comparison between uninfected/treated and infected/treated explants. Significant differences were analyzed using (*;#) One-Way ANOVA with Dunnett’s multiple comparison post-test and (&) Unpaired Student´s t-test (two-tailed). Differences were considered as statistically significant when P < 0.05.
Since the concentrations of 32 and 64 µg/mL of chalcones had the same effectiveness in control T. gondii proliferation in villous explants, we assessed the cytokine profile release when explants were treated with the highest possible concentration (64 µg/mL). In relation to MIF secretion, a down-regulation was observed in infected/untreated explants in comparison to uninfected/untreated villi (&P = 0.0139) (Fig. 8B). However, a significant reduction was not detected in treated experimental conditions (Fig. 8B). Regarding TNF-α, we noticed that in the absence of infection, the levels of this cytokine were downmodulated by the S + P, C2, C4 and C9 treatments in relation to untreated explants (****P < 0.0001 for S + P, C2 and C4; **P = 0.030 for C9). In response to T. gondii infection, TNF-α levels were upregulated in infected samples in comparison to uninfected controls except for the S + P group. Data from infected/untreated group (M) (&&P = 0.0018), C2, (&&P = 0.0060), C4 (&&P = 0.0014) and C9 (&&&P = 0.0001) are shown in Fig. 8C. All chalcones, as well as S + P, were able to reduce the TNF-α release in comparison to infected/untreated villi (****P < 0.0001; ***P = 0.0003; ****P < 0.0001; ***P = 0.0005, respectively). Infected S + P-treated explants did not alter the TNF-α levels in relation to uninfected SP-treated control (Fig. 8C). The IL-4, IL-6, IL-8 and IL-10 releases by placental villous explants were also measured, but IL-4 was not detected and IL-6, IL-8 and IL-10 levels did not present significant differences (data not shown). Taken together, these results demonstrate the efficacy of chalcones to control T. gondii in human placental explants, which was accompanied by the downregulation of TNF-α production.
Discussion
Congenital toxoplasmosis remains as a major global public health concern, particularly in developing countries where preventive measures and early diagnostic tools are often limited. Despite the widespread prevalence of T. gondii infection, the conventional treatment based on sulfadiazine and pyrimethamine can trigger severe side effects and its effectiveness is still limited in the latent phase of infection12. These challenges highlight the mandatory urgency to develop therapies with less toxicity and greater efficiency against the parasite.
In this context, experimental models of placental environment, such as BeWo cells and human placental explants, are essential tools for researching host-pathogen interactions at the maternal–fetal interface32,34,38,42. These models allow the direct observation of cellular and molecular interactions of placental environment, significantly contributing to the understanding of congenital diseases, such toxoplasmosis, and supporting the preclinical evaluation of novel therapeutic strategies. The present study evaluated the potential of prenylated chalcones (C2, C4, and C9) as alternative agents for the control of T. gondii growth in experimental models of human maternal-fetal interface. Specifically, we investigated their effects on T. gondii infection in human trophoblast cells (BeWo) and placental explants, focusing on parasite invasion, intracellular proliferation, ultrastructural alterations, and host immune response modulation.
Regarding BeWo cells assays, our study demonstrated that non-cytotoxic doses of C2, C4 and C9 were capable of control T. gondii intracellular proliferation. Based on IC50, CC50 and SI calculations, we observed that C9 presented the greatest therapeutic potential, since it had the highest SI value (4.76). Besides, it was found that chalcones maintained their ability to restrict parasite growth, even after treatment removal, suggesting irreversible damage on parasite biology.
T. gondii invades host cells through a specialized invasion mechanism mediated by apical complex structures, which includes rhoptries, micronemes and dense granules43,44. The rhoptries and micronemes secrete essential proteins for the adhesion and penetration of the parasite into the host cell, culminating in the formation of a parasitophorous vacuole45. The importance of these structures is huge, as they allow the parasite to invade and replicate into the host cells, perpetuating the infection and contributing to the pathogenesis of toxoplasmosis. In order to assess whether chalcones were able to impact these essential steps for the onset of infection, we performed assays with chalcone-pretreated parasites. The pre-treatment of tachyzoites for 1 h with C4 and C9, but not C2, reduced the total number of parasites adhered to cells, as well as the number of cells with adhered parasites. In addition, all tested chalcones inhibited the invasion and the subsequent proliferation of this parasite.
In sequence, we sought to investigate the possible direct effect of chalcones on the parasite ultrastructure. Therefore, both scanning (SEM) and transmission electron microscopy (TEM) were performed. The parasite damages caused by the 24-hour treatment with C4 and C9 were confirmed by TEM images, which showed the formation of vacuole-like structures inside the parasites and surrounding the parasitophorous vacuole. Intracellular parasites treated with C9 exhibited the most significant damage, with a greater qualitative number of vacuole-like structures and organelle disruption. Furthermore, the SEM demonstrated significant impairment in T. gondii surfaces when tachyzoites pretreated with C2, C4 and C9 displayed irregular rough surface, plasma membrane rupture with hole-like structures, torsion and substantial changes in the characteristic shape of the parasites. These ultrastructural changes in tachyzoites can be associated with the irreversible antiparasitic role of these compounds, configuring potent mechanisms of action of these prenylated chalcones. C2 was the compound that induced minor changes in parasite ultrastructure, although a significant function in downregulating the parasite replication has been promoted. We can speculate that C2 may impair essential parasite functions that are not detectable in assays focused on morphological aspects. Molecular targets in T. gondii with high affinity to C2 must be investigated to explore alternative mechanisms of action of this specific prenylated chalcone. Thus, additional experiments are necessary to elucidate its precise mechanism of action.
Contributing to our findings, current literature has already reported a similar activity of synthetic chalcones in other protozoans. De Mello et al. (2016) noticed several ultrastructural and morphological changes in promastigotes of Leishmania (Viannia) braziliensis, since the authors observed significant changes in mitochondrial ultrastructure, cytoplasmic vacuolization, cell shrinkage, cytoplasmic condensation and rounded shape of the parasites46. Also, Alonso et al. (2020) discovered that a novel chalcone derivative demonstrated activity against Leishmania (Leishmania) amazonensis by altering the parasite´s membrane fluidity47. In the scenario of toxoplasmosis, an isolated compound derived from the flavonoid family triggered a conformational change in the size and format of tachyzoites with depressions on the surface30.
T. gondii infection can induce the production of reactive oxygen species (ROS) by the host. During infection, host phagocytic cells generate ROS as a defense mechanism to destroy the parasite44. However, T. gondii has developed strategies to evade these defenses, including producing its own antioxidant enzymes, such as cytosolic peroxiredoxins TgPRX1 and TgPRX2 and mitochondrial peroxiredoxin TgPRX3, superoxide dismutases TgSOD2 and TgSOD3, antioxidant glutathione-S-transferase (TgGST), glutaredoxin (TgGrx) and catalase44,48. Although the ROS production plays a crucial role on infection control, improper regulation can lead to tissue damage and inflammation, requiring an appropriate balance. In order to investigate whether chalcones could modulate the production of ROS in BeWo cells, we measured it in cells submitted to different experimental conditions. It was observed that the infection induced a potent increment in ROS levels, regardless of the presence of chalcones. However, C9 was able to decrease the ROS levels induced by infection. Thus, it is possible to conclude that only C9 was capable to modulate ROS production in BeWo cells.
Cytokines are also important mediators in the control of T. gondii infection on trophoblast cells36,49,50,51. In this sense, we measured the release of cytokines in BeWo cell supernatants as an additional mechanism to explain the anti-T. gondii activity mediated by chalcones. As result, we detected that C2, C4 and C9 did not alter significantly the IL-6 levels in infected BeWo cells, although this cytokine is considered an important molecule to T. gondii control in the placental environment34,38,41,52. In relation to MIF production, it is important to highlight that the parasite itself produces a molecule structurally similar to human MIF, which binds to the same receptor, recruiting immune system cells and stimulating them to produce pro-inflammatory cytokines such as TNF-α, IL-8 and IL-12. Although seemingly contradictory, studies have demonstrated that T. gondii can use this as a tool to infect these cells and disseminate the infection53,54,55. In agreement, our data demonstrated an increase in MIF secretion in all infected conditions. On the other hand, infected cells treated with the conventional treatment or C4 downregulated the MIF release in comparison to the untreated group, demonstrating a regulatory mechanism to minimize the infection spread.
Regarding IL-8, C2 and C4 were potent inducers of this cytokine release in the absence and presence of infection. The role of IL-8 during infection by T. gondii in maternal-fetal interface is still unclear. Nejad et al. (2011) detected high levels of IL-8 in pregnant women with elevated rates of IgM, characterizing an acute infection56. As mentioned above, IL-8 has been associated as an important molecule for the activation of cellular mechanisms that help the spread of the parasite in the host53. In accordance with this information, our previous studies demonstrated that IL-8 was associated to the favoring of T. gondii growth in human trophoblast and monocytes51,57. Therefore, we cannot associate the high levels of IL-8 with the parasite control in BeWo cells. The mechanisms triggered by chalcones to reach this control prevented parasite proliferation even in the presence of high levels of IL-8.
To complement our in vitro findings, we also tested the anti-T. gondii effect of chalcones in human villous placental explants from the third trimester. Similarly to BeWo cells, our experiments demonstrated that C2, C4 and C9 were all capable to control T. gondii intracellular proliferation in non-toxic doses for explants. Based on MTT, LDH, morphological analysis and β-galactosidase proliferation assays, we selected the explants treated with 64 µg/mL of chalcones to analyze the cytokine profile. It was found that all tested chalcones did not modify the MIF production but downregulated the TNF-α levels. Previous studies already proved the role of TNF-α in the immune response against T. gondii. It is believed that this cytokine acts synergistically with IFN-y to improve the activity of macrophages in inhibiting T. gondii proliferation58. Additionally, it was been reported that high levels of TNF-α were associated with the increase in acute-phase proteins in mice, which are required for the T. gondii infection defense59. Also, our previous studies related the important role of TNF to reduce T. gondii infection in human trophoblast cells and villous explants36,38. While adequate levels of TNF-α play a crucial role in controlling T. gondii infection by activating immune cells and promoting the production of other pro-inflammatory cytokines, excessive production can cause significant damage to host cells. Onda et al. (2015) related that TNF downmodulation in primary trophoblast cells was associated to preeclampsia pathogenesis control60.
Preeclampsia is a severe complication of pregnancy, characterized especially by hypertension. An imbalance between angiogenic and antiangiogenic factors can trigger endothelial dysfunction, and it represents a key point in the mechanism of this condition61. Interestingly, Sofalcone, a chalconic drug, induced the reduction of antiangiogenic protein (sFlt-1) in human primary trophoblasts and downmodulated TNF, one of the most important cytokines involved in the preeclampsia pathogenesis. The authors concluded that chalcones were important to maintain a successful pregnancy and can be a good target for the treatment of preeclampsia to improve the angiogenesis and downmodulate TNF60,61. Hence, we hypothesized that chalcones reduced the production of TNF-α by infected villi in relation to the infected/untreated control group to avoid tissue destruction, at the same time that they modulated the immune response to maintain this cytokine in effective levels to combat the infection spread. Taken together, prenylated chalcones avoid a potent proinflammatory profile, which benefits the maintenance of a tolerant immune profile necessary for a successful gestation and, at the same time, reduce parasite infection when trigger important ultrastructural changes in tachyzoites.
In conclusion, our results demonstrated that C2, C4 and C9 chalcones were able to control T. gondii infection in both in vitro and ex vivo experimental models with low toxicity. We supposed that the irreversibly anti-T. gondii activity promoted by these chalcones is related mainly by a direct effect on parasites. Taking these findings together, we provided scientific evidence of the potential use of C2, C4 and C9 as an alternative source of treatment for prevent congenital toxoplasmosis, as well as chalcones as a promising scaffold for the design of new anti-T. gondii agents.
Methods
Cell culture and parasite maintenance
Human villous trophoblast cells (BeWo lineage) were acquired from the American Type Culture Collection (ATCC, CCL-98, Manassas, VA, USA) and maintained in RPMI 1640 medium (Cultilab, Campinas, SP, Brazil) supplemented with 100 U/mL penicillin (Sigma Chemical Co., St. Louis, MO, USA), 100 µg/mL streptomycin (Sigma) and 10% heat-inactivated fetal bovine serum (FBS) (Cultilab) in a humidified atmosphere at 37 °C and 5% CO262.
T. gondii tachyzoites (highly virulent RH strain, 2F1 clone) expressing the β-galactosidase gene were maintained by serial passages in BeWo cell culture in RPMI 1640 medium supplemented with penicillin and streptomycin and 2% FBS at 37 °C and 5% CO236.
Chalcones
Prenylated chalcones (C2, C4, and C9) were obtained according to simple and well-established protocol reported in our previous works63,64. Synthesis of chalcones C2 and C4 was carried out in two steps (Fig. 9A). Syringaldehyde was submitted to O-prenylation reaction, using bromide isoprenyl, potassium carbonate and acetone. This step furnished 4-O-prenylated syringaldehyde. In the second step, 4-O-isoprenylated syringaldehyde was condensed with 4’-methoxyacetophenone and 3’,4’-dimethoxyacetophenone to produce chalcones C2 and C4, respectively. For this step, reagents were solubilized in ethanol with potassium hydroxyde as basis. Chalcone C9 was prepared in two steps (Fig. 9B). Isovanillin was O-isoprenylated with isoprenyl bromide in the presence of potassium carbonate and acetone; yielding 3-O-prenylated isovanillin. 3-O-Prenylated isovanillin was submitted to condensation with 3’,4’-dimethoxyacetophenone, with potassium hydroxyde and ethanol, leading to compound C9. Compounds C2, C4 and C9 were maintained at − 80 °C in library of compounds at the Laboratory of Antibiotics and Chemotherapeutics, São Paulo State University, Brazil. To obtain the storage dilutions, the lyophilized samples were solubilized in dimethyl sulfoxide (DMSO) and stored at a concentration of 50,000 µg/mL at − 80 °C.
Cell viability assay
To evaluate the cytotoxicity of chalcones in BeWo cells, the MTT colorimetric assay [(3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltertrazolim bromide)] was performed according to Mosmann protocol65. BeWo cells at 3.0 × 104/well were seeded in 96-well microplates. After overnight adhesion, the cells were treated with two-fold serial dilutions of C2, C4 and C9 (ranging from 0.5 to 128 µg/mL) for 24 h at 37 °C and 5% CO2. The control group received only RPMI culture medium with 10% FBS and, for the vehicle control, BeWo cells were treated with DMSO 0.25% in RPMI medium, which corresponds to the DMSO percentage present in the highest concentration of tested chalcones (128 µg/mL). Subsequently, the supernatants were carefully removed and a solution of MTT reagent (5 mg/mL, 10 µL; Sigma) plus 90 µL of supplemented RPMI medium was added to cell monolayers followed by incubation for 4 h at 37 °C and 5% CO2. Next, the wells received a solution of 10% sodium dodecyl sulfate (SDS; Sigma) and 50% N,N-dimethyl formamide (DMF; Sigma). MTT reduction was measured at 570 nm absorbance using a spectrophotometer (Versa Max ELISA Microplate Reader, Molecular Devices, Sunnyvale, CA, USA) and cell viability was reported in percentages (Cell viability %), with the absorbance of cells incubated only with medium considered to be 100% viable. At least three independent experiments with eight replicates were performed.
T. gondii intracellular proliferation assay
In order to establish the best concentrations for the next experiments, we assessed whether C2, C4 and C9 would be capable of controlling the parasite intracellular proliferation using the β-galactosidase colorimetric assay, as previously described49. For this purpose, BeWo cells at 3.0 × 104/well were seeded in 96-well microplates, allowed to adhere overnight and then infected with T. gondii tachyzoites at a multiplicity of infection (MOI) of 3:1 for 3 h at 37 °C and 5% CO2. After this, the cells were carefully rinsed with sterile 1X phosphate-buffered saline (PBS) to remove the remaining extracellular parasites and treated with two-fold serial dilutions of C2, C4 and C9 (ranging from 0.5 to 16 µg/mL for C2 and C4 and 0.5 to 32 µg/mL for C9) for 24 h at 37 °C and 5% CO2. The association of sulfadiazine and pyrimethamine (S + P) was used as a positive control to simulate conventional treatment for toxoplasmosis (positive control). The concentration of S + P (200 µg/mL and 8 µg/mL, respectively) was chosen based on published data, since it has been reported as non-toxic for BeWo cells41. Infected cells incubated with only RPMI medium were used as negative control representing non-inhibited parasite growth. Parasite quantification on cell samples was performed by the β-galactosidase reaction using the chlorophenol red-β-D-galactopyranoside reagent substrate (CPRG; Roche Diagnostics, Mannheim, Germany). Afterwards, the absorbance (570 nm) was measured using a spectrophotometer (Versa Max ELISA Microplate Reader, Molecular Devices), and the parasite intracellular proliferation was expressed in percentage (T. gondii proliferation %). The number of tachyzoites was calculated in comparison to a standard curve with free tachyzoites (ranging from 1 × 106 to 15,625 × 103 parasites). The efficiency of each treatment condition was defined by comparison with the negative control (100% parasite proliferation). At least three independent experiments with eight replicates were performed.
Reversibility assay
After selecting non-cytotoxic concentrations of C2, C4 and C9 that showed an inhibitory effect on the T. gondii intracellular proliferation, we investigated whether the antiparasitic effect would be maintained even after treatment removal. The reversibility assay was performed as previously described34. BeWo cells were seeded at 3.0 × 104 cells/well in 96-well microplates and after adhesion were infected with T. gondii tachyzoites at a MOI of 3:1 in supplemented RPMI medium. Parasites were left to invade the host cells for 3 h and then rinsed to remove the unattached ones. In the first part of the experiment, the infected cells were treated with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL, respectively, positive control) or with culture medium only (untreated group, negative control) for 24 h at 37 °C and 5% CO2. After 24 h treatment, the supernatant was removed and the parasite intracellular proliferation was measured. In parallel, the mentioned treatments were removed, replaced by a treatment-free culture medium allowing the parasites to grow for an additional 24 h and then the parasite growth was measured. In both situations, the T. gondii intracellular proliferation was quantified using the β-galactosidase assay and presented in percentage (T. gondii proliferation %). The reversibility of the treatment was assessed by comparing the condition 24 h after treatment with the condition 24 h after treatment removal. Three independent experiments with eight replicates were performed.
Adhesion assay
To investigate whether the chalcones are able to impair parasite adhesion to host cells, we proceeded with an adhesion assay, as previously described34. BeWo cells were seeded at a density of 1.0 × 105 cells in 24-well microplates containing 13-mm coverslips on each well. After overnight adhesion, cells were fixed with paraformaldehyde (4%) for 30 min at room temperature and washed three times with 1x PBS. T. gondii tachyzoites at a MOI of 3:1 were pretreated with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL) or culture medium only (control group) for 1 h at 37 °C and 5% CO2. Chalcone-pretreated tachyzoites were rinsed and resuspended in a treatment-free culture medium before incubation with previous fixed BeWo cells for 3 h at 37 °C and 5% CO2. After removal of non-adherent parasites with sterile 1× PBS, the adhered parasites were fixed as mentioned above. The coverslips were incubated with rabbit polyclonal primary anti-T. gondii antibody (Abcam #20530; Waltham, MA, USA) (diluted 1:500 in PGN-0.01% saponin solution) for 1 h, rinsed three times with sterile 1× PBS. Next, coverslips were incubated with Alexa Fluor 488-conjugated anti-rabbit IgG (diluted 1:500 in PGN-0.01% saponin solution) (Invitrogen, #A11008; Waltham, MA, USA), tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin (Sigma) (diluted 1:50 in PGN + saponin) and the cell nucleus marker TO-PRO-3 Iodide (Life Technologies, Waltham, MA, USA) (diluted 1:500 in PGN + saponin) for an additional 1 h in the dark at room temperature to label T. gondii tachyzoites, F-actin and nuclei, respectively. The coverslips were washed three times with sterile 1x PBS and mounted on glass slides. Afterwards, samples were analyzed using a confocal fluorescence microscopy (Zeiss, LSM 510 Meta, Oberkochen, Germany) with an inverted microscope (Zeiss Axiovert 200 M, Oberkochen, Germany). The results were expressed by the number of BeWo cells with adhered parasites and the total number of attached parasites per cell in a total of 20 fields chosen randomly. Two independent experiments with four replicates were performed.
Invasion and intracellular proliferation of chalcone-pretreated parasites
The parasite invasion and subsequent intracellular proliferation were also evaluated by pretreating the tachyzoites34,41. This experiment proposes an investigation of a possible direct effect of chalcones on tachyzoites. Cell culture-derived tachyzoites of T. gondii at a MOI of 3:1 were pre-incubated with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL) or S + P (200 + 8 µg/mL, respectively) for 1 h at 37 °C and 5% CO2. Parasites preincubated only with culture medium were used as negative control group. Next, the pretreated parasites were centrifuged to treatment removal, resuspended in fresh medium and placed in 96-well culture microplates containing previously adhered BeWo cells at 3.0 × 104 cells/well. Parasite interaction with host cells was allowed to occur for 3 h at 37 °C and 5% CO2. For the invasion analysis, the supernatant was removed, infected cell monolayers were carefully rinsed to remove extracellular parasites and quantification of invaded parasites was performed immediately. For intracellular proliferation analysis, we perform the same protocol as described above, but after the 3-hour invasion, a fresh culture medium was added to infected cell monolayers for an additional 24 h at 37 °C and 5% CO2. In both experimental approaches, the number of intracellular parasites was quantified using the β-galactosidase assay, as described above. Three independent experiments with eight replicates were performed.
Transmission Electron microscopy (TEM) and scanning Electron microscopy (SEM)
To further investigate the anti-Toxoplasma effect of chalcones, we observed the ultrastructure of T. gondii tachyzoites treated with C2, C4 and C9 by the scanning electron microscopy (SEM)66,67 and transmission electron microscopy (TEM), as previously described41with minor modifications.
For TEM, BeWo cells were seeded at 1.0 × 106 cells/well in 6-well microplates and after an overnight adhesion, the cells were then infected with T. gondii tachyzoites at a MOI of 3:1 in supplemented RPMI medium for 3 h at 37 °C and 5% CO2. Infected cells were rinsed and incubated with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL) or culture medium only (negative control) for 24 h at 37 °C and 5% CO2. Next, cells were harvested and fixed in Karnovsky solution containing 2% paraformaldehyde and glutaraldehyde in a 0.1 M sodium cacodylate buffer (pH 7.4) for 24 h. Samples were incubated for 1 h in 1% osmium tetroxide (OsO4) in cacodylate solution, followed by agar addition. The samples were maintained at 4 °C for approximately 2 days to finally be dehydrated in increasing concentrations of ethanol and propylene oxide, and then included in Epon resin. Ultrathin sections were stained with uranyl acetate and lead citrate and analyzed using a transmission electron microscope (Hitachi, TM 3000, CIQTEK, Hefei, Anhui, China).
To perform SEM, cell culture-derived tachyzoites at 1.0 × 106 parasites/1,000 µl were pretreated or not with C2, C4, C9 and S + P (same experimental procedure described above). Next, the pretreated parasites were centrifuged to treatment removal, rinsed with 0.1 M sodium cacodylate buffer and fixed in Karnovsky solution for 2 h at 4 °C. After two successive washes with sodium cacodylate buffer, the parasites were post-fixed in 1% OsO4 in cacodylate solution for 1 h at room temperature. Then, the fixed tachyzoites were rinsed over again with sodium cacodylate buffer and deposited in 24-well plates containing 13-mm coverslips. After overnight drying, the coverslips were dehydrated in increasing concentrations of ethanol and followed by analyses in scanning electron microscope (Tescan, VEGA 3 LMU, CIQTEK).
Measurement of intracellular reactive oxygen species (ROS)
To verify the impact of chalcones in the intracellular ROS production, we measured the ROS in BeWo cells based on the intracellular peroxide-dependent oxidation of 2′,7′-dichlorodihydrofuorescein diacetate (H2DCF-DA) (Invitrogen) to form the fluorescent compound 2′,7′-dichlorofuorescein (DCF), as previously described68,69with some modifications. In brief, BeWo cells at 3.0 × 104/well were seeded in black 96-well microplates with clear bottoms (Costar REF# 3603, New York city, New York, USA). After overnight adhesion, cells were then infected or not with T. gondii tachyzoites at a MOI of 3:1 for 3 h, rinsed and then treated with C2 (8 µg/mL), C4 (8 µg/mL), C9 (32 µg/mL), S + P (200 + 8 µg/mL) or culture medium only for 24 h at 37 °C and 5% CO2. Following, the culture supernatants from all conditions were collected and stored at -80 °C for further cytokine measurement. Cell monolayers were incubated with 150 µL of H2DCF-DA (10 µM; diluted in 1× PBS containing 10% FBS, Sigma) for 45 min under darkness at 37 °C and 5% CO2. For positive control of ROS production, four wells received hydrogen peroxide (H2O2). Last, cells were again rinsed with 1× PBS and the microplate submitted to an excitation (488 nm) and emission (550 nm) wavelength in the multi-well scanning spectrophotometer (Versa Max ELISA Microplate Reader, Molecular Devices). The data were presented as Median Fluoresce Intensity (MFI). Four independent experiments with twelve replicates were performed.
Human third-trimester villous explants culture
To complement our in vitro findings, we proceed with an ex vivo experimental model of the human maternal-fetal interface to assess the antiparasitic effects of the chalcones52,70. Briefly, chorionic villous explants were collected from human placentas (36 to 40 weeks of pregnancy, N = 3) after elective cesarean section deliveries at the Clinic Hospital of the Universidade Federal de Uberlândia (HC-UFU), MG, Brazil. The placentas were from patients without comorbidities, such as pre-eclampsia, chronic hypertension, infectious disease (e.g., toxoplasmosis, Chagas disease), chorioamnionitis, chronic renal disease, cardiac disease, connective tissue disease, pre-existing diabetes mellitus, gestational diabetes mellitus, and other pathological manifestations. Terminal chorionic villi containing five to seven free tips per explant were dissected up to 1 h after placenta collection. The placental tissues were then added to 96-well microplates (one villus per well) in 200 µL/well of a fresh RPMI medium supplemented with 10% FBS for 24 h at 37 °C under a humidified atmosphere containing 5% CO2 until the experimental procedure.
Viability assay in human explants
Tissue viability of villous explants under chalcone treatments was performed through MTT and lactate dehydrogenase (LDH) assays, in accordance to published protocols52,65,71. After dissection and overnight incubation, as mentioned above, villous explants were exposed to 24 h with chalcones treatment using two-fold serial dilutions of C2, C4 and C9 (ranging from 0.5 to 128 µg/mL). As vehicle control, we treated the villi with DMSO (0.25%) in RPMI medium, which corresponds to the highest percentage of DMSO present in the 128 µg/mL of the chalcones. As negative control of viability, villous explants were treated with culture medium only. Following 24 h of incubation, the culture supernatants were collected, and LDH concentration was measured according to the manufacturer’s instructions (Lactate Dehydrogenase LDH UV, Ref K014-2, Quibasa Química Básica Ltda, Belo Horizonte, MG, Brazil) at 340 nm by a microplate reader (Versa Max ELISA Microplate Reader, Molecular Devices). This assay is based on the consumption and decrease of absorption of NADH. LDH released into the culture medium was expressed in U/L of LDH enzymatic activity and was used as a marker for tissue integrity.
Tissue viability was also assessed by the MTT assay. After 24 h of treatment, the supernatants were carefully removed and replaced by a solution of MTT reagent (5 mg/mL, 20 µL) plus 180 µL of supplemented RPMI medium followed by an incubation for 4 h. Subsequently, formazan crystal resulting from MTT reduction was solubilized overnight by the addition of 100 µL PBS containing SDS 10% and DMF 50%. MTT reduction was measured on the next day at 570 nm absorbance using a spectrophotometer and tissue viability was reported in percentages (viability % by MTT incorporation), with the absorbance of villi incubated only with culture medium considered to be 100% viable. Furthermore, we performed morphological analysis of chalcone-treated villi to corroborate the viability assays. Villi tissue sections were stained with hematoxylin/eosin and examined using a light microscope (BX40 Olympus, Tokyo, Japan). One experiment with eight replicates was performed for each viability methodology.
T. gondii intracellular proliferation in human explants
To quantify T. gondii intracellular proliferation in human villous explants, the β-galactosidase colorimetric assay was performed32,34,38. Villi previously collected and cultured in 96-well microplates were infected or not with T. gondii tachyzoites (1 × 106 parasites per well/200 µL) and incubated for 24 h at 37 °C and 5% CO2. Next, the villi were treated for an additional 24 h with C2, C4 or C9 (32 and 64 µg/mL) based on tissue viability data (LDH and MTT assays). Sulfadiazine and pyrimethamine (S + P) were used as positive control at concentrations of 150 µg/mL and 200 µg/mL, respectively41. Infected villi incubated with RPMI medium in the absence of any treatment was used as negative control, representing non-inhibited parasite growth. After 24 h of incubation, the supernatants were collected and stored at -80 °C, as well as the placental villi. The same experimental conditions were applied for uninfected and treated villous explants. The supernatants were collected for further cytokine measurement and villous explants for protein content determination using Bradford reagent and T. gondii intracellular proliferation by β-galactosidase assay with minor modifications.
Frozen villous explants were homogenized by the addition of 150 µL of radioimmunoprecipitation assay buffer (RIPA) [50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1% (w/v) sodium deoxycholate and 0.1% (w/v) SDS, pH 7.5] containing protease inhibitor cocktail (Complete, Roche Diagnostic). The homogenate was centrifuged at 21,000×g for 15 min at 4 °C, and the supernatants were collected. To measure the total amount of protein using the Bradford method, 2 µL of supernatants was diluted in 18 µL of 1X PBS in 96-well microplates, followed by the addition of Bradford reagent (Sigma). T. gondii intracellular proliferation was carried out using β-galactosidase assay, as mentioned above, by incubating 20 µL of supernatants from each sample with 160 µL of assay buffer (1× PBS, β-mercaptoethanol and MgCl2) and 40 µL of CPRG (chlorophenol red-β-D-galactopyranoside; Roche). The absorbance (570 nm) was measured by a spectrophotometer plate reader (Versa Max ELISA Microplate Reader, Molecular Devices). The number of T. gondii tachyzoites was normalized according to the total protein concentration (µg/mL) of each villous obtained by Bradford assay, and expressed by the number of parasites per µg of tissue32,34,38. T. gondii proliferation in villous explants was expressed in percentage (T. gondii proliferation %) and the number of tachyzoites calculated in comparison to a standard curve of free tachyzoites (ranging from 1 × 106 to 15.625 × 103 parasites). The efficiency of each treatment condition was measured by comparing it with the negative control (100% parasite proliferation). Three independent experiments with eight replicates were performed.
Cytokine measurement
The cytokine release in supernatants of BeWo cells and villous explants was measured using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). Assays for IL-4, IL-6, IL-8, IL-10, TNF-α (OpTEIA, BD Bioscience, San Diego, CA, USA) and MIF (Duoset R&D Systems, Minneapolis, MN, USA) were performed according to the manufacturer’s instructions. Cytokine concentrations for experiments with BeWo cells were expressed in pg/mL. Cytokine concentration in the villous experiments was normalized using total protein content (mg/mL) of each villous, obtained by the Bradford method and results were expressed as pg/mg of tissue. The limits of detection of each cytokine were determined from standard curves: IL-4 and IL-6 (4.7 pg/mL); IL-8 IL-10, TNF-α and MIF (7.8 pg/mL).
Statistical analysis
Data were first checked for normal distribution and expressed as means ± standard error of mean (SEM), using GraphPad Prism Software version 8.0.1 (GraphPad Software Inc., San Diego, CA, USA). Significance differences were compared to controls by using one-way analysis of variance (ANOVA) followed by Dunnett´s multiple comparisons post-test for the parametric data. Nonparametric data were analyzed by the Kruskal–Wallis test and Dunn’s multiple comparison post-test. Differences between two groups were assessed either by unpaired Student’s t test or by Mann-Whitney´s test, for parametric or non-parametric data, respectively. Data were considered statistically significant when P < 0.05.
Ethical approval
The present research protocol using human tissue samples was performed in accordance with relevant guidelines and regulations, being approved by the Ethics Committee of the Universidade Federal de Uberlândia, MG, Brazil, with approval number 5.812.532. Informed consent was obtained from all the study participants. The participants were well-informed in depth about the study procedure before starting the study and they participated in the study without any external influence. In cases of subjects were under 18, a consent term was acquired from parent and/or legal guardian.
Data availability
Correspondence and requests for materials should be addressed to B.F.B.
References
Dubey, J. P., Lago, E. G., Gennari, S. M., Su, C. & Jones, J. L. Toxoplasmosis in humans and animals in brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 139, 1375–1424. https://doi.org/10.1017/s0031182012000765 (2012).
Montoya, J. G., Liesenfeld, O. & Toxoplasmosis Lancet 363, 1965– (1976). https://doi.org/10.1016/s0140-6736(04)16412-x (2004).
Ahmed, M., Sood, A. & Gupta, J. Toxoplasmosis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 255, 44–50. https://doi.org/10.1016/j.ejogrb.2020.10.003 (2020).
Kodjikian, L. et al. Vertical transmission of toxoplasmosis from a chronically infected immunocompetent woman. Pediatr. Infect. Dis. J. 23, 272–274. https://doi.org/10.1097/01.inf.0000115949.12206.69 (2004).
Li, X. L., Wei, H. X., Zhang, H., Peng, H. J. & Lindsay, D. S. A Meta analysis on risks of adverse pregnancy outcomes in Toxoplasma gondii infection. PLoS One. 9, e97775. https://doi.org/10.1371/journal.pone.0097775 (2014).
Moncada, P. A. & Montoya, J. G. Toxoplasmosis in the fetus and newborn: An update on prevalence, diagnosis and treatment. Expert Rev. Anti Infect. Ther. 10, 815–828. https://doi.org/10.1586/eri.12.58 (2012).
Montoya, J. G. & Remington, J. S. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47, 554–566. https://doi.org/10.1086/590149 (2008).
Ben-Harari, R. R., Goodwin, E. & Casoy, J. Adverse event profile of Pyrimethamine-Based therapy in toxoplasmosis: A systematic review. Drugs R D 17, 523–544. https://doi.org/10.1007/s40268-017-0206-8 (2017).
Aspinall, T. V., Joynson, D. H. M., Guy, E., Hyde, J. E. & Sims, P. F. G. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J. Infect. Dis. 185, 1637–1643. https://doi.org/10.1086/340577 (2002).
Montazeri, M. et al. Drug resistance in Toxoplasma gondii. Front. Microbiol. 9, 2587. https://doi.org/10.3389/fmicb.2018.02587 (2018).
Silva, L. A., Reis-Cunha, J. L., Bartholomeu, D. C. & Vítor, R. W. A. Genetic polymorphisms and phenotypic profiles of sulfadiazine-resistant and sensitive Toxoplasma gondii isolates obtained from newborns with congenital toxoplasmosis in Minas gerais, Brazil. PLoS One. 12, e0170689. https://doi.org/10.1371/journal.pone.0170689 (2017).
Black, M. W. & Boothroyd, J. C. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64, 607–623. https://doi.org/10.1128/mmbr.64.3.607-623.2000 (2000).
Mamede, L., Ledoux, A., Jansen, O. & Frédérich, M. Natural phenolic compounds and derivatives as potential antimalarial agents. Planta Med. 86, 585–618. https://doi.org/10.1055/a-1148-9000 (2020).
Lucas, L. A. et al. Antiparasitic properties of Propolis extracts and their compounds. Chem. Biodivers. 18, e2100310. https://doi.org/10.1002/cbdv.202100310 (2021).
Soto-Sánchez, J. Bioactivity of natural polyphenols as antiparasitic agents and their biochemical targets. Mini Rev. Med. Chem. 22, 2661–2677. https://doi.org/10.2174/1389557522666220404090429 (2022).
Xiao, J., Gao, M., Diao, Q. & Gao, F. Chalcone derivatives and their activities against drug-resistant cancers: An overview. Curr. Top. Med. Chem. 21, 348–362. https://doi.org/10.2174/1568026620666201022143236 (2021).
Khan, S. A. & Asiri, A. M. Green synthesis, characterization and biological evaluation of novel chalcones as anti-bacterial agents. Arab. J. Chem. 10, S2890–S2895. https://doi.org/10.1016/j.arabjc.2013.11.018 (2017).
Xu, M., Wu, P., Shen, F., Ji, J. & Rakesh, K. P. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 91, 103133. https://doi.org/10.1016/j.bioorg.2019.103133 (2019).
de Oliveira, A. S. et al. Novel trypanocidal thiophen-chalcone cruzain inhibitors: Structure- and ligand-based studies. Future Med. Chem. 14, 795–808. https://doi.org/10.4155/fmc-2022-0013 (2022).
Trein, M. R. et al. Anti-Trichomonas vaginalis activity of chalcone and amino-analogues. Parasitol. Res. 118, 607–615. https://doi.org/10.1007/s00436-018-6164-4 (2019).
Rudrapal, M. et al. Chalcone scaffolds, bioprecursors of flavonoids: Chemistry, bioactivities, and pharmacokinetics. Molecules 26, 7177. https://doi.org/10.3390/molecules26237177 (2021).
Ni, L., Meng, C. Q. & Sikorski, J. A. Recent advances in therapeutic chalcones. Expert Opin. Ther. Pat. 14, 1669–1691. https://doi.org/10.1517/13543776.14.12.1669 (2004).
Oliveira, L. R. et al. Phenolic chalcones as agents against Trichomonas vaginalis. Bioorg. Chem. 141, 106888. https://doi.org/10.1016/j.bioorg.2023.106888 (2023).
Zheoat, A. M. et al. Antitrypanosomal and antileishmanial activity of Chalcones and flavanones from Polygonum salicifolium. Pathogens 10, 1–9. https://doi.org/10.3390/pathogens10020175 (2021).
Cuellar, J. E., Quiñones, W., Robledo, S., Gil, J. & Durango, D. Coumaro-chalcones synthesized under solvent-free conditions as potential agents against malaria, leishmania and trypanosomiasis. Heliyon 8, e08939. https://doi.org/10.1016/j.heliyon.2022.e08939 (2022).
Espinoza-Hicks, J. C. et al. Novel prenyloxy chalcones as potential leishmanicidal and trypanocidal agents: Design, synthesis and evaluation. Eur. J. Med. Chem. 167, 402–413. https://doi.org/10.1016/j.ejmech.2019.02.028 (2019).
González, L. A. et al. Effect of substituents in the A and B rings of chalcones on antiparasite activity. Arch. Pharm. (Weinheim) 353, e2000157. https://doi.org/10.1002/ardp.202000157 (2020).
dos Santos, A. T. L. et al. Synthesis of Chalcones and their antimicrobial and drug potentiating activities. Microb. Pathog. 180, 106129. https://doi.org/10.1016/j.micpath.2023.106129 (2023).
Satokata, A. A. C. et al. Chalcones With potential antibacterial and antibiofilm activities against periodontopathogenic bacteria. Anaerobe 76, 102588. https://doi.org/10.1016/j.anaerobe.2022.102588 (2022).
Si, H. et al. Licochalcone A: An effective and low-toxicity compound against Toxoplasma gondii in vitro and in vivo. Int. J. Parasitol. Drugs Drug Resist. 8, 238–245. https://doi.org/10.1016/j.ijpddr.2018.02.006 (2018).
Jiang, L. et al. Discovery and evaluation of chalcone derivatives as novel potential anti-Toxoplasma gondii agents. Eur. J. Med. Chem. 234, 114244. https://doi.org/10.1016/j.ejmech.2022.114244 (2022).
Martínez, A. F. F. et al. Leaf hydroalcoholic extract and oleoresin from Copaifera multijuga control Toxoplasma gondii infection in human trophoblast cells and placental explants from third-trimester pregnancy. Front. Cell. Infect. Microbiol. 13, 1113896. https://doi.org/10.3389/fcimb.2023.1113896 (2023).
de Fernandes, M. BjussuLAAO-II, an L-amino acid oxidase from Bothrops jararacussu snake venom, impairs Toxoplasma gondii infection in human trophoblast cells and villous explants from the third trimester of pregnancy. Microbes Infect. 25, 105123. https://doi.org/10.1016/j.micinf.2023.105123 (2023).
Teixeira, S. C. et al. Copaifera spp. oleoresins impair Toxoplasma gondii infection in both human trophoblastic cells and human placental explants. Sci. Rep. 10, 15158. https://doi.org/10.1038/s41598-020-72230-0 (2020).
Teixeira, S. C. et al. Polyalthic acid and oleoresin from Copaifera trapezifolia Hayne reduce Toxoplasma gondii growth in human villous explants, even triggering an anti-inflammatory profile. Exp. Parasitol. 250, 108534. https://doi.org/10.1016/j.exppara.2023.108534 (2023).
Barbosa, B. F. et al. IL10, TGF beta1, and IFN gamma modulate intracellular signaling pathways and cytokine production to control Toxoplasma gondii infection in bewo trophoblast cells. Biol. Reprod. 92, 82. https://doi.org/10.1095/biolreprod.114.124115 (2015).
Oliveira, J. G. et al. BeWo trophoblasts are unable to control replication of Toxoplasma gondii, even in the presence of exogenous IFN-γ. Placenta 27, 691–698. https://doi.org/10.1016/j.placenta.2005.06.006 (2006).
de Souza, G. et al. Trypanosoma Cruzi P21 recombinant protein modulates Toxoplasma gondii infection in different experimental models of the human Maternal–Fetal interface. Front. Immunol. 14, 1243480. https://doi.org/10.3389/fimmu.2023.1243480 (2023).
Teixeira, S. C. et al. Rottlerin impairs early and late steps of Toxoplasma gondii infection in human trophoblast cells and villous explants. Chem. Biol. Interact. 384, 110716. https://doi.org/10.1016/j.cbi.2023.110716 (2023).
Rosini, A. M. et al. LPS-mediated activation of TLR4 controls Toxoplasma gondii growth in human trophoblast cell (Bewo) and human villous explants in a dependent-manner of TRIF, Myd88, NF-Κb and cytokines. Tissue Cell. 78, 101907. https://doi.org/10.1016/j.tice.2022.101907 (2022).
da Silva, R. J. et al. Enrofloxacin and toltrazuril are able to reduce Toxoplasma gondii growth in human bewo trophoblastic cells and villous explants from human third trimester pregnancy. Front. Cell. Infect. Microbiol. 7, 340. https://doi.org/10.3389/fcimb.2017.00340 (2017).
de Araújo, T. E. et al. Experimental models of maternal-fetal interface and their potential use for nanotechnology applications. Cell. Biol. Int. 44, 36–50. https://doi.org/10.1002/cbin.11222 (2020).
Dubremetz, J. F., Garcia-Réguet, N., Conseil, V. & Fourmaux, M. N. Invited review apical organelles and host-cell invasion by apicomplexa. Int. J. Parasitol. 28, 1007–1013. https://doi.org/10.1016/S0020-7519(98)00076-9 (1998).
Zhu, W., Li, J., Pappoe, F., Shen, J. & Yu, L. Strategies developed by Toxoplasma gondii to survive in the host. Front. Microbiol. 10, 899. https://doi.org/10.3389/fmicb.2019.00899 (2019).
Hunter, C. A. & Sibley, L. D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778. https://doi.org/10.1038/nrmicro2858 (2012).
de Mello, T. F. P. et al. Ultrastructural and morphological changes in Leishmania (Viannia) Braziliensis treated with synthetic chalcones. Exp. Parasitol. 160, 23–30. https://doi.org/10.1016/j.exppara.2015.11.005 (2016).
Alonso, L. et al. Antileishmanial activity of the chalcone derivative LQFM064 associated with reduced fluidity in the parasite membrane as assessed by EPR spectroscopy. Eur. J. Pharm. Sci. 151, 105407. https://doi.org/10.1016/j.ejps.2020.105407 (2020).
Kwok, L. Y., Schlüter, D., Clayton, C. & Soldati, D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol. Microbiol. 51, 47–61. https://doi.org/10.1046/j.1365-2958.2003.03823.x (2004).
Barbosa, B. F. et al. Susceptibility to Toxoplasma gondii proliferation in bewo human trophoblast cells is dose-dependent of macrophage migration inhibitory factor (MIF), via ERK1/2 phosphorylation and prostaglandin E2 production. Placenta 35, 152–162. https://doi.org/10.1016/j.placenta.2013.12.013 (2014).
de Souza, G. et al. Cyclooxygenase (COX)-2 modulates Toxoplasma gondii infection, immune response and lipid droplets formation in human trophoblast cells and villous explants. Sci. Rep. 11, 12709. https://doi.org/10.1038/s41598-021-92120-3 (2021).
Milian, I. C. B. et al. Increased Toxoplasma gondii intracellular proliferation in human extravillous trophoblast cells (HTR8/Svneo line) is sequentially triggered by MIF, ERK1/2, and COX-2. Front. Microbiol. 10, 852. https://doi.org/10.3389/fmicb.2019.00852 (2019).
Castro-Filice, L. S. et al. Azithromycin is able to control Toxoplasma gondii infection in human villous explants. J. Transl Med. 12, 132. https://doi.org/10.1186/1479-5876-12-132 (2014).
Sommerville, C. et al. Biochemical and immunological characterization of Toxoplasma gondii macrophage migration inhibitory factor. J. Biol. Chem. 288, 12733–12741. https://doi.org/10.1074/jbc.m112.419911 (2013).
Harker, K. S., Ueno, N. & Lodoen, M. B. Toxoplasma gondii dissemination: A parasite’s journey through the infected host. Parasite Immunol. 37, 141–149. https://doi.org/10.1111/pim.12163 (2015).
Ghosh, S., Jiang, N., Farr, L., Ngobeni, R. & Moonah, S. Parasite-produced MIF cytokine: Role in immune evasion, invasion, and pathogenesis. Front. Immunol. 10, 1995. https://doi.org/10.3389/fimmu.2019.01995 (2019).
Nejad, M. R. et al. The evaluation of interleukin-8 chemokine in chronic and acute Toxoplasma gondii infection. Gastroenterol. Hepatol. Bed Bench. 4, 34–37 (2011).
Pereira, A. C. A. et al. Cyclooxygenase (COX)-2 inhibitors reduce Toxoplasma gondii infection and upregulate the pro-inflammatory immune response in Calomys callosus rodents and human monocyte cell line. Front. Microbiol. 10, 225. https://doi.org/10.3389/fmicb.2019.00225 (2019).
Chang, H. R., Grau, G. E. & Pechère, J. C. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology 69, 33–37 (1990).
Atmaca, N. & Atmaca, H. T. The correlation of TNF alpha levels with acute phase proteins in acute Toxoplasma gondii infection in mice. Exp. Parasitol. 239, 108311. https://doi.org/10.1016/j.exppara.2022.108311 (2022).
Onda, K. et al. Sofalcone upregulates the nuclear factor (erythroid-derived 2)-like 2/heme oxygenase-1 pathway, reduces soluble Fms-like tyrosine kinase-1, and quenches endothelial dysfunction: Potential therapeutic for preeclampsia. Hypertension 65, 855–862. https://doi.org/10.1161/hypertensionaha.114.04781 (2015).
Steegers, E. A. P., Von Dadelszen, P., Duvekot, J. J. & Pijnenborg, R. Pre-eclampsia. Lancet 376, 631–644. https://doi.org/10.1016/s0140-6736(10)60279-6 (2010).
Barbosa, B. F., Silva, D. A. O., Costa, I. N., Mineo, J. R. & Ferro, E. A. V. BeWo trophoblast cell susceptibility to Toxoplasma gondii is increased by Interferon-gamma, interleukin-10 and transforming growth factor-Β1. Clin. Exp. Immunol. 151, 536–545. https://doi.org/10.1111/j.1365-2249.2007.03583.x (2008).
Zeraik, M. L. et al. Identification of a prenyl chalcone as a competitive lipoxygenase inhibitor: Screening, biochemical evaluation and molecular modeling studies. Molecules 26, 2205. https://doi.org/10.3390/molecules26082205 (2021).
Passalacqua, T. G. et al. Synthesis and evaluation of novel prenylated chalcone derivatives as anti-leishmanial and anti-trypanosomal compounds. Bioorg. Med. Chem. Lett. 25, 3342–3345. https://doi.org/10.1016/j.bmcl.2015.05.072 (2015).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65, 55–63. https://doi.org/10.1016/0022-1759(83)90303-4 (1983).
Elazab, S. T. & Arafa, F. M. Anti-Toxoplasma activities of some Egyptian plant extracts: An in vitro study. Acta Parasitol. 67, 1800–1806. https://doi.org/10.1007/s11686-022-00633-2 (2022).
de Portes, J. A new type of pterocarpanquinone that affects Toxoplasma gondii tachyzoites in vitro. Vet. Parasitol. 186, 261–269. https://doi.org/10.1016/j.vetpar.2011.11.008 (2012).
Kian, D. et al. Trypanocidal activity of copaiba oil and kaurenoic acid does not depend on macrophage killing machinery. Biomed. Pharmacother. 103, 1294–1301. https://doi.org/10.1016/j.biopha.2018.04.164 (2018).
Costa, M. S. et al. Increased ROS generation causes apoptosis-like death: Mechanistic insights into the Anti-Leishmania activity of a potent Ruthenium(II) complex. J. Inorg. Biochem. 195, 1–12. https://doi.org/10.1016/j.jinorgbio.2019.03.005 (2019).
De Gomes, O. Effect of macrophage migration inhibitory factor (MIF) in human placental explants infected with Toxoplasma gondii depends on gestational age. Am. J. Pathol. 178, 2792–2801. https://doi.org/10.1016/j.ajpath.2011.02.005 (2011).
Vaidya, S., Walsh, S. W., Gerk, M. & S. & Application of human placental villous tissue explants to study ABC transporter mediated efflux of 2,4-dinitrophenyl-S-glutathione. Curr. Pharm. Biotechnol. 12, 814–823. https://doi.org/10.2174/138920111795470976 (2011).
Acknowledgements
The authors gratefully acknowledge the financial support by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, APQ-01629-22), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 305791/2022-2), and research productivity grant from Universidade do Estado de Minas Gerais (PQ/UEMG). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
B.F.B. and M.P. designed the experiments. M.P., L.L., G.S., A.R., M.O., D.S., J.J., N.S., R.O., I.D., G.F., M.B., O.S., V.J., T.F., and R.A. performed the experiments. M.P., L.R., A.G., S.T. and B.F.B. analyzed the data. B.F.B., L.R. and E.F. provided the reagents. M.P., S.T. and B.F.B. discussed the findings. B.F.B., S.T., L.R. and E.F. reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests and no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Paschoalino, M., Júnior, V.S., Soares, O.H.L. et al. Prenylated chalcone analog-mediated Inhibition of Toxoplasma gondii growth in human trophoblast cell line and villous explants. Sci Rep 15, 23539 (2025). https://doi.org/10.1038/s41598-025-08764-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08764-y