Abstract
Gastric cancer ranks among the most prevalent forms of cancer worldwide. Recent rapid advancements in diagnostic methods, neoadjuvant or adjuvant therapies, and surgical procedures have significantly improved survival rates for patients with gastric cancer. Nonetheless, these benefits have not yet reached the majority of individuals affected. Previous research has indicated that USP2, a component of the ubiquitin system, plays a crucial role in reshaping the proteome and enhancing the prognosis of diseases. However, the current understanding of USP2 expression and the associated pathways in gastric cancer remains unclear. The differential expression of USP2 was examined in pan-cancer, with a particular focus on its expression in gastric cancer cells and patients. Additionally, the impact of USP2 on the proliferation, migration, and apoptosis of gastric cancer cells was explored via CCK8, transwell, and invasion assays. RNA sequencing was employed to investigate pathways associated with USP2, and RT-qPCR and western blotting were utilized to confirm the expression of related pathway genes and proteins. The prognostic value of a model derived from USP2 expression was assessed and validated. USP2 expression was significantly reduced in gastric cancer cells and patient samples (p < 0.05). Patients with low USP2 expression are primarily associated with genetic variations, neoantigen loads, microsatellite instability (MSI) scores, and immune cell infiltration (p < 0.05). The overexpression of USP2 suppresses proliferation, migration, and cell cycle progression while enhancing apoptosis in GC cells. Concurrently, we identified 865 genes whose expression was downregulated. KEGG and GSEA enrichment analyses revealed significant suppression of the focal adhesion and ECM receptor interaction pathways following USP2 overexpression. A genomic model derived from USP2 was constructed and validated for its reliability in predicting patient prognosis. The expression of USP2 was positively correlated with sensitivity to small-molecule drugs, including entinostat, SB590885, and PF-562,271. USP2 acts as a negative regulator of gastric cancer progression. Consequently, USP2 has the potential to be utilized as a therapeutic target to improve the clinical prognosis and survival rates of patients.
Similar content being viewed by others
Introduction
The prognosis for patients with gastric cancer is closely linked to the stage at which it is diagnosed1. Surgery is still the main treatment for gastric cancer, but owing to the low rate of early diagnosis, most gastric cancers are already diagnosed in the middle to late stages2. Although several drugs, such as HER2 and PD-L13, have been marketed as therapeutic targets, some patients do not benefit from them. There is an urgent need to explore new therapeutic approaches to improve the prognosis of patients with gastric cancer4.
As a member of the USP (ubiquitin-specific protease) family, USP2 is located predominantly in the cytoplasmic and perinuclear regions of cells and is broadly distributed in human tissues5. In epithelial cells that have not undergone transformation, high levels of USP2 expression can confer oncogenic potential. To date, the majority of USP2 downstream targets identified are key oncoproteins, and its role in tumors appears to be predominantly procarcinogenic, primarily via the direct regulation of these downstream target proteins6. For example, USP2 interacts with early EGFR transporters and performs deubiquitination modifications, which prevent EGFR from being transported to lysosomes for degradation. This process enhances growth signaling and facilitates the malignant proliferation of tumor cells7. USP2 also influences the P53 signaling pathway by stabilizing MDM2 and MDM48 and modulates the activation of β-catenin in the Wnt/β-catenin signaling pathway9. Furthermore, USP2 hinders tumor cell apoptosis by deubiquitinating and stabilizing FASN10, a pivotal enzyme in fatty acid synthesis, and promotes tumor cell proliferation by deubiquitinating and stabilizing CyclinD111, a critical protein in the cell cycle. USP2 facilitates the binding of R-SMAD to the TGF-β receptor by detaching the K33-linked polyubiquitin chain from the receptor12. The deubiquitinating enzyme USP2 has been implicated in various processes. Its mechanism of action has been thoroughly investigated, yet the role of USP2 in gastric cancer remains unclear.
In this study, we focused our investigation on the influence of USP2 on gastric cancer. We investigated the effect of USP2 on gastric cancer cells through in vitro experiments, analyzed the underlying mechanisms via differential and functional enrichment of RNA sequencing data, and developed a differential genomic model to predict and confirm the survival and prognosis of cancer patients. We are confident that our results will offer deeper insights into USP2 research and assist in identifying new potential targets for the treatment of gastric cancer.
Materials and methods
Pancancer analyses
We aggregated gene expression data for gastric cancer from The Cancer Genome Atlas (TCGA) via the UCSC Xena database (https://xenabrowser.net/datapages/), which is available through the GDC data portal13. The dataset included transcriptomic data for 408 samples, presented in log2 (FPKM + 1) format, and was processed for data cleaning and compilation via the R package dplyr to derive a gene expression matrix. Furthermore, we downloaded methylation data and copy number variation (CNV) data from this database. We utilized Pearson’s correlation test to assess the associations between USP2 and the aforementioned factors in a pancancer analysis.
Clinical specimens, data sources and immunohistochemistry
Formalin-fixed paraffin-embedded samples, including primary cancer samples, corresponding to normal tissue adjacent to the cancer for immunohistochemistry (IHC), were obtained from 40 GC patients who underwent surgery between 2022 and 2023 at Henan Provincial Cancer Hospital (Ethics Review Number: 2020012). The tissue sections were incubated with a USP2 antibody (manufacturer: Abcam; catalog number: ab187881). The expression level of USP2 was determined by counting the percentage of positive immunoreactive cells and assessing the intensity of cell staining. The percentage of positive immunoreactive cells determined by USP2 IHC was defined as “0–25%”, “25–50%”, or “50–75%”. The intensity of USP2 IHC staining was defined as “no signal color in the cytoplasm, cytosol or nucleus and interstitium”, “pale yellow color in the cytoplasm, cytosol or nucleus and interstitium”, or “no signal color in the cytoplasm, cytosol or nucleus and interstitium”. The final IHC score is the product of the results of the two indicators. The IHC results were judged by “the positive cell score"*” the intensity of the staining color score”, with higher scores indicating higher antibody expression. All samples were reviewed by two independent and experienced pathologists who were blinded to the identities of the samples.
Cell line culture small-interfering RNA (siRNA) and overexpression of USP2
The human GC cell lines GES1, HGC27, MKN45, MGC-803, and SNU668 were procured from ATCC (Manassas, VA, USA) and subsequently cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. These cells were maintained in a dehumidified incubator (with 5% CO2) at a constant temperature of 37 °C.
We utilized polymerase chain reaction (PCR) technology to amplify the USP2 gene and clone it into a eukaryotic expression vector. For the transfection experiments, 1 × 105 HGC27 and MGC803 cells were inoculated into 6-well plates with the USP2 overexpression vector or empty vector, and the effect was validated via western blotting with an anti-human USP2 antibody. Negative controls (transfected with the empty vector) and positive controls (overexpressing USP2) were established. For transfection experiments, 1 × 105 HGC27 were inoculated in 6-well plates and then transfected with 10 nM siRNA-NC or 10 nM siRNA-USP2. Transfection was performed using the kit (Ribobio, Guangzhou 510663, China), and the transfection effect has been verified by western blot.
Cell experiments
Proliferation Assay: Cancer cell lines (HGC27 and MGC-803) were seeded into 96-well plates at a concentration of 8,000 cells per well. The following day, 100 µL of a 10% Cell Counting Kit-8 (CCK-8) solution was added to each well. The supernatant was then transferred to a new 96-well plate for incubation, and the optical density (OD) at 450 nm was measured via a microplate reader (Bomei Biotechnology, Nanning, China). Each experimental group utilized five replicate wells, and the results were based on two separate experiments.
Transwell Assays: For the migration assay, 2 × 104 cells were seeded into the upper chamber of the transwell insert and suspended in 200 µL of serum-free medium. The lower chamber was filled with 800 µL of basal medium supplemented with 20% fetal bovine serum (FBS). After a 24-h incubation period, the cells were analyzed via crystal violet staining.
Invasion Assay: The upper surface of the membrane was initially coated with a mixture of 40 µL of 1:8 diluted Matrigel and culture medium. Subsequently, 4 × 104 cells were suspended in 200 µL of serum-free medium, while 800 µL of basal medium containing 20% FBS was placed in the lower chamber. Following a 24-hour incubation at 37 °C, the cells were stained with crystal violet.
For the apoptosis assays, GC cells were dual-stained with Annexin V-FITC/PI via the eBioscience Annexin V apoptosis detection kit (88-8005-74; Thermo Fisher Scientific, USA). The cells were resuspended at a density of 1 × 106 cells/mL in binding buffer. A 100 µL aliquot of this suspension was combined with 5 µL of FITC Annexin V and 5 µL of PI in culture tubes. After a 15-minute incubation in the dark, 400 µL of binding buffer was added to each tube for analysis by flow cytometry. All experiments were performed in triplicate for three independent trials.
RNA sequencing and functional enrichment analysis
The RNA sample preparations utilized 1 µg of total RNA per sample as the input material. To generate sequencing libraries, we followed the manufacturer’s guidelines for the NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA), incorporating index codes to attribute sequences to individual samples. The index-coded samples were then clustered via a cBot Cluster Generation System with the TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s protocol. Following cluster generation, the library preparations were sequenced on an Illumina NovaSeq platform, resulting in the production of 150 bp paired-end reads. The raw reads were subsequently mapped to the hg38 human genome, and featureCounts v1.5.0-p3 was employed to determine the number of reads aligned to each gene.
The differential expression between HGC27-OE and HGC27-NC cells was evaluated via the DESeq2 package version 1.16.1. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of the DEGs were conducted via clusterProfiler R software14. Employing the gene set designated “c2.cp.kegg.v7.5.1.symbols.gmt” as a reference15, we utilized the gene set enrichment analysis (GSEA) algorithm to explore the disparities in activated pathways between HGC27-OE and HGC27-NC cells. Findings were considered statistically significant when the p value was less than 0.05.
Quantitative reverse-transcription polymerase chain reaction (qRT‒PCR) and Western blot (WB)
To extract total RNA, the cell lines were pretreated. cDNA was subsequently synthesized with the Prime Script RT Kit (TaKaRa, Dalian, China), which strictly adhered to the manufacturer’s guidelines. For quantitative reverse transcription polymerase chain reaction (qRT‒PCR), total RNA was isolated via the RNeasy Mini Kit (Qiagen). A 7500 real-time PCR system was utilized to conduct qRT‒PCR, thereby analyzing the expression levels of target genes (primers shown in Supplementary Table S1). Transcriptional levels of the GAPDH gene were used for normalization during statistical analysis. The relative mRNA expression levels of the target genes were determined via the CT method.
Human gastric cancer cell lines were harvested via RIPA buffer, and the protein concentrations were quantified via a BCA protein assay kit. Proteins were subsequently separated via SDS‒PAGE, transferred onto a membrane, blocked, and then incubated with primary and secondary antibodies (antibodies shown in the Supplementary Table S1).
The exploitation of a USP2-differential genomic prognostic model and nomogram
To identify USP2 differential genes, patients with STAD were divided into high- and low-USP2-expressing subgroups according to the median values of USP2 expression. The limma package16 for R was used to screen for differentially expressed genes (DEGs) between the two subgroups. The significance criteria for USP2-associated genes were set at |fold variation| >1.5 and adjusted P values < 0.05, and 434 DEGs were screened.
We first used univariate Cox regression analysis to initially screen 66 DEGs related to prognosis and then further screened them via the LASSO regression method. A total of 11 genes were ultimately entered into the multivariate Cox regression analysis and integrated as differential genomic models for USP2. The formula used to calculate the risk score for each sample was risk score=\(\:{\sum\:}_{k-1}^{n}\:{exp}_{gene}*{HR}_{gene}\), and the samples were divided into high- and low-risk groups on the basis of the median risk score. Risk score associations were plotted via the ggrisk package17, survival status and expression heatmaps of USP2-related signature genes on the basis of the risk score. Kaplan‒Meier curves for overall survival (OS) between the high- and low-risk subgroups were generated via the survivor and survminer packages, followed by a log-rank test. Thereafter, receiver operating characteristic (ROC) curves for 1-, 3- and 5-year survival were created via the survival ROC package18. In addition, the USP2 differential genomic prognostic model was externally validated in the GSE62254 cohort.
The relationships between clinical indicators (age, sex, stage, T, N, M and risk score) and OS in GC patients were assessed and validated via the TCGA and GSE62254 databases. The predictive power of the screened clinical prognostic models was similarly assessed via ROC curves and nomogram prediction models via multivariate analysis. Furthermore, calibration curves were used to assess the degree of agreement between actual survival probabilities and those predicted by the nomogram.
Prediction of drug response
The Genomics of Drug Sensitivity in Cancer (GDSC) initiative (https://www.cancerrxgene.org/) offers insights into drug sensitivity and is derived from approximately 75,000 experiments involving 138 anticancer agents tested across 700 different cancer cell lines19. The half-maximal inhibitory concentration (IC50), which indicates the drug response, was estimated via the pRRophetic package20. To identify potential drugs for the treatment of GC, we uploaded the up- and downregulated DEGs to the cMAP database21 and matched them to small molecule drugs. Three important small-molecule drugs and their enrichment scores are then listed. Correlation scores (−100 to 100) were obtained on the basis of the enrichment of DEGs in the reference gene expression profile. Small-molecule drugs with higher enrichment scores are more likely to reverse gene expression in GC.
Statistical analysis
Statistical analyses were conducted via R software (version 4.2.1; available at https://www.r-project.org/) along with its associated packages. Group comparisons were performed via the unpaired Student’s t test or the Mann‒Whitney test. A confidence interval of 95% was established; thus, p values less than 0.05 were considered statistically significant.
Results
Low expression of USP2 in gastric cancer cells and patients
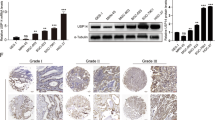
We conducted a pancancer analysis in TCGA to determine the expression of USP2, which revealed that USP2 was downregulated in almost all tumors, especially in LUAAD, COAD, COADREAD, BRCA, STES, KIRP, KIPAN, STAD, PRAD, HNSC, KIRC, LUSC, THCA, READ, BLCA and KICH (p < 0.05, Fig. 1A). To further elucidate the expression level of USP2 in gastric cancer, we found that the expression of USP2 was downregulated in tumor tissues (p < 0.001, Fig. 1B) in the TCGA and GEO databases and compared the expression of USP2 in gastric cancer and adjacent normal tissues. Moreover, the results were consistent in early gastric cancer tissues and paired adjacent normal tissues. We subsequently analyzed the mRNA and protein expression of USP2 in a gastric epithelial histiocytic cell line (GSE-1) and four gastric cancer cell lines (including HGC27, MKN45, MGC803 and SNU668) and found that the expression of these genes was significantly downregulated (p < 0.05, Fig. 1C and D) in gastric cancer cells, particularly in MGC803 and HGC27 cells. Finally, we also found that the IHC score of USP2 in 26 gastric cancer patients was lower than that in their paired tissues (p < 0.001, Fig. 1E). Considering the above results, we can conclude that USP2 is expressed at low levels in both gastric cancer cells and patients.
Low expression of USP2 in gastric cancer cells and patients. (A) The expression of USP2 in the TCGA pancancer and GEO datasets (B) and GEO datasets: GSE66229: 100 paratumorous tissues; 300 GC tissues; GSE208099: 8 M cancers (intramucosal gastric cancer), 8 SM cancers (submucosal invasion ≥ 500 μm), and 8 adjacent normal tissues (AN). The mRNA (C) and protein (D) expression of USP2 in four GC cell lines and a gastric epithelial histiocytic cell line. GC cell lines: MKN45, SNU668, HGC27, and MGC803; gastric epithelial histiocytic cell line: GSE-1. (E) Comparison of USP2 expression in carcinoma mucosa and paired primary GCs via immunohistochemistry; T: tumor; P: paratumorous tissue; scale bar: 200 mm.
Influence of the differential expression of USP2 on the genome and immune cell infiltration
In the preceding section, it was determined that USP2 is differentially expressed in both gastric cancer tissues and adjacent noncancer tissues. Utilizing the median expression level of USP2, we categorized TCGA-STAD patients into two groups: those with high and low USP2 expression. This stratification enabled us to delve deeper into the impact of the differential expression of USP2 on genetic variations and immune cell infiltration. Notably, the low-USP2 expression group was predominantly associated with missense mutations in TTN, TP53, and MUC16. (Fig. 2A). The tumor mutational burden (TMB) (p < 0.001, Fig. 2B) and neoantigen load (p < 0.001, Fig. 2C) were both greater in the group with low USP2 expression than in the group with high USP2 expression. Furthermore, tumor microsatellite instability (MSI) scores, as measured by the MANTIS score, differed significantly between the high and low USP2 expression groups (p < 0.001, Fig. 2D). In terms of immune cell infiltration, the low-USP2 expression group presented increased levels of CD8 T cells, activated memory CD4 + T cells, M1 macrophages, and neutrophils, whereas the levels of naive B cells, resting memory CD4 + T cells, regulatory T cells, and resting monocytes and mast cells were reduced (p < 0.05, Fig. 2E). In conclusion, the reduced expression of USP2 in gastric cancer tissues appears to be positively correlated with gene mutations and may enhance the immune infiltration of certain immune cell types.
Influence of differential expression of USP2 on genomic variation and immune cell infiltration in TCGA-STAD. Differences in gene variation (A), mutational load (B), neoantigen load (C), and MANTIS score: microsatellite instability (MSI) score. (D) Differences in immune cell infiltration (E) between patients with high and low USP2 expression.
Overexpression of USP2 significantly inhibits GC cell characteristics
To examine the impact of USP2 on gastric cancer cells, we engineered USP2-overexpressing (OE) cells and USP2-negative control (NC) cells from both the HGC27 and MGC803 gastric cancer lines. Compared with those in NC cells, the protein expression levels of USP2 were elevated in OE cells (Fig. 3A). Employing cell proliferation as a metric, the OD450 value reduced in both the HGC27 and MGC803 lines (p < 0.001, Fig. 3B), while rose with siRNA interference in HGC27 cells (p < 0.001, Supplementary Fig. 1 A). Furthermore, the migratory capacity of gastric cancer (GC) cells (HGC27 and MGC803) was notably diminished in both the wound healing (p < 0.001, Fig. 3C) and transwell (p < 0.001, Fig. 3D) assays upon USP2 overexpression or elevated in siRNA-HGC27 cells (p < 0.001, Supplementary Fig. 1B and C). Compared with that of the NC cells, the apoptosis rate of the GC cells significantly increased (p < 0.001, Fig. 3E) or decreased in siRNA-HGC27 cells (p < 0.001, Supplementary Fig. 1D). In conclusion, the overexpression of USP2 in gastric cancer cells not only diminishes their proliferation and migration capabilities but also enhances their degree of apoptosis, markedly suppressing tumor cell characteristics.
USP2 overexpression inhibits proliferation, migration, and the cell cycle and promotes apoptosis in GC cells. (A) The protein level of USP2 in HGC27 and MGC803 cells overexpressing USP2. (B) Cell proliferation analysis of HGC27 and MGC803 cells at different times (0 h, 24 h, 48 h, and 72 h). Wound healing assay (C) and transwell assay (D) in HGC27 and MGC803 cells overexpressing USP2. (E) Apoptosis of HGC27 and MGC803 cells overexpressing USP2.
Overexpression of USP2 inhibits focal adhesion and the ECM pathway
The RNAseq analysis revealed 2898 genes with differential expression in HGC27 cells overexpressing USP2 (OE cells) compared with normal control (NC) cells, with threshold of |fold-change|>2 and an adjusted P-value < 0.01. The volcano plot indicated that 2033 of these genes were upregulated, whereas 865 were downregulated (Fig. 4A, Supplementary Table S2).
Upon further investigation into their biological processes via GO analysis (Fig. 4B), we observed that the 865 downregulated genes were frequently linked to processes such as cell junction assembly, positive regulation of protein localization, axonogenesis, and cell-substrate adhesion, among others. KEGG pathway enrichment analysis revealed significant associations with cytoskeletal structures in muscle cells, focal adhesion, axon guidance, and ECM-receptor interactions (Fig. 4C, Supplementary Fig. S2). Correspondingly, the GSEA enrichment results demonstrated marked suppression of focal adhesion and ECM receptor interaction pathways following USP2 overexpression in HGC27 cells (nominal p-value < 0.001 and normalized enrichment score < −1.5; Fig. 4D). We stratified patients from the TCGA-STAD dataset into high and low USP2 expression groups and identified 434 differentially expressed genes (DEGs) (Supplementary Table S3). Subsequent GO and KEGG functional enrichment analyses also revealed enrichment in pathways related to focal adhesion and ECM receptor interactions (Supplementary Fig. S3).
A heatmap was generated to illustrate the expression of differentially expressed genes associated with the focal adhesion pathway (Fig. 4E, Supplementary Table S2), revealing a significant reduction in expression when USP2 was overexpressed. To substantiate the impact of USP2 on the focal adhesion pathway, we monitored the protein levels of FAK, p-FAK, AKT, p-AKT, Src, p-Src, ERK, and p-ERK in cells with overexpressing USP2 (OE cells). The findings indicated that FAK pathway protein levels were diminished in OE HGC27 cells, with a more pronounced decrease observed in MGC803 cells (Fig. 4F), and increased in siRNA-HGC27 cells (Supplementary Fig. 4E). To delve deeper into the effect of USP2 on ECM receptor interaction-related differentially expressed genes, we identified 10 genes that exhibited significant downward trend in both HGC27 OE cells and MGC803 OE cells (p < 0.05, Fig. 4G), and increased in siRNA-HGC27 cells (p < 0.05, Supplementary Fig. 4 F). Notably, USP2 appears to modulate the invasive and migratory potential of gastric cancer cells via the focal adhesion and ECM receptor interaction pathways.
USP2 overexpression inhibits focal adhesion and the ECM pathway in GC cells. (A) Volcano plot of RNA-seq data from the NC and OE groups of HGC27 cells. OE group: overexpression of USP2; NC group: control group transfected with empty plasmid. The results of the GO-BP (B) and KEGG (C) functional enrichment analyses of the downregulated genes. (D) GSEA enrichment plot of the focal adhesion and ECM receptor interaction pathways. (E) Heatmap of DEGs in the ECM receptor interaction pathway. (F) Protein levels of focal adhesion pathway proteins in HGC27 and MGC803 cells. (G) mRNA levels of DEGs in the ECM receptor interaction pathway in HGC27 and MGC803 cells.
Establishing a reliable USP2-differential gene prognostic model for predicting GC patients
We identified 434 DEGs (Supplementary Table S3) between the high and low groups on the basis of USP2 expression levels in the TCGA-STAD cohort, and a total of 66 significantly prognosis-related genes were obtained through univariate risk analysis (Supplementary Table S4). To obtain a USP2-derived prognostic model, we used LASSO regression analysis to define the final 11 related genes (Supplementary Fig. S4, Supplementary Table S4). On this basis, a multivariate Cox regression model was used to establish a risk score system with the formula: risk score = 0.005*\(\:{exp}_{\text{P}\text{T}\text{G}\text{I}\text{S}}\) − 0.0914*\(\:{exp}_{\text{F}\text{R}\text{Z}\text{B}}\) − 0.0383*\(\:{exp}_{\text{C}\text{X}\text{C}\text{L}3}\) + 0.0994*\(\:{exp}_{\text{S}\text{F}\text{R}\text{P}2}\) + 0.0038*\(\:{exp}_{\text{S}\text{L}\text{I}\text{T}3}\) − 0.014*\(\:{exp}_{\text{T}\text{M}\text{O}\text{D}1}\) − 0.0047*\(\:{exp}_{\text{C}\text{H}\text{R}\text{D}\text{L}2}\) + 0.0985*\(\:{exp}_{\text{S}\text{N}\text{C}\text{G}}\) + 0.2277*\(\:{exp}_{\text{R}\text{G}\text{S}5}\) − 0.1271*\(\:{exp}_{\text{C}\text{Y}\text{B}\text{R}\text{D}1}\) − 0.0969*\(\:{exp}_{\text{I}\text{T}\text{G}\text{B}4}\). By averaging, we divided the GC patients into high- and low-risk subgroups according to the median risk score, and overall survival was significantly greater in the high-risk subgroup than in the low-risk subgroup (p < 0.0001, Fig. 5A). The ROC curves confirmed that the USP2-differential genomic model had better validity in estimating the probability of OS at 1-, 3- and 5-years (0.632, 0.638 and 0.749, Fig. 5B, respectively).
Univariate Cox regression analysis revealed that age, stage, T stage, N stage, M stage, and the USP2 differential riskscore were significantly correlated with GC prognosis (Fig. 5C). Age and the USP2 differential riskscore emerged as independent prognostic indicators for GC (Fig. 5D). By integrating these three independent prognostic indicators, we utilized a nomogram to predict the survival outcomes of patients with GC. The USP2 differential risk score was found to contribute most significantly to the prediction of OS at 1-, 3-, and 5-years. The ROC curve further confirmed its desirable efficacy in predicting patient survival outcomes (Fig. 5E). Additionally, we assessed the predictive performance of the nomogram via calibration curves and demonstrated that the 1-year survival rates predicted by the nomogram closely aligned with the actual survival rates (Fig. 5F).
The USP2-differential genomic model was externally validated in the GSE66229, GSE26899 and GSE13861 cohort. Our findings indicate that risk scores are predictive of favorable OS outcomes (Fig. 6A, E, I). The area under the curve (AUC) values for the USP2-differential genomic model at 1, 3, and 5 years (0.608, 0.665, and 0.668, respectively) demonstrated the model’s accuracy in distinguishing between the high- and low-risk groups (Fig. 6B, F, J). Univariate and multivariate Cox regression analyses in the validation database identified the USP2 differential risk score as independent prognostic indicators for GC patients (Fig. 6C, D, G, H, K, L). In summary, our constructed USP2-differential genomic prognostic model exhibited robust performance in both the training and validation datasets.
Exploitation and establishment of a USP2 differential prognostic model for the prediction of GC patients in TCGA. (A) Kaplan-Meier curves of OS for high- and low-GC subgroups. (B) ROC curves for 1-, 3-, and 5-years OS outcomes in accordance with the USP2 differential risk score. (C) Univariate and multivariate (D) Cox regression analyses were conducted to determine the associations of clinical features and the USP2-differential risk score with GC survival outcome. (E) A prognostic nomogram was exploited by integrating independent prognostic indicators (age, sex, and the USP2 differential risk score) to estimate the 1-, 3-, and 5-years survival probabilities. (F) Calibration plots showing the associations of the predicted 1-year OS with the actual survival duration.
Verification of the prognostic significance of the USP2-differential genomic model with GSE66229, GSE26899 and GSE13861. (A-C) Kaplan-Meier curves of OS for high- and low- GC subpopulations. (D-F) Time-dependent ROC analysis of the USP2-differential genomic model showing the overall survival of patients with GC patients. Univariate (G-I) and multivariate (J-L) Cox regression models were used to determine the correlations between clinical characteristics and the USP2 differential riskscore.
Screening of small molecule drugs and assessment of drug sensitivity
Targeted therapies are pivotal in the management of gastric cancer (GC), and genetic alterations that contribute to the heterogeneity of GC have been linked to the efficacy of these treatments. However, reliable predictive biomarkers for these targeted therapies remain scarce. The findings of this study could aid in the development of novel GC-targeted drugs. We observed that the estimated IC50 values for bexarotene and lincitinib were notably lower in the high-risk group than in the low-risk group, indicating that the high-risk subgroup may be more sensitive to sunitinib, erlotinib, and lapatinib. Conversely, the low-risk group presented significantly lower estimated IC50 values for afatinib, suggesting a greater likelihood of response to this drug (Fig. 7A). We evaluated whether USP2 expression was correlated with drug sensitivity and utilized the cMAP database to identify small molecule drugs. By integrating these results, we selected four small-molecule drugs with absolute enrichment scores exceeding 90 for further demonstration (Fig. 7B). The expression of USP2 was significantly correlated with the sensitivity to entinostat, SB590885, and PF-562,271 (Fig. 7C). In the aforementioned experiments, the risk groups demonstrated differential sensitivity to various drugs, which could guide the selection of chemotherapy agents on the basis of USP2 expression in gastric cancer patients, thereby advancing the personalization of gastric cancer treatment.
Association of USP2 with drug sensitivity in gastric cancer. (A) Comparison of the estimated IC50 values of afatinib, erlotinib, lapatinib, and sunitinib between the high- and low-risk score GC subgroups. (B) Four small-molecule drugs with their corresponding enrichment fractions in cMAP. (C) Scatter plots displaying the correlation of USP2 expression with the drug sensitivity of four small-molecule drugs in gastric cancer.
Discussion
The ubiquitin–proteasome system (UPS) governs 80–90% of the degradation of intracellular proteins and plays a pivotal role in regulating cellular functions and preserving protein homeostasis. This system is integral to the regulation of numerous critical biological processes in eukaryotic cells, such as the cell cycle, stress response, signal transduction, transcriptional activation, and DNA repair22. Thus, blocking the UPS might offer a novel tactic for combating and overcoming chemotherapy resistance in cancerous tumors23. USP2, a unique representative of the USP family, has been revealed by studies to exhibit aberrant expression and to contribute to carcinogenesis in multiple malignant tumors24. However, evidence concerning the effects and mechanisms of USP2 in gastric cancer cells is still lacking. Therefore, the present study focused on the functional role of USP2 in gastric cancer. In this investigation, we performed a thorough examination of the molecular profile, tumorigenic function, and pharmacogenomic traits of USP2 within the context of a pancancer analysis, with a special focus on gastric cancer (GC).
Our data suggest that USP2 expression is markedly reduced in the majority of cancer types, including gastric cancer, as evidenced by immunoblotting and qPCR analyses in cells and immunohistochemistry in tissues. Nevertheless, conflicting observations have emerged regarding the validation of USP2 levels. Studies have indicated that USP2 expression is notably elevated in patients with bladder cancer25, ovarian cancer26, rectal cancer27, and prostate cancer28. Conversely, various studies have indicated that USP2 is notably diminished in tumor tissues or cells, including renal clear cell carcinoma29, nested cell lymphoma30, cutaneous T-cell lymphoma (CTCL)31, and glioblastoma12. This reduction is currently thought to be due primarily to the varying subtypes of tumors and the different isomers of USP2. USP2 expression is reduced in invasive ductal carcinoma but elevated in estrogen receptor-positive, progesterone receptor-positive, triple-negative breast cancer, as well as in distant metastasis sites32. USP2b is regarded as the primary subtype of USP2 in the liver33 and is recognized as the predominant isomer of USP2 in hepatic tissues. The expression of USP2 at both the mRNA and protein levels is notably lower in primary hepatocellular carcinoma (HCC) tumor tissues than in surrounding and normal liver tissues. The expression pattern of USP2b mirrors total USP2 expression34. In contrast, increased levels of USP2-AS1 are closely linked to a poorer prognosis35. Despite the widespread clinical application of HER2- and PD-L1 targeted therapies for the management of gastric cancer, the majority of patients remain resistant due to tumor heterogeneity or genetic mutations and thus continue to await the advent of novel therapeutic agents36. Our subsequent analysis revealed that the upregulation of USP2 is correlated with immune cell infiltration and factors influencing the immune microenvironment of cancer. Moreover, USP2 downregulation was found to be significantly correlated with gene mutations, tumor mutational burden (TMB), and microsatellite instability (MSI) across various cancer types. These findings suggest that USP2 may function as a predictive biomarker for immunotherapy efficacy. Collectively, these data underscore the pivotal role of USP2 in the carcinogenesis of gastric cancer.
The impact of USP2 on tumor cell division, proliferation, differentiation, and senescence has been substantiated by numerous studies. Correspondingly, our findings demonstrate that the overexpression of USP2 significantly suppresses proliferation, induces apoptosis, and reduces the invasive and migratory capacities of HGC27 and MGC803 gastric cancer cells. Qu et al.37. reported that USP2 overexpression upregulated MMP2, thereby facilitating the migration and invasion of breast cancer cells. In contrast, the silencing of USP2 markedly diminished these effects. Furthermore, the overexpression of USP2 has been shown to inhibit the proliferation, migration, and invasive potential of renal cancer cells (A498 and CAKi-1)29. Zhang et al.38. reported that knockout of USP2 promotes ubiquitin-mediated degradation of SKP2 and restrains the growth of lung cancer cells. Similarly, USP2 can deubiquitinate and stabilize the CCNA1 protein, thereby promoting the cell cycle progression of bladder cancer cells39, and deubiquitinate and stabilize FAS expression, inhibiting apoptosis in prostate cancer cells40. In summary, the manipulation of USP2 profoundly influences various characteristics of tumor cells, including proliferation, invasion, migration, and apoptosis.
To further elucidate the underlying mechanisms by which USP2 influences the characteristics of gastric cancer cells, our investigation revealed that the focal adhesion and ECM-receptor interaction pathways are downregulated in these cells, as evidenced by RNA-Seq analysis. This finding was corroborated by data from TCGA and validated through Western blot and RT-qPCR experiments. Extensive research has demonstrated that focal adhesion-mediated cell-ECM interactions are pivotal in tumor progression and invasion41,42. Focal adhesions are specialized sites on the cell membrane that facilitate connection to the extracellular matrix via integrin heterodimers43,44. These structures act as conduits for cell-ECM junctions, being recruited to adhesive plaques and undergoing autophosphorylation upon activation of integrin receptors by the ECM45,46. Notably, compared with their normal tissue counterparts, tumor cells exhibit a marked increase in the expression of core adhesion signaling receptors and specific integrin receptors47, a trend that was also observed in our data. And a study also shown that USP2 regulates T cell activation and epithelial cell ECM network repair in myeloid cells of ulcerative colitis48. Additionally, research indicates that USP2 expression increases in angiotensin II-induced neonatal rat primary cardiac fibroblasts. Furthermore, inhibition of USP2 suppresses CF proliferation, collagen synthesis, and cell cycle progression49. Based on these findings, we propose that USP2 may indirectly regulate focal adhesion and ECM-receptor interaction pathways.
Conclusion
In summary, the overexpression of USP2 appears to significantly impede the invasive and migratory potential of gastric cancer cells via the modulation of focal adhesion and ECM receptor interaction pathways, meriting further in-depth investigation.
Data availability
The datasets analyzed for this study can be found in the TCGA-STAD project (http://www.cancer.gov/tcga) and GEO (https://www.ncbi.nlm.nih.gov/geo/query). Original data referenced in the study are included in the article/supplementary materials, and further inquiries can be directed to the corresponding author.
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249 (2021).
Hoshi, H. Management of gastric adenocarcinoma for general surgeons. Surg. Clin. North. Am. 100 (3), 523–534 (2020).
Ilson, D. H. Advances in the treatment of gastric cancer. Curr. Opin. Gastroenterol. 36 (6), 525–529 (2020).
Högner, A. & Moehler, M. Immunotherapy in gastric Cancer. Curr. Oncol. 29 (3), 1559–1574 (2022).
Kitamura, H. & Hashimoto, M. USP2-Related cellular signaling and consequent pathophysiological outcomes. Int J. Mol. Sci 22(3). (2021).
Sacco, J. J., Coulson, J. M., Clague, M. J. & Urbé, S. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life. 62 (2), 140–157 (2010).
Jeong, P. et al. Assess the expression of ubiquitin specific protease USP2a for bladder cancer diagnosis. BMC Urol. 15, 80 (2015).
Wang, C. L. et al. Ubiquitin-specific protease 2a stabilizes MDM4 and facilitates the p53-mediated intrinsic apoptotic pathway in glioblastoma. Carcinogenesis 35 (7), 1500–1509 (2014).
Kim, J., Alavi Naini, F., Sun, Y. & Ma, L. Ubiquitin-specific peptidase 2a (USP2a) deubiquitinates and stabilizes β-catenin. Am. J. Cancer Res. 8 (9), 1823–1836 (2018).
Kim, J., Kim, W. J., Liu, Z., Loda, M. & Freeman, M. R. The ubiquitin-specific protease USP2a enhances tumor progression by targeting Cyclin A1 in bladder cancer. Cell. Cycle. 11 (6), 1123–1130 (2012).
Qie, S. & Diehl, J. A. Cyclin D degradation by E3 ligases in cancer progression and treatment. Semin Cancer Biol. 67 (Pt 2), 159–170 (2020).
Tu, Y. et al. Loss of deubiquitylase USP2 triggers development of glioblastoma via TGF-β signaling. Oncogene 41 (18), 2597–2608 (2022).
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38 (6), 675–678 (2020).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16 (5), 284–287 (2012).
Liberzon, A. et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell. Syst. 1 (6), 417–425 (2015).
Ritchie, M. E. et al. Smyth GK: Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 (7), e47 (2015).
Jiang, H., Xu, S. & Chen, C. A ten-gene signature-based risk assessment model predicts the prognosis of lung adenocarcinoma. BMC Cancer. 20 (1), 782 (2020).
Kamarudin, A. N., Cox, T. & Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med. Res. Methodol. 17 (1), 53 (2017).
Yang, W. et al. Genomics of drug sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 41 (Database issue), D955–961 (2013).
Geeleher, P., Cox, N. & Huang, R. S. pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS One. 9 (9), e107468 (2014).
Yang, K. et al. CMAP: complement map database. Bioinformatics 29 (14), 1832–1833 (2013).
Park, J., Cho, J. & Song, E. J. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch. Pharm. Res. 43 (11), 1144–1161 (2020).
Narayanan, S. et al. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist. Updat. 48, 100663 (2020).
Zhang, S. et al. Targeting the deubiquitinase USP2 for malignant tumor therapy (Review). Oncol Rep 50(4). (2023).
Xu, F. et al. The role of deubiquitinase USP2 in driving bladder cancer progression by stabilizing EZH2 to epigenetically silence SOX1 expression. Transl Oncol. 49, 102104 (2024).
Yang, Y. et al. [Differential expression of USP2, USP14 and UBE4A between ovarian serous cystadenocarcinoma and adjacent normal tissues]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 23 (6), 504–506 (2007).
Li, D., Bao, J., Yao, J. & Li, J. LncRNA USP2-AS1 promotes colon cancer progression by modulating Hippo/YAP1 signaling. Am. J. Transl Res. 12 (9), 5670–5682 (2020).
Janssen, E. M. et al. Analysis of patient preferences in lung Cancer - Estimating acceptable tradeoffs between treatment benefit and side effects. Patient Prefer Adherence. 14, 927–937 (2020).
Meng, X. et al. Downregulation of ubiquitin-specific protease 2 possesses prognostic and diagnostic value and promotes the clear cell renal cell carcinoma progression. Ann. Transl Med. 8 (6), 319 (2020).
Davis, M. I. et al. Small molecule Inhibition of the Ubiquitin-specific protease USP2 accelerates Cyclin D1 degradation and leads to cell cycle arrest in colorectal Cancer and mantle cell lymphoma models. J. Biol. Chem. 291 (47), 24628–24640 (2016).
Wei, T. et al. Ubiquitin-specific protease 2 decreases p53-dependent apoptosis in cutaneous T-cell lymphoma. Oncotarget 7 (30), 48391–48400 (2016).
Metzig, M. et al. An RNAi screen identifies USP2 as a factor required for TNF-α-induced NF-κB signaling. Int. J. Cancer. 129 (3), 607–618 (2011).
Gousseva, N. & Baker, R. T. Gene structure, alternate splicing, tissue distribution, cellular localization, and developmental expression pattern of mouse deubiquitinating enzyme isoforms Usp2-45 and Usp2-69. Gene Expr. 11 (3–4), 163–179 (2003).
Nadolny, C. et al. Dysregulation and activities of ubiquitin specific peptidase 2b in the pathogenesis of hepatocellular carcinoma. Am. J. Cancer Res. 11 (10), 4746–4767 (2021).
Chen, S. P. et al. LncRNA USP2-AS1 promotes hepatocellular carcinoma growth by enhancing YBX1-Mediated HIF1α protein translation under hypoxia. Front. Oncol. 12, 882372 (2022).
Stanton, S. E., Castle, P. E., Finn, O. J., Sei, S. & Emens, L. A. Advances and challenges in cancer Immunoprevention and immune interception. J Immunother Cancer 12(3). (2024).
Qu, Q. et al. USP2 promotes cell migration and invasion in triple negative breast cancer cell lines. Tumour Biol. 36 (7), 5415–5423 (2015).
Zhang, F., Zhao, Y. & Sun, Y. USP2 is an SKP2 deubiquitylase that stabilizes both SKP2 and its substrates. J. Biol. Chem. 297 (4), 101109 (2021).
Statement of removal. Cell. Cycle 14(5):784. (2015).
Graner, E. et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 5 (3), 253–261 (2004).
Shen, J. et al. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J. Exp. Clin. Cancer Res. 37 (1), 175 (2018).
Zhang, P. et al. CPNE8 promotes gastric Cancer metastasis by modulating focal adhesion pathway and tumor microenvironment. Int. J. Biol. Sci. 18 (13), 4932–4949 (2022).
Lee, B. Y., Timpson, P., Horvath, L. G. & Daly, R. J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 146, 132–149 (2015).
Sulzmaier, F. J., Jean, C. & Schlaepfer, D. D. FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer. 14 (9), 598–610 (2014).
Tan, X. et al. Focal adhesion kinase: from biological functions to therapeutic strategies. Exp. Hematol. Oncol. 12 (1), 83 (2023).
Lietha, D. et al. Structural basis for the autoinhibition of focal adhesion kinase. Cell 129 (6), 1177–1187 (2007).
Quispe, P. A., Lavecchia, M. J. & León, I. E. Focal adhesion kinase inhibitors in the treatment of solid tumors: preclinical and clinical evidence. Drug Discov Today. 27 (2), 664–674 (2022).
An, R. et al. USP2 promotes experimental colitis and bacterial infections by inhibiting the proliferation of myeloid cells and remodeling the extracellular matrix network. Cell. Insight. 1 (4), 100047 (2022).
Xu, Q. et al. Ubiquitin-specific protease 2 regulates Ang II-induced cardiac fibroblasts activation by up-regulating Cyclin D1 and stabilizing β-catenin in vitro. J. Cell. Mol. Med. 25 (2), 1001–1011 (2021).
Acknowledgements
YJL, JWW, and XL conceived, designed, and directed this study; JWW and XL performed the bioinformatics validation and experiments; XML, MKH and YCW analyzed the data; XL, KNL and JWW interpreted the data and wrote the manuscript; YJL, XBC, ZL and JWW revised the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
This study was funded by the Henan Provincial Middle-aged and Young People’s Health Science, Technology Innovation Excellent Youth Talent Project (YXKC2021053) and the Henan Medical Science and Technology Research and Development Program Project (SBGJ202402034).
Author information
Authors and Affiliations
Contributions
YJL, JWW, and XL conceived, designed, and directed this study; JWW and XL performed the bioinformatics validation and experiments; XML, MKH and YCW analyzed the data; XL, KNL and JWW interpreted the data and wrote the manuscript; YJL, XBC, ZL and JWW revised the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study was approved by the Institutional Review Board of the Affiliated Cancer Hospital of Zhengzhou University (Ethics Review Number: 2020012) and conformed to the provisions of the Declaration of Helsinki. Informed Consent: All patients provided written informed consent. Registry and registration no. of studies/trials: N/A. Animal studies: N/A.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Li, X., Lian, K. et al. The downregulation of ubiquitin-specific peptidase 2 indicates a poor prognosis and promotes the progression of gastric cancer through focal adhesion and ECM pathway signaling. Sci Rep 15, 33308 (2025). https://doi.org/10.1038/s41598-025-08881-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08881-8