Abstract
The prevalence rates of microbial infections in wounds present a medical challenge, as damaged skin is inherently more susceptible to colonization/infection by pathogens. Deep eutectic solvents are a type of environmentally friendly cost-effective, and efficient solvent used in the extraction of bioactive compounds from plant materials. The study investigates the preparation of a xerogel formulation incorporating Nigella sativa extract, utilizing deep eutectic solvents that comprise chitosan and gallic acid-grafted gelatin, with the potential to enhance wound healing through antimicrobial and antioxidant actions. The xerogel has been evaluated for physicochemical characteristics by using Fourier transform infrared spectroscopy, scanning electron microscopy, and different chemical analyses. Mechanical properties of the film has confirmed that this formulation would be suitable for dressing wounds that require conformability. It is indicated from the disk diffusion test that the film showed efficacy in inhibition of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. Preliminary studies have shown this formulation has structural integrity, a cell-friendly nature, is remarkably antioxidant-active, and exhibited antimicrobial activity hence possibly application in pharmaceutical fields. The results will add to our understanding of how DES can effectively be used in enhancing the bioactivity of natural extracts in polymeric matrices and help pave a way for advanced applications in health-related products.
Similar content being viewed by others
Introduction
Screening of natural products for their pharmacological potential has gained great impetus in recent times, especially those related to herbal extracts1,2,3,4,5. Nigella sativa (N. sativa), commonly known as black seed, kalonji, or Shoniz in Persian, is primarily attributed to the bioactive compounds present in it, such as thymoquinone (TQ), carvacrol, and p-cymene6. These constituents have been well documented for their antioxidant, anti-inflammatory, and antimicrobial activities; thus, N. sativa is an important candidate for developing novel pharmacological agents1,2,6.
The design of formulations using N. sativa extract has emerged, with different approaches already being tried to improve their in vivo bioavailability and, hence, the efficacy of its active principles6. Among all the recent approaches, deep eutectic solvents (DESs) have been widely adopted for extraction and formulation studies2. DESs are regarded as efficient extractants in various chemical analyses, including those of natural products, pharmaceuticals, and other bioactive compounds2,7. DESs can be used for the separation of valuable components from complex mixtures using an inherently greener alternative to traditional extraction methods. These solvents are prepared by a simple mixture of hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD)2,7,8. The complexation between HBA and HBD yields a green, nontoxic solvent, which is long-term stable and has a low melting point2,7,9. They are preferred over conventional ionic liquids and toxic organic solvents. DESs have the ability to dissolve a wide range of compounds while being eco-friendly and lower cost in comparison to conventional organic solvents2,7,10,11.This technique also preserves the integrity of sensitive bioactive compounds and enables the preparation of stable formulations like xerogel that could be used in topical applications1,2,11. Xerogel represents a unique class of materials resulting from the sol-gel processes, being highly porous in structure and featuring an extensive internal surface area12,13,14. These are the products resulting from the process of drying a gel by evaporation of the liquids contained within it, while keeping a rigid framework that retains essentially the same morphology as the original gel. Due to this unique architecture, xerogel allows for a good deal of void space that may be useful in various applications, especially in drug delivery and biomedical fields12,14. This may indicate longer therapeutic efficacy, reducing the frequency of application and increasing patient compliance accordingly. The incorporation of N. sativa extract in xerogel formulations may further enhance its antioxidant and antimicrobial properties, thereby offering better therapeutic benefits. Bioactive compounds of N. sativa, particularly thymoquinone, have been reported for their effective antioxidant activity15,16. These compounds could provide improved stability and bioavailability through the incorporation in a xerogel matrix; therefore, enhancing antioxidant effects2,11,15. By embedding such extracts in a xerogel matrix, it is conceivable to formulate a sustained-release antimicrobial system that may offer protection against microbial growth for extended periods1,15.
Chitosan (CS), a biodegradable and biocompatible polysaccharide derived from chitin, has been extensively studied for wound dressing applications due to its inherent antimicrobial properties, mucoadhesiveness, and ability to promote tissue regeneration12,14,17. Recent advances in CS-based wound dressings have focused on incorporating bioactive compounds to enhance functionality. For example: Karami et al. (2023) developed a CS/xerogel film containing Thymus pubescens essential oil, demonstrating antimicrobial activity against wound pathogens but limited antioxidant capacity12. Li et al. (2022) fabricated quercetin-loaded CS/PVA xerogel films with antioxidant properties, though their mechanical flexibility was insufficient for dynamic wound sites14. Barzegar et al. (2021) engineered CS/PVA nanofibers with Satureja mutica oil, which showed improved antimicrobial effects but required complex electrospinning techniques17. Despite these advancements, challenges persist in achieving a balance between mechanical robustness, sustained bioactive release, and multifunctionality (e.g., combined antimicrobial/antioxidant effects). Many existing CS dressings also rely on organic solvents for herbal extract incorporation, which may degrade thermolabile compounds like TQ2,11.
Our study introduces a groundbreaking approach by combining deep DES with N. sativa extract within a CS-based xerogel matrix. This innovative method enhances the bioactivity and stability of the extract, preserving sensitive compounds like thymoquinone and improving their bioavailability compared to conventional extraction methods. The formulation offering synergistic antimicrobial, antioxidant, and mechanical properties. This system demonstrates dual functionality with significant antimicrobial and antioxidant effects, enhanced flexibility for wound conformability, and sustained release of bioactive compounds. Methodological advancements include optimized DES parameters and ultrasonication for efficient extraction and uniform distribution of bioactive compounds within the xerogel matrix. The outcomes show broad-spectrum antimicrobial activity, biocompatibility, and dose-dependent antioxidant activity, addressing gaps in current wound dressings. This work is the first to report a DES-assisted N. sativa xerogel film with dual therapeutic effects, providing a scalable and biocompatible solution for wound care that bridges natural medicine and material science. This is principally important in wound dressings or topical formulations, for which infection control may be of paramount importance6,14,15. Among other factors, the physicochemical properties of these formulations are one of the most important factors that determines their stability and efficacy. Therefore, such systems require an in-depth study using a variety of characterization techniques.
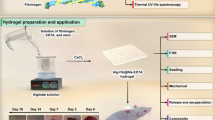
This research aimed to prepare a xerogel formulation containing N. sativa extract using DES and explore its physicochemical properties alongside its antioxidant and antimicrobial activities. By clarifying these characteristics, we hope to contribute valued insights into the potential applications of N. sativa-based xerogel in the pharmaceutical and cosmetic fields for future research in herbal medicine. This research has been approved by the Ethics Committee of Shiraz University of Medical Sciences: http://ethics.research.ac.ir/IR.SUMS.MED.REC.1403.437.
Result and discussion
Physicochemical properties of CS/GGA/Ns xerogel film
FTIR analysis
The chemical composition of the hydrogel was analyzed using FTIR spectroscopy, with the results depicted in Fig. 1. A broadband around 3300 cm−1 was observed, attributed to the combined stretching vibrations of N–H and O–H bonds from CS, Gel, and GA. These vibrations are further amplified by the alcoholic O–H groups in the N. sativa EX and cross-linker, signifying the effective integration of all components into the hydrogel network18,19,20. The aliphatic C–H stretching vibrations produced distinctive peaks between 2920 cm1, and 2870 cm−1 in different materials. These peaks arise from the molecular frameworks of CS, Gel, cross-linker, and constituents of N. sativa EX, reflecting the structural roles of these components within the hydrogel11,18,19. Around 1600 cm−1, the spectrum exhibits a significant band attributed to the C = O stretching vibrations in GA and Gel, which overlaps with the NH₂ groups of CS and Gel. Additionally, the C = C bonds and aromatic rings from the N. sativa EX contribute to this region, highlighting the interaction between the extract and the hydrogel matrix11,18,19. Another characteristic peak appears near 1530 cm−1, corresponding to the C–N stretching and amide N–H bending modes, which are fundamental to the structures of CS and Gel. This indicates the formation of amide linkages and confirms the chemical interactions between the xerogel’s building blocks17,18. The aromatic and functional components of the N. sativa EX also influence the spectral region around 1070 cm−1, associated with C–O stretching vibrations and the structural framework of CS, Gel, and GA. These shifts suggest specific molecular interactions, highlighting the successful integration of the extract within the hydrogel matrix11,18,21. In the present study, the FTIR analysis confirms the effective assembly of the CS/GGA/Ns xerogel film, where the unique contributions of N. sativa EX are evident in the spectral modifications. This aligns with prior studies showcasing GA’s potential to self-assemble and incorporates into similar formulations, enhancing their structural and functional properties.
SEM
The SEM images (Fig. 2) revealed distinct differences in the surface texture and morphology of the xerogel films containing N. sativa EX compared to the blank film (CS/GGA). As observed, the blank film showed a compact and homogeneous structure (Fig. 2A). The film with N. sativa EX exhibited a significantly porous structure (Fig. 2B), which may enhance its potential for drug delivery by increasing the surface area for interactions. This porosity suggests that the phytochemicals from N. sativa EX influence the polymer matrix, leading to unique surface characteristics. The observed pores on the surface of the xerogel film containing N. sativa EX could be attributed to the incorporation of phytochemicals from the extract, which may disrupt the polymer matrix during the drying process, resulting in more porous structure.
Additionally, the interaction between the extract and the polymer components could facilitate the formation of voids or cavities as the solvent evaporates, leading to increased porosity. This enhanced porosity is significant as it may improve the film’s functionality, particularly in applications related to drug delivery or bioactivity, by providing a larger surface area for interaction and absorption12,22. Notable morphological changes were observed, with the presence of N. sativa EX likely enhancing or altering the structural integrity of the xerogel. The blank film serves as a control, providing a basis for comparison to understand how the extract modifies the properties of the xerogel. Unique microstructures were identified that may correlate with functional properties such as mechanical strength or bioactivity. Additionally, the presence of pores can enhance the adhesion of the xerogel film to the wound bed, ensuring better contact and facilitating faster healing12.
Differential scanning calorimetry analysis `
Differential scanning calorimetry (DSC) analysis was performed to evaluate the thermal behavior of the hydrogel films that indicated in Fig. 3. The thermogram of pure CS showed no prominent thermal transitions within the range of 40–240 °C, indicating its amorphous nature and absence of significant thermal events. Upon incorporation of Gel and GA (CS/GGA), a distinct endothermic peak appeared at approximately 170 °C, which is attributed to increased molecular interactions and partial crosslinking within the biopolymeric matrix. Interestingly, the addition of the plant extract caused a slight shift of the endothermic peak to 165 °C (CS/GGA/Ns), indicating that the extract did not change the phase transition. This shift may be attributed to intermolecular hydrogen bonding resulting from the phytochemical constituents present in the media. These findings confirm that the inclusion of GA and N. sativa EX did not influences the thermal properties of the films, indicating successful structural modification and enhanced interaction among the hydrogel components.
Mechanical properties
Based on the mechanical properties presented in Table 1, the investigations of the two types of films, the CS/GGA, and CS/GGA/Ns xerogel films, indicated significant differences. The blank hydrogel exhibits a low elongation at break (1.3%), indicating brittleness and a lack of flexibility, while the CS/GGA/Ns film shows a higher elongation at break (1.54%), suggesting that the addition of N. sativa EX enhanced flexibility and ductility, making it more suitable for applications requiring stretchability. The ultimate tensile strength is higher for the blank xerogel at 17.75 MPa when compared with CS/GGA/Ns xerogel film at 12.13 MPa. Therefore, although the blank hydrogel withstood a higher stress value before failure, it possessed lower flexibility than the modified film. The Young’s modulus of the blank hydrogel is 6.5 MPa, reflecting its stiffness, while the CS/GGA/Ns film has a Young’s modulus of 5.9 MPa, suggesting a slight reduction in stiffness with the incorporation of N. sativa extract, consistent with the observed increase in elongation at break. These mechanical properties indicate that while the CS/GGA/Ns film has demonstrated improved flexibility, it sacrificed some tensile strength in comparison to the blank hydrogel and may potentially be better suited for other applications that require conformability, such as wound dressings or drug delivery systems12.
Water contact angle
It is important to maintain a balance between the hydrophilicity and hydrophobicity of materials that come into contact with the skin and mucosal surfaces of the human body31. The wettability of the produced film was evaluated by performing water contact angle measurements to assess the influence of N. sativa EX. As indicated in Fig. 4, the addition of N. sativa EX increased the water contact angle from 70.94° ± 0.32 to 73.20° ± 1.12. Additionally, the successful incorporation of N. sativa EX did not significantly alter the surface hydrophilicity. The hydrophilicity of the blank film (Fig. 4A) is due to the presence of hydrophilic functional groups, such as hydroxyl groups. This leads to a small water contact angle. These findings underscore the promising prospects of this biomaterial in the field of wound care and antimicrobial therapy. Pereira et al. developed thin hydrogel films composed of alginate and Aloe vera gel for wound healing applications, in which the water contact angle was in the range of 30.25–52.52, highlighting the hydrophilic properties of the films38.
pH measurement
It has already been mentioned that the pH of the stratum corneum lies between 4.0 and 4.5, whereas that of the overlying viable epidermis lies between 5.0 and 7.035. Therefore, to ensure safe application on the skin, a topical preparation should have a pH between these two values. The films with a concentration of acidic or basic functional groups determine their final application, and can consequently lead to damage to patients36. In the present study, the surface pH for CS/GGA/Ns xerogel film was 5.08 ± 0.09, which implies that the xerogel film is non-irritating when applied to the superficial surfaces37. During a superficial microbial infection, the pH of these surfaces may vary depending on the severity and specific type of infection.
Swelling of CS/GGA/Ns xerogel film
Swelling ratio is one of the most important characteristics of wound dressings since they have the ability to absorb large amounts of exudate from the wound surface, preventing or reducing infection32. Moreover, study of swelling degree for superficial application films is important due to enhanced bio-adhesion properties and makes drug release easier. The pH of medium, temperature, ionic strength, composition of the solvent, functional groups, and dissociation constant of the polymeric constituents are considered significant parameters for the level of swelling of films12. Therefore, in the recent study, swelling value was investigated at two pHs: pH = 5.0 (near to pHs of normal superficial skin)12 and pH = 7.0 (correlated with wounded tissue)12,39.
As shown in Fig. 5, both the blank and CS/GGA/Ns films exhibited higher swelling at pH 7.0 (280–637%) than at pH 5.0 (270–577%) after 24 h, with pH 7.0 also showing faster initial hydration after 1 h. Near-neutral pH reduced ionization of CS (weak base) and Gel (amphoteric), weakening electrostatic repulsion but favoring network hydration, while acidic pH (5.0) increased protonation and chain expansion without matching long-term swelling. The CS/GGA/Ns film’s marginally superior performance suggests that the N. sativa EX enhances water retention via polar groups (− OH, −COOH) or modified porosity, though further study is needed to elucidate its role.
Overall, these results suggest that swelling behavior of synthesized hydrogel is influenced by pH variation due to variations in ionization states of its components, which affect the electrostatic interactions in the network structure.
In vitro cell culture studies
The MTT assay was conducted to assess the cytocompatibility of CS/GGA/Ns xerogel film towards the 3T3 fibroblast cell proliferation on days 1, 3, and 5. As shown in Fig. 6, a steady upward trend can be observed across all treatment groups over time. Notably, the absorbance values on day 5 were significantly higher compared to earlier time points, with a statistical significance (p value < 0.05). This indicates that the cells were not only surviving but were actively proliferating in response to the treatment. Based on these findings, the inclusion of N. sativa EX within the polymeric matrix (CS/GGA) exhibited no cytotoxic effect on 3T3 fibroblasts, with cell viability and proliferation rates comparable to the control group (p > 0.05 at all-time points). The absorbance values increased steadily over time in both the CS/GGA/Ns film and control groups, indicating normal cell proliferation without adverse effects from the xerogel components.
These results have significant implications for the development of biocompatible materials for applying in tissue engineering and regenerative medicine. The positive effect of N. sativa EX on fibroblast proliferation indicates its potential role as a bioactive component in enhancing the performance of biomaterials. The ability of CS/GGA/Ns xerogel film to support cell growth without inducing cytotoxicity positions it as a promising candidate for future applications in wound healing and tissue regeneration.
To evaluate the safety profile of the N. sativa EX alone, an MTT assay was conducted across a range of concentrations (10, 33 and 100% w/v). Notably, no significant cytotoxicity was observed, with cell viability consistently > 98% (p > 0.05 vs. untreated controls). Even at applied concentrations (33% DES), viability remained > 95%, confirming the solvent’s biocompatibility. In contrast, extreme concentrations (100% DES) induced toxicity (viability dropped to ~ 65%, p < 0.001), though this threshold is higher than residual levels detected in the final xerogel film (33% w/w). These findings confirm that the N. sativa DES EX while not removed during processing, poses no cytotoxic risk at biologically relevant concentrations, aligning with its classification as a green solvent.
Antimicrobial activity
The antimicrobial activity of the CS/GGA/Ns xerogel film was evaluated using one of the most conventional techniques, the disk diffusion method, which is used to determine microbial susceptibility to various agents. This study exposed the standard microbial strains to xerogel film solutions in the range of 4–32 µg/mL; subsequently, the inhibition zones around each disk were measured. The results demonstrated varying degrees of antimicrobial efficacy across different concentrations for each microbial strain (Table 2). The findings indicate that the CS/GGA/Ns xerogel film exhibited significant antimicrobial activity against all tested strains, particularly at higher concentrations. This suggests a dose-dependent relationship in which larger inhibition zones correlate with higher concentrations (Fig. 7). This pattern is consistent with the general trend in antimicrobial susceptibility tests, thus confirming the effectiveness of the xerogel films against C. albicans, and enhancing their multifunctionality in combating both bacterial and fungal infections. These findings provide insight into the potential use of CS/GGA/Ns xerogel films as antimicrobial agents in various fields, such as medical devices and wound dressings, where infection control is crucial. Further studies may investigate the mechanisms behind such antimicrobial activity and/or the long-term stability and efficacy of these films in real applications. The main reasons for the antimicrobial action of CS/GGA/Ns xerogel films involve several mechanisms associated with the properties of CS, a key component in these films. One significant mode of action is the interaction of CS with microbial cell membranes, leading to the disruption of the cell membrane structure12,17,22,23.
Antioxidant activity
The antioxidant activity of the developed xerogel film formulation containing GGA, CS, and N. sativa EX was assessed using DPPH was performed at four different concentrations: 4, 8, 16, and 32 µg/mL. The antioxidant activities demonstrated a concentration-dependent increase in activity (Fig. 8), peaking at 32 µg/mL (90.6%). Comparative analysis indicated that the blank hydrogel (CS and GGA without N. sativa EX) showed lower inhibition percentages across all concentrations. Statistical analysis confirmed that the differences in antioxidant activities were significant (p-value < 0.05), underscoring the critical role of N. sativa EX in enhancing antioxidant properties. Additionally, phenolic hydroxyl groups of GA can scavenge reactive oxygen species and disrupt the cycle of new radical generation. They act as antioxidants and may prevent the oxidation of lipids, DNA, proteins, and enzymes participating in the radicals formation16. In general, the synthesized xerogel formulation exhibits promising antioxidant activity, suggesting potential applications in biomedical fields that warrant additional studies into the fundamental mechanisms.
Comparative analysis with commercial wound dressings
To contextualize the potential clinical utility of the developed CS/GGA/Ns xerogel film, Table 3 compares its key properties with three widely used commercial wound dressings: (1) DuoDERM®, (2) Tegaderm™ CHG, and (3) Aquacel®. The comparison encompasses critical wound care parameters, including material composition, antimicrobial/antioxidant activity, mechanical performance, swelling capacity, and clinical applications. This analysis highlights the advantages of the xerogel film, particularly its dual antimicrobial-antioxidant functionality derived from natural constituents, while also identifying areas for further optimization to match established commercial benchmarks.
Conclusions
This study provides the potential of utilizing deep eutectic solvents to extract bioactive compounds from natural sources, Nigella sativa, toward formulate them into the xerogel film platform. Wound care is considered one of the major clinical challenges, as it can lead to wound infections. Simultaneously, the emergence of multi-drug-resistant microorganisms has worsened the wound healing process. To address these issues, a chitosan-based xerogel film containing Nigella sativa extract was formulated, showcasing effective antimicrobial properties against different bacteria species and Candida albicans. The antioxidant activity of this formulation was another advantage of this novel product, suggesting its application as a reliable wound dressing. The xerogel film was also found to be biocompatible when exposed to 3T3 fibroblast cell lines, demonstrating the safety of the developed formulation. Regarding mechanical properties, the incorporation of the Nigella sativa extract increased the flexibility of the synthesized film, which can be beneficial when in contact with the irritated wound site. The findings provide valuable insights into developing innovative health-related products that leverage the antimicrobial and antioxidant properties of natural extracts, paving the way for future advancements in herbal medicine and pharmaceutical applications. All of these properties underscore the importance of integrating natural extracts into biocompatible matrices to develop effective therapeutic solutions for managing wounds and preventing infections. However, in vivo studies are required to further assess the effectiveness of this formulation.
Materials and methods
Material
All materials were of analytical grade. Ammonium acetate (AA), 1, 1-diphenyl-2-picrylhydrazyl (DPPH), ethyl acetate, methanol, Mueller-Hinton Agar (MHA), Sabouraud Dextrose Agar (SDA), and aluminum plate silica gel 60 F254 (20 × 10 cm) were purchased from Merck Company (Darmstadt, Germany). The chitosan [CS, CAS Number: 448869, low molecular weight (LMW: 75.55 ± 0.01 kDa), purity: ≥ 93% (w/w), 75–85% deacetylated, and Sigma-Aldrich], RPMI-1640 were purchased from Sigma-Aldrich Company. The edible bovine gelatin (Gel), gallic acid (GA), and dimethyl sulfoxide (DMSO), along with Folin-Ciocalteau reagent and toluene, were obtained from BDH and Fisher Scientific Company, respectively. Lactic acid (LA) was sourced from Rhone Poulenc (France). Formic acid, ethanol, sodium carbonate, normal saline, 3T3 mouse fibroblast cell line (Pasteur Institute of Iran, Tehran), and distilled water (D.W.) were available in the laboratory. 3-(4, 5-dimethylthiazol-2-yl)−2, 5 diphenyl tetrazolium bromide (MTT), Dulbecco’s modified Eagle’s medium nutrient mixture F-12 (DMEM/F12), and fetal bovine serum (FBS) were purchased from Gibco, BRL (Eggenstein, Germany).The N. sativa plant seeds were purchased from Hayar Teb Company, a supplier of medicinal herbs.
Preparation of deep eutectic solvent (DES)
The DES was synthesized by heating AA and LA as the HBA and HBD components, respectively. The molar ratios of 1:1, 1:2, and 1:3 were examined for HBA and HBD, respectively. The components of each ratio were combined in a tightly sealed flask and stirred at 50 ºC until a uniform and colorless liquid was achieved7,27,28.
Extraction process
The optimized parameters of the extraction process using DES solvent were selected according to the previous studies7,8,28,29, with modifications made to further enhance the results. Briefly, the powdered N. sativa seeds (0.25 g) was mixed with 3 mL of DES solvent. The solvent included 19.41% water to decrease viscosity. In the following, the extraction process was carried out using ultrasonic waves for 122 min at a temperature of 35 ºC. To increase contact between the solvents and the plant material, the tube was vortexed every 30 min. Finally, the obtained N. sativa extract (N. sativa EX) was filtered and kept at −20 ºC until analysis.
Fabrication of the xerogel films
For the synthesis of the desired xerogel film, gallic acid-grafted gelatin (GGA) was prepared by dissolving Gel (0.5 g/mL), and GA (0.01 g/mL) in D.W., to initiate the grafting reaction. Various ratios of CS, GGA and N. sativa EX were tested. After conducting several experiments, CS (0.05 g/mL, with acetic acid 2%) and GGA (2% w/v) solutions were combined in a 1:1 ratio. Mixing the CS/GGA solution in a final volume of 10 mL was followed by stirring for 1 h. Subsequently, the N. sativa EX (33.3%, v/v) was added to the polymeric solution while stirring for 24 h continuously during homogenization. Similarly, the blank xerogel film (CS/GGA) was prepared using CS and GGA (without N. sativa EX) for comparison with the CS/GGA/Ns xerogel film as the final formulation. The obtained precursor solutions were poured into a specific mold and were allowed to remain in the refrigerator for gelation for (24 h) and subsequently dried at room temperature.
Characterization of the CS/GGA/Ns xerogel film
Fourier-transform infrared spectroscopy (FTIR) was conducted in attenuated total reflectance (FTIR-ATR) transmittance mode using a Bruker Tensor II instrument (Germany). Spectra were obtained at a resolution of 4 cm−1 with 32 scans in the range of 4000–400 cm−1. This technique verified the possible interactions among various components of the xerogel films and confirmed the successful formulation of the CS/GGA/Ns xerogel film. The morphology of the xerogel films was determined using a scanning electron microscope (SEM, TESCAN-Vega 3, Czech Republic). Before the examination, the xerogel films were coated with gold using a sputter coater (Quorum Technologies, England) for 120 s.
Differential scanning calorimetery
Differential scanning calorimeter (DSC Q600) was used to study the phase transition of the mixture CS/GGA/Ns (1:2:0.1:0.03 mmol) at a heating rate of 10 °C min−1 under an argon atmosphere. The conductivity of this DES was measured by Metrohm (SWISS MADE, model 644 conductivity meter).
Mechanical properties of the CS/GGA/Ns xerogel film
The mechanical properties of the synthesized films, including tensile strength, elongation at break, and Young’s modulus, were determined using a Z010 Zwick/Roell Universal tensile strength device at a crosshead speed of 0.1 mm/s, by ASTM D882-02 (ASTM, 2002). Films were cut into strips with dimensions of 10 × 60 mm (three specimens for each film). The Young’s modulus (MPa) was acquired from the initial linear slope of the stress-strain curves. The parameters of elongation at break and ultimate tensile strength (MPa) were evaluated by the following formulae (1) and (2):
(1) Elongation at the break = (Length at the breakpoint (m))/(Initial length (m)) ×100.
(2) Ultimate tensile strength = (Maximum load at the breakpoint (N))/(Cross-sectional area of the film (m2)). The experiment was done three times and the mean values ± SD were reported.
Water contact angle measurement
Contact angle measurements were performed to quantify the surface hydrophilicity, thereby providing insights into the film’s bio-adhesion properties. The hydrophilicity of the CS/GGA/Ns xerogel film was assessed through a water contact angle test using a CAG200 Contact Angle instrument. Based on the sessile drop method, 25 µL of D.W. droplets were carefully dispensed onto various locations of the sample surfaces, followed by immediate image capture. The average contact angles were determined based on the shape of the water droplets. The experiment was done three times and the mean values along with their corresponding standard deviations (mean ± SD) were reported31.
Determination of pH value
For measuring the surface pH of CS/GGA/Ns xerogel film, a pH meter (HANNA, pH 211, microprocessor pH meter, Australia) was used. The pH meter was previously standardized using a buffer solution at pH 7.0 and pH 10.0. The CS/GGA/Ns xerogel film was cut into a circular shape and kept on a Petri dish containing D.W to swell for 30 min at room temperature. Then, pH was measured by contacting the surface of the glass electrode with the swelled xerogel film. The experiment was done three times and the mean values ± SD were reported30.
Swelling studies
Gravimetrically, the swelling behavior of dried films was investigated at various buffer solutions (pH = 5.0 and pH = 7.0) for 1 and 24 h at room temperature. The dried films were weighed initially before being immersed in buffer solution (WD). At specific time intervals, swollen films were removed, dried on filter paper to evaporate excess water, and weighed (WW). The experiment was conducted in three times, and finally, the extent of swelling was calculated using the following equation:
Where WD and WW are weights of films in initial (dry) and swollen states, respectively32. Water solubility is extremely important in ensuring the moisture and humidity levels of wound dressings by minimizing infection33.
In vitro cell culture studies
Cytotoxicity of the obtained CS/GGA/Ns xerogel film was quantitatively determined using the MTT assay. The 3T3 mouse fibroblast cell line (Pasteur Institute of Iran, Tehran) was cultured in the DMEM/F12 medium containing 10% (v/v) FBS, 100 unit/mL of penicillin, and 100 µg/mL of streptomycin in a humidified incubator at 37 °C with 5% CO2. Sterilized CS/GGA/Ns xerogel film concentrations of 8, 16, and 32 µg/mL were added to each well (three replicates). No treatment was applied to one well, which served as a control group. At each time point, (1, 3, and 5 days after cell seeding), the culture medium was removed, and 0.2 mL of MTT solution (0.5 mg/mL) was added to each well, incubated at 37 ˚C for 4 h in a dark incubator. Then, the solution was discarded, and 0.1 mL of DMSO was added to each well to dissolve the formed formazan crystals. After that, the absorbance of the samples was read with microplate reader (wavelength: 570 nm) from Biotech Instruments23. The experiment was done three times and the mean values ± SD were reported.
Antimicrobial activity
The antimicrobial efficiency of CS/GGA/Ns xerogel film was performed using the disk diffusion method according to a protocol from the Clinical Laboratory Standards Institute protocol (CLSI M44-A2). All microbial strains, including Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans, were cultured overnight and adjusted to a 0.5 McFarland standard for uniformity. Mueller-Hinton agar plates were prepared and inoculated with the microbial suspension. The xerogel film solutions were prepared at 4, 8, 16, and 32 µg/mL concentrations; then, the blank disk (without any treatment) was immersed in the specified xerogel solutions for 30 min, followed by placing on the inoculated agar plates. After incubation at 37 °C for 24 h, the zones of inhibition around each disk were measured using a ruler. The results were recorded to estimate the antimicrobial effectiveness of each concentration, allowing a comparison of dose-dependent activity and providing valued insights into the efficiency of these xerogel films against specific microbial strains. Additionally, the N. sativa EX was analyzed independently, along with control disks containing fluconazole and co-trimoxazole drugs40,41. The experiment was done three times and the mean values ± SD were reported.
Activity of DPPH radical scavenging
The antioxidant capacity of the developed xerogel film was evaluated by the free radical scavenging DPPH method. The CS/GGA/Ns xerogel film was cut and homogenized using a tissue grinder. In this study, the xerogel film solutions were prepared at concentrations of 4, 8, 16, and 32 µg/mL. The assay was conducted by adding 100 µL of the DPPH ethanolic solution (0.3 mM) to samples of each concentration in each well of 96-well cell culture plates. DPPH solution served as a standard in this experiment. Subsequently, the samples were mixed and incubated in a dark environment for half an hour. Finally, the absorbance of the samples was recorded using a UV-VIS plate reader at 517 nm (BMG Spectro Nano plate reader, Germany). The degradation of DPPH was calculated using the following equation: (3):
Where A sample is the absorbance of the (DPPH + ethanol + xerogel), and A0 is the absorbance of the control (DPPH + ethanol)40,42. The experiment was done three times and the mean values ± SD were reported.
Statistical analyses
The data obtained in this study were subjected to analysis using GraphPad Prism 8 software (San Diego, CA, United States). The results are presented as mean values ± standard deviation (SD). Data analysis was performed using the One-way ANOVA method and t-test, with statistical significance determined by a p-value less than 0.05. All experiments were conducted in triplicate for robustness and consistency.
Data availability
The data used to support the findings of this study were supplied by Vice-Chancellor for Research of Shiraz University of Medical Sciences under license. Requests for data access should be made to Zahra Zareshahrabadi, zare_shahrabadi@sums.ac.ir and zahrazarem368@gmail.com.
References
Sharifi, M. & Bahrami, S. H. Review on application of herbal extracts in biomacromolecules-based nanofibers as wound dressings and skin tissue engineering. Int. J. Biol. Macromol., : p. 133666. (2024).
Ling, J. K. U. & Hadinoto, K. Deep eutectic solvent as green solvent in extraction of biological macromolecules: A review. Int. J. Mol. Sci. 23 (6), 3381 (2022).
Moshaverinia, M. et al. Antimicrobial and Anti-Biofilm activities of Thymus fallax essential oil against oral pathogens. Biomed. Res. Int. 2022 (1), 9744153 (2022).
Kazemi, A. et al. Peppermint and menthol: a review on their biochemistry, Pharmacological activities, clinical applications, and safety considerations. Crit. Rev. Food Sci. Nutr., : pp. 1–26. (2023).
Meysami, M. et al. Efficacy of short term topical Malva Sylvestris L. cream in pediatric patients with atopic dermatitis: a randomized double-blind placebo-controlled clinical trial. Endocr. Metabolic Immune Disorders-Drug Targets (Formerly Curr. Drug Targets-Immune Endocr. Metabolic Disorders). 21 (9), 1673–1678 (2021).
Rashwan, H. K. et al. Black Cumin seed (Nigella sativa) in inflammatory disorders: therapeutic potential and promising molecular mechanisms. Drugs Drug Candidates. 2 (2), 516–537 (2023).
Smith, E. L., Abbott, A. P. & Ryder, K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 114 (21), 11060–11082 (2014).
Abbott, A. P. et al. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun., 2003(1): pp. 70–71 .
Aydin, F., Yilmaz, E. & Soylak, M. A simple and novel deep eutectic solvent based ultrasound-assisted emulsification liquid phase Microextraction method for malachite green in farmed and ornamental aquarium fish water samples. Microchem. J. 132, 280–285 (2017).
García, A. et al. Extraction of phenolic compounds from Virgin Olive oil by deep eutectic solvents (DESs). Food Chem. 197, 554–561 (2016).
Shafodino, F. S., Lusilao, J. M. & Mwapagha, L. M. Phytochemical characterization and antimicrobial activity of Nigella sativa seeds. PloS One. 17 (8), e0272457 (2022).
Karami, F. et al. Chitosan-based emulgel and xerogel film containing Thymus pubescens essential oil as a potential wound dressing. Carbohydr. Polym. 318, 121156 (2023).
Bilanovic, D., Starosvetsky, J. & Armon, R. H. Preparation of biodegradable xanthan–glycerol hydrogel, foam, film, aerogel and xerogel at room temperature. Carbohydr. Polym. 148, 243–250 (2016).
Li, X. et al. Antibacterial, antioxidant and biocompatible nanosized quercetin-PVA xerogel films for wound dressing. Colloids Surf., B. 209, 112175 (2022).
Majdalawieh, A. F. & Fayyad, M. W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int. Immunopharmacol. 28 (1), 295–304 (2015).
Zahrani, N. A. A., El-Shishtawy, R. M. & Asiri, A. M. Recent developments of Gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 204, 112609 (2020).
Barzegar, S. et al. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 597, 120288 (2021).
Nurzynska, A. et al. Curdlan-based hydrogels for potential application as dressings for promotion of skin wound healing—Preliminary in vitro studies. Materials 14 (9), 2344 (2021).
Das, A., Uppaluri, R. & Das, C. Feasibility of poly-vinyl alcohol/starch/glycerol/citric acid composite films for wound dressing applications. Int. J. Biol. Macromol. 131, 998–1007 (2019).
Rezvanian, M. et al. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 97, 131–140 (2017).
Adelakun, O. E., Oyelade, O. J. & Olanipekun, B. F. Use of Essential Oils in Food Preservation, in Essential Oils in Food Preservation, Flavor and Safetyp. 71–84 (Elsevier, 2016).
Torabiardekani, N. et al. Encapsulation of Zataria multiflora essential oil in Polyvinyl alcohol/chitosan/gelatin thermo-responsive hydrogel: synthesis, physico-chemical properties, and biological investigations. Int. J. Biol. Macromol. 243, 125073 (2023).
Azadi, S. et al. Antifungal activity of Fe3O4@ SiO2/Schiff-base/Cu (II) magnetic nanoparticles against pathogenic Candida species. Sci. Rep. 14 (1), 5855 (2024).
Zare, M. R. et al. Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 603, 120698 (2021).
Zareshahrabadi, Z. et al. Magnetic Chitosan nanoparticles loaded with amphotericin B: synthesis, properties and potentiation of antifungal activity against common human pathogenic fungal strains. Int. J. Biol. Macromol. 222, 1619–1631 (2022).
Jangjou, A. et al. Time to conquer fungal infectious diseases: employing nanoparticles as powerful and versatile antifungal nanosystems against a wide variety of fungal species. Sustainability 14 (19), 12942 (2022).
Di Muzio, S. et al. Binary mixtures of choline acetate and tetrabutylammonium acetate with natural organic acids by vibrational spectroscopy and molecular dynamics simulations. J. Phys. Chem. B. 128 (3), 857–870 (2024).
Georgantzi, C. et al. Combination of lactic acid-based deep eutectic solvents (DES) with β-cyclodextrin: performance screening using ultrasound-assisted extraction of polyphenols from selected native Greek medicinal plants. Agronomy 7 (3), 54 (2017).
Ijardar, S. P., Singh, V. & Gardas, R. L. Revisiting the physicochemical properties and applications of deep eutectic solvents. Molecules 27 (4), 1368 (2022).
Bassi, P. & Kaur, G. Bioadhesive vaginal drug delivery of Nystatin using a derivatized polymer: development and characterization. Eur. J. Pharm. Biopharm. 96, 173–184 (2015).
Altaf, F. et al. Synthesis and characterization of pva/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 29, 156–174 (2021).
Tamahkar, E. et al. A novel multilayer hydrogel wound dressing for antibiotic release. J. Drug Deliv. Sci. Technol. 58, 101536 (2020).
Chuysinuan, P. et al. Development of gelatin hydrogel pads incorporated with Eupatorium adenophorum essential oil as antibacterial wound dressing. Polym. Bull. 76 (2), 701–724 (2019).
Aitboulahsen, M. et al. Gelatin/pectin-based film incorporated with essential oils: functional characteristics and shelf life extension of tilapia fillets under refrigeration. J. Food Saf. 40 (3), e12774 (2020).
Rippke, F. et al. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus aureus. Am. J. Clin. Dermatol. 5, 217–223 (2004).
Sisson, K. et al. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules 10 (7), 1675–1680 (2009).
Gajdošová, M. et al. Evaluation of mucoadhesive oral films containing Nystatin. J. Appl. Biomed. 14 (4), 247–256 (2016).
Pereira, R. et al. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 52, 221–230 (2013).
Enache, A. C. et al. Evaluation of physically and/or chemically modified Chitosan hydrogels for proficient release of insoluble Nystatin in simulated fluids. Gels 8 (8), 495 (2022).
Hashempur, M. H. et al. Enrichment of creatine-gelatin cryogel with Zataria multiflora essential oil and titanium dioxide nanoparticles as a potential wound dressing. Mater. Today Chem. 38, 102069 (2024).
Conte, J. et al. Development of biopolymer films loaded with fluconazole and thymol for resistant vaginal candidiasis. Int. J. Biol. Macromol. 275, 133356 (2024).
Liang, Y. et al. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 556, 514–528 (2019).
Acknowledgements
The data used to support the findings of this study were supplied by Vice-Chancellor for Research of Shiraz University of Medical Sciences under license. Requests for data access should be made to Zahra Zareshahrabadi, zare_shahrabadi@sums.ac.ir and zahrazarem368@gmail.com.
Funding
This study was extracted from the thesis of Ali Sabili [Grant No. 30492] and financially supported by the Vice-Chancellor for Research of Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
M.H.H: Conceptualization, Data curation, Methodology, Investigation and Writing- Original draft preparation. A.S: Methodology and Investigation. F. K. & K.Z.: Software, Methodology and Validation. A.V., K. S. & S. Sh.: Methodology and Investigation. Z. Z.SH.: Project administration, Conceptualization, Methodology, Writing- Original draft and Writing–review & editing.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hashempur, M.H., Sabili, A., Karami, F. et al. Synthesize, antioxidant and antimicrobial properties of a chitosan xerogel film with Nigella Sativa extract. Sci Rep 15, 24635 (2025). https://doi.org/10.1038/s41598-025-09153-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09153-1