Abstract
Measuring skeletal muscle mass index using bioelectrical impedance analysis or dual-energy X-ray absorptiometry is challenging for home care patients and those with implanted devices. Contrastingly, ultrasound measurement of skeletal muscle mass is a simple and non-invasive method. We aimed to investigate quadriceps muscle cross-sectional area measured using a newly developed ultrasonic muscle imaging system to serve as a diagnostic tool for sarcopenia. The cross-sectional study including 510 independent adults aged 65 years and older (180 men; median age, 75 years) was performed between October 2021 and December 2022. Quadriceps muscle cross-sectional area of the dominant leg was measured, and the average value was analyzed for correlation with body composition indices measured using the bioelectrical impedance analysis method, muscle strength and physical performance tests. Quadriceps muscle cross-sectional area showed a good correlation with skeletal muscle mass index (R = 0.78, P < 0.001), handgrip strength (R = 0.70, P < 0.001), and knee extension strength (R = 0.68, P < 0.001). This study demonstrated that quadriceps muscle cross-sectional area using a newly developed ultrasonic system shows a strong correlation with skeletal muscle mass index and muscle strength. These findings suggest that this system could be an alternative to bioelectrical impedance analysis for the diagnosis of sarcopenia.
Similar content being viewed by others
Introduction
Sarcopenia has gained significant attention because of its association with reduced life expectancy, increased risk of falls and fractures, and a decline in activities of daily living1. Sarcopenia is defined as “age-related loss of skeletal muscle mass accompanied by a decrease in muscle strength and/or reduced physical performance2.” According to the Asian Working Group for Sarcopenia (AWGS) 20192, an assessment of the skeletal muscle mass index (SMI) using either bioelectrical impedance analysis (BIA) or dual-energy X-ray absorptiometry (DXA) is recommended for the diagnosis of definite sarcopenia. However, the hardware required is not appropriate to measure SMI in home care services3. In contrast, ultrasound measurement is a simple and non-invasive method used to evaluate skeletal muscle mass3,4; it is easily available to patients with implanted devices or those receiving home care services. However, the usefulness of conventional ultrasonography to evaluate skeletal muscle mass remains limited because its reliability is lower than that of BIA or DXA5. In conventional ultrasonography, measurements are typically limited to muscle thickness, cross-sectional area (CSA), echo intensity, pennation angle, and fascicle length. Moreover, CSA measurements in previous studies have generally been limited to a single muscle, such as the rectus femoris or vastus lateralis, rather than the entire quadriceps5. The limitation is not with ultrasonography itself but rather with the conventional method, which captures only a portion of the muscle cross-section. In contrast, the newly developed ultrasonic muscle imaging system allows for the simplified visualization of the entire quadriceps CSA in a single operation, rather than focusing solely on muscle thickness6.
As for other devices to measure muscle mass, in the European Working Group on Sarcopenia in Older People2 (EWGSOP2) guidelines, magnetic resonance imaging (MRI) and computed tomography (CT) were broadly reported as gold standards for non-invasive assessment7. The newly developed ultrasonic muscle imaging system demonstrates a good correlation between quadriceps muscle cross-sectional area (QMCSA) and the value using the CT6. The advantage of this ultrasound device is high reproducibility, which is equivalent to CT measurement without radiation exposure. However, there were several limitations to the previous study, including the patient characteristics and numbers to investigate the muscle mass in the geriatric study6. Thus, it remains unknown whether QMCSA measurement using this ultrasonic system is useful to estimate skeletal muscle mass in community-dwelling older adults, who are important study participants in investigations regarding subjects for primary sarcopenia.
We hypothesized that the newly developed ultrasound muscle imaging system could serve as a novel diagnostic tool for sarcopenia, providing an alternative to conventional methods such as BIA and/or DXA. We aimed to investigate the use of this ultrasound device in QMCSA measurement to estimate lower SMI and whether QMCSA measurement could serve as an alternative diagnostic tool for sarcopenia in community-dwelling older adults. We simultaneously performed QMCSA measurements using this system and body composition analysis using BIA in a cohort study of older adults living in a rural area of Japan.
Methods
Study participants
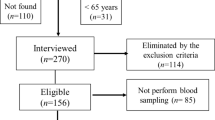
This cross-sectional study was part of a cohort study named “Frail Elderly in the Sasayama-Tamba Area”8,9. We included adults aged 65 years and older, who were independent community-dwelling or required long-term care of less than or equal to first degree living in the Tamba-Sasayama area, a rural area in Hyogo Prefecture, Japan, between October 2021 and December 2022. A detailed sampling tree is represented in Supporting information, Fig. S1.
Measures
The following characteristic factors were assessed: age, sex, height, body weight, blood pressure, body mass index (BMI), comorbid conditions, and body composition, measured using InBody770 (Biospace Japan, Osaka, Japan). Muscle strength and physical performance tests included gait speed (GS), handgrip strength (HGS), knee extension strength (KES), and the 5-chair-stand test (5CS). GS was determined by measuring the time taken to walk 10 m at a normal pace from the start of the movement without deceleration; velocity was converted to meters per second (m/s)10. HGS was assessed using a digital dynamometer (TKK 5101 Grip-D; Takei, Tokyo, Japan)9. KES was measured with the dominant leg during isometric contraction of the knee extensor in the sitting position, with the knee maintained at 90°, using a handheld dynamometer (Sakai Medical. Co., Ltd., Tokyo, Japan)11. For the 5CS, participants were asked to stand up and sit down five times as rapidly as possible, and the time taken from the initial sitting position to the final standing position at the end of the fifth stand was recorded12.
QMCSA
The newly developed ultrasonic muscle imaging system uses technology for panorama synthesis sector B-mode images by applying it to the thigh and moving it in one direction along the skin (Furuno Electric Co., Ltd., Nishinomiya, Japan)6. The device is portable and conveniently employed with 10 cm of width and a weight of approximately 260 g (Fig. 1a). In addition, a clearly visible image is shown when evaluating a cross-sectional muscle image similar to that of CT6. The participants were evaluated in a sitting position with the knee joint flexed at 90°. The same examiner lightly placed the probe perpendicular to the midpoint of the thigh of the patient’s dominant leg (greater trochanter and lateral epicondyle of the femur) and scanned the circumference (two-thirds of the anterior thigh) using the probe (Fig. 1b). The method of determining the measurement site differed from the previous study13. When performing measurements in a sitting position, identifying the inguinal region can be difficult, particularly in women and individuals with obesity. In contrast, the greater trochanter is easily palpable, making it a more reliable anatomical landmark. This study defined the dominant leg based on a questionnaire asking, “Which foot do you use to kick a ball?” The foot chosen by the participant was considered the dominant leg. To ensure consistency in probe angle, an attachment that allows the probe to be positioned perpendicularly to the quadriceps muscle was used. Regarding the applied pressure, a generous amount of gel was used to minimize compression of the measurement site. The probe was slid smoothly over the skin with care to avoid excessive pressure. It is determined by the device whether the pressure is appropriate or not. Information was sent wirelessly to a Microsoft Surface Pro tablet (Microsoft Japan, Tokyo, Japan) (Fig. 1c) placed nearby and a cross-sectional muscle image (Fig. 1d) was automatically displayed within approximately 15 s. The quadriceps muscle boundaries were marked automatically on the obtained image for CSA measurement (Fig. 1d). The final decision was made by the physician who was not involved in the BIA measurement, and if necessary, the modifications were made manually. Finally, we measured the QMCSA (cm2). We performed three measurements, and the average value was recorded. According to a previous report by Matsui et al., the reproducibility of QMCSA measurement by three examiners is excellent (intra-examiner intraclass correlation coefficient (ICC) [1, 1]: 0.993, 0.996, and 0.997; inter-examiner intraclass correlation coefficient [1, 2]: 0.993)6.
A newly developed ultrasonic muscle imaging system. (a) The probe, palm-size and wireless. (b) Measurement of QMCSA, on the midpoint of the thigh of the patient’s dominant leg in a sitting position. The probe was gently and slowly slid for approximately 10 s around two-thirds of the anterior thigh. (c) The PC tablet connected with Bluetooth. (d) Image of QMCSA obtained using the system. Quadriceps plots were automatically detected using machine learning. QMCSA: quadriceps muscle cross-sectional area.
We also measured the QMCSA of 35 young, healthy volunteers, along with their body composition indices, using the InBody 770. The volunteers consisted of students from Hyogo Medical University as well as dentists and physical therapists from Hyogo Medical University Hospital.
Ethical approvals
This study was approved by the Ethics Review Board of Hyogo Medical University (approval number: Rinhi0342) and conducted in accordance with the principles of the Declaration of Helsinki. The participants were informed of the study objectives and methods and provided written informed consent.
Diagnosis of lower SMI, sarcopenia, and frailty
The participants were divided into two groups: low SMI and normal SMI. Participants with an SMI of less than 7.0 kg/m2 for men and 5.7 kg/m2 for women were classified into the low SMI group based on the AWGS 2019 criteria2. The participants were also divided into non-sarcopenia and sarcopenia groups. Finally, frailty status was diagnosed based on the Kihon Checklist (KCL), a self-administered questionnaire with 25 questions14,15. The response options were “negative” (score: 1) and “positive” (score: 0), with a total score of 25. Referring to Satake et al., scores of 8–25, 4–7, and 0–3 denoted frailty, prefrailty, and robustness, respectively14,15.
Statistical analysis
Data are expressed as either mean ± standard deviation, percentages, or median (interquartile range: IQR). The Mann–Whitney U-test was used to compare the two groups. Pearson’s correlation analysis was used to examine the correlation between each measurement. The QMCSA cutoff value was calculated to predict low SMI, sarcopenia, or frailty by performing receiver operating characteristic (ROC) curve and area under the curve (AUC) analyses. QMCSA with the highest Youden’s index (sensitivity + specificity − 1) was determined to be the optimal cutoff point. Statistical significance was set at P < 0.05. All statistical analyses were performed using IBM SPSS ver. 27 (IBM Japan, Tokyo, Japan).
Results
Characteristics of participants
We measured the QMCSA in 519 older adults. Of these, nine individuals were excluded due to severe cardiovascular, renal, hepatic disease, uncontrolled diabetes mellitus, and missing data. Therefore, 510 individuals, consisting of 180 men and 330 women, with a median age of 75 years, were included in the final analysis (Table 1 and Supporting information, Fig. S1). Women were younger and had significantly higher systolic and diastolic blood pressure and faster 5CS time, whereas men were significantly taller, with heavier body weight, higher BMI, higher muscle mass, greater muscle strength, higher incidence of cardiovascular diseases, and a history of cancer (Table 1). There was no difference in GS between men and women. The proportion of sarcopenia was significantly higher in men than in women; however, no sex-based differences were found in the proportions of robust, pre-frail, and frail cases (Table 1).
Relationship between QMCSA and body composition
The QMCSA used in the analysis was calculated as the average of these three measurements. The ICC [1.1] was 0.991 (95% Confidence Interval 0.960–0.999), suggesting that this device has a reasonable level of reproducibility. Regarding the relationship between QMCSA and body composition by BIA, QMCSA showed a strong correlation with both SMI and muscle mass of the extremities and trunk (Table 2). The correlation coefficient between the QMCSA and appendicular skeletal muscle (ASM) and between QMCSA and SMI were 0.80 and 0.78 (P < 0.001 and P < 0.001, respectively) (Fig. 2). The correlation coefficient was significantly higher in men than in women (Table 2 and Supporting Information, Fig. S2). In evaluating the correlation between QMCSA and age, there was a significant correlation between the two values in men (R = − 0.50, P < 0.001), but a very weak correlation was found in women (R = − 0.22, P < 0.001) (Fig. 3).
Relationship between QMCSA and physical function
In terms of muscle strength, strong correlations were observed between QMCSA and HGS (R = 0.70, P < 0.001) (Fig. 4a) and KES (R = 0.69, P < 0.001) (Fig. 4c). However, no significant correlations between QMCSA and GS (R = 0.08, P = 0.064), and 5CS (R = -0.04, P = 0.417) were seen (Supporting Information, Figs. S3a,c). In terms of the correlation between SMI and each muscle strength and physical performance test, strong correlations were observed between SMI and HGS (R = 0.71, P < 0.001) (Fig. 4b) and KES (R = 0.60, P < 0.001) (Fig. 4d). However, no significant correlations between SMI and GS (R = -001, P = 0.885), and 5CS (R = 0.08, P = 0.077) were seen (Supporting Information, Figs. S3b,d). When evaluating the relationship between QMCSA, HGS, and KES based on gender, a similar correlation was found in both men and women, but the correlation coefficients in women were always lower than those in men (Supplemental Information, Figs. S4 and S5).
The median age of the young, healthy volunteers was 23 years, with 21 men (60.0%) and 14 women (40.0%). As shown in Supporting Information Fig. S6a, there was a strong correlation between QMCSA and SMI (R = 0.94, P < 0.001). We found strong correlations between QMCSA and HGS (R = 0.79, P < 0.001) and between QMCSA and KES (R = 0.67, P < 0.001) in young volunteers (Supporting Information, Fig. S6b,c).
QMCSA values between the normal and lower SMI groups and between non-frail and frail groups
In terms of QMCSA between the normal and the lower SMI groups, the low SMI group exhibited significantly lower QMCSA than that of the normal SMI group in both men and women (Fig. 5a). In the ROC curves, the AUC values were 0.81 in men and 0.76 in women (Men: cutoff value 56.73 cm2, sensitivity 0.55, specificity 0.93. Women: cutoff value 42.19 cm2, sensitivity 0.55, specificity 0.86.) (Fig. 5b). There was a significant difference in the QMCSA between the non-sarcopenia and sarcopenia groups. The sarcopenia group exhibited significantly lower QMCSA than that of the non-sarcopenia group in both men and women. (Fig. 5c). The ROC curve of QMCSA as a predictor of sarcopenia showed an AUC of 0.85 in men and 0.69 in women (Men: cutoff value 48.55 cm2, sensitivity 0.82, specificity 0.77, Women: cutoff value 39.12 cm2, sensitivity 0.59, specificity 0.73.) (Fig. 5d).
QMCSA values in the normal and lower SMI groups and in the non-sarcopenia and sarcopenia groups. (a) QMCSA value in either normal or lower SMI group divided by sex. (b) ROC curve of QMCSA as a predictor of lower SMI. (c) QMCSA value in either non-sarcopenia or sarcopenia group divided by sex. (d) ROC curve of QMCSA as a predictor of sarcopenia. ROC receiver operating characteristic.
Finally, all participants were divided into a non-frail group and a frail group according to their KCL scores. The non-frail group included robust and pre-frail. There was a significant difference in the QMCSA between non-frail and frail groups. The frail group exhibited significantly lower QMCSA than that of the non-frail group in both men and women (Fig. 6a). The ROC curve of QMCSA as a predictor of frailty showed an AUC of 0.78 in men and 0.59 in women (Men: cutoff value 51.83 cm2, sensitivity 0.85, specificity 0.65, Women: cutoff value 40.01 cm2, sensitivity 0.63, specificity 0.55.) (Fig. 6b).
Discussion
QMCSA measurement using a newly developed ultrasonic muscle imaging system showed a strong correlation with SMI measured by the Inbody770, HGS, and KES (Table 2 and Figs. 2 and 4). The AUC for predicting low SMI or sarcopenia was favorable, and the correlation between QMCSA and muscle strength was equivalent to that between SMI and muscle strength (Figs. 4 and 5). Taken together with a previous paper reported by Matsui et al.6, the results of this study strongly suggest that measuring the QMCSA using a newly developed ultrasonic muscle imaging system can be an alternative to BIA or DXA methods to diagnose sarcopenia.
An MRI-based cross-sectional study of participants aged 18–88 years found a steeper age-related decline in lower-body muscle mass than in the upper body. In individuals aged 65 and older, thigh muscle mass accounts for about 30% of total skeletal muscle mass16, making it a good proxy for whole-body muscle. In addition, larger muscles have a smaller coefficient of variation than smaller ones17. Therefore, we assessed QMCSA using this system.
Currently, CT and MRI are the only other methods to assess skeletal muscle mass in addition to the DXA and BIA methods in EWGSOP27,18. However, their cost and lack of defined cutoff values limit their clinical application3. Therefore, it is extremely important to find an alternative method for estimating muscle mass that can be implemented in clinical settings5. The application of ultrasound in the treatment of sarcopenia has been extensively studied. Mounting evidence demonstrates that muscle ultrasonography is a reliable tool for estimating muscle mass and may serve as a potential screening method for sarcopenia3,19,20,21,22,23,24,25,26,27,28. However, to obtain a strong correlation, multi-site measurements or complex equations that incorporate factors such as height and body weight can be cumbersome and time consuming21,22,24,25,26,28. When measurements are taken at a single site, the correlation coefficient tends to be weak3,19,20,23,27. However, in this study, the ultrasonic system enabled the measurement of QMCSA in only 30 s, demonstrating a strong correlation with SMI, HGS, and KES. These results indicate that this system is superior to previous models and has the potential to diagnose sarcopenia quickly and accurately.
Our study revealed a stronger correlation between QMCSA and both SMI and muscle strength in young volunteers than in older adults (Table 2, Figs. 2 and 4, and Supporting Information Fig. S6). In addition, the correlation coefficients between QMCSA and both SMI and muscle strength were stronger in men than in women (Table 2 and Supplemental Information, Figs. S4 and S5). We speculate that several factors contribute to generational and gender-based differences. First, muscle mass is greater in men than in women29,30,31,32, and fat mass is greater in women16,19,30. In addition, the components of skeletal muscle change with aging, and the decrease in skeletal muscle with aging is particularly pronounced in men30,33,34. Second, there are intrinsic differences in muscle composition between sexes. Women exhibit higher levels of intramuscular fat deposits and fibrosis, which may be influenced by sex-hormone depletion of sex hormones33. Consequently, it was speculated that stronger correlations would be observed either in young volunteers or men than in older adults or women.
SMI is a skeletal muscle parameter adjusted for squared height. However, in this study, we did not adjust QMCSA for height. While muscle mass is often normalized by height16,33, this may lead to misinterpretation when assessing specific muscles via CT, MRI, or ultrasound because these methods provide cross-sectional images. Additionally, height decreases with age due to vertebral fractures, disc height loss, and spinal degeneration35, potentially leading to muscle mass overestimation in older adults19. Our analysis also showed that the correlation between SMI and non-adjusted QMCSA was stronger than that between SMI and adjusted QMCSA.
In addition to the usefulness of this system as a diagnostic tool for sarcopenia, it can provide information to predict frailty status in older adults diagnosed based on the KCL (Fig. 6). The concept of frailty includes sarcopenia as a physical domain and indicates vulnerability to stress in older adults from a broader viewpoint36. The KCL was originally developed to screen older adults requiring nursing care14,15. The KCL assesses several domains including instrumental activities of daily living, mobility, falling, nutrition, oral function, social withdrawal, cognitive function, and depression14,15. The KCL has been proven to show a good correlation with the Japanese version of the Cardiovascular Health Study on the diagnosis of frailty in older adults14. Therefore, the KCL was used to evaluate the frailty status in this study. Since the KCL includes items that reflect physical function and nutritional status in older adults, it was speculated that this explains the good AUC value between the QMCSA and frailty in our results (Fig. 6). The fact that this system is useful not only for sarcopenia, but also for frailty diagnosis suggests that it helps medical treatment in older patients during home medical care and facility entrance. Thus, this system can be widely used for the medical treatment of sarcopenia and frailty and it was concluded that it can contribute to the development of this field.
Study limitations
This study had several limitations. First, there may have been a selection bias due to the nature of voluntary participation in the medical check-up. This study was conducted in a small town of rural Japan. The inclusion criteria required the participants to be capable of independent living and be able to visit the survey location. As a result, those who attended the check-up may have had higher levels of daily life activities, which could introduce a selection bias and affect the research results. The second limitation arises from the inability to standardize the timing of the measurement. We conducted body composition measurements using BIA and QMCSA assessments simultaneously. However, we cannot exclude the possibility that the variable timing of these measurements may have influenced the results of this study37,38. Third, we did not assess muscle quality in this study. Recent studies have suggested that ultrasonography has the potential to assess muscle quality by evaluating muscle echo intensity39,40,41,42. We obtained data on muscle echo intensity in the study participants. Therefore, we aim to clarify whether the estimation of muscle quality using muscle echo intensity increases the precision of QMCSA measurements in future investigations. Fourth, this study did not include an assessment of calf circumference (CC), which is recognized as a screening tool for sarcopenia by the AWGS 2019. Moreover, the usefulness of BMI-adjusted CC and the calf muscle circumference index that was adjusted by subcutaneous fat thickness has been reported43,44,45. Therefore, future studies comparing QMCSA with CC-based indices will be necessary to clarify the diagnostic value of QMCSA. Finally, we showed different cutoff values of the QMCSA in the ROC analysis for individual indices, such as low SMI, sarcopenia, and frailty. However, we believe that these values should be standardized before clinical use. To achieve this, it is necessary to measure the QMCSA in a larger group of individuals with normal SMI, low SMI, sarcopenia, or frailty. Unifying these results would allow for the establishment of an appropriate cutoff value for the QMCSA in older adults.
Conclusion
QMCSA measured using the newly developed ultrasonic muscle imaging system showed a strong correlation with muscle strength, such as HGS and KES, and skeletal muscle mass index measured using the bioelectrical impedance analysis method, indicating the usefulness of this system in diagnosing sarcopenia. In addition, QMCSA measurement may serve as a diagnostic aid in predicting the coexistence of frailty and exhibit great potential in various clinical settings, such as patients receiving home care services or those with implanted devices.
Data availability
Data supporting the findings of this study are available upon request from the corresponding authors. The data are not publicly available because of privacy and ethical restrictions.
References
Wiedmer, P. et al. Sarcopenia-molecular mechanisms and open questions. Ageing Res. Rev. 65, 101200. https://doi.org/10.1016/j.arr.2020.101200 (2021).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300–307. https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Tang, X. Y., Huang, L., Yue, J. R. & Qiu, L. Quantitative estimation of muscle mass in older adults at risk of sarcopenia using ultrasound: A cross-sectional study. Quant. Imag. Med. Surg. 12, 2498–2508. https://doi.org/10.21037/qims-21-685 (2022).
Tagliafico, A. S., Bignotti, B., Torri, L. & Rossi, F. Sarcopenia: How to measure, when and why. Radiol. Med. 127, 228–237. https://doi.org/10.1007/s11547-022-01450-3 (2022).
Ticinesi, A., Meschi, T., Narici, M. V., Lauretani, F. & Maggio, M. Muscle ultrasound and sarcopenia in older individuals: A clinical perspective. J. Am. Med. Dir. Assoc. 18, 290–300. https://doi.org/10.1016/j.jamda.2016.11.013 (2017).
Matsui, Y. et al. Evaluation of quadriceps muscle cross-sectional area using an ultrasonic diagnostic equipment with a wide field of view. PLoS ONE 19, e0311043. https://doi.org/10.1371/journal.pone.0311043 (2024).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Matsuzawa, R. et al. Serum creatinine-cystatin C based screening of sarcopenia in community dwelling older adults: A cross-sectional analysis. J. Frailty Aging 13, 116–124. https://doi.org/10.14283/jfa.2024.13 (2024).
Tsuji, S. et al. Low back pain is closely associated with frailty but not with sarcopenia: Cross-sectional study of rural Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 21, 54–59. https://doi.org/10.1111/ggi.14100 (2021).
Guralnik, J. M. et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A Biol. Sci. Med. Sci. 55, M221-231. https://doi.org/10.1093/gerona/55.4.m221 (2000).
Wang, C. Y., Olson, S. L. & Protas, E. J. Test-retest strength reliability: Hand-held dynamometry in community-dwelling elderly fallers. Arch. Phys. Med. Rehabil. 83, 811–815. https://doi.org/10.1053/apmr.2002.32743 (2002).
Buatois, S. et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J. Am. Geriatr. Soc. 56, 1575–1577. https://doi.org/10.1111/j.1532-5415.2008.01777.x (2008).
Thomaes, T. et al. Reliability and validity of the ultrasound technique to measure the rectus femoris muscle diameter in older CAD-patients. BMC Med. Imaging 12, 7. https://doi.org/10.1186/1471-2342-12-7 (2012).
Satake, S. et al. Validity of the Kihon checklist for assessing frailty status. Geriatr. Gerontol. Int. 16, 709–715. https://doi.org/10.1111/ggi.12543 (2016).
Satake, S., Shimokata, H., Senda, K., Kondo, I. & Toba, K. Validity of total kihon checklist score for predicting the incidence of 3-year dependency and mortality in a community-dwelling older population. J. Am. Med. Dir. Assoc. 18(552), e551-552. https://doi.org/10.1016/j.jamda.2017.03.013 (2017).
Janssen, I., Heymsfield, S. B., Wang, Z. M. & Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 1985(89), 81–88. https://doi.org/10.1152/jappl.2000.89.1.81 (2000).
Bemben, M. G. Use of diagnostic ultrasound for assessing muscle size. J. Strength Cond. Res. 16, 103–108 (2002).
Heymsfield, S. B., Adamek, M., Gonzalez, M. C., Jia, G. & Thomas, D. M. Assessing skeletal muscle mass: Historical overview and state of the art. J. Cachexia Sarcopenia Muscle 5, 9–18. https://doi.org/10.1007/s13539-014-0130-5 (2014).
Hida, T. et al. Ultrasound measurement of thigh muscle thickness for assessment of sarcopenia. Nagoya J. Med. Sci. 80, 519–527. https://doi.org/10.18999/nagjms.80.4.519 (2018).
Isaka, M. et al. The muscle thickness assessment using ultrasonography is a useful alternative to skeletal muscle mass by bioelectrical impedance analysis. Clin. Interv. Aging 17, 1851–1861. https://doi.org/10.2147/CIA.S385469 (2022).
Isaka, M. et al. The usefulness of an alternative diagnostic method for sarcopenia using thickness and echo intensity of lower leg muscles in older males. J. Am. Med. Dir. Assoc. 20(1185), e1181-1185. https://doi.org/10.1016/j.jamda.2019.01.152 (2019).
Kara, M. et al. STAR-sonographic thigh adjustment ratio: A golden formula for the diagnosis of sarcopenia. Am. J. Phys. Med. Rehabil. 99, 902–908. https://doi.org/10.1097/PHM.0000000000001439 (2020).
Neira Álvarez, M. et al. Muscle assessment by ultrasonography: Agreement with dual-energy X-ray absorptiometry (DXA) and relationship with physical performance. J. Nutr. Health Aging 25, 956–963. https://doi.org/10.1007/s12603-021-1669-4 (2021).
Rustani, K., Kundisova, L., Capecchi, P. L., Nante, N. & Bicchi, M. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch. Gerontol. Geriat. 83, 151–154. https://doi.org/10.1016/j.archger.2019.03.021 (2019).
Sanada, K., Kearns, C. F., Midorikawa, T. & Abe, T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur. J. Appl. Physiol. 96, 24–31. https://doi.org/10.1007/s00421-005-0061-0 (2006).
Yamada, M. et al. Differential characteristics of skeletal muscle in community-dwelling older adults. J. Am. Med. Dir. Assoc. 18(807), e809–e807. https://doi.org/10.1016/j.jamda.2017.05.011 (2017).
Yoshida, T., Watanabe, Y., Yokoyama, K., Kimura, M. & Yamada, Y. Thigh muscle thickness on ultrasonography for diagnosing sarcopenia: The Kyoto-Kameoka study. Geriatr. Gerontol. Int. 24(Suppl 1), 156–161. https://doi.org/10.1111/ggi.14714 (2024).
Zhu, S. et al. The correlation of muscle thickness and pennation angle assessed by ultrasound with sarcopenia in elderly Chinese community dwellers. Clin. Interv. Aging 14, 987–996. https://doi.org/10.2147/CIA.S201777 (2019).
Baumgartner, R. N., Waters, D. L., Gallagher, D., Morley, J. E. & Garry, P. J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 107, 123–136. https://doi.org/10.1016/s0047-6374(98)00130-4 (1999).
Gallagher, D. et al. Weight stability masks sarcopenia in elderly men and women. Am. J. Physiol. Endocrinol. Metab. 279, E366-375. https://doi.org/10.1152/ajpendo.2000.279.2.E366 (2000).
Hida, T. et al. Sarcopenia and physical function are associated with inflammation and arteriosclerosis in community-dwelling people: The Yakumo study. Mod. Rheumatol. 28, 345–350. https://doi.org/10.1080/14397595.2017.1349058 (2018).
Hida, T. et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur. Spine J. 25, 3424–3431. https://doi.org/10.1007/s00586-015-3805-5 (2016).
Kasai, T. et al. Sex- and age-related differences in mid-thigh composition and muscle quality determined by computed tomography in middle-aged and elderly Japanese. Geriatr. Gerontol. Int. 15, 700–706. https://doi.org/10.1111/ggi.12338 (2015).
Yuki, A. et al. Relationship between low free testosterone levels and loss of muscle mass. Sci. Rep. 3, 1818. https://doi.org/10.1038/srep01818 (2013).
Dey, D. K., Rothenberg, E., Sundh, V., Bosaeus, I. & Steen, B. Height and body weight in the elderly. I. A 25-year longitudinal study of a population aged 70 to 95 years. Eur. J. Clin. Nutr. 53, 905–914. https://doi.org/10.1038/sj.ejcn.1600852 (1999).
Fried, L. P. et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146-156. https://doi.org/10.1093/gerona/56.3.m146 (2001).
Kyle, U. G. et al. Bioelectrical impedance analysis–part I: Review of principles and methods. Clin. Nutr. 23, 1226–1243. https://doi.org/10.1016/j.clnu.2004.06.004 (2004).
Rösler, A., Lehmann, F., Krause, T., Wirth, R. & von Renteln-Kruse, W. Nutritional and hydration status in elderly subjects: Clinical rating versus bioimpedance analysis. Arch. Gerontol. Geriatr. 50, e81-85. https://doi.org/10.1016/j.archger.2009.06.007 (2010).
Baek, S. H. et al. Usefulness of muscle ultrasound in appendicular skeletal muscle mass estimation for sarcopenia assessment. PLoS ONE 18, e0280202. https://doi.org/10.1371/journal.pone.0280202 (2023).
Stringer, H. J. & Wilson, D. The role of ultrasound as a diagnostic tool for sarcopenia. J. Frailty Aging 7, 258–261. https://doi.org/10.14283/jfa.2018.24 (2018).
Watanabe, Y., Ikenaga, M., Yoshimura, E., Yamada, Y. & Kimura, M. Association between echo intensity and attenuation of skeletal muscle in young and older adults: A comparison between ultrasonography and computed tomography. Clin. Interv. Aging 13, 1871–1878. https://doi.org/10.2147/CIA.S173372 (2018).
Young, H. J., Jenkins, N. T., Zhao, Q. & McCully, K. K. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 52, 963–971. https://doi.org/10.1002/mus.24656 (2015).
Miyahara, S., Maeda, K., Yasuda, A., Satake, S. & Arai, H. The potential of body mass index-adjusted calf circumference as a proxy for low muscle mass in the global leadership initiative on malnutrition criteria. Clin. Nutr. 43, 225–230. https://doi.org/10.1016/j.clnu.2024.10.025 (2024).
Sato, R. et al. Measurement of the calf muscle circumference is useful for diagnosing sarcopenia in older adults requiring long-term Care. Ann. Geriatr. Med. Res. 29, 58–65. https://doi.org/10.4235/agmr.24.0126 (2025).
Sato, R. et al. Screening for sarcopenia using calf muscle circumference in older adults requiring long-term care. J. Nutr. Health Aging 28, 100006. https://doi.org/10.1016/j.jnha.2023.100006 (2024).
Acknowledgements
We would like to thank the medical staff at Sasayama Medical Center, Hyogo Medical University, for their assistance. We acknowledge the free offer of the newly developed ultrasonic muscle imaging system by Furuno Electric Co., Ltd. We also express our appreciation to Mr. Shunichi Nishi, Mr. Tatsuo Arai, and Mr. Ryoichi Suetoshi at Furuno Electrics Co., Ltd. for providing supportive information.
Funding
This study was supported in part by JSPS KAKENHI (grant number: 22K19496) (2022–2024) and the National Center for Geriatrics and Gerontology (Choujyu 20-1, 21-18) (2022–2023) (Dr. Shinmura), and Grant-in-Aid for Graduate Students, School of Medicine, Hyogo Medical University (2024) (Dr. Onishi). The funders had no role in the study design, data collection and analysis, manuscript preparation, or the decision to publish this study.
Author information
Authors and Affiliations
Contributions
Study concept and design: M. O., T. I., S. T., and K. Shinmura.; Acquisition of data: M. O., S. T., K. Shojima, H. Y., Y. N., K. T., T. M., Y. W., H. K., M. S., R. M., K. N., and K. Shinmura.; Analysis and interpretation of data: M. O. and K. Shinmura.; Drafting of the manuscript: M. O. and K. Shinmura.; Critical revision of the manuscript for important intellectual content: T. I., S. T., K. Shojima, H. Y., Y. N., K. T., T. M., Y. W., H. K., M. S., R. M., K. N., and T. T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Onishi, M., Iseki, T., Tsuji, S. et al. Usefulness of a newly developed ultrasonic system to estimate skeletal muscle mass and muscle strength in community-dwelling older adults. Sci Rep 15, 24893 (2025). https://doi.org/10.1038/s41598-025-09430-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09430-z