Abstract
Antimicrobial resistance has caused tremendous loss of life, health, and economic property, and Enterobacteriaceae bacteria have been classified by the World Health Organization as one of the key pathogens for which drug development is urgently needed. There is a lack of in-depth research on spatial effect analysis of Escherichia coli (E. coli) by researchers. The study was analyzed using spatial effect analysis with data from the China Antibiotic Resistance Surveillance System covering the prevalence of third-generation cephalosporins-resistant E. coli (3GCREC), carbapenem-resistant E. coli (CREC) and quinolone-resistant E. coli (QREC) in 30 provinces during 2014–2023. Spatial Durbin model and effect decomposition were used to determine the spatial effects of E. coli and their influencing factors, including Ambient temperature, Particulate matter (PM2.5), Precipitation, Absolute Humidity, Hospital beds, Physicians, Health facility, and GDP per capita. 3GCREC, CREC, and QREC showed significant spatial autocorrelation and regional differences (p < 0.001). The results of the time-fixed spatial Durbin model showed that ambient temperature, PM2.5, hospital beds, and healthcare facilities had significant effects on antimicrobial resistance of different E. coli strains with spatial spillover effects. Direct and indirect effects of ambient temperature, PM2.5, and healthcare facilities on CREC and QREC were determined by effect decomposition. The prevalence of different E. coli strains exhibits spatial autocorrelation, with provinces in close geographical proximity often having more similar resistance rates. More attention should be paid to areas with higher E. coli prevalence and interregional dynamics. The effects of ambient temperature, PM2.5, hospital beds, and healthcare facilities on E. coli prevalence should be fully recognized.
Similar content being viewed by others
Introduction
Escherichia coli (E. coli) is one of the causes of the highest number of deaths due to antimicrobial resistance (AMR)1, and E. coli has been labeled by WHO as one of the key pathogens requiring urgent drug development2,3. China is the world’s second-largest consumer of antibiotics and one of the countries in the world with serious problems of antibiotic abuse and AMR4,5,6. According to the China antibiotic resistance surveillance system (CARSS), the resistance rate of 3GCREC has increased from more than 10% in 2005 to 48.9% in 2023; the resistance rate of QREC is even more than 50% and shows high spatial and temporal heterogeneity7.
Many studies of E. coli have shown the dangers of their presence8,9,10. Abdullah conducted a systematic review of the current status of global antibiotic resistance and its influencing factors, discussed the impact of environmental E. coli on water quality and public health, and described the biological and non-biological factors affecting the growth and survival of E. coli11. In recent years, there has been a growing body of research on AMR in China11,12,13. One notable study is WB Li’s research on AMR in 101 countries. The study explored the impact of socioeconomic and environmental factors on AMR and revealed that the gap in AMR between high-income countries and low- and middle-income countries is widening under different socioeconomic conditions13. In another ecological study, WB Li investigated the impact of rising ambient temperature on AMR in E. coli in China. The findings revealed that for every 1℃ increase in ambient temperature, the resistance of 3GCREC, CREC, and QREC increased by 2.71%, 32.92%, and 1.81%, respectively11. However, to date, no studies have conducted spatial effect analyses of AMR from a spatial perspective, and the regional distribution of high-resistance areas and their impact on adjacent provinces remain unexplored.
Theoretically, bacterial activity is inextricably linked to temperature14. Researchers found that E. coli and Staphylococcus aureus have significantly different sensitivities to antibiotics at different temperatures15,16. Rodriguez found that higher minimum temperatures were associated with increased antibiotic resistance. For every 10 °C increase in temperature across regions, antibiotic resistance increased by approximately 5%, 3.5%, and 3% for E. coli, Klebsiella pneumoniae, and S. aureus, respectively17. Another study in the United States showed that a 10 °C increase in minimum temperature across the region was associated with a 4.2% increase in antibiotic resistance in E. coli18.
Health outcomes are known to be influenced by common development indicators. Collignon showed that the proportion of antibiotic-resistance was positively correlated with the ratio of private to public health expenditure and with rising temperatures19. Overcrowded living conditions and low incomes have also been mentioned as being associated with a high prevalence of antibiotic resistance bacterial infections20,21. Evidence shows that gross domestic product (GDP) per capita, out-of-pocket healthcare expenditures, and physician density are associated with antibiotic resistance in 3GCREC, Staphylococcus aureus, and Klebsiella pneumoniae22,23. The correlation between antibiotic resistance and environmental factors should not be overlooked24. Fisman found that humidity, monthly precipitation, and temperature were all associated with gram-negative bacterial bloodstream infections in hospitalized patients25. In health care, the quality of governance, education and health facilities is closely linked to inter-regional differences in antibiotic resistance and antibiotic consumption patterns26,27,28.
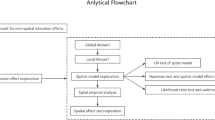
As we know, this study is the first time used panel data to construct a spatial econometric model of AMR in E. coli in China. From the perspective of regional differences, we conducted a spatial effect analysis to explore its spatial distribution characteristics and clustering patterns. We used spatial effect analysis to estimate the effects and spatial correlations of environmental, health service, and socioeconomic factors on the AMR in E. coli, as well as their spatial correlations, to fill the gap in this field and provide theoretical support and scientific basis for controlling the growth of AMR in China.
Results
Descriptive statistics
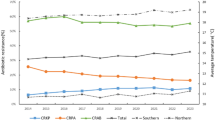
We conducted a descriptive analysis of the prevalence of 3GCREC, CREC, and QREC from 2014 to 2023. The average annual detection rate of drug-resistant bacteria for 3GCREC and QREC is around 50%, and the CREC is lower, with an overall distribution of less than 2%. Table 1 shows the detailed data. Figure 1 shows the detection rates of 3GCREC, CREC, and QREC in mainland China in 2023. We used the natural breakpoint method to classify them and labelled them with progressive colours.
Unadjusted linear analysis showed a linear relationship between temperature and the detection rate of drug-resistant bacteria, and divided China into two regions based on geographical location: the Southern and Northern, and the linear relationship and distribution are shown in Figs. 2, 3 and 4. CREC showed a stable positive relationship with temperature (ρ = 0.205, p < 0.001), and QREC showed a significant negative relationship with temperature (ρ = − 0.525, p < 0.001).
Table 2 demonstrates the independent, dependent, and control variables of this study. The average values of GCREC and QREC are 0.53 (0.41–0.74) and 0.51 (0.42–0.68), respectively, and CREC is 0.02 (0.01–0.06). The average ambient temperature is 14.9 ℃ (5.0–25.8), and the annual average concentration of PM2.5 is 45 (13–124).
Panel test results for non-spatial interaction effects
We conducted a panel data regression analysis of the independent and dependent variables. Because the detection rates of 3GCREC, CREC, and QREC are data in percentages, they are processed by taking the natural logarithm.
The model results indicate that ambient temperature has a significant impact on 3GCREC (coefficient = − 0.004, p < 0.01), CREC (coefficient = 0.047, p < 0.001), and QREC (coefficient = − 0.01, p < 0.001). Additionally, the model results indicate that PM2.5, Precipitation, Humidity, and Hospital beds capacity have significant effects (p < 0.05). The multicollinearity problem is defined as variance inflation factor (VIF) > 10, and the regression results show that it does not have this problem, complies with the basic assumptions of regression analysis (Table 3).
Global moran’ index
The global Moran’ I is generally used to assess the autocorrelation of spatial panel data across the study area. As shown in Table 4, the global Moran I indices of QREC were all significant (p < 0.001), whereas the spatial autocorrelation of CRECs for 2016–2023 is also significant; there is significant spatial autocorrelation for some years of the 3GCREC.
Local moran’ index
The local Moran’ Index assesses the local spatial autocorrelation between each spatial cell and its neighbors. It can help identify spatial clusters and outliers. The Moran’s I scatter plot of 3GCREC, CREC, and QREC show that most of the cities are concentrated in Quadrant 1 and Quadrant 3. This trend becomes more obvious with the passing of the year, showed significant correlation (p < 0.05). The Moran’s I scatter plots for 3GCREC, CREC, and QREC from 2014 to 2023 are provided in the Supplemental File.
Local Indicators of Spatial Association (LISA) map is a visualization tool for revealing the association patterns between local areas and their neighbors in spatial panel data, Among them, “high–high” clustering and “low–low” clustering refers to areas where high/low value observation points are surrounded by other high/low value observation points, while “high–low” and “low–high” outliers indicate obvious anomalies in the prevalence rates of neighboring areas. Figures 5, 6 and 7 shows that 3GCREC, CREC, and QREC have different aggregation characteristics. 3GCREC shows a scattered distribution of clusters, the “high-high” aggregation phenomenon gradually changes from East China to Northeast China; CREC is more aggregated in Northwest China and Eastern coastal provinces, such as “high–high” aggregation phenomenon in Xinjiang and Qinghai, and “low–low” aggregation phenomenon in Eastern coastal areas, and its distribution is more stable; the distribution of QREC agglomeration is relatively stable, with “high–high” aggregation phenomenon in Northeast China and Shandong Province, and obvious “low–low” agglomeration in Southwest China and South China.
Spatial regression model exploration
Lagrange multiplier test
The study first conducted a spatial panel data analysis of 3GCREC, CREC, and QREC, conceptualising spatial relationships using an inverse distance method, assigning spatial matrix values using the Euclidean distance method, and standardising the data. Based on the Lagrange multiplier (LM) test results, we ultimately chose SDM to capture the spatial effects of 3GCREC, CREC, and QREC. When the analysis results pass the LM test, this indicates significant spatial autocorrelation in the model, and the error term rather than the dependent variable itself is more likely to be affected by spatial autocorrelation. The robust LM test model results for 3GCREC, CREC, and QREC showed that 3GCREC and QREC present significance. Therefore, the LM test results indicate that for 3GCREC, CREC, and QREC, the corresponding SDM, SLM, and SDM should be selected for further analysis, respectively. Although the LM-robust test of QREC was not significant, taking into account the LM test, the model’s explanatory power (R2), and the broader applicability of the SDM compared to SLM and SEM, SDM has still been chosen to capture the effect of spatial of QREC (Table 5).
Likelihood ratio test
LR test is a commonly used statistical test to assess the fit of a model or to test the constraints of a model. When the LR test is significant, SDM cannot degenerate into SEM or SAM. When both tests are significant, or both are not, it can be judged by comparing the magnitude of their chi-square statistics. The results of the LR test showed that both LR-SAM and LR-SEM results were significant for 3GCREC and QREC, and SDM cannot degenerate to SEM or SAM (p < 0.05). Therefore, the LR test results support the use of the SDM model for subsequent analysis of 3GCREC, CREC, and QREC (Table 6).
Hausman test
After completing the estimation of the spatial regression model, the appropriateness of the model selection can be determined using the Hausman test. This test centers on testing for systematic differences in the estimation results of the fixed effects (FE) model versus the random effects (RE) model to determine which model is more appropriate for the data analysis.
Although the Hausman test supports the suitability of QREC for random effects modeling, a comparison can still be attempted using an FE Model. The results show that 3GCREC and QREC are more suitable for the time fixed Effect. In addition, academics believe that scholars mostly choose fixed effects for analysis even when the Hausman test results are insignificant, so this study still chooses the time fixed-SDM model for spatial effect analysis of 3GCREC and QREC (Table 7).
Empirical analysis
Analysis of model results
Through exploration of spatial regression models, we ultimately selected the SDM model for analysis. We also selected the time fixed effect in fixed-effects analysis for subsequent spatial effect analysis of 3GCREC and QREC to control for heterogeneity caused by unmeasured confounding factors. As shown in Table 8, the analytical results of the time-fixed SDM model show that the effects of Temperature, PM2.5, Hospital beds, and Health facilities on 3GCREC, CREC, and QREC reached a significant level.
The spatial values of 3GCREC did not reach a significant level, while CREC and QREC showed significant positive spatial spillover effects (p < 0.05), indicating a positive impact on surrounding areas.
Results of Spatial effect decomposition
If the SDM is used, it is necessary to decompose the direct effect (local area impact), indirect effect (spatial spillover effect), and total effect. Table 9 presents the results of the decomposition of spatial effects on 3GCREC, CREC, and QREC.
For 3GCREC, local PM2.5 (Coef. = 0.002, p < 0.001), precipitation (Coef. = 0.001, p < 0.05), hospital beds (Coef. = 0.003, p < 0.01), and medical facilities (Coef. = 0.005, p < 0.01) dominate the total effect (total effect = direct effect + indirect effect), and the spillover effect of surrounding PM2.5 on 3GCREC is not significant (p > 0.05).
For CREC, the overall effect exhibits a coexistence of ‘local positive drive and peripheral negative inhibition.’ Local temperature (Coef. = 0.042, p < 0.001), PM2.5 (Coef. = 0.003, p < 0.001), precipitation (Coef. = 0.002, p < 0.05), and hospital beds (Coef. = 0.017, p < 0.001) offset by the negative spillover effects of surrounding ambient temperature and Health facilities. Attention should be paid to interregional resource competition.
For QREC, the overall effect is primarily influenced by the negative spillover effects of surrounding ambient temperature and medical facilities, indicating that an increase in surrounding ambient temperature (Coef. = − 0.039, p < 0.001) and improved Health facilities (Coef. = − 0.034, p < 0.001) may exert a significant inhibitory effect on local QREC, while the positive spillover effect of PM2.5 (Coef. = 0.005, p < 0.01) is relatively weak.
Discussion
In this study, we conducted the first spatial panel data analysis of the AMR in E. coli in China. By constructing SDM, the spatial distribution of the AMR in E. coli was explored in depth from both the overall and local dimensions, and the effects of environmental factors, socioeconomic factors, and health service factors of the AMR in E. coli were considered. We identified significant spatial autocorrelation and spatial spillover effects among E. coli strains. From the dispersed distribution of 3GCREC resistance to the differential East–West distribution of CREC to the differential North-South distribution of QREC, different strains of E. coli exhibit different distribution patterns, and there is significant regional heterogeneity. These findings provide additional support to the existing evidence revealed in previous studies18,29,30.
Temperature has a significant effect on AMR in E. coli, with CREC and QREC showing a significant negative impact on neighboring regions. Most pathogenic bacteria (e.g., E. coli, Klebsiella pneumoniae) grow best at temperatures between 20 and 37 ℃. Southern China has high average temperatures throughout the year, and extreme heat in summer (often exceeding 35 ℃) may exceed the physiological tolerance range of these bacteria, easily triggering the direct inhibitory effect of high temperatures on drug-resistant bacteria31,32. In contrast, the average temperatures in Central and Northern China are relatively low, and the drug resistance rates are high, showing a clustered distribution. The effects were equally validated in cross-seasonal and cross-regional analyses29,30. In North America, Europe, the Middle East, Australia, and Asia, infection rates were higher in the summer months29,33,34,35. It has been suggested that temperature or seasonality may influence environmental growth and horizontal gene transfer of resistant strains10,36. Perhaps this can explain the role of temperature-responsive genes in antibiotic responses from a microscopic perspective37,38.
Environmental factors such as Precipitation and PM2.5 have a significant impact on AMR in E. coli. In neighbouring regions, PM2.5 has a significant positive impact on CREC, which may promote an increase in CREC prevalence. The indirect effect of PM2.5 on AMR in E. coli was found by effect decomposition. This is consistent with the findings of other researchers39. Pollutants in particulate matter (e.g., PM2.5) have been shown to contain a wide range of antibiotic-resistant bacteria and antibiotic-resistant genes40. PM2.5 increases the permeability of cell membranes, improves the efficiency of lateral gene transfer, and accelerates the evolution and exchange of antibiotic components in bacterial pathogens41,42,43, which may lead to an increase in antibiotic resistance44. The North China and Northeast regions are areas of China with relatively developed heavy industry, and air pollution, especially PM2.5, is more severe than in other regions11. Airborne pollutants are transferred to the ground with precipitation, thereby affecting the probability of human exposure.
In the effect decomposition, the direct impact of precipitation on 3GCREC, CREC, and QREC reached a significant level. Same as further studies, Yin et al. found that the middle and lower reaches of urban rivers were contaminated with more antibiotic-resistant genes than the headwaters, whereas no significant spatial distributional differences were found in soil samples because water bodies are more susceptible to human activities than soil45. In Vellore, India, children living in flood-prone urban informal settlements have recently been shown to be significantly more susceptible to enteric infections during rainfall events46. However, it has been shown that antibiotic resistance also varies with humidity and precipitation12 and that the concentration of E. coli cultured in rainwater discharge is ten times higher in wet weather than in dry weather47.
Adequate healthcare resources and services may help mitigate the potential harm of E. coli through good hygiene and infection control practices48. In our spatial effect analysis of AMR in E. coli,, we verified the important influence of healthcare facilities on AMR in E. coli and also emphasized the negative impact of AMR in E. coli in adjacent regions. An increase in the number of healthcare facilities inevitably leads to an increase in antibiotic use49,50, and hospitals are also a major site for the spread of antibiotic resistance51. Some regions (East China and North China) have advanced and concentrated medical resources, and population movement between neighbouring regions also creates conditions for the accumulation and distribution of E. coli resistance. While an increase in healthcare facilities may enhance antibiotic accessibility, the absence of standardized management can lead to the misuse of antibiotics for prophylactic purposes (using antibiotics for the common cold), thereby accelerating the development of drug-resistant bacteria13. In the years following the new healthcare reform, China lacked attention to the issue of antibiotic resistance, with severe antibiotic overuse in hospitals and inadequate implementation of pathogen control and infection prevention measures, leading to an increase in antibiotic resistance. With the development of hospitals, improved management systems, and strengthened public health campaigns in urban areas, resistance to E. coli has gradually been brought under control4,11.
Higher economic levels often mean better infrastructure, higher levels of governance, and more healthcare spending, but also more antibiotic use19,23,52,53,54. However, our results did not find a significant effect of GDP per capita on 3GCREC, CREC, and QREC. The study included OOP metrics in the preparatory phase to explore the effect on AMR in E. coli. Still, none of the results found an influential relationship between socioeconomic factors and AMR in E. coli. The reason for this result may be that the dependent variables mainly come from the test data of patient samples from secondary and tertiary public hospitals, which may weaken the impact of socioeconomic factors on AMR in E. coli. Future research based on data from rural hospitals may further reveal the relationship between them. China is a vast country with varying socioeconomic development and healthcare resources, as well as different antibiotic prescribing habits and resistance patterns12.
Certain limitations must not be overlooked. First, the limitations in obtaining variable data mean that this study cannot cover longer continuous time periods, and there is a lack of comprehensive control variable data to explain these relationships. Unmeasured confounding factors or ecological studies may influence the causal relationships between variables, thereby reducing the credibility of this study. Second, provincial-level data have a higher data granularity, which may increase random errors and data bias. The data for the dependent variable primarily come from secondary and tertiary hospitals and do not include primary healthcare institutions. Additionally, the lack of specific data on antibiotic use results in a certain degree of underrepresentation. Finally, this survey focused solely on 3GCREC, CREC, and QREC; further research is needed to comprehensively understand the resistance of other common antibiotic-resistant bacteria.
Method
Data sources
According to the National Bacterial Resistance Surveillance Report published annually by the CARSS (https://www.carss.cn)7, the detection rates of 3GCREC, QREC, and the resistance rate of Escherichia coli to carbapenems (CREC) were obtained from 2014 to 2023 to represent their AMR. The report began reporting in 2014, with 10 years of data reported to date. Hospitals involved in the report include those from 31 provinces, municipalities, and autonomous regions in China. We did not consider Tibet because CARSS did not report the detection rate of CRGN regarding Tibet from 2014 to 2016; Hong Kong, Macau, and Taiwan Province were also not included in our study. The 2014–2023 panel dataset used in this study had 900 observations. It contains AMR of three types of E. coli from 30 provinces and regions in China.
In 2014, CARSS membership included 1,429 hospitals from 31 provinces, cities, and autonomous regions across the country, of which 1,334 hospitals reported data. By 2023, membership in the CARSS core network has grown to 2,000 hospitals, of which 1,997 have reported data. Among the member units reporting data are 581 secondary hospitals and 1416 tertiary hospitals. After data review, 1,941 hospitals were included in the data analysis, of which 565 (29.1%) were secondary hospitals and 1,376 (70.9%) were tertiary hospitals (Table 10).
Variables
Dependent variables
E. coli is a gram-negative bacillus that lives mainly in the intestinal tract of humans and animals and is part of the normal intestinal flora. With the widespread use of antibiotics,Escherichia coli has become increasingly resistant9. Many strains of Escherichia coli have become resistant to a variety of commonly used antibiotics, and even the emergence of superbugs resistant to carbapenems and other antibiotics of the “last line of defense” has brought great challenges to clinical treatment9.
The prevalence of 3GCREC, CREC, and QREC was determined according to the annual detection rate with E. coli as the dependent variable in this study. In the WHO 2024 Bacterial Priority Pathogens List, 3GCREC is a critical priority, underscoring the need for targeted policies and interventions against this threat3. CREC was included in the WHO’s list of bacteria urgently needing new antibiotics in 2017 at the critical highest priority level55. Carbapenem antibiotics are important for the treatment of serious infections and are considered one of the last resort. The emergence of CREC has made the treatment of infections extremely difficult. QREC can form biofilms, which increases their ability to survive in the environment and resistance to disinfectants, making prevention and control difficult. The treatment of QREC infections is difficult and lengthy, consuming many healthcare resources and prevention and control costs.
Independent variables
The average ambient temperature of the provinces, municipalities and autonomous regions was used as the independent variable in this study. These data are obtained from the China Statistical Yearbook 2015–2024 (https://www.stats.gov.cn/sj/ndsj/)56.
Control variables
Previous studies have shown that socioeconomic conditions, health services, and environmental factors are related to the growth of AMR9,57. This study selected 7 indicators, including particulate matter (PM2.5), precipitation, absolute humidity, hospital beds, physician, healthcare facility, and GDP per capita (GDP) as control variables based on the summarization of the relevant literature and research29,39,43,58. The data for each control variable were obtained from the 2015–2024 China Statistical Yearbook56 (Table 11; Fig. 8).
Spatial regression model
Based on the theory of spatial econometrics, the preliminary model includes:
(1) Spatial Lag Model (SLM)
ρrepresents the spatial autocorrelation coefficient. W denotes the spatial weight matrix. W(DR) is the spatial lag term of the dependent variable DR. X is the matrix that includes the independent variables PM2.5, precipitation, absolute humidity, hospital beds, physician, healthcare facility, and GDP. β are the regression coefficients for the independent variables. \(\epsilon\) is the error term.
(2) Spatial Error Model (SEM).
u is the error term. λ is the spatial error coefficient. Wu is the spatial lag of the error term. µ is the disturbance term.
(3) Spatial Durbin Model (SDM)
ρ and θ are the coefficients for the spatial lags of the dependent variable and the independent variables, respectively. W(DR) and W(X) are the spatial lag terms for the dependent variable and the independent variables, respectively.
Statistical methods
We used SPSS 25.0 to collect detailed data on the corresponding dependent, independent, and control variables and analyzed the data descriptively. Stata 17.0 was used to calculate Moran’s I index and Moran’s scatter plot for the spatial autocorrelation test for 3GCREC, CREC, and QREC. The appropriate spatial regression model was selected using the Lagrange multiplier test, likelihood ratio test, and Hausman test. Distribution maps and LISA plots were drawn using ArcGIS Pro.
Conclusion
This study employed spatial econometric models to conduct spatial panel data analysis and spatial durbin model analysis on E. coli. In recent years, AMR in E. coli has been on the rise in China, different strains exhibit different spatial clustering patterns, and provinces geographically close to one another often exhibit more similar resistance rates. Additionally, the study identified that temperature, PM2.5, precipitation, hospital bed availability, and the distribution of medical facilities may influence AMR and potentially generate cross-regional spillover effects. The report also noted that provinces in the Northeast, North China, and Central China regions exhibit significantly higher resistance rates. Policymakers should pay more attention to regions with high AMR in E. coli and the dynamics between regions. It is necessary to develop joint prevention and control strategies for provinces with high antibiotic resistance, such as Shandong, Henan, and Hubei, and strengthen policy coordination between adjacent regions in order to control and reduce AMR in China.
Data availability
Data Availability Statement: All data are publicly available on the official website. For full access to data needs, please contact Prof. Xinping Zhang to get it. Email: xpzhang602@hust.edu.cn.
References
GBD 2021 AMR Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404 (10459), 1199–1226 (2024).
Jesudason, T. WHO publishes updated list of bacterial priority pathogens. Lancet Microbe. 5 (9), 100940 (2024).
WHO. WHO bacterial priority pathogens list. : Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. World Health Organization. 2024. (2024).
Liang, C. et al. Trends and correlation between antibacterial consumption and carbapenem resistance in gram-negative bacteria in a tertiary hospital in China from 2012 to 2019. BMC Infect. Dis. 21 (1), 444 (2021).
Tang, Q. et al. Control of antibiotic resistance in China must not be delayed: the current state of resistance and policy suggestions for the government, medical facilities, and patients. Biosci. Trends. 10 (1), 1–6 (2016).
Zhang, Q. Q., Ying, G. G., Pan, C. G., Liu, Y. S. & Zhao, J. L. Comprehensive evaluation of antibiotics emission and fate in the river basins of china: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 49 (11), 6772–6782 (2015).
CARSS. National Bacterial Drug Resistance Surveillance Report 2023 (Brief Version). China Antibiotic resistance Surveillance System. (2024).
Dhanji, H. et al. Isolation of fluoroquinolone-resistant O25b:H4-ST131 Escherichia coli with CTX-M-14 extended-spectrum β-lactamase from UK river water. J. Antimicrob. Chemother. 66 (3), 512–516 (2011).
Jang, J. et al. Pathogenic Escherichia coli strains producing extended-spectrum β-lactamases in the Yeongsan river basin of South Korea. Environ. Sci. Technol. 47 (2), 1128–1136 (2013).
Walsh, T. R., Weeks, J., Livermore, D. M. & Toleman, M. A. Dissemination of NDM-1 positive bacteria in the new Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect. Dis. 11 (5), 355–362 (2011).
Li, W. et al. Estimating the effect of increasing ambient temperature on antimicrobial resistance in china: a nationwide ecological study with the difference-in-differences approach. Sci. Total Environ. 882, 163518 (2023).
Li, W. et al. Association between antibiotic resistance and increasing ambient temperature in china: an ecological study with nationwide panel data. Lancet Reg. Health West. Pac. 30, 100628 (2023).
Li, W. et al. Changing climate and socioeconomic factors contribute to global antimicrobial resistance. Nat. Med. 31(6), 1798-1808 (2025).
Rochelle-Newall, E., Nguyen, T. M., Le, T. P., Sengtaheuanghoung, O. & Ribolzi, O. A short review of fecal indicator bacteria in tropical aquatic ecosystems: knowledge gaps and future directions. Front. Microbiol. 6, 308 (2015).
Al-Nabulsi, A. A. et al. Effects of osmotic pressure, acid, or cold stresses on antibiotic susceptibility of Listeria monocytogenes. Food Microbiol. 46, 154–160 (2015).
Mira, P., Lozano-Huntelman, N., Johnson, A., Savage, V. M. & Yeh, P. Evolution of antibiotic resistance impacts optimal temperature and growth rate in Escherichia coli andStaphylococcus epidermidis. J. Appl. Microbiol. 133 (4), 2655–2667 (2022).
Rodríguez-Verdugo, A., Lozano-Huntelman, N., Cruz-Loya, M., Savage, V. & Yeh, P. Compounding effects of climate warming and antibiotic resistance. iScience 23 (4), 101024 (2020).
MacFadden, D. R., McGough, S. F., Fisman, D., Santillana, M. & Brownstein, J. S. Antibiotic resistance increases with local temperature. Nat. Clim. Change 8 (6), 510–514 (2018).
Collignon, P. & Beggs, J. J. Socioeconomic enablers for contagion: factors impelling the antimicrobial resistance epidemic. Antibiotic (Basel) 8(3).86 (2019).
Blakiston, M. R. & Freeman, J. T. Population-level exposures associated with MRSA and ESBL-E. coli infection across district health boards in Aotearoa new zealand: an ecological study. N. Z. Med. J. 133 (1510), 62–69 (2020).
Holmes, A. H. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387 (10014), 176–187 (2016).
Barchitta, M. et al. Antibiotic consumption and resistance during a 3-Year period in sicily, Southern Italy. Int. J. Environ. Res. Public. Health 16(13). 2253 (2019).
Collignon, P., Beggs, J. J., Walsh, T. R., Gandra, S. & Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet. Health. 2 (9), e398–e405 (2018).
Chandran, A. & Mazumder, A. Erratum for Chandran and mazumder, pathogenic potential, genetic diversity, and population structure of Escherichia coli strains isolated from a Forest-Dominated watershed (Comox Lake) in British columbia, Canada. Appl. Environ. Microbiol. 82 (2), 767 (2016).
Fisman, D. et al. Geographical variability in the likelihood of bloodstream infections due to gram-negative bacteria: correlation with proximity to the equator and health care expenditure. PLoS One. 9 (12), e114548 (2014).
Alividza, V. et al. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect. Dis. Poverty. 7 (1), 76 (2018).
Alsan, M. et al. Out-of-pocket health expenditures and antimicrobial resistance in low-income and middle-income countries: an economic analysis. Lancet Infect. Dis. 15 (10), 1203–1210 (2015).
Alvarez-Uria, G., Gandra, S. & Laxminarayan, R. Poverty and prevalence of antimicrobial resistance in invasive isolates. Int. J. Infect. Dis. 52, 59–61 (2016).
Kaba, H. E. J., Kuhlmann, E. & Scheithauer, S. Thinking outside the box: association of antimicrobial resistance with climate warming in Europe—a 30 country observational study. Int. J. Hyg. Environ. Health. 223 (1), 151–158 (2020).
McGough, S. F., MacFadden, D. R., Hattab, M. W., Mølbak, K. & Santillana, M. Rates of increase of antibiotic resistance and ambient temperature in europe: a cross-national analysis of 28 countries between 2000 and 2016. Euro. Surveill 25(45).1900414 (2020).
Cruz-Loya, M. et al. Antibiot. Shift Temp. Response Curve Escherichia coli Growth Msystems 6(4).e0022821 (2021).
Guyot, S., Pottier, L., Ferret, E., Gal, L. & Gervais, P. Physiological responses of Escherichia coli exposed to different heat-stress kinetics. Arch. Microbiol. 192 (8), 651–661 (2010).
McDonald, L. C., Banerjee, S. N. & Jarvis, W. R. Seasonal variation of acinetobacter infections: 1987–1996. Nosocomial infections surveillance system. Clin. Infect. Dis. 29 (5), 1133–1137 (1999).
Perencevich, E. N. et al. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect. Control Hosp. Epidemiol. 29 (12), 1124–1131 (2008).
Smith, T. L., Pullen, G. T., Crouse, V., Rosenberg, J. & Jarvis, W. R. Bloodstream infections in pediatric oncology outpatients: a new healthcare systems challenge. Infect. Control Hosp. Epidemiol. 23 (5), 239–243 (2002).
Cardoso, K. et al. DnaK and GroEL are induced in response to antibiotic and heat shock in acinetobacter baumannii. J. Med. Microbiol. 59 (Pt 9), 1061–1068 (2010).
Cruz-Loya, M. et al. Stressor interaction networks suggest antibiotic resistance co-opted from stress responses to temperature. Isme J. 13 (1), 12–23 (2019).
Grilo, M. L., Pereira, A., Sousa-Santos, C., Robalo, J. I. & Oliveira, M. Climatic alterations influence bacterial growth, biofilm production and antimicrobial resistance profiles in Aeromonas spp. Antibiot. (Basel) 10(8).1008 (2021).
Zhou, Z. et al. Association between particulate matter (PM2.5) air pollution and clinical antibiotic resistance: a global analysis. Lancet Planet. Health. 7 (8), e649–e659 (2023).
Gat, D., Mazar, Y., Cytryn, E. & Rudich, Y. Origin-dependent variations in the atmospheric microbiome community in Eastern mediterranean dust storms. Environ. Sci. Technol. 51 (12), 6709–6718 (2017).
Li, L. et al. Municipal solid waste treatment system increases ambient airborne bacteria and antibiotic resistance genes. Environ. Sci. Technol. 54 (7), 3900–3908 (2020).
Wang, Y. et al. Estimating causal effects of Long-Term PM2.5 exposure on mortality in new Jersey. Environ. Health Perspect. 124 (8), 1182–1188 (2016).
Xie, S., Gu, A. Z., Cen, T., Li, D. & Chen, J. The effect and mechanism of urban fine particulate matter (PM(2.5)) on horizontal transfer of plasmid-mediated antimicrobial resistance genes. Sci. Total Environ. 683, 116–123 (2019).
Xie, J. et al. Bacteria and antibiotic resistance genes (ARGs) in PM(2.5) from china: implications for human exposure. Environ. Sci. Technol. 53 (2), 963–972 (2019).
Yin, F. H., Hao, W. L., Zhang, H. N., Miao, J. J. & Shi, H. M. Pollution status and Spatial distribution of antibiotic resistance genes in urban surface water and surrounding soil media. J. Environ. Chem. Eng. 12(5).831 (2024).
Berendes, D. M. et al. Associations between open drain flooding and pediatric enteric infections in the MAL-ED cohort in a low-income, urban neighborhood in vellore, India. BMC Public. Health. 19 (1), 926 (2019).
Ahmed, W. et al. Precipitation influences pathogenic bacteria and antibiotic resistance gene abundance in storm drain outfalls in coastal sub-tropical waters. Environ. Int. 116, 308–318 (2018).
Jang, J. et al. Environmental Escherichia coli: ecology and public health implications-a review. J. Appl. Microbiol. 123 (3), 570–581 (2017).
Ayukekbong, J. A., Ntemgwa, M. & Atabe, A. N. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control. 6, 47 (2017).
Fleming-Dutra, K. E. et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. Jama 315 (17), 1864–1873 (2016).
Haak, B. W. & Wiersinga, W. J. Uncovering hidden antimicrobial resistance patterns within the hospital microbiome. Nat. Med. 26 (6), 826–828 (2020).
Masiero, G., Filippini, M., Ferech, M. & Goossens, H. Socioeconomic determinants of outpatient antibiotic use in Europe. Int. J. Public Health. 55 (5), 469–478 (2010).
Maugeri, A., Barchitta, M., Puglisi, F. & Agodi, A. Socio-economic, governance and health indicators shaping antimicrobial resistance: an ecological analysis of 30 European countries. Glob. Health. 19 (1), 12 (2023).
Savoldi, A. et al. Gross National income and antibiotic resistance in invasive isolates: analysis of the top-ranked antibiotic-resistant bacteria on the 2017 WHO priority list. J. Antimicrob. Chemother. 74 (12), 3619–3625 (2019).
WHO. WHO Publishes List of bacteria for Which New Antibiotics Are Urgently Needed (World Health Organization, 2017).
National Bureau of Statistics. China Statistical Yearbook (National Bureau of Statistics, 2024).
Zhen, X. et al. Socioeconomic factors contributing to antibiotic resistance in china: a panel data analysis. Antibiotics (Basel) 10(8).994 (2021).
Cassini, A. et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 19 (1), 56–66 (2019).
Funding
This research was funded by the National Natural Science Foundation of China (72074085).
Author information
Authors and Affiliations
Contributions
Xinping Zhang was responsible for the study conception and review; Ruxin Kou and Haixia Wang wrote the manuscript; Dongdong Zou and Jinwen Hu were responsible for the data processing and analysis of the manuscript; Yuanyang Wu and Qianqian Xu participated in the literature collection and data compilation of this manuscript. All authors have read and agreed to submit the final version. Xinping Zhang is the corresponding author: xpzhang602@hust.edu.cn.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
Not applicable.
Clinical trial number
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kou, R., Wang, H., Zou, D. et al. Spatial panel data analysis of antimicrobial resistance in Escherichia coli in China. Sci Rep 15, 23753 (2025). https://doi.org/10.1038/s41598-025-09085-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09085-w